Supplementation of an Anthocyanin-Rich Elderberry (Sambucus nigra L.) Extract in FVB/n Mice: A Healthier Alternative to Synthetic Colorants
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
Stability of the Aqueous Extract
2.2. Experimental Design
Animals
2.3. Hematological Analysis
2.3.1. Microhematocrit
2.3.2. Serum Biochemistry
2.4. Comet Assay
2.5. Hepatic and Renal Histology
2.6. Hepatic and Renal Oxidative Stress
2.7. Statistical Analysis
3. Results
3.1. General Findings
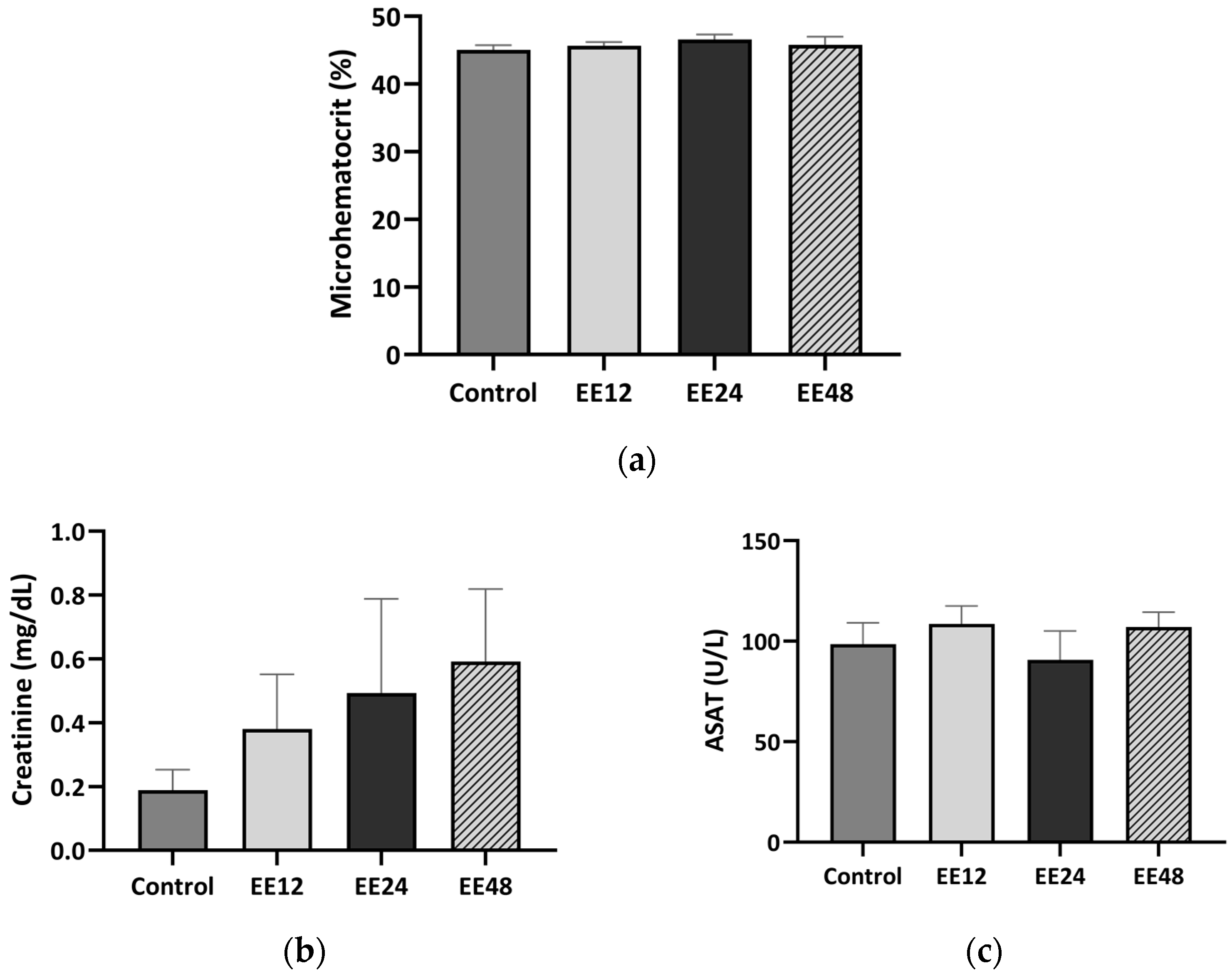
3.2. Haematological Analysis
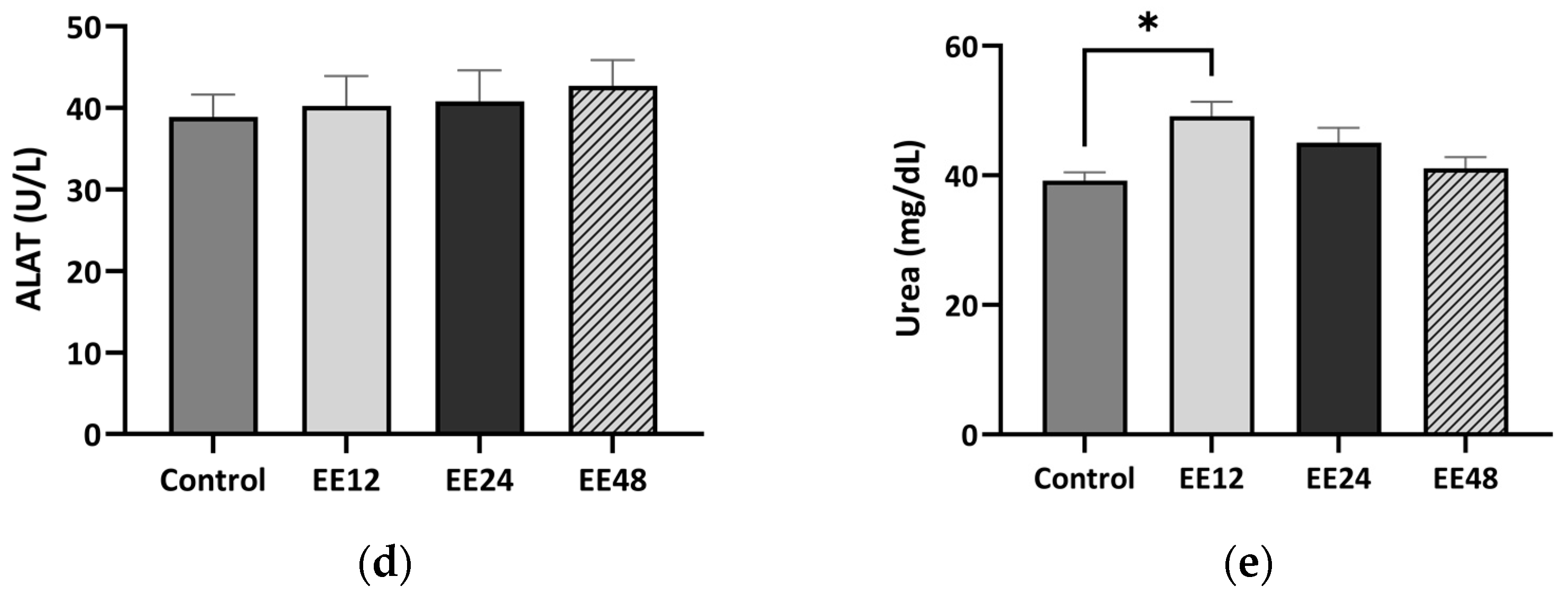
3.3. Hepatic and Renal Histology
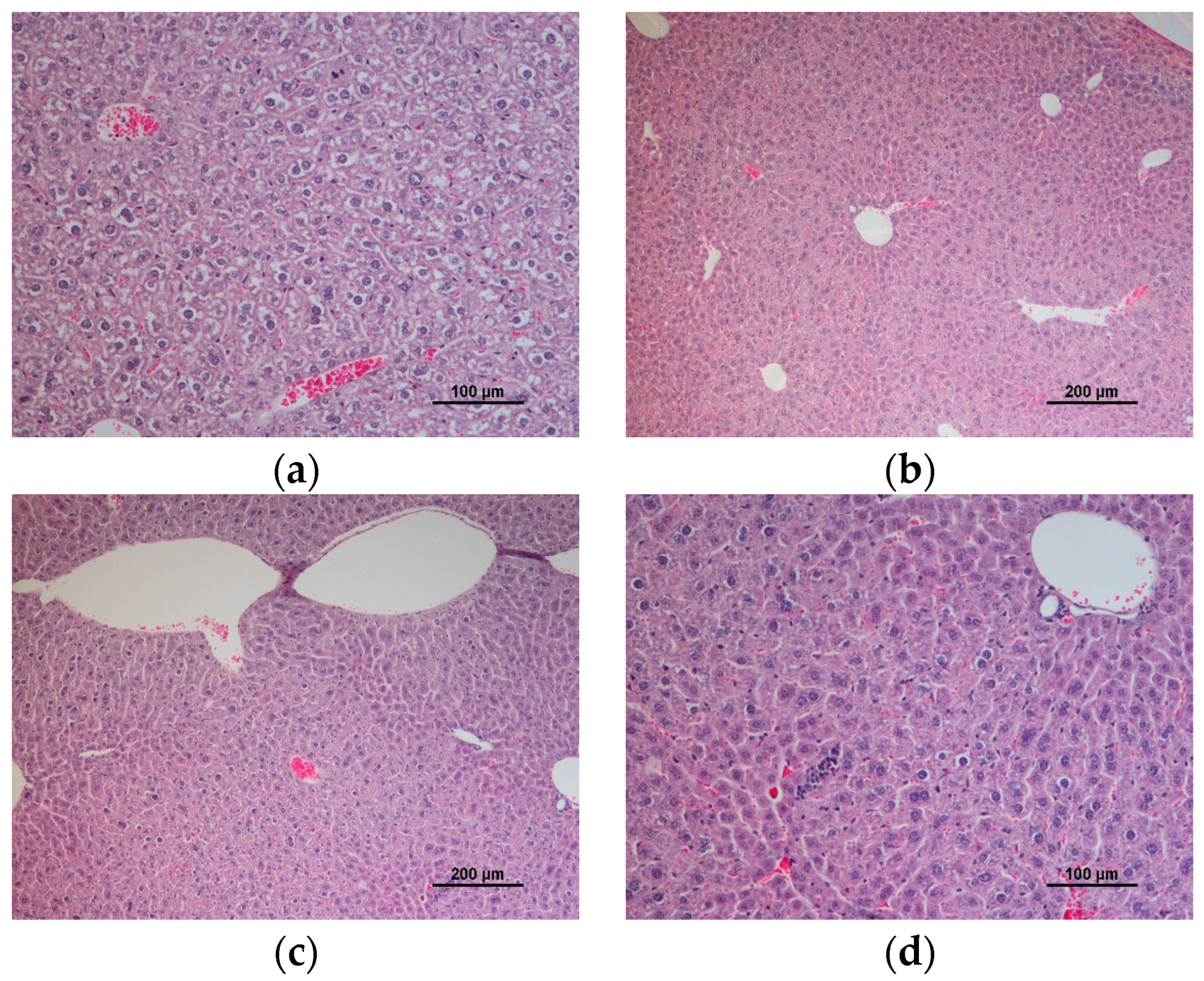
3.4. Comet Assay
3.5. Liver and Kidney Oxidative Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schmitzer, V.; Veberic, R.; Stampar, F. European Elderberry (Sambucus Nigra L.) and American Elderberry (Sambucus Canadensis L.): Botanical, Chemical and Health Properties of Flowers, Berries and Their Products. In Berries: Properties, Consumption and Nutrition; Nova Biomedical: Waltham, MA, USA, 2012; pp. 127–148. [Google Scholar]
- Salvador, Â.C.; Silvestre, A.J.D.; Rocha, M.S. Sambucus Nigra L.: A Potential Source of Healthpromoting Components. Front. Nat. Prod. Chem. 2016, 2, 343–392. [Google Scholar]
- Młynarczyk, K.; Walkowiak-Tomczak, D.; Łysiak, G.P. Bioactive Properties of Sambucus Nigra L. as a Functional Ingredient for Food and Pharmaceutical Industry. J. Funct. Foods 2018, 40, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Ağalar, H.G. Elderberry (Sambucus Nigra L.). In Nonvitamin and Nonmineral Nutritional Supplements; Elsevier: Amsterdam, The Netherlands, 2019; pp. 211–215. ISBN 978-0-12-812491-8. [Google Scholar]
- Atkinson, M.D.; Atkinson, E. Sambucus Nigra L. J. Ecol. 2002, 90, 895–923. [Google Scholar] [CrossRef]
- Veberic, R.; Jakopic, J.; Stampar, F.; Schmitzer, V. European Elderberry (Sambucus Nigra L.) Rich in Sugars, Organic Acids, Anthocyanins and Selected Polyphenols. Food Chem. 2009, 114, 511–515. [Google Scholar] [CrossRef]
- Sidor, A.; Gramza-Michałowska, A. Advanced Research on the Antioxidant and Health Benefit of Elderberry (Sambucus Nigra) in Food—A Review. J. Funct. Foods 2015, 18, 941–958. [Google Scholar] [CrossRef]
- Charlebois, D.; Byers, P.L.; Finn, C.E.; Thomas, A.L. Elderberry: Botany, Horticulture, Potential. Horticultural Reviews; Janick, J., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; Volume 37, pp. 213–280. ISBN 978-0-470-54367-2. [Google Scholar]
- Barros, L.; Dueñas, M.; Carvalho, A.M.; Ferreira, I.C.F.R.; Santos-Buelga, C. Characterization of Phenolic Compounds in Flowers of Wild Medicinal Plants from Northeastern Portugal. Food Chem. Toxicol. 2012, 50, 1576–1582. [Google Scholar] [CrossRef]
- Vlachojannis, C.; Zimmermann, B.F.; Chrubasik-Hausmann, S. Quantification of Anthocyanins in Elderberry and Chokeberry Dietary Supplements. Phytother. Res. 2015, 29, 561–565. [Google Scholar] [CrossRef]
- Cejpek, K.; Maloušková, I.; Konečný, M.; Velíšek, J. Antioxidant Activity in Variously Prepared Elderberry Foods and Supplements. Czech J. Food Sci. 2009, 27, S45–S48. [Google Scholar] [CrossRef]
- Małgorzata, D.; Aleksandra, P.; Monika, T.; Ireneusz, K. A New Black Elderberry Dye Enriched in Antioxidants Designed for Healthy Sweets Production. Antioxidants 2019, 8, 257. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Silva, A.M.; Nunes, F.M. Sambucus Nigra L. Fruits and Flowers: Chemical Composition and Related Bioactivities. Food Rev. Int. 2020, 38, 1–29. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and Anthocyanins: Colored Pigments as Food, Pharmaceutical Ingredients, and the Potential Health Benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Mateus, N.; de Freitas, V. Anthocyanins as Food Colorants. In Anthocyanins: Biosynthesis, Functions, and Applications; Winefield, C., Davies, K., Gould, K., Eds.; Springer: New York, NY, USA, 2009; pp. 284–304. ISBN 978-0-387-77335-3. [Google Scholar]
- Saxena, S.; Raja, A.S.M. Natural Dyes: Sources, Chemistry, Application and Sustainability Issues. In Roadmap to Sustainable Textiles and Clothing: Eco-Friendly Raw Materials, Technologies, and Processing Methods; Muthu, S.S., Ed.; Textile Science and Clothing Technology; Springer: Singapore, 2014; pp. 37–80. ISBN 978-981-287-065-0. [Google Scholar]
- Da Silva, R.F.R.; Barreira, J.C.M.; Heleno, S.A.; Barros, L.; Calhelha, R.C.; Ferreira, I.C.F.R. Anthocyanin Profile of Elderberry Juice: A Natural-Based Bioactive Colouring Ingredient with Potential Food Application. Molecules 2019, 24, 2359. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Feng, R.; Wang, S.Y.; Bowman, L.; Lu, Y.; Qian, Y.; Castranova, V.; Jiang, B.-H.; Shi, X. Cyanidin-3-Glucoside, a Natural Product Derived from Blackberry, Exhibits Chemopreventive and Chemotherapeutic Activity. J. Biol. Chem. 2006, 281, 17359–17368. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.; Chung, E.Y.; Jang, H.-Y.; Hong, O.-Y.; Chae, H.S.; Jeong, Y.-J.; Kim, S.-Y.; Kim, B.-S.; Yoo, D.J.; Kim, J.-S.; et al. Anti-Cancer Effect of Cyanidin-3-Glucoside from Mulberry via Caspase-3 Cleavage and DNA Fragmentation in Vitro and in Vivo. Anticancer Agents Med. Chem. 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Ning, S. Cyanidin-3-glucoside Attenuates the Angiogenesis of Breast Cancer via Inhibiting STAT3/VEGF Pathway. Phytother. Res. 2019, 33, 81–89. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Han, X.; Guo, J.; Guo, Y.; Yin, M.; Liu, G.; Huang, W.; Zhan, J. Cyanidin-3-Glucoside Attenuates High-Fat and High-Fructose Diet-Induced Obesity by Promoting the Thermogenic Capacity of Brown Adipose Tissue. J. Funct. Foods 2018, 41, 62–71. [Google Scholar] [CrossRef]
- Bastos, C.; Barros, L.; Dueñas, M.; Calhelha, R.C.; Queiroz, M.J.R.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Chemical Characterisation and Bioactive Properties of Prunus Avium L.: The Widely Studied Fruits and the Unexplored Stems. Food Chem. 2015, 173, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Nascimento-Gonçalves, E.; Silva, J.; Oliveira, P.A.; Ferreira, R.; Antunes, L.; Arantes-Rodrigues, R.; Faustino-Rocha, A.I. Implementation of Human Endpoints in a Urinary Bladder Carcinogenesis Study in Rats. In Vivo 2017, 31. [Google Scholar] [CrossRef]
- Forbes, D.; Blom, H.J.M.; Kostomitsopoulos, N.; Moore, G.; Perretta, G. FELASA EUROGUIDE. On the Accomodation and Care of Animals Used for Experimental and Other Scientific Purposes; The Royal Society of Medicine Press Ltd.: London, UK, 2007; ISBN 978-1-85315-751-6. [Google Scholar]
- Collins, A.R. The Comet Assay for DNA Damage and Repair: Principles, Applications, and Limitations. Mol. Biotechnol. 2004, 26, 249–261. [Google Scholar] [CrossRef]
- Marques, A.; Ferreira, J.; Abreu, H.; Pereira, R.; Pinto, D.; Silva, A.; Gaivão, I.; Pacheco, M. Comparative Genoprotection Ability of Wild-Harvested vs. Aqua-Cultured Ulva Rigida Coupled with Phytochemical Profiling. Eur. J. Phycol. 2021, 56, 105–118. [Google Scholar] [CrossRef]
- Martins, T.; Oliveira, P.A.; Pires, M.J.; Neuparth, M.J.; Lanzarin, G.; Félix, L.; Venâncio, C.; Pinto, M.d.L.; Ferreira, J.; Gaivão, I.; et al. Effect of a Sub-Chronic Oral Exposure of Broccoli (Brassica Oleracea L. Var. Italica) By-Products Flour on the Physiological Parameters of FVB/N Mice: A Pilot Study. Foods 2022, 11, 120. [Google Scholar] [CrossRef]
- Ribeiro-Silva, C.M.; Faustino-Rocha, A.I.; Gil da Costa, R.M.; Medeiros, R.; Pires, M.J.; Gaivão, I.; Gama, A.; Neuparth, M.J.; Barbosa, J.V.; Peixoto, F.; et al. Pulegone and Eugenol Oral Supplementation in Laboratory Animals: Results from Acute and Chronic Studies. Biomedicines 2022, 10, 2595. [Google Scholar] [CrossRef] [PubMed]
- Martins, T.; Matos, A.F.; Soares, J.; Leite, R.; Pires, M.J.; Ferreira, T.; Medeiros-Fonseca, B.; Rosa, E.; Oliveira, P.A.; Antunes, L.M. Comparison of Gelatin Flavors for Oral Dosing of C57BL/6J and FVB/N Mice. J. Am. Assoc. Lab. Anim. Sci. 2022, 61, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, P.; Ferreira, T.; Nascimento-Gonçalves, E.; Seixas, F.; Gil da Costa, R.M.; Martins, T.; Neuparth, M.J.; Pires, M.J.; Lanzarin, G.; Félix, L.; et al. Dietary Supplementation with Chestnut (Castanea Sativa) Reduces Abdominal Adiposity in FVB/n Mice: A Preliminary Study. Biomedicines 2020, 8, 75. [Google Scholar] [CrossRef] [PubMed]
- Sachse, B.; Meinl, W.; Glatt, H.; Monien, B.H. Ethanol and 4-Methylpyrazole Increase DNA Adduct Formation of Furfuryl Alcohol in FVB/N Wild-Type Mice and in Mice Expressing Human Sulfotransferases 1A1/1A2. Carcinogenesis 2016, 37, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Porras-Mija, I.; Chirinos, R.; García-Ríos, D.; Aguilar-Galvez, A.; Huaman-Alvino, C.; Pedreschi, R.; Campos, D. Physico-Chemical Characterization, Metabolomic Profile and in Vitro Antioxidant, Antihypertensive, Antiobesity and Antidiabetic Properties of Andean Elderberry (Sambucus Nigra Subsp. Peruviana). J. Berry Res. 2020, 10, 193–208. [Google Scholar] [CrossRef]
- Chrubasik, C.; Maier, T.; Dawid, C.; Torda, T.; Schieber, A.; Hofmann, T.; Chrubasik, S. An Observational Study and Quantification of the Actives in a Supplement with Sambucus Nigra and Asparagus Officinalis Used for Weight Reduction. Phytother. Res. 2008, 22, 913–918. [Google Scholar] [CrossRef]
- Wu, T.; Qi, X.; Liu, Y.; Guo, J.; Zhu, R.; Chen, W.; Zheng, X.; Yu, T. Dietary Supplementation with Purified Mulberry (Morus Australis Poir) Anthocyanins Suppresses Body Weight Gain in High-Fat Diet Fed C57BL/6 Mice. Food Chem. 2013, 141, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Wallig, M.A.; Janovitz, E.B. Chapter 4—Morphologic Manifestations of Toxic Cell Injury. In Haschek and Rousseaux’s Handbook of Toxicologic Pathology, 3rd ed.; Haschek, W.M., Rousseaux, C.G., Wallig, M.A., Eds.; Academic Press: Boston, MA, USA, 2013; pp. 77–105. ISBN 978-0-12-415759-0. [Google Scholar]
- Hou, Z.; Qin, P.; Ren, G. Effect of Anthocyanin-Rich Extract from Black Rice ( Oryza Sativa L. Japonica ) on Chronically Alcohol-Induced Liver Damage in Rats. J. Agric. Food Chem. 2010, 58, 3191–3196. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Y.; Zhou, Q.; Luo, C.-L.; Deng, A.-P.; Zhang, Z.-C.; Zhang, J.-L. Characterization and Hepatoprotective Activity of Anthocyanins from Purple Sweet Potato (Ipomoea Batatas L. Cultivar Eshu No. 8). J. Food Drug Anal. 2017, 25, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Curtis, P.J.; Kroon, P.A.; Hollands, W.J.; Walls, R.; Jenkins, G.; Kay, C.D.; Cassidy, A. Cardiovascular Disease Risk Biomarkers and Liver and Kidney Function Are Not Altered in Postmenopausal Women after Ingesting an Elderberry Extract Rich in Anthocyanins for 12 Weeks. J. Nutr. 2009, 139, 2266–2271. [Google Scholar] [CrossRef]
- Salvador, Â.; Król, E.; Lemos, V.; Santos, S.; Bento, F.; Costa, C.; Almeida, A.; Szczepankiewicz, D.; Kulczyński, B.; Krejpcio, Z.; et al. Effect of Elderberry (Sambucus Nigra L.) Extract Supplementation in STZ-Induced Diabetic Rats Fed with a High-Fat Diet. Int. J. Mol. Sci. 2016, 18, 13. [Google Scholar] [CrossRef]
- Medeiros-Fonseca, B.; Mestre, V.F.; Colaço, B.; Pires, M.J.; Martins, T.; Gil da Costa, R.M.; Neuparth, M.J.; Medeiros, R.; Moutinho, M.S.S.; Dias, M.I.; et al. Laurus Nobilis (Laurel) Aqueous Leaf Extract’s Toxicological and Anti-Tumor Activities in HPV16-Transgenic Mice. Food Funct. 2018, 9, 4419–4428. [Google Scholar] [CrossRef]
- Gounden, V.; Bhatt, H.; Jialal, I. Renal Function Tests. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Opris, R.; Tatomir, C.; Olteanu, D.; Moldovan, R.; Moldovan, B.; David, L.; Nagy, A.; Decea, N.; Kiss, M.L.; Filip, G.A. The Effect of Sambucus Nigra L. Extract and Phytosinthesized Gold Nanoparticles on Diabetic Rats. Colloids Surf. B Biointerfaces 2017, 150, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, S.; Gaivão, I. Natural Ingredients Common in the Trás-Os-Montes Region (Portugal) for Use in the Cosmetic Industry: A Review about Chemical Composition and Antigenotoxic Properties. Molecules 2021, 26, 5255. [Google Scholar] [CrossRef] [PubMed]
- Olejnik, A.; Olkowicz, M.; Kowalska, K.; Rychlik, J.; Dembczyński, R.; Myszka, K.; Juzwa, W.; Białas, W.; Moyer, M.P. Gastrointestinal Digested Sambucus Nigra L. Fruit Extract Protects In Vitro Cultured Human Colon Cells against Oxidative Stress. Food Chem. 2016, 197, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Santos, P.; Badim, H.; Salvador, Â.C.; Silvestre, A.J.D.; Santos, S.A.O.; Rocha, S.M.; Sousa, A.M.; Pereira, M.O.; Wilson, C.P.; Rocha, C.M.R.; et al. Chemical Characterization of Sambucus Nigra L. Flowers Aqueous Extract and Its Biological Implications. Biomolecules 2021, 11, 1222. [Google Scholar] [CrossRef]
- Domínguez, R.; Zhang, L.; Rocchetti, G.; Lucini, L.; Pateiro, M.; Munekata, P.E.S.; Lorenzo, J.M. Elderberry (Sambucus Nigra L.) as Potential Source of Antioxidants. Characterization, Optimization of Extraction Parameters and Bioactive Properties. Food Chem. 2020, 330, 127266. [Google Scholar] [CrossRef]
- Silva, P.; Ferreira, S.; Nunes, F.M. Elderberry (Sambucus Nigra L.) by-Products a Source of Anthocyanins and Antioxidant Polyphenols. Ind. Crop. Prod. 2017, 95, 227–234. [Google Scholar] [CrossRef]
- Hassimotto, N.M.; Lajolo, F.M. Antioxidant Status in Rats after Long-Term Intake of Anthocyanins and Ellagitannins from Blackberries: Anthocyanin Intake Improves Antioxidant Status in Rats. J. Sci. Food Agric. 2011, 91, 523–531. [Google Scholar] [CrossRef]
- Fernández-Pachón, M.S.; Berná, G.; Otaolaurruchi, E.; Troncoso, A.M.; Martín, F.; García-Parrilla, M.C. Changes in Antioxidant Endogenous Enzymes (Activity and Gene Expression Levels) after Repeated Red Wine Intake. J. Agric. Food Chem. 2009, 57, 6578–6583. [Google Scholar] [CrossRef]
- Amchova, P.; Kotolova, H.; Ruda-Kucerova, J. Health Safety Issues of Synthetic Food Colorants. Regul. Toxicol. Pharmacol. 2015, 73, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Amaya, D.B. Update on Natural Food Pigments—A Mini-Review on Carotenoids, Anthocyanins, and Betalains. Food Res. Int. 2019, 124, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Rhim, J.-W. Anthocyanin Food Colorant and Its Application in PH-Responsive Color Change Indicator Films. Crit. Rev. Food Sci. Nutr. 2021, 61, 2297–2325. [Google Scholar] [CrossRef] [PubMed]


| Groups | Food (g) | Drink (mL) | ||
|---|---|---|---|---|
| Initial | Final | Initial | Final | |
| Control | 22.14 | 23.02 | 27.90 | 29.33 |
| EE12 | 19.99 | 25.27 | 26.12 | 26.60 |
| EE24 | 22.51 | 21.57 | 27.09 | 28.12 |
| EE48 | 21.47 | 21.60 | 22.08 | 27.12 |
| Group/Organ | Control | EE12 | EE24 | EE48 |
|---|---|---|---|---|
| Thymus | 1.92 ± 0.129 | 1.71 ± 0.259 | 2.02 ± 0.194 | 2.29 ± 0.255 |
| Heart | 4.69 ± 0.148 a | 5.50 ± 0.186 b | 4.50 ± 0.147 a | 4.49 ± 0.156 a |
| Lungs | 6.92 ± 0.349 | 7.37 ± 0.314 | 7.13 ± 0.257 | 7.78 ± 0.494 |
| Spleen | 3.89 ± 0.279 | 4.15 ± 0.239 | 4.08 ± 0.245 | 3.94 ± 0.344 |
| Liver | 54.56 ± 1.293 | 49.61 ± 0.904 | 50.50 ± 2.287 | 51.64 ± 2.171 |
| Left Kidney | 6.00 ± 0.118 a | 6.99 ± 0.114 b | 6.95 ± 0.196 b | 6.51 ± 0.271 a,b |
| Right Kidney | 6.05 ± 0.308 | 7.36 ± 0.134 | 6.33 ± 0.456 | 6.77 ± 0.330 |
| Left Adrenal | 0.42 ± 0.102 | 0.46 ± 0.126 | 0.47 ± 0.076 | 0.48 ± 0.101 |
| Right Adrenal | 0.32 ± 0.075 | 0.45 ± 0.064 | 0.29 ± 0.069 | 0.34 ± 0.060 |
| Control | EE12 | EE24 | EE48 | |
|---|---|---|---|---|
| Liver | ||||
| Normal | 1/5 (20.0%) a | 5/6 (83.3%) b | 4/6 (66.7%) a,b | 4/6 (66.7%) a,b |
| Hydropic changes (HC) | 4/5 (80.0%) a | 1/6 (16.7%) b | 2/6 (33.3%) a,b | 2/6 (33.3%) a,b |
| HC general | 4/5 (80.0%) a | 0/6 (0.0%) b | 0/6 (0.0%) b | 0/6 (0.0%) b |
| HC centrilobular | 0/5 (0.0%) | 1/6 (16.7%) | 2/6 (33.3%) | 2/6 (33.3%) |
| Inflammation | ||||
| Absent | 3/5 (60.0%) | 5/6 (83.3%) | 3/6 (50.0%) | 3/6 (50.0%) |
| Chronic focal hepatitis | 2/5 (40.0%) | 1/6 (16.7%) | 3/6 (50.0%) | 3/6 (50.0%) |
| Kidney | ||||
| Normal | 5/5 (100.0%) | 6/6 (100.0%) | 5/6 (83.3%) | 6/6 (100.0%) |
| Chronic interstitial nephritis | 0/0 (0.0%) | 0/0 (0.0%) | 1/6 (16.7%) | 0/0 (0.0%) |
| Biochemical Parameters | Control | EE12 | EE24 | EE48 |
|---|---|---|---|---|
| Liver | ||||
| ROS | 1212 (1115–1623) | 1649 (1330–1729) | 2051 (1678–3046) | 1896 (1408–2308) |
| SOD | 231 (169–271) a | 250 (239–255) a | 274 (233–322) a,b | 333 (290–382) b |
| CAT | 5956 (5047–6608) a | 4001 (3197–4482) a,b | 3906 (2960–5081) a,b | 2925 (2241–3612) b |
| GPx | 0.41 ± 0.2 | 0.46 ± 0.13 | 0.61 ± 0.19 | 0.3 ± 0.27 |
| GST | 0.17 ± 0.01 | 0.18 ± 0.02 | 0.2 ± 0.03 | 0.19 ± 0.02 |
| GSH | 14.58 ± 3.4 | 14.71 ± 1.86 | 13.33 ± 5.55 | 12.95 ± 2.68 |
| GSSG | 270 ± 63.6 | 272.9 ± 138.5 | 339.7 ± 109 | 318.8 ± 141.6 |
| OSI | 0.05 ± 0.01 | 0.03 ± 0.02 | 0.04 ± 0.004 | 0.04 ± 0.01 |
| MDA | 205.1 ± 45.1 | 189.8 ± 23.8 | 207.0 ± 60.3 | 176.0 ± 28.6 |
| Kidney | ||||
| ROS | 1460 ± 323 | 1545 ± 338 | 1429 ± 443 | 1337 ± 322 |
| SOD | 161 (139–188) a | 203 (178–271) a,b | 333 (267–432) a,b | 341 (305–374) b |
| CAT | 78.55 ± 7.19 | 76.05 ± 10.12 | 77.37 ± 12.21 | 77.89 ± 13.25 |
| GPx | 0.15 (0.13–0.22) | 0.08 (0.04–0.13) | 0.07 (0.05–0.1) | 0.08 (0.04–0.11) |
| GST | 0.058 ± 0.01 | 0.045 ± 0.002 | 0.058 ± 0.01 | 0.045 ± 0.002 |
| GSH | 148.1 ± 31.60 | 131.4 ± 16.69 | 146.2 ± 35.49 | 132.8 ± 21.63 |
| GSSG | 18.18 ± 13.02 | 23.07 ± 14.43 | 18.54 ± 8.78 | 14.93 ± 6.07 |
| OSI | 5.83 ± 1.92 | 8.01 ± 2.75 | 9.57 ± 5.12 | 10.6 ± 5.21 |
| MDA | 5600 ± 1189 | 4960 ± 728 | 5501 ± 1378 | 5040 ± 864 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azevedo, T.; Ferreira, T.; Ferreira, J.; Teixeira, F.; Ferreira, D.; Silva-Reis, R.; Neuparth, M.J.; Pires, M.J.; Pinto, M.d.L.; Gil da Costa, R.M.; et al. Supplementation of an Anthocyanin-Rich Elderberry (Sambucus nigra L.) Extract in FVB/n Mice: A Healthier Alternative to Synthetic Colorants. Appl. Sci. 2022, 12, 11928. https://doi.org/10.3390/app122311928
Azevedo T, Ferreira T, Ferreira J, Teixeira F, Ferreira D, Silva-Reis R, Neuparth MJ, Pires MJ, Pinto MdL, Gil da Costa RM, et al. Supplementation of an Anthocyanin-Rich Elderberry (Sambucus nigra L.) Extract in FVB/n Mice: A Healthier Alternative to Synthetic Colorants. Applied Sciences. 2022; 12(23):11928. https://doi.org/10.3390/app122311928
Chicago/Turabian StyleAzevedo, Tiago, Tiago Ferreira, João Ferreira, Filipa Teixeira, Diana Ferreira, Rita Silva-Reis, Maria João Neuparth, Maria João Pires, Maria de Lurdes Pinto, Rui M. Gil da Costa, and et al. 2022. "Supplementation of an Anthocyanin-Rich Elderberry (Sambucus nigra L.) Extract in FVB/n Mice: A Healthier Alternative to Synthetic Colorants" Applied Sciences 12, no. 23: 11928. https://doi.org/10.3390/app122311928
APA StyleAzevedo, T., Ferreira, T., Ferreira, J., Teixeira, F., Ferreira, D., Silva-Reis, R., Neuparth, M. J., Pires, M. J., Pinto, M. d. L., Gil da Costa, R. M., Bastos, M. M. S. M., Medeiros, R., Félix, L., Venâncio, C., Dias, M. I., Gaivão, I., Barros, L., & Oliveira, P. A. (2022). Supplementation of an Anthocyanin-Rich Elderberry (Sambucus nigra L.) Extract in FVB/n Mice: A Healthier Alternative to Synthetic Colorants. Applied Sciences, 12(23), 11928. https://doi.org/10.3390/app122311928















