Inhibitory Effect of Coumarins and Isocoumarins Isolated from the Stems and Branches of Acer mono Maxim. against Escherichia coli β-Glucuronidase
Abstract
:1. Introduction
2. Results
2.1. Isolated Compounds from the Stems and Branches of A. mono Maxim.
2.2. Inhibitory Activity of Isolated Compounds against β-Glucuronidase
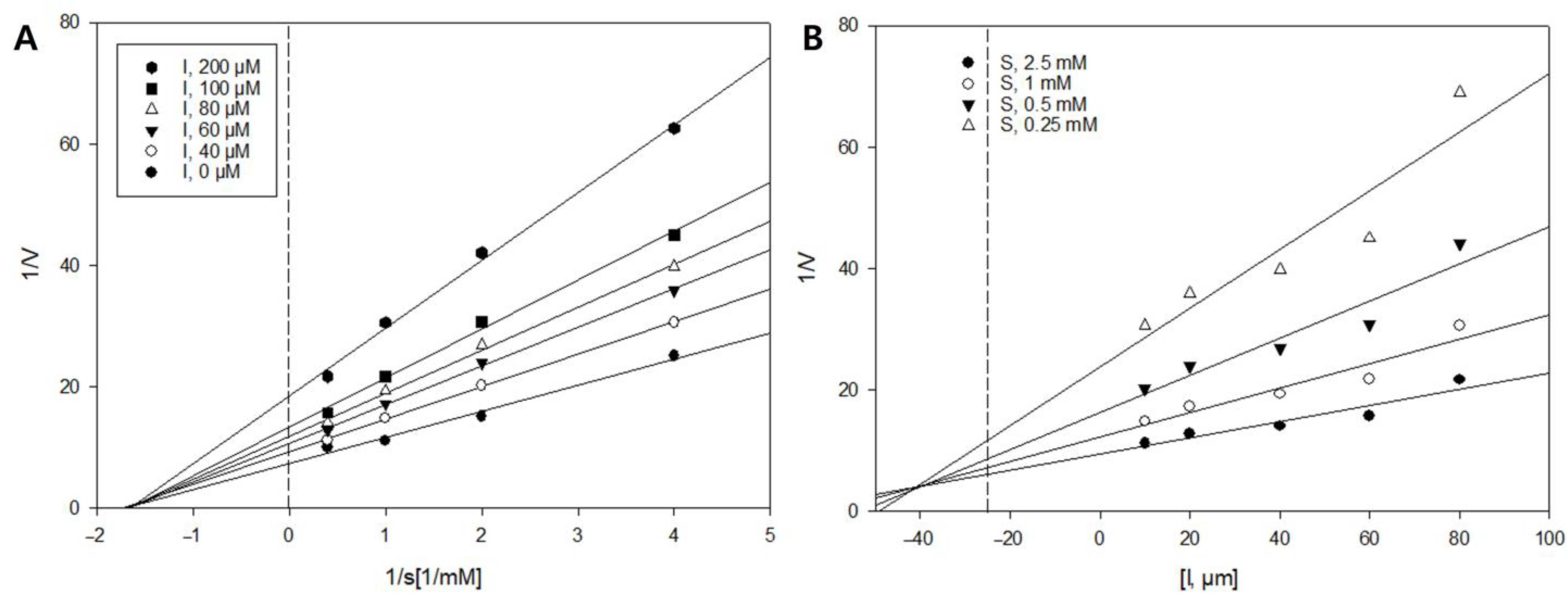
2.3. Enzyme Kinetics of Compound 1 against β-Glucuronidase
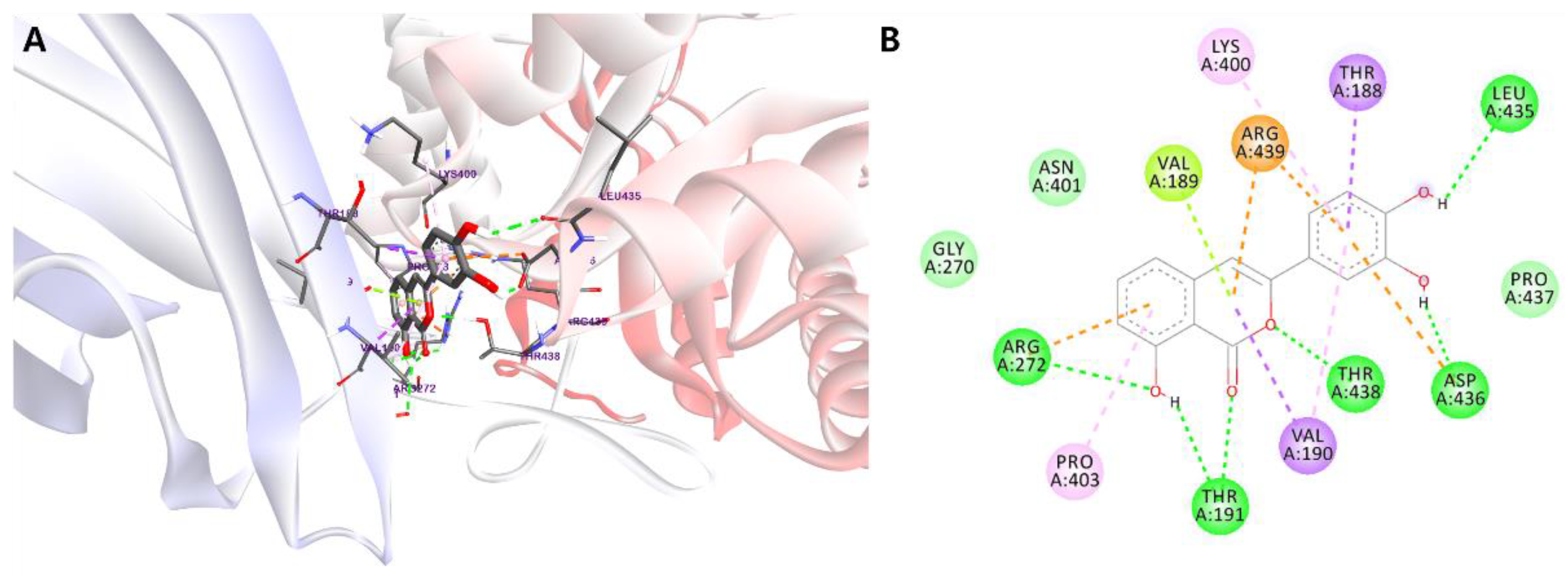
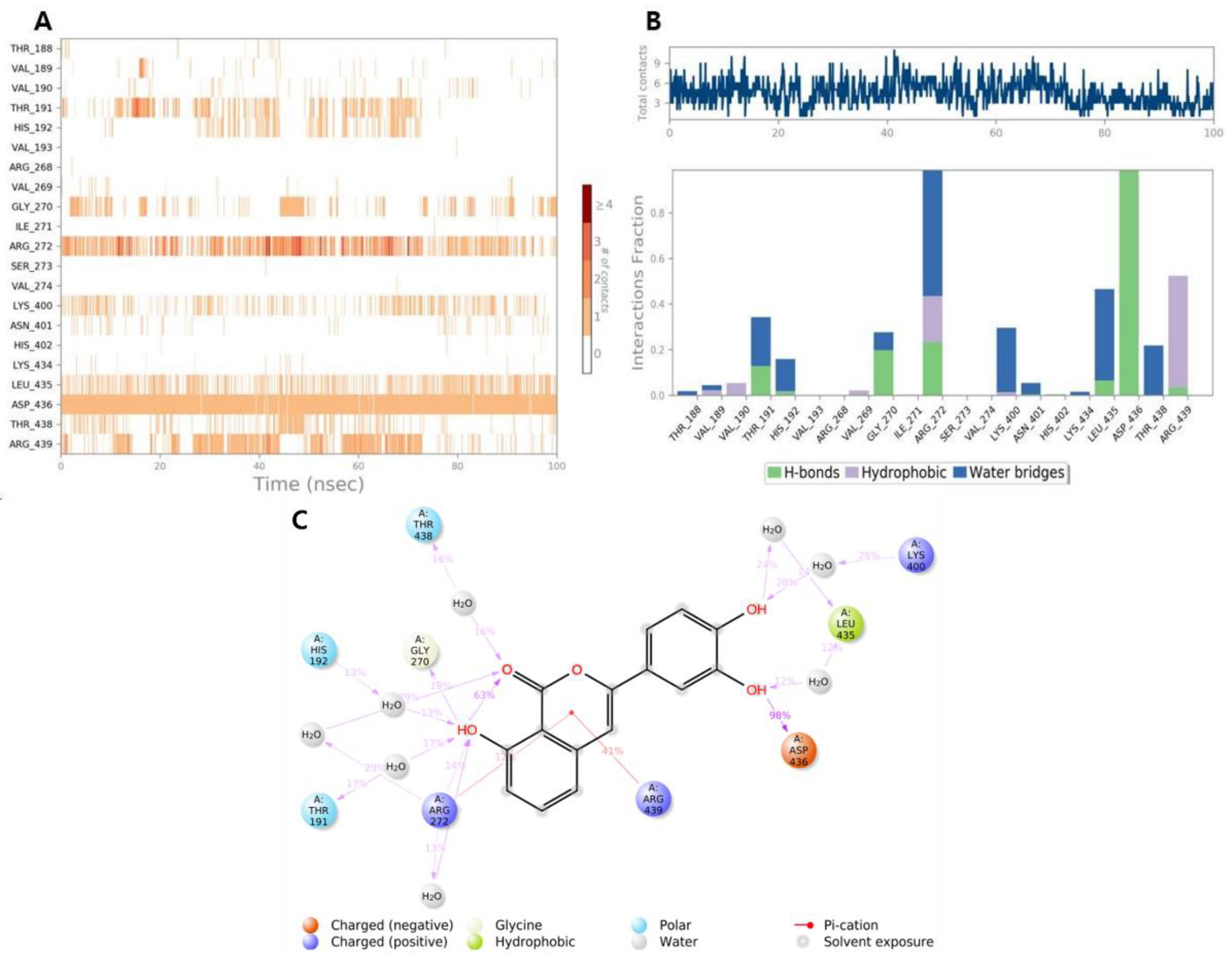
2.4. Molecular Docking Studies
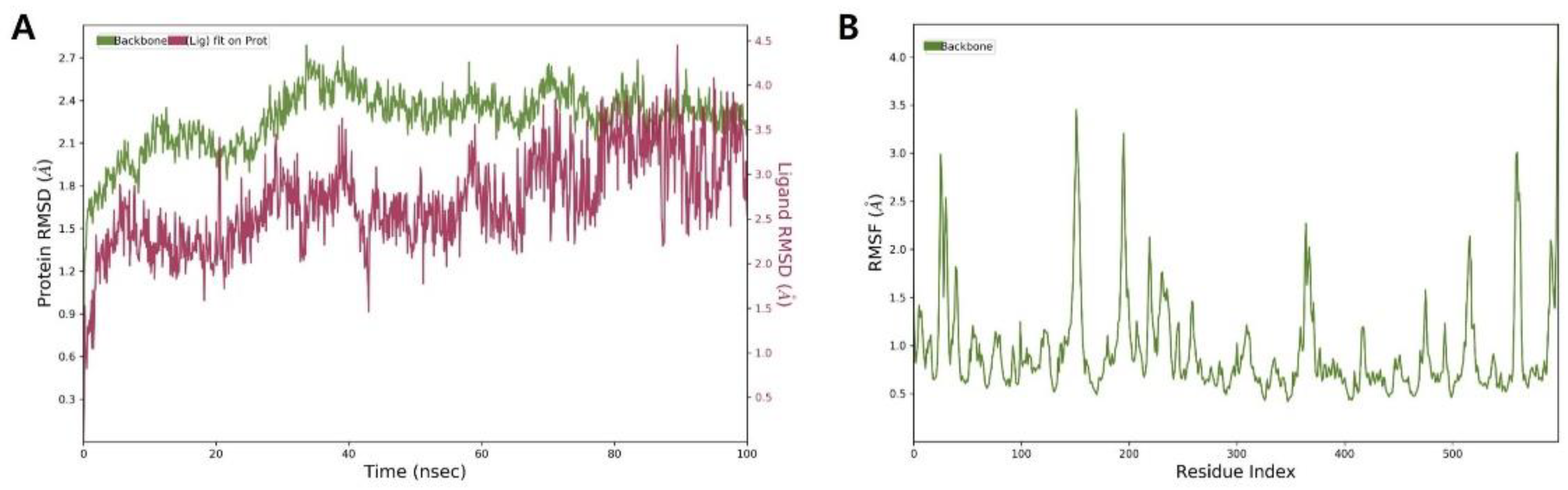
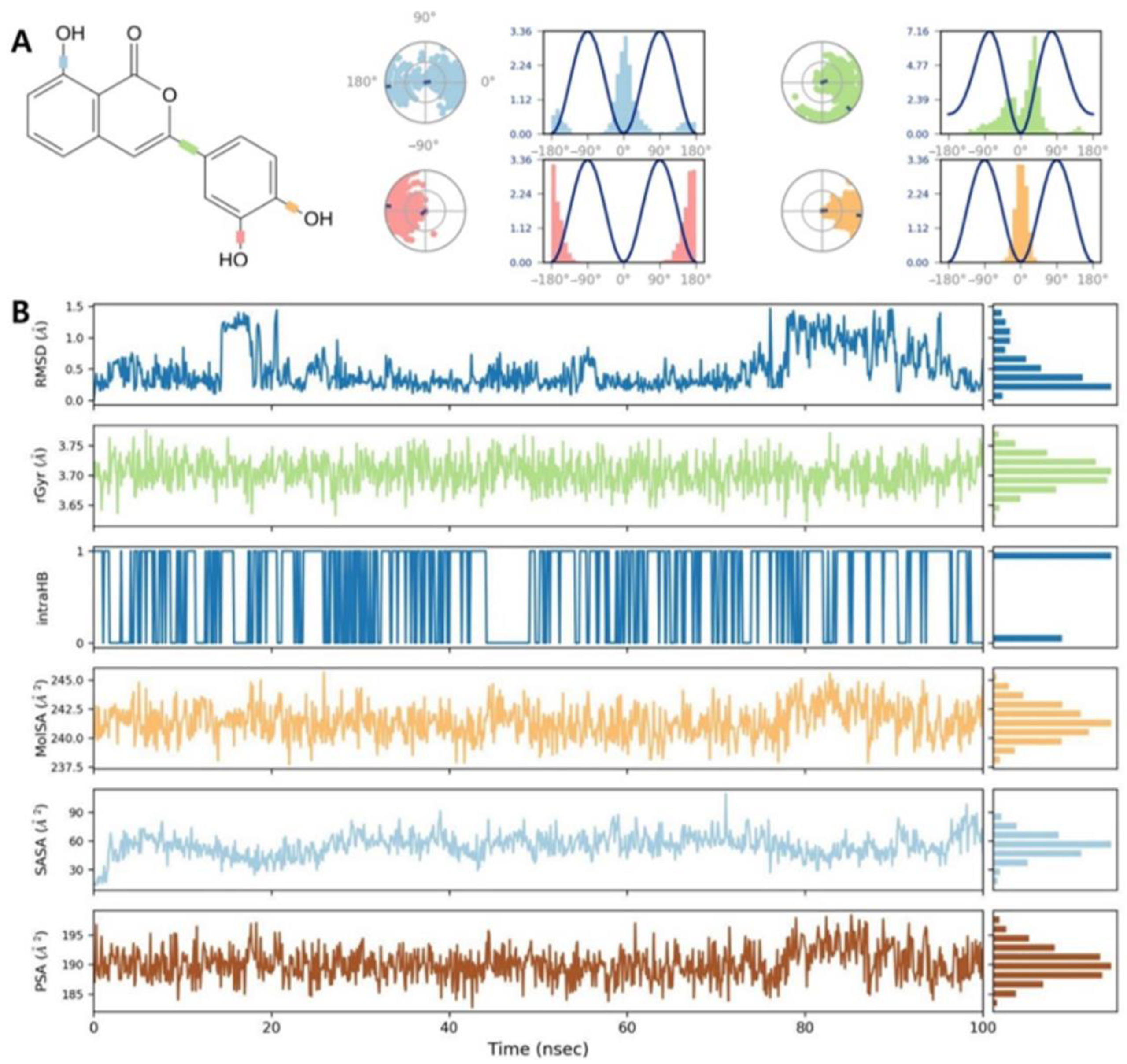
2.5. Molecular Dynamics Simulation of β-Glucuronidase Inhibition
3. Discussion
4. Materials and Methods
4.1. Experimental Procedures
4.2. Chemicals and Reagents
4.3. Plant Material
4.4. Extraction and Isolation
4.5. β-Glucuronidase Inhibition Assay
4.6. β-Glucuronidase Kinetics Assay
4.7. Molecular Docking
4.8. Molecular Dynamics Simulation
4.9. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bano, B.; Arshia; Khan, K.M.; Kanwal; Fatima, B.; Taha, M.; Ismail, N.H.; Wadood, A.; Ghufran, M.; Perveen, S. Synthesis, in vitro β-glucuronidase inhibitory potential and molecular docking studies of quinolines. Eur. J. Med. Chem. 2017, 139, 849–864. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.; Baharudin, M.S.; Ismail, N.H.; Selvaraj, M.; Salar, U.; Alkadi, K.A.A.; Khan, K.M. Synthesis and in silico studies of novel sulfonamides having oxadiazole ring: As β-glucuronidase inhibitors. Bioorg. Chem. 2017, 71, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Kaliannan, K.; Donnell, S.O.; Murphy, K.; Stanton, C.; Kang, C.; Wang, B.; Li, X.-Y.; Bhan, A.K.; Kang, J.X. Decreased tissue omega-6/omega-3 fatty acid ratio prevents chemotherapy-induced gastrointestinal toxicity associated with alterations of gut microbiome. Int. J. Mol. Sci. 2022, 23, 5332. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Drendel, W.B.; Chen, Z.-w.; Mathews, F.S.; Sly, W.S.; Grubb, J.H. Structure of human β-glucuronidase reveals candidate lysosomal targeting and active-site motifs. Nat. Struct. Mol. Biol. 1996, 3, 375–381. [Google Scholar] [CrossRef]
- Wallace, B.D.; Wang, H.; Lane, K.T.; Scott, J.E.; Orans, J.; Koo, J.S.; Venkatesh, M.; Jobin, C.; Yeh, L.-A.; Mani, S.; et al. Alleviating cancer drug toxicity by inhibiting a bacterial enzyme. Science 2010, 330, 831–835. [Google Scholar] [CrossRef] [Green Version]
- Jacobson, R.H.; Zhang, X.J.; DuBose, R.F.; Matthews, B.W. Three-dimensional structure of β-galactosidase from E. coli. Nature 1994, 369, 761–766. [Google Scholar] [CrossRef]
- Phong, N.V.; Zhao, Y.; Min, B.S.; Yang, S.Y.; Kim, J.A. Inhibitory activity of bioactive phloroglucinols from the rhizomes of Dryopteris crassirhizoma on Escherichia coli β-glucuronidase: Kinetic analysis and molecular docking studies. Metabolites 2022, 12, 938. [Google Scholar] [CrossRef]
- Weissmann, G.; Zurier, R.B.; Spieler, P.J.; Goldstein, I.M. Mechanisms of lysosomal enzyme release from leukocytes exposed to immune complexes and other particles. J. Exp. Med. 1971, 134, 149–165. [Google Scholar] [CrossRef]
- Goldin, B.R.; Gorbach, S.L. The relationship between diet and rat fecal bacterial enzymes implicated in colon cancer. J. Natl. Cancer Inst. 1976, 57, 371–375. [Google Scholar] [CrossRef]
- George, J. Elevated serum β-glucuronidase reflects hepatic lysosomal fragility following toxic liver injury in rats. Biochem. Cell Biol. 2008, 86, 235–243. [Google Scholar] [CrossRef]
- Yang, J.; Tao, L.; Yang, J.; Wu, C.; Sun, W. Integrated conservation of Acer yangbiense: A case study for conservation methods of plant species with extremely small populations. Plants People Planet, 2022, in press. [CrossRef]
- Bi, W.; Gao, Y.; Shen, J.; He, C.; Liu, H.; Peng, Y.; Zhang, C.; Xiao, P. Traditional uses, phytochemistry, and pharmacology of the genus Acer (maple): A review. J. Ethnopharmacol. 2016, 189, 31–60. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Lee, M.K.; Kim, Y.C. Protective activities of stilbene glycosides from Acer mono leaves against H2O2-induced oxidative damage in primary cultured rat hepatocytes. J. Agric. Food. Chem. 2005, 53, 4182–4186. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Sung, S.H.; Kim, Y.C. Two new hepatoprotective stilbene glycosides from Acer mono leaves. J. Nat. Prod. 2005, 68, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Bae, K.H. Medicinal Plants of Korea; Kyo-Hak Publishing Co.: Seoul, Korea, 2000. [Google Scholar]
- Park, C.-H.; Son, H.-U.; Son, M.; Lee, S.-H. Protective effect of Acer mono Max. sap on water immersion restraint stress-induced gastric ulceration. Exp. Ther. Med. 2011, 2, 843–848. [Google Scholar] [CrossRef] [Green Version]
- Adam, K.P.; Becker, H. Phenanthrenes and other phenolics from in vitro cultures of Marchantia polymorpha. Phytochemistry 1993, 35, 139–143. [Google Scholar] [CrossRef]
- Myung, D.-B.; Han, H.-S.; Shin, J.-S.; Park, J.Y.; Hwang, H.J.; Kim, H.J.; Ahn, H.S.; Lee, S.H.; Lee, K.-T. Hydrangenol isolated from the leaves of Hydrangea serrata attenuates wrinkle formation and repairs skin moisture in uvb-irradiated hairless mice. Nutrients 2019, 11, 2354. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.G.; Lee, Y.; Hur, H.-G.; Lim, Y.; Ahn, J.-H. Production of three O-methhylated esculetins with Escherichia coli expressing O-methyltransferase from poplar. Biosci. Biotechnol. Biochem. 2006, 70, 1269–1272. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.-B.; Zheng, X.-D.; Jian, Y.-F.; Liu, J.-X.; Zhu, J.-H. Constituents of the root of Anemone tomentosa. Arch. Pharm. Res. 2011, 34, 1097. [Google Scholar] [CrossRef]
- Garcez, F.R.; Garcez, W.S.; Martins, M.; Cruz, A.C. A bioactive lactone from Nectandra gardneri. Planta Med. 1999, 65, 775. [Google Scholar] [CrossRef]
- Jin, W.; Thuong, P.T.; Su, N.D.; Min, B.S.; Son, K.H.; Chang, H.W.; Kim, H.P.; Kang, S.S.; Sok, D.E.; Bae, K. Antioxidant activity of cleomiscosins A and C isolated from Acer okamotoanum. Arch. Pharm. Res. 2007, 30, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Ray, A.B.; Konno, C.; Oshima, Y.; Hikino, H. Cleomiscosin D, a coumarino-lignan from seeds of Cleome viscosa. Phytochemistry 1988, 27, 636–638. [Google Scholar] [CrossRef]
- Karunairatnam, M.C.; Levvy, G.A. The inhibition of β-glucuronidase by saccharic acid and the role of the enzyme in glucuronide synthesis. Biochem. J. 1949, 44, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Yung-Chi, C.; Prusoff, W.H. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973, 22, 3099–3108. [Google Scholar] [CrossRef]
- Huang, W.; Lu, S.; Huang, Z.; Liu, X.; Mou, L.; Luo, Y.; Zhao, Y.; Liu, Y.; Chen, Z.; Hou, T.; et al. Allosite: A method for predicting allosteric sites. Bioinformatics 2013, 29, 2357–2359. [Google Scholar] [CrossRef] [Green Version]
- Tian, H.; Jiang, X.; Tao, P. PASSer: Prediction of allosteric sites server. Mach. Learn. Sci. Technol. 2021, 2, 035015. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kumar, P.; Sarvagalla, S.; Bharadwaj, T.; Nayak, N.; Coumar, M.S.; Giri, R.; Garg, N. Functional inhibition of c-Myc using novel inhibitors identified through “hot spot” targeting. J. Biol. Chem. 2022, 298, 101898. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.-w.; Liao, M.-l.; Meng, X.-l.; Somero, G.N. Structural flexibility and protein adaptation to temperature: Molecular dynamics analysis of malate dehydrogenases of marine molluscs. Proc. Natl. Acad. Sci. USA 2018, 115, 1274–1279. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Zeng, C.; Massiah, M.A. Molecular dynamics simulation reveals insights into the mechanism of unfolding by the A130T/V mutations within the MID1 zinc-binding Bbox1 domain. PLoS ONE 2015, 10, e0124377. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.-Y.; Chen, C.-Y.; Lin, T.-C.; Yeh, L.-F.; Hsieh, W.-C.; Gao, S.; Burnouf, P.-A.; Chen, B.-M.; Hsieh, T.-J.; Dashnyam, P.; et al. Entropy-driven binding of gut bacterial β-glucuronidase inhibitors ameliorates irinotecan-induced toxicity. Commun. Biol. 2021, 4, 280. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Grigera, J.R.; Straatsma, T.P. The missing term in effective pair potentials. J. Phys. Chem. 1987, 91, 6269–6271. [Google Scholar] [CrossRef]
- Zrieq, R.; Ahmad, I.; Snoussi, M.; Noumi, E.; Iriti, M.; Algahtani, F.D.; Patel, H.; Saeed, M.; Tasleem, M.; Sulaiman, S.; et al. Tomatidine and patchouli alcohol as inhibitors of SARS-CoV-2 enzymes (3CLpro, PLpro and NSP15) by molecular docking and molecular dynamics simulations. Int. J. Mol. Sci. 2021, 22, 10693. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug. Deliv. Rev. 2012, 64, 4–17. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Z.; Cao, J.; Zhang, Q.; Lin, L. The drug likeness analysis of anti-inflammatory clerodane diterpenoids. Chin. Med. 2020, 15, 126. [Google Scholar] [CrossRef]
- Daina, A.; Zoete, V. A BOILED-Egg to predict gastrointestinal absorption and brain penetration of small molecules. ChemMedChem 2016, 11, 1117–1121. [Google Scholar] [CrossRef]

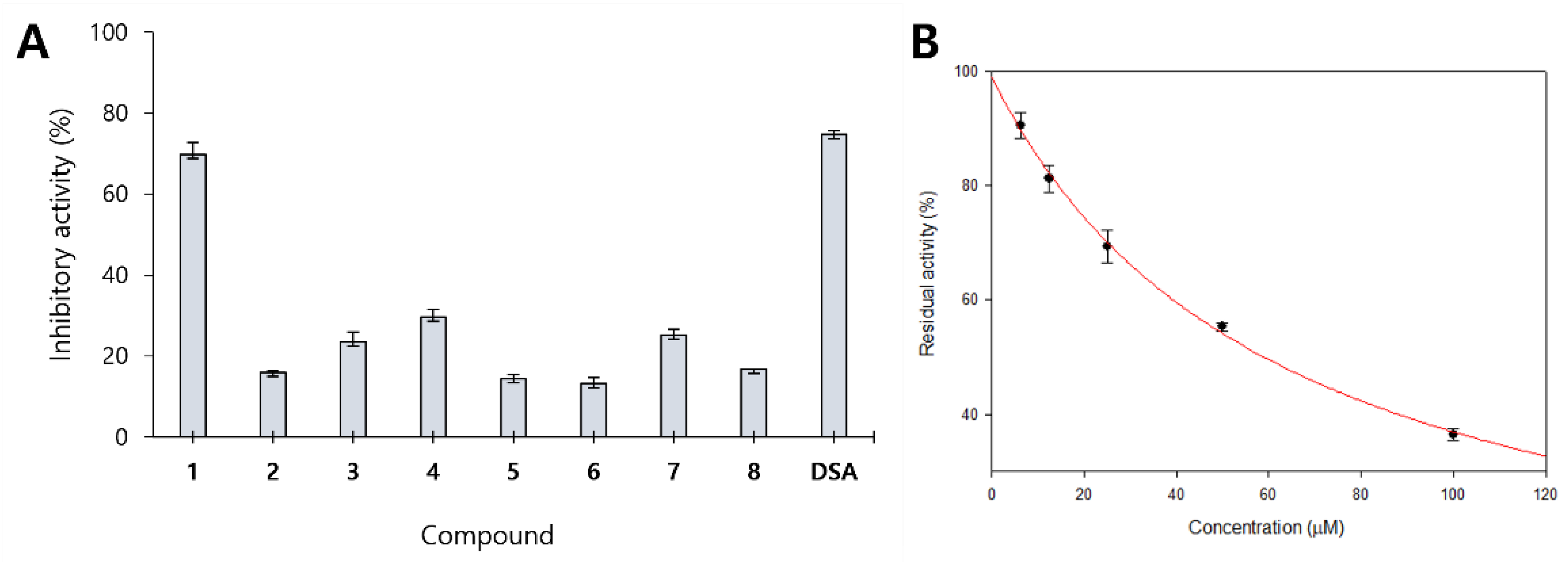

| Compounds | Inhibition against β-Glucuronidase | |
|---|---|---|
| Inhibition% (100 μM) | IC50 (μM) 1 | |
| 1 | 69.85 ± 2.93 | 58.83 ± 1.36 |
| 2 | 15.89 ± 0.55 | >100 |
| 3 | 23.46 ± 2.47 | >100 |
| 4 | 29.61 ± 1.83 | >100 |
| 5 | 14.34 ± 1.10 | >100 |
| 6 | 13.17 ± 1.46 | >100 |
| 7 | 25.21 ± 1.46 | >100 |
| 8 | 16.73 ± 0.09 | >100 |
| DSA 2 | 74.70 ± 0.95 | 24.56 ± 1.15 |
| Comp. | Binding Energy (kcal/mol) | Hydrogen Bonds | van der Waals Interactions | Hydrophobic Interactions | Electrostatic Interactions | Others |
|---|---|---|---|---|---|---|
| 1 | −8.35 | Thr191 Arg272 Leu435 Thr438 | Gly270 Asn401 Pro437 | Thr188 (π−σ) Val190 (π−σ) Lys400 (π−alkyl) Pro403 (π−alkyl) | Arg439 (π−cation) | Val189 (π−lone pair) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phong, N.V.; Min, B.S.; Yang, S.Y.; Kim, J.A. Inhibitory Effect of Coumarins and Isocoumarins Isolated from the Stems and Branches of Acer mono Maxim. against Escherichia coli β-Glucuronidase. Appl. Sci. 2022, 12, 10685. https://doi.org/10.3390/app122010685
Phong NV, Min BS, Yang SY, Kim JA. Inhibitory Effect of Coumarins and Isocoumarins Isolated from the Stems and Branches of Acer mono Maxim. against Escherichia coli β-Glucuronidase. Applied Sciences. 2022; 12(20):10685. https://doi.org/10.3390/app122010685
Chicago/Turabian StylePhong, Nguyen Viet, Byung Sun Min, Seo Young Yang, and Jeong Ah Kim. 2022. "Inhibitory Effect of Coumarins and Isocoumarins Isolated from the Stems and Branches of Acer mono Maxim. against Escherichia coli β-Glucuronidase" Applied Sciences 12, no. 20: 10685. https://doi.org/10.3390/app122010685
APA StylePhong, N. V., Min, B. S., Yang, S. Y., & Kim, J. A. (2022). Inhibitory Effect of Coumarins and Isocoumarins Isolated from the Stems and Branches of Acer mono Maxim. against Escherichia coli β-Glucuronidase. Applied Sciences, 12(20), 10685. https://doi.org/10.3390/app122010685








