X-ray Image Enhancement Based on Adaptive Gradient Domain Guided Image Filtering
Abstract
1. Introduction
2. Related Works
Gradient Domain Guided Image Filtering
3. The Proposed Method
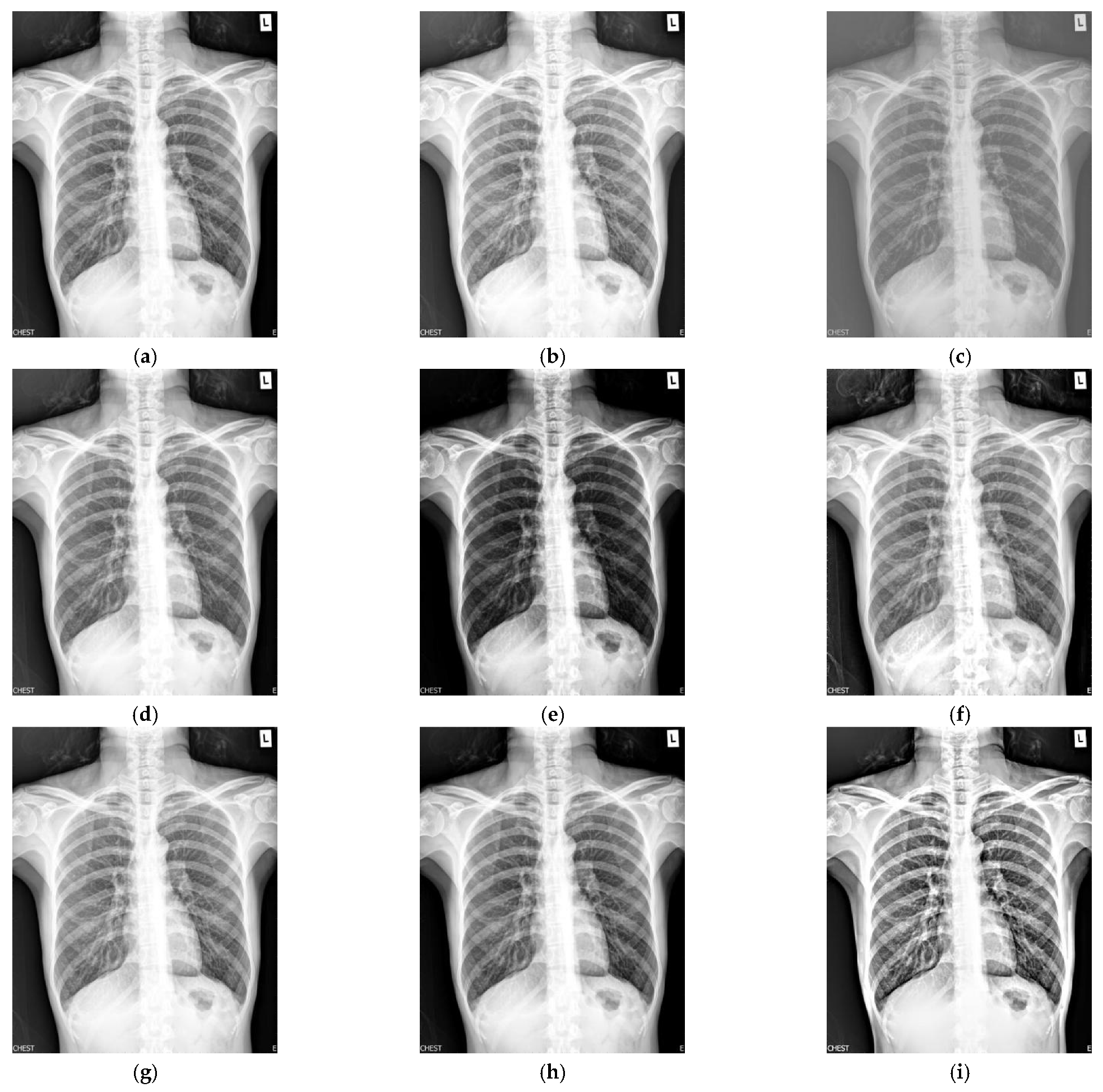
4. Experimental Results
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, C.; Manh, H. X-ray enhancement based on component attenuation, contrast adjustment, and image fusion. IEEE Trans. Image Process. 2019, 28, 127–141. [Google Scholar] [CrossRef]
- Hussain, L. Lung cancer prediction using robust machine learning and image enhancement methods on extracted gray-level co-occurrence matrix features. Appl. Sci. 2022, 12, 6517. [Google Scholar] [CrossRef]
- Yao, L.; Lin, Y.; Muhammad, S. An improved multi-scale image enhancement method based on retinex theory. J. Med. Imaging Health Inform. 2018, 8, 122–126. [Google Scholar] [CrossRef]
- Guo, Q.; Jia, Z.; Yang, J.; Nikola, K. Contrast enhancement of medical images using fuzzy set theory and nonsubsampled shearlet transform. Int. J. Imaging Syst. Technol. 2019, 29, 110–117. [Google Scholar]
- Kansal, S.; Purwar, S.; Tripathi, R. Image contrast enhancement using unsharp masking and histogram equalization. Multimed. Tools Appl. 2018, 77, 26919–26938. [Google Scholar] [CrossRef]
- Kallel, F.; Sahnoun, M.; Hamida, A. CT scan contrast enhancement using singular value decomposition and adaptive gamma correction. Signal Image Video Process. 2018, 12, 905–913. [Google Scholar] [CrossRef]
- Joseph, J.; Anoop, B.; Williams, J. A modified unsharp masking with adaptive threshold and objectively defined amount based on saturation constraints. Multimed. Tools Appl. 2019, 78, 11073–11089. [Google Scholar] [CrossRef]
- Rahman, Z.; Jobson, D.J.; Woodell, G. Multi-scale retinex for color image enhancement. In Proceedings of the International Conference on Image Processing, Lausanne, Switzerland, 19 September 1996; pp. 1003–1006. [Google Scholar]
- Zuiderveld, K. Contrast limited adaptive histograph equalization. In Graphic Gems IV; Academic Press Professional: San Diego, CA, USA, 1994; pp. 474–485. [Google Scholar]
- Subramani, B.; Veluchamy, M. MRI brain image enhancement using brightness preserving adaptive fuzzy histogram equalization. Int. J. Imaging Syst. Technol. 2018, 28, 217–222. [Google Scholar] [CrossRef]
- Singh, K.; Kapoor, R.; Sinha, S. Enhancement of low exposure images via recursive histogram equalization algorithms. Optik 2015, 126, 2619–2625. [Google Scholar] [CrossRef]
- Ashiba, H.; Mansour, H.; Ahmed, H. Enhancement of IR images using histogram processing and the undecimated additive wavelet transform. Multimed. Tools Appl. 2019, 78, 11277–11290. [Google Scholar] [CrossRef]
- Selesnick, I.; Baraniuk, R.; Kingsbury, N. The dual-tree complex wavelet transform. IEEE Signal Process. Mag. 2005, 22, 123–151. [Google Scholar] [CrossRef]
- Do, M.; Vetterli, M. The contourlet transform: An efficient directional multiresolution image representation. IEEE Trans. Image Process. 2005, 14, 2091–2106. [Google Scholar] [CrossRef] [PubMed]
- Cunha, A.; Zhou, J.; Do, M. The nonsubsampled contourlet transform: Theory, design, and applications. IEEE Trans. Image Process. 2006, 15, 3089–3101. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Labate, D.; Lim, W. Edge analysis and identification using the continuous shearlet transform. Appl. Comput. Harmon. Anal. 2009, 27, 24–46. [Google Scholar] [CrossRef]
- Easley, G.; Labate, D.; Lim, W. Sparse directional image representations using the discrete shearlet transform. Appl. Comput. Harmon. Anal. 2008, 25, 25–46. [Google Scholar] [CrossRef]
- Yang, M.; Tang, G.; Liu, X. Low-light image enhancement based on retinex theory and dual-tree complex wavelet transform. Optoelectron. Lett. 2018, 14, 470–475. [Google Scholar] [CrossRef]
- Feng, P.; Pan, Y.; Wei, B. Enhancing retinal image by the contourlet transform. Pattern Recognit. Lett. 2007, 28, 516–522. [Google Scholar] [CrossRef]
- Li, L.; Si, Y.; Jia, Z. A novel brain image enhancement method based on nonsubsampled contourlet transform. Int. J. Imaging Syst. Technol. 2018, 28, 124–131. [Google Scholar] [CrossRef]
- Li, L.; Si, Y.; Wang, L. Brain image enhancement approach based on singular value decomposition in nonsubsampled shearlet transform domain. J. Med. Imaging Health Inform. 2020, 10, 1785–1794. [Google Scholar] [CrossRef]
- Zhao, T.; Zhang, S. X-ray image enhancement based on nonsubsampled shearlet transform and gradient domain guided filtering. Sensors 2022, 22, 4074. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Sun, J.; Tang, X. Guided image filtering. IEEE Trans. Pattern Anal. Mach. Intell. 2013, 35, 1397–1409. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zheng, J.; Zhu, Z. Weighted guided image filtering. IEEE Trans. Image Process. 2015, 24, 120–129. [Google Scholar]
- Kou, F.; Chen, W.; Wen, C. Gradient domain guided image filtering. IEEE Trans. Image Process. 2015, 24, 4528–4539. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Han, B.; Li, J. Weighted guided image filtering with steering kernel. IEEE Trans. Image Process. 2020, 29, 500–508. [Google Scholar] [CrossRef]
- Ochotorena, C.; Yamashita, Y. Anisotropic guided filtering. IEEE Trans. Image Process. 2020, 29, 1397–1412. [Google Scholar] [CrossRef]
- Lu, Z.; Long, B.; Li, K. Effective guided image filtering for contrast enhancement. IEEE Signal Process. Lett. 2018, 25, 1585–1589. [Google Scholar] [CrossRef]
- Yin, H.; Gong, Y.; Qiu, G. Side window guided filtering. Signal Process. 2019, 165, 315–330. [Google Scholar] [CrossRef]
- Chen, B.; Wu, S. Weighted aggregation for guided image filtering. Signal Image Video Process. 2020, 14, 491–498. [Google Scholar] [CrossRef]
- Zhang, X.; He, C. Robust double-weighted guided image filtering. Signal Process. 2022, 199, 108609. [Google Scholar] [CrossRef]
- Funt, B.; Ciurea, F.; Mccann, J. Retinex in MATLABTM. J. Electron. Imaging 2004, 13, 48–57. [Google Scholar] [CrossRef]
- Zuo, C.; Chen, Q.; Sui, X. Range limited bi-histogram equalization for image contrast enhancement. Optik 2013, 124, 425–431. [Google Scholar] [CrossRef]
- Al-Ameen, Z. A new algorithm for improving the low contrast of computed tomography images using tuned brightness controlled single-scale retinex. Scanning 2015, 37, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Al-Ameen, Z. Contrast enhancement of medical images using statistical methods with image processing concepts. In Proceedings of the 2020 6th International Engineering Conference, Sustainable Technology and Development (IEC) 2020, Erbil, Iraq, 26–27 February 2020; pp. 169–173. [Google Scholar]
- Al-Ameen, Z. Contrast enhancement of digital images using an improved Type-II fuzzy set-based algorithm. Traitem. Signal 2021, 38, 39–50. [Google Scholar] [CrossRef]
- Kumar, B.S. Image fusion based on pixel significance using cross bilateral filter. Signal Image Video Process. 2015, 9, 1193–1204. [Google Scholar] [CrossRef]
- Zhan, K.; Shi, J.; Teng, J. Linking synaptic computation for image enhancement. Neurocomputing 2017, 238, 1–12. [Google Scholar] [CrossRef]





| AG | H | ALC | SF | MG | |
|---|---|---|---|---|---|
| McCann | 6.3692 | 7.6512 | 0.4712 | 10.9025 | 5.3625 |
| RLBHE | 3.8103 | 6.9851 | 0.1225 | 6.2601 | 3.2202 |
| RESIHE | 6.5199 | 7.6378 | 0.5963 | 10.9708 | 5.5011 |
| TBCSSR | 7.7360 | 6.4739 | 0.9298 | 13.2405 | 6.5689 |
| GDGIF | 8.0281 | 7.6542 | 0.9125 | 12.7309 | 6.7635 |
| SMIPC | 6.2976 | 7.7175 | 0.6225 | 10.8886 | 5.3096 |
| FuzzyII | 6.8285 | 7.4938 | 0.7476 | 11.8243 | 5.7914 |
| Proposed | 10.3812 | 7.7013 | 1.4911 | 17.6055 | 8.8347 |
| AG | H | ALC | SF | MG | |
|---|---|---|---|---|---|
| McCann | 5.1281 | 7.4184 | 0.4665 | 12.5124 | 4.3633 |
| RLBHE | 2.3495 | 6.0856 | 0.4310 | 6.7396 | 1.9789 |
| RESIHE | 5.3859 | 7.1987 | 0.8933 | 13.3430 | 4.6023 |
| TBCSSR | 5.3069 | 5.8050 | 1.2322 | 15.4840 | 4.5424 |
| GDGIF | 6.9491 | 7.3688 | 1.3773 | 15.1471 | 5.8878 |
| SMIPC | 5.2131 | 7.2701 | 0.8633 | 13.1491 | 4.4709 |
| FuzzyII | 5.1984 | 7.0568 | 0.9300 | 14.1044 | 4.4660 |
| Proposed | 7.5621 | 7.1856 | 1.8069 | 20.1044 | 6.5175 |
| AG | H | ALC | SF | MG | |
|---|---|---|---|---|---|
| McCann | 4.0123 | 7.5435 | 0.2870 | 8.9464 | 3.4352 |
| RLBHE | 2.2445 | 6.7002 | 0.0932 | 4.8208 | 1.8789 |
| RESIHE | 4.5401 | 7.3486 | 0.4647 | 9.6568 | 3.8846 |
| TBCSSR | 3.9534 | 5.8991 | 0.5479 | 9.4282 | 3.3742 |
| GDGIF | 5.1827 | 7.5822 | 0.8479 | 11.0629 | 4.4008 |
| SMIPC | 4.0469 | 7.4819 | 0.4668 | 9.1315 | 3.4699 |
| FuzzyII | 4.0409 | 7.3498 | 0.5045 | 9.2729 | 3.4821 |
| Proposed | 6.6297 | 7.5951 | 0.8829 | 12.9503 | 5.6974 |
| AG | H | ALC | SF | MG | |
|---|---|---|---|---|---|
| McCann | 4.9747 | 7.3960 | 0.2901 | 9.1737 | 4.0981 |
| RLBHE | 2.4721 | 6.6451 | 0.0514 | 4.2870 | 2.0405 |
| RESIHE | 5.4976 | 7.6038 | 0.4383 | 9.8437 | 4.5322 |
| TBCSSR | 6.1722 | 6.6784 | 0.6021 | 11.0394 | 5.1153 |
| GDGIF | 7.0291 | 7.6321 | 0.6090 | 11.4703 | 5.7992 |
| SMIPC | 4.9663 | 7.6440 | 0.3823 | 9.1813 | 4.0798 |
| FuzzyII | 5.5589 | 7.4430 | 0.4732 | 10.0969 | 4.6027 |
| Proposed | 7.8310 | 7.7382 | 0.8196 | 13.7265 | 6.5771 |
| AG | H | ALC | SF | MG | |
|---|---|---|---|---|---|
| McCann | 4.5097 | 7.1685 | 0.2709 | 8.4758 | 3.7301 |
| RLBHE | 2.9646 | 6.4171 | 0.1004 | 5.3958 | 2.4631 |
| RESIHE | 5.3505 | 7.1441 | 0.4545 | 9.7358 | 4.4233 |
| TBCSSR | 5.8109 | 6.2229 | 0.5695 | 10.6472 | 4.8123 |
| GDGIF | 6.3404 | 7.2809 | 0.5814 | 10.5298 | 5.2334 |
| SMIPC | 4.5190 | 7.2783 | 0.3798 | 8.5168 | 3.7244 |
| FuzzyII | 5.0117 | 7.1125 | 0.4527 | 9.3992 | 4.1508 |
| Proposed | 7.1445 | 7.3520 | 0.8475 | 13.3944 | 5.9647 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Lv, M.; Ma, H.; Jia, Z.; Yang, X.; Yang, W. X-ray Image Enhancement Based on Adaptive Gradient Domain Guided Image Filtering. Appl. Sci. 2022, 12, 10453. https://doi.org/10.3390/app122010453
Li L, Lv M, Ma H, Jia Z, Yang X, Yang W. X-ray Image Enhancement Based on Adaptive Gradient Domain Guided Image Filtering. Applied Sciences. 2022; 12(20):10453. https://doi.org/10.3390/app122010453
Chicago/Turabian StyleLi, Liangliang, Ming Lv, Hongbing Ma, Zhenhong Jia, Xinghua Yang, and Weiyi Yang. 2022. "X-ray Image Enhancement Based on Adaptive Gradient Domain Guided Image Filtering" Applied Sciences 12, no. 20: 10453. https://doi.org/10.3390/app122010453
APA StyleLi, L., Lv, M., Ma, H., Jia, Z., Yang, X., & Yang, W. (2022). X-ray Image Enhancement Based on Adaptive Gradient Domain Guided Image Filtering. Applied Sciences, 12(20), 10453. https://doi.org/10.3390/app122010453







