Effects of Primary Processing and Pre-Salting of Baltic Herring on Contribution of Digestive Proteases in Marinating Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Baltic Herring and Marinating Methods
2.2. Total Nitrogen, Lipids, Salt, pH, Acidity and Meat Moisture Analysis
2.3. Extractions and Activity Assay of Proteases
2.4. Non-Protein Nitrogen Fraction Analysis
2.5. Texture Profile Analysis (TPA)
2.6. Sensory Profiling
2.7. Microbiological Analysis
2.8. Statistical Analysis
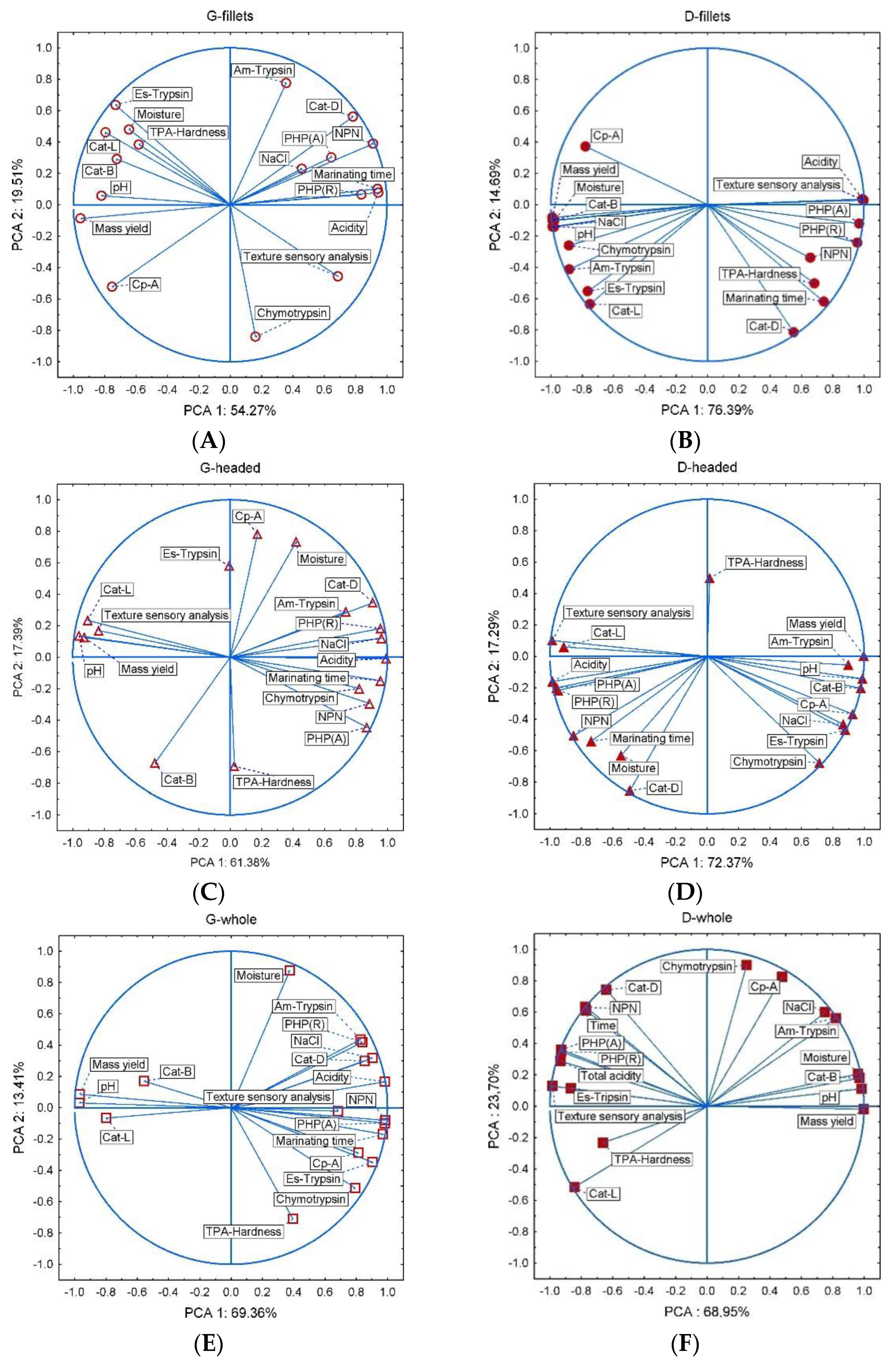
3. Results and Discussion
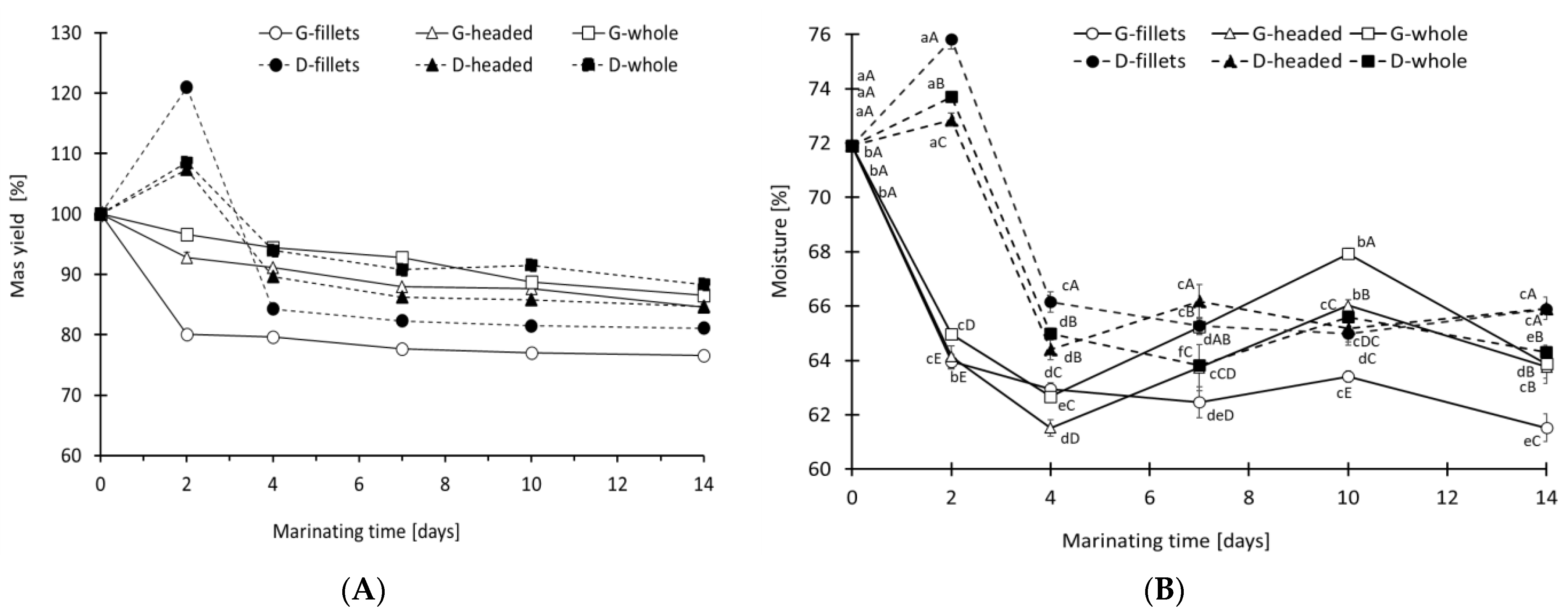
3.1. Mass Yield, pH and Salt Concentration
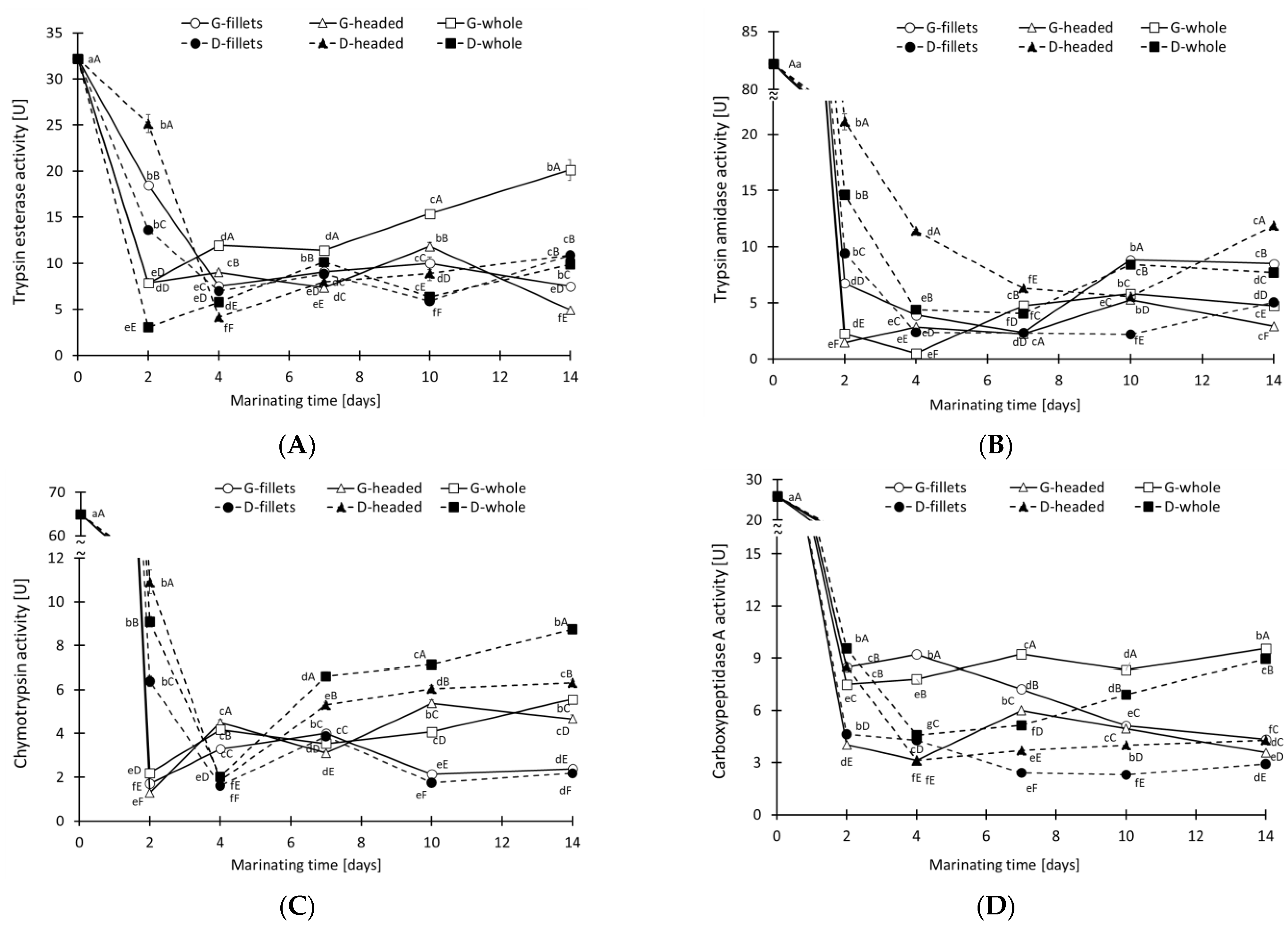
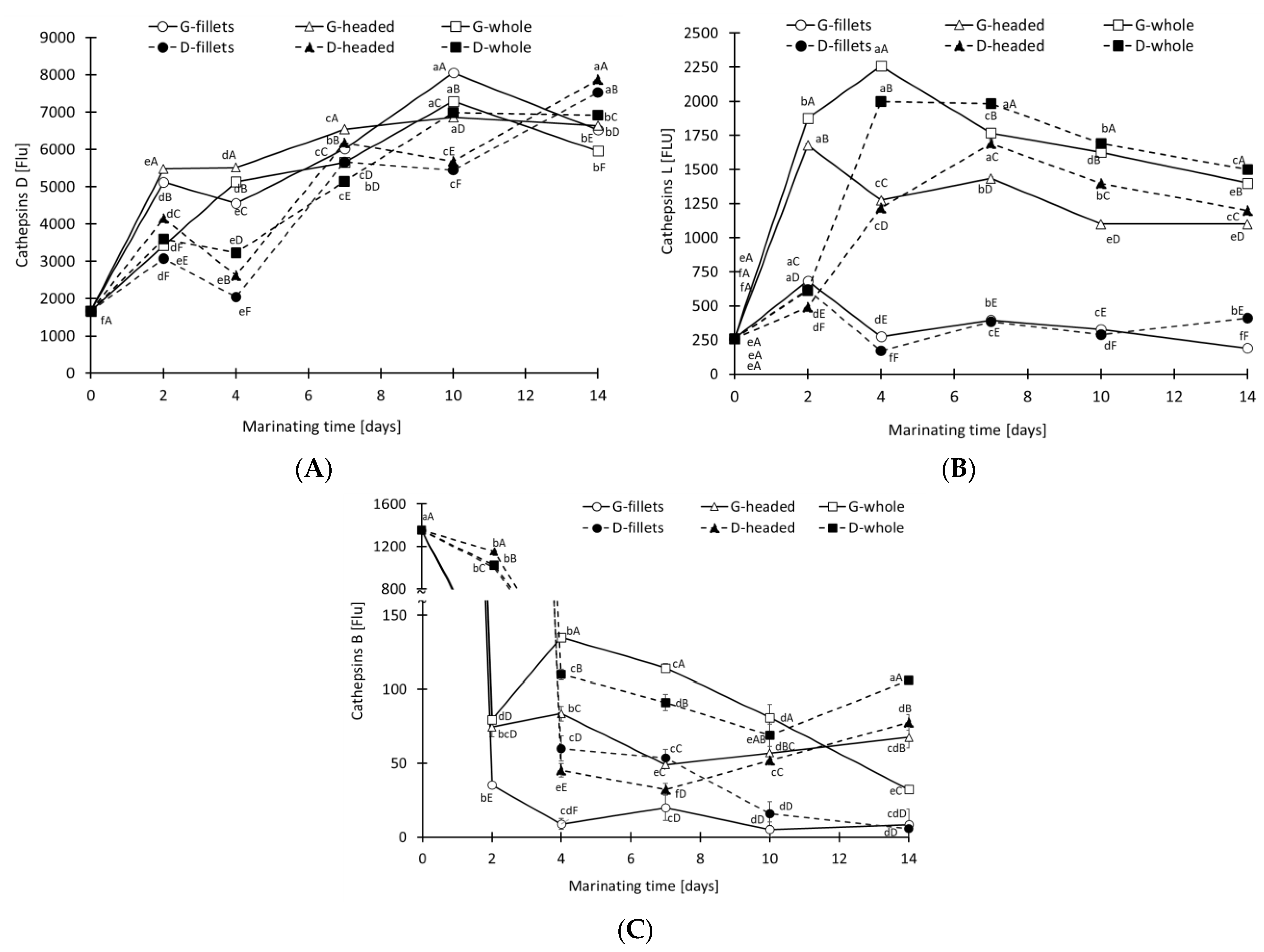
3.2. Activity of Digestive Proteases and Cathepsins
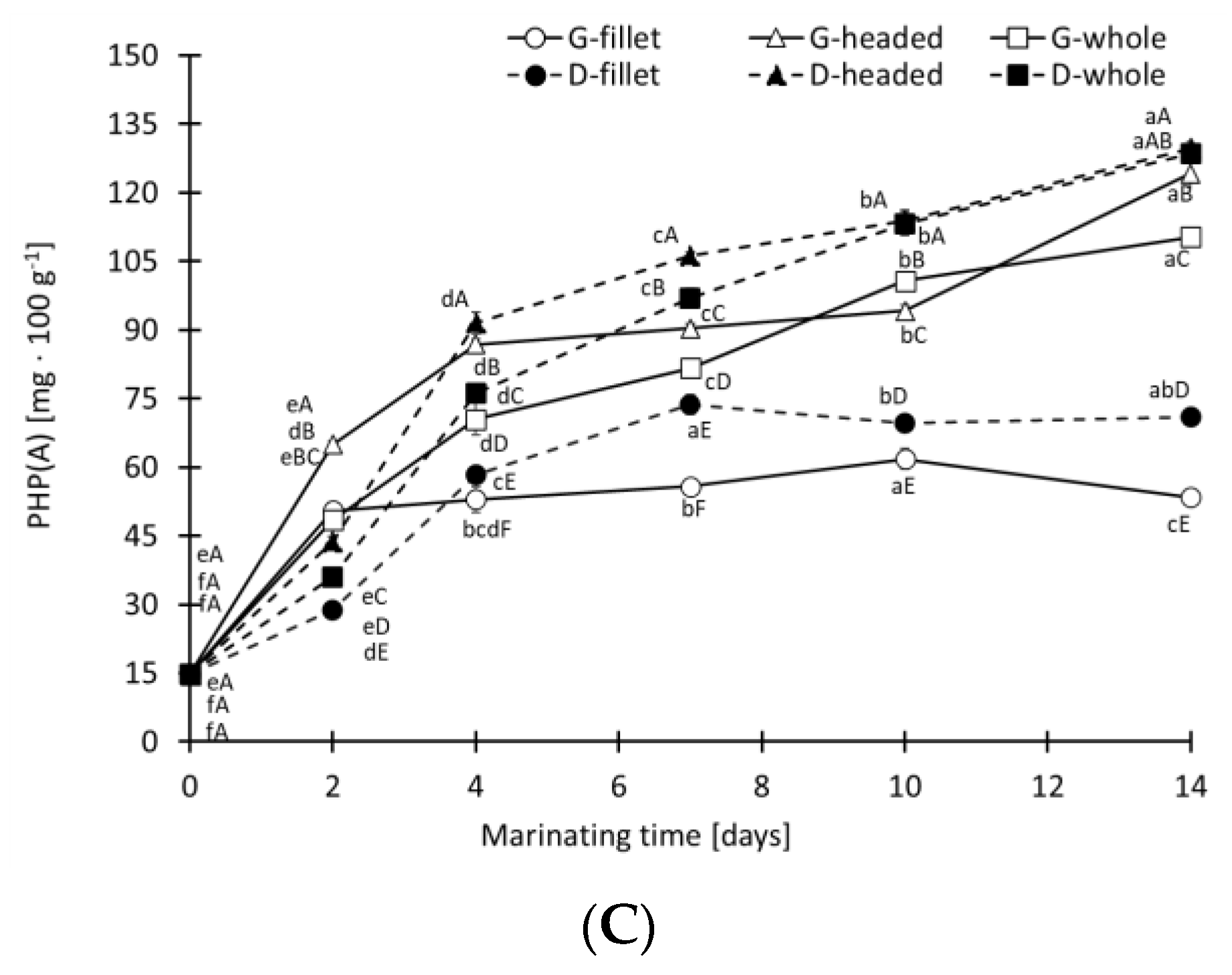
3.3. Concentration of Protein Hydrolysis Products (PHP)
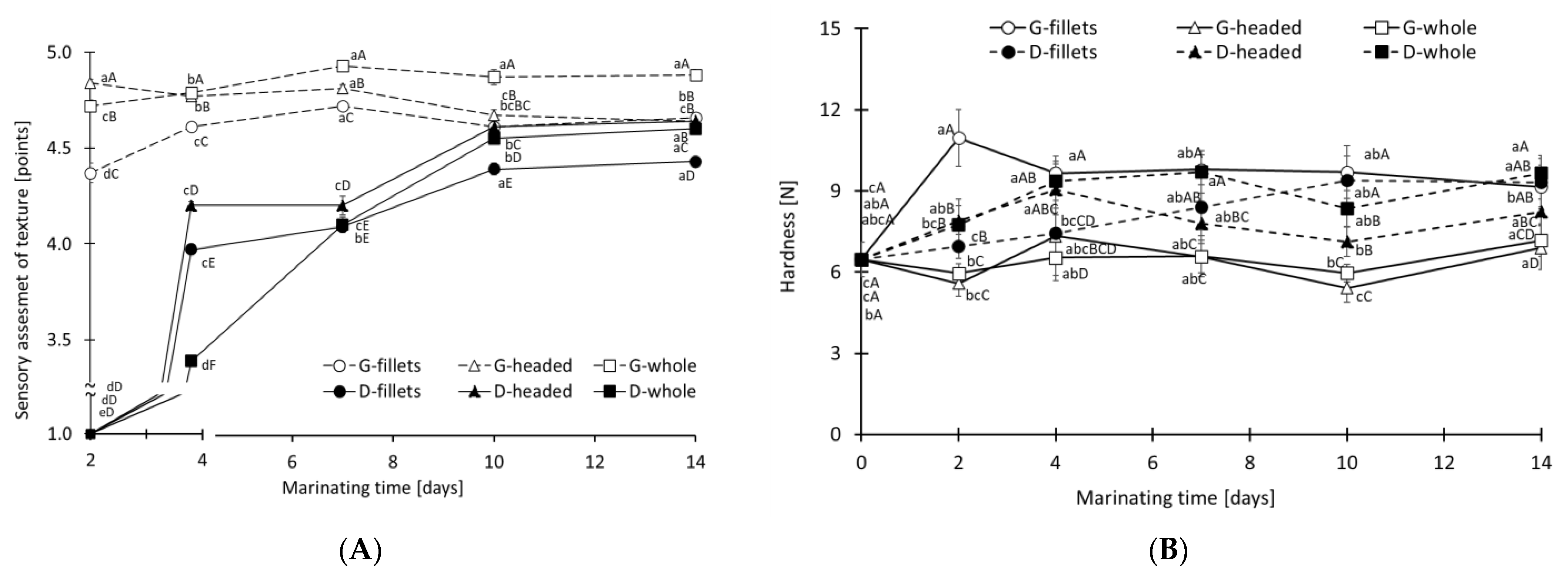
3.4. Hardness-TPA and Sensory Assessment of Marinated Meat
3.5. Microbiota of Herring
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hyldig, G.; Jørgensen, B.M.; Undeland, I.; Olsen, R.E.; Jónsson, A.; Nielsen, H.H. Sensory Properties of Frozen Herring (Clupea harengus) from Different Catch Seasons and Locations. J. Food Sci. 2012, 77, S288–S293. [Google Scholar] [CrossRef] [PubMed]
- Szymczak, M. Effect of technological factors on the activity and losses of cathepsins B, D and L during the marinating of Atlantic and Baltic herrings. J. Sci. Food Agric. 2017, 97, 1488–1496. [Google Scholar] [CrossRef] [PubMed]
- Szymczak, M.; Kamiński, P.; Felisiak, K.; Szymczak, B.; Dmytrów, I.; Sawicki, T. Effect of constant and fluctuating temperatures during frozen storage on quality of marinated fillets from Atlantic and Baltic herrings (Clupea harengus). LWT-Food Sci. Technol. 2020, 133, 109961. [Google Scholar] [CrossRef]
- Gringer, N.; Osman, A.; Nielsen, H.H.; Undeland, I.; Baron, C.P. Chemical Characterization, Antioxidant and Enzymatic Activity of Brines from Scandinavian Marinated Herring Products. J. Food Process. Technol. 2014, 5, 346. [Google Scholar] [CrossRef] [Green Version]
- Szymczak, M.; Felisiak, K.; Tokarczyk, G.; Szymczak, B. The reuse of brine to enhance the ripening of marine and freshwater fish resistant to marinating. Int. J. Food Sci. Technol. 2019, 54, 1151–1159. [Google Scholar] [CrossRef]
- Benjakul, S.; Yarnpakdee, S.; Senphan, T.; Halldorsdottir, S.M.; Kristinsson, G. Fish Protein Hydrolysates: Production, Bioactivities, and Applications; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014. [Google Scholar]
- Kamiński, P.; Szymczak, M.; Szymczak, B. The effect of previous storage condition on the diffusion and contribution of digestive proteases in the ripening of marinated Baltic herring (Clupea harengus). LWT-Food Sci. Technol. 2022, 165, 113723. [Google Scholar] [CrossRef]
- Szymczak, M. Comparison of physicochemical and sensory changes in fresh and frozen herring (Clupea harrengus L.) during marinating. J. Sci. Food Agric. 2011, 91, 68–74. [Google Scholar] [CrossRef]
- Laub-Ekgreen, M.H.; Martinez-Lopez, B.; Frosch, S.; Jessen, F. The influence of processing conditions on the weight change of single herring (Clupea harengus) fillets during marinating. Food Res. Int. 2018, 108, 331–338. [Google Scholar] [CrossRef]
- Babikova, J.; Hoeche, U.; Boyd, J.; Noci, F. Nutritional, physical, microbiological, and sensory properties of marinated Irish sprat. Int. J. Gastron. Food Sci. 2020, 22, 100277. [Google Scholar] [CrossRef]
- Mansur, M.A.; Uddin, M.N.; Rahman, S.; Horner, W.F.A.; Uga, S. Northern Europe Processing Technique, Nutritional Composition, Quality and Safety, Flavour Compounds, Bio-Chemical Change During Pickle Curing of North Sea Herring (Clupea harengus) of Britain. Oceanogr. Fish. Open Access J. 2018, 6, 555679. [Google Scholar] [CrossRef]
- Felberg, H.S.; Hagen, L.; Slupphaug, G.; Batista, I.; Nunes, M.L.; Olsen, R.L.; Martinez, I. Partial characterisation of gelatinolytic activities in herring (Clupea harengus) and sardine (Sardina pilchardus) possibly involved in post-mortem autolysis of ventral muscle. Food Chem. 2010, 119, 675–683. [Google Scholar] [CrossRef]
- Engvang, K.; Nielsen, H.H. In situ activity of chymotrypsin in sugar-salted herring during cold storage. J. Sci. Food Agric. 2000, 80, 1277–1283. [Google Scholar] [CrossRef]
- Gudmundur, S.; Nielsen, H.H.; Torstein, S.; Reinhard, S.; Jörg, O.; Joop, L.; Gudný, G. Frozen herring as raw material for spice-salting. J. Sci. Food Agric. 2000, 80, 1319–1324. [Google Scholar]
- Kilinc, B.; Cakli, S. Chemical, enzymatical and textural changes during marination and storage period of sardine (Sardina pilchardus) marinades. Eur. Food Res. Technol. 2005, 221, 821–827. [Google Scholar] [CrossRef]
- Nielsen, D.; Hyldig, G. Influence of handling procedures and biological factors on the QIM evaluation of whole herring (Clupea harengus L.). Food Res. Int. 2004, 37, 975–983. [Google Scholar] [CrossRef]
- Erlanger, B.F.; Kokowsky, N.; Cohen, W. The preparation and properties of two new chromogenic substrates of trypsin. Arch. Biochem. Biophys. 1961, 95, 271–278. [Google Scholar] [CrossRef]
- Hummel, B.C.W. A modified spectrophotometric determination of chymotrypsin, trypsin and thrombin. Can. J. Biochem. Physiol. 1959, 37, 1393–1399. [Google Scholar] [CrossRef]
- Mock, W.L.; Liu, Y.; Stanford, D.J. Arazoformyl Peptide Surrogates as Spectrophotometric Kinetic Assay Substrates for Carboxypeptidase A. Anal. Biochem. 1996, 239, 218–222. [Google Scholar] [CrossRef]
- Kołakowski, E. Analysis of proteins, peptides, and amino acids in foods. In Methods of Analysis of Food Components and Additives; Ötles, S., Ed.; CRC, Taylor & Francis Group: Boca Raton, FL, USA, 2005; pp. 59–96. [Google Scholar]
- Felisiak, K.; Szymczak, M. Use of Rapid Capillary Zone Electrophoresis to Determine Amino Acids Indicators of Herring Ripening during Salting. Foods 2021, 10, 2518. [Google Scholar] [CrossRef]
- ISO 6887-1:2017-05; Microbiology of the Food Chain—Preparation of Test Samples, Starting Suspension and Decimal Dilutions for Microbiological Tests—Part 1: General Rules for the Preparation of Starting Suspension and Decimal Dilutions. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 4833-2:2013-12; Microbiology of the Food Chain. Horizontal Method for the Determination of the Number of Microorganisms. Part 2. Determination of the Number by Surface Culture at 20 °C. International Organization for Standardization: Geneva, Switzerland, 2013.
- ISO 21527-1:2009; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Yeasts and Moulds—Part 1: Colony Count Technique in Products with Water Activity Greater than 0,95. International Organization for Standardization: Geneva, Switzerland, 2008.
- ISO 21528-1:2017-08; Microbiology of the Food Chain. Horizontal Method for Detection and Enumeration of Enterobacteriaceae. Part 1: Detection of Enterobacteriaceae. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 4833-1:2013; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 1: Colony Count at 30 Degrees C by the Pour Plate Technique. International Organization for Standardization: Geneva, Switzerland, 2013.
- Pielak, M.; Czarniecka-Skubina, E.; Trafiałek, J.; Głuchowski, A. Contemporary Trends and Habits in the Consumption of Sugar and Sweeteners—A Questionnaire Survey among Poles. Int. J. Environ. Res. Public Health 2019, 16, 1164. [Google Scholar] [CrossRef] [Green Version]
- Kolanowski, W.; Trafialek, J.; Drosinos, E.H.; Tzamalis, P. Polish and Greek young adults’ experience of low quality meals when eating out. Food Control 2020, 109, 106901. [Google Scholar] [CrossRef]
- Trafiałek, J.; Zwolinski, M.; Kolanowski, W. Assessing hygiene practices during fish selling in retail stores. Br. Food J. 2016, 118, 2053–2067. [Google Scholar] [CrossRef]
- Szymczak, M.; Kołakowski, E. Losses of nitrogen fractions from herring to brine during marinating. Food Chem. 2012, 132, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Sharedeh, D.; Gatellier, P.; Astruc, T.; Daudin, J.-D. Effects of pH and NaCl levels in a beef marinade on physicochemical states of lipids and proteins and on tissue microstructure. Meat Sci. 2015, 110, 24–31. [Google Scholar] [CrossRef]
- Szymczak, M.; Kołakowski, E.; Felisiak, K. Influence of salt concentration on properties of marinated meat from fresh and frozen herring (Clupea harengus L.). Int. J. Food Sci. Technol. 2012, 47, 282–289. [Google Scholar] [CrossRef]
- Kołodziejska, I.; Sikorski, Z.E. Muscle cathepsins of marine fish and invertebrates. Pol. J. Food Nutr. Sci. 1995, 45, 3–10. [Google Scholar]
- Talukdar, R.; Sareen, A.; Zhu, H.; Yuan, Z.; Dixit, A.; Cheema, H.; George, J.; Barlass, U.; Sah, R.; Garg, S.K.; et al. Release of Cathepsin B in Cytosol Causes Cell Death in Acute Pancreatitis. Gastroenterology 2016, 151, 747–758.e5. [Google Scholar] [CrossRef] [Green Version]
- Zaidi, N.; Kalbacher, H. Cathepsin E: A mini review. Biochem. Biophys. Res. Commun. 2008, 367, 517–522. [Google Scholar] [CrossRef]
- Özden, Ö. Changes in amino acid and fatty acid composition during shelf-life of marinated fish. J. Sci. Food Agric. 2005, 85, 2015–2020. [Google Scholar] [CrossRef]
- Felberg, H.S.; Slizyté, R.; Mozuraityte, R.; Dahle, S.W.; Olsen, R.L.; Martinez, I. Proteolytic activities of ventral muscle and intestinal content of North Sea herring (Clupea harengus) with full and emptied stomachs. Food Chem. 2009, 116, 40–46. [Google Scholar] [CrossRef]
- Johnston, I.A.; Solberg, C.; Johnston, I.A. Activity of aspargate (cathepsin D), cysteine proteases (cathepsins B, B + L, and H), and matrix metallopeptidase (collagenase) and their influence on protein and water-holding capacity of muscle in commercially farmed Atlantic halibut (Hippoglossus hippoglossuss L.). J. Agric. Food Chem. 2008, 56, 5953–5959. [Google Scholar]
- Umetsu, H.; Ichishima, E. Mechanism of digestion of bitter peptide from a fish protein concentrate by wheat carboxypeptidase. J. Food Sci. Technol. 1985, 32, 281–287. [Google Scholar] [CrossRef]
- Broncano, J.; Timón, M.; Parra, V.; Andrés, A.; Petrón, M. Use of proteases to improve oxidative stability of fermented sausages by increasing low molecular weight compounds with antioxidant activity. Food Res. Int. 2011, 44, 2655–2659. [Google Scholar] [CrossRef]
- Logrén, N.; Hiidenhovi, J.; Kakko, T.; Välimaa, A.-L.; Mäkinen, S.; Rintala, N.; Mattila, P.; Yang, B.; Hopia, A. Effects of Weak Acids on the Microbiological, Nutritional and Sensory Quality of Baltic Herring (Clupea harengus membras). Foods 2022, 11, 1717. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamiński, P.; Szymczak, M.; Szymczak, B. Effects of Primary Processing and Pre-Salting of Baltic Herring on Contribution of Digestive Proteases in Marinating Process. Appl. Sci. 2022, 12, 10877. https://doi.org/10.3390/app122110877
Kamiński P, Szymczak M, Szymczak B. Effects of Primary Processing and Pre-Salting of Baltic Herring on Contribution of Digestive Proteases in Marinating Process. Applied Sciences. 2022; 12(21):10877. https://doi.org/10.3390/app122110877
Chicago/Turabian StyleKamiński, Patryk, Mariusz Szymczak, and Barbara Szymczak. 2022. "Effects of Primary Processing and Pre-Salting of Baltic Herring on Contribution of Digestive Proteases in Marinating Process" Applied Sciences 12, no. 21: 10877. https://doi.org/10.3390/app122110877
APA StyleKamiński, P., Szymczak, M., & Szymczak, B. (2022). Effects of Primary Processing and Pre-Salting of Baltic Herring on Contribution of Digestive Proteases in Marinating Process. Applied Sciences, 12(21), 10877. https://doi.org/10.3390/app122110877






