Anodic Alumina Membranes: From Electrochemical Growth to Use as Template for Fabrication of Nanostructured Electrodes
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
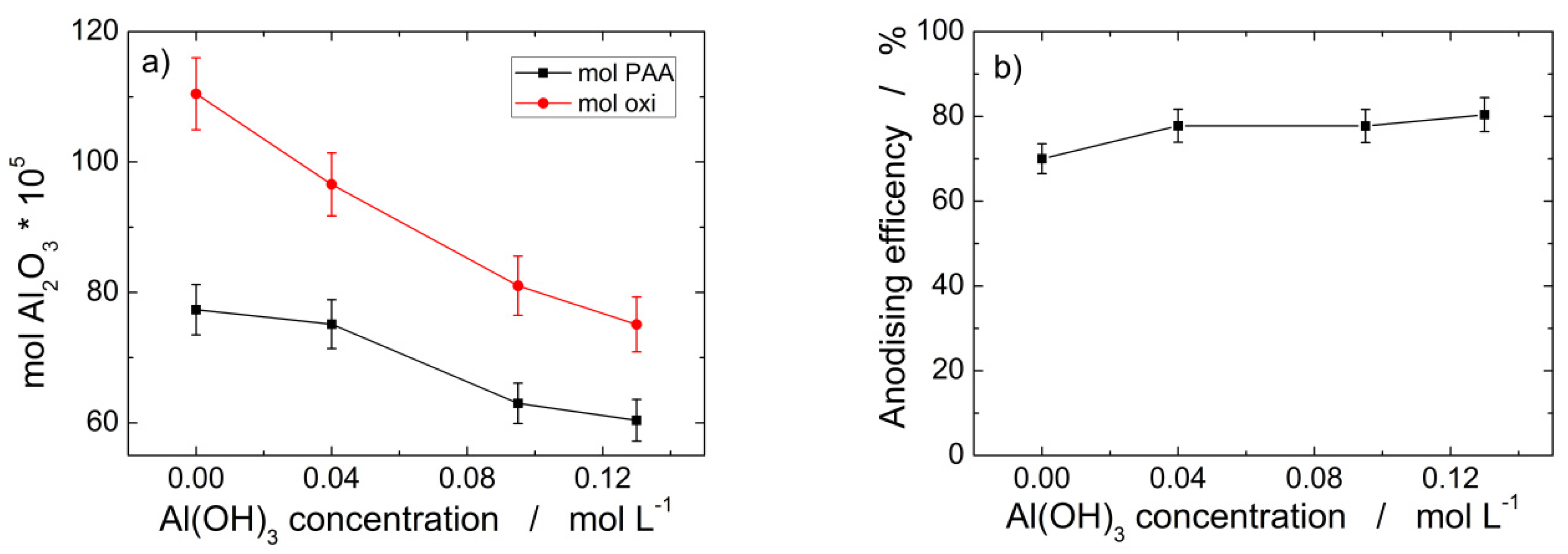
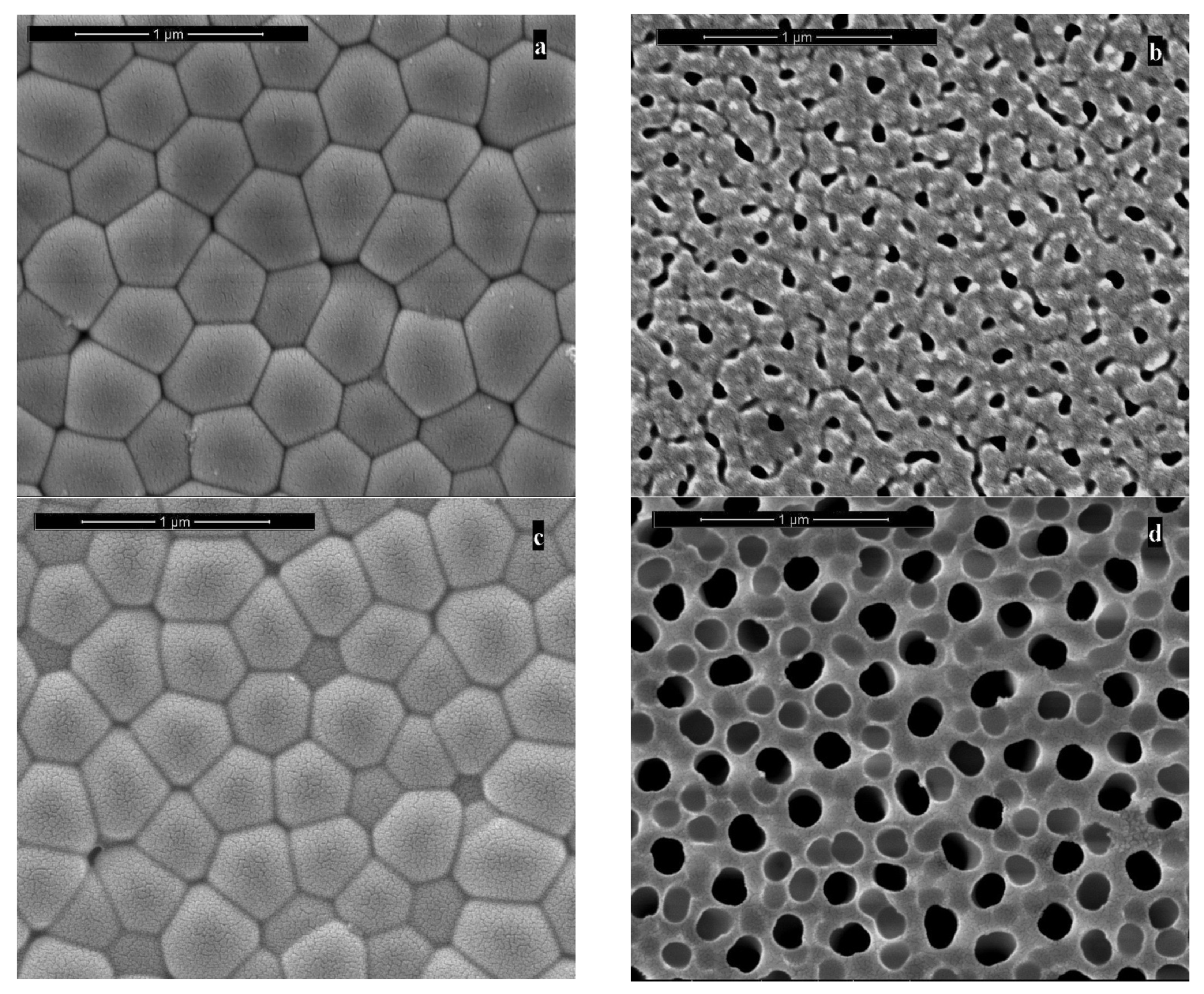
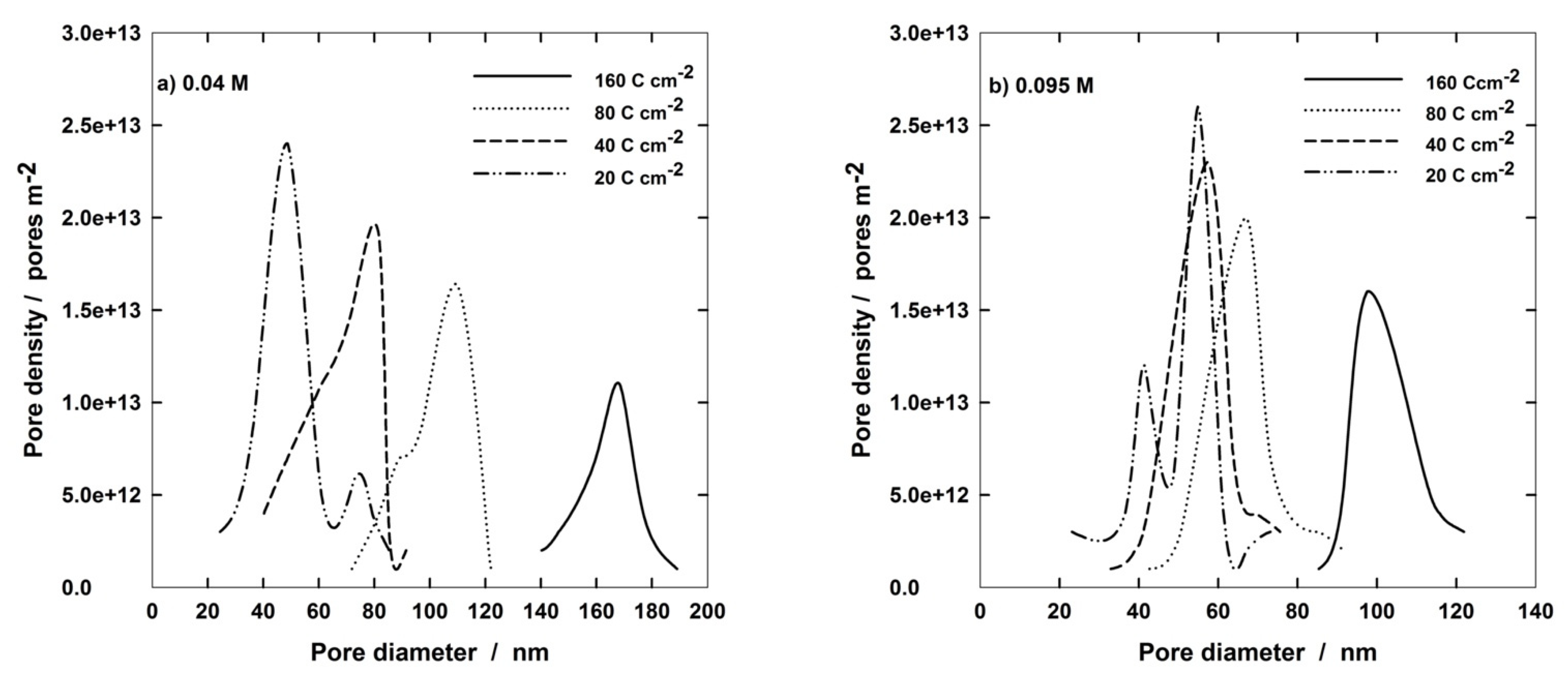
3.1. Fabrication and Characterization of AAM
3.2. Fabrication and Characterization of Nanostructures
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Keller, F.; Hunter, M.S.; Robinson, D.L. Structural Features of Oxide Coatings on Aluminum. J. Electrochem. Soc. 1953, 100, 411. [Google Scholar] [CrossRef]
- Dell’Oca, C.J.; Pulfrey, D.L.; Young, L. Anodic Oxide Films. In Physics of Thin Films; Elsevier: Amsterdam, The Netherlands, 1971; Volume 6, pp. 1–79. ISBN 978-0-12-533006-0. [Google Scholar] [CrossRef]
- Diggle, J.W.; Downie, T.C.; Goulding, C.W. Anodic oxide films on aluminum. Chem. Rev. 1969, 69, 365–405. [Google Scholar] [CrossRef]
- Henley, V.F. Anodic Oxidation of Aluminium and Its Alloys: The Pergamon Materials Engineering Practice Series; Elsevier Science: Kent, UK, 2014; ISBN 978-1-4831-4733-8. Available online: http://qut.eblib.com.au/patron/FullRecord.aspx?p=1838701 (accessed on 15 September 2021).
- Thompson, G.E.; Wood, G.C. Porous anodic film formation on aluminium. Nature 1981, 290, 230–232. [Google Scholar] [CrossRef]
- Wood, G.C.; O’Sullivan, J.P. The anodizing of aluminium in sulphate solutions. Electrochim. Acta 1970, 15, 1865–1876. [Google Scholar] [CrossRef]
- Garcia-Vergara, S.J.; Skeldon, P.; Thompson, G.E.; Habazaki, H. A flow model of porous anodic film growth on aluminium. Electrochim. Acta 2006, 52, 681–687. [Google Scholar] [CrossRef]
- Oh, J.; Thompson, C.V. The role of electric field in pore formation during aluminum anodization. Electrochim. Acta 2011, 56, 4044–4051. [Google Scholar] [CrossRef]
- Poinern, G.E.J.; Ali, N.; Fawcett, D. Progress in Nano-Engineered Anodic Aluminum Oxide Membrane Development. Materials 2011, 4, 487–526. [Google Scholar] [CrossRef]
- Patermarakis, G.; Plytas, J. A novel theory interpreting the extremes of current during potentiostatic anodising of Al and the mechanisms of normal and abnormal growth of porous anodic alumina films. J. Electroanal. Chem. 2016, 769, 97–117. [Google Scholar] [CrossRef]
- Poznyak, A.; Knörnschild, G.; Karoza, A.; Norek, M.; Pligovka, A. Peculiar Porous Aluminum Oxide Films Produced via Electrochemical Anodizing in Malonic Acid Solution with Arsenazo-I Additive. Materials 2021, 14, 5118. [Google Scholar] [CrossRef]
- Cheng, C.; Ngan, A.H.W. Modelling and simulation of self-ordering in anodic porous alumina. Electrochim. Acta 2011, 56, 9998–10008. [Google Scholar] [CrossRef]
- Ono, S.; Saito, M.; Asoh, H. Self-ordering of anodic porous alumina formed in organic acid electrolytes. Electrochim. Acta 2005, 51, 827–833. [Google Scholar] [CrossRef]
- Lee, W.; Schwirn, K.; Steinhart, M.; Pippel, E.; Scholz, R.; Gösele, U. Structural engineering of nanoporous anodic aluminium oxide by pulse anodization of aluminium. Nat. Nanotechnol. 2008, 3, 234–239. [Google Scholar] [CrossRef]
- Lee, W.; Park, S.-J. Porous Anodic Aluminum Oxide: Anodization and Templated Synthesis of Functional Nanostructures. Chem. Rev. 2014, 114, 7487–7556. [Google Scholar] [CrossRef]
- Cai, Y.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. A smart membrane with antifouling capability and switchable oil wettability for high-efficiency oil/water emulsions separation. J. Membr. Sci. 2018, 555, 69–77. [Google Scholar] [CrossRef]
- Dai, J.; Singh, J.; Yamamoto, N. Nonbrittle nanopore deformation of anodic aluminum oxide membranes. J. Am. Ceram. Soc. 2018, 101, 2170–2180. [Google Scholar] [CrossRef]
- Pakizeh, E.; Moradi, M. Kramers-Kronig method for determination of optical properties of PZT nanotubes fabricated by sol-gel method and porous anodic alumina with high aspect ratio. Int. J. Mod. Phys. B 2018, 32, 1850096. [Google Scholar] [CrossRef]
- Aw, M.S.; Bariana, M.; Losic, D. Nanoporous Alumina: Fabrication, Structure, Properties and Applications, 1st ed.; Losic, D., Santos, A., Eds.; Springer Series in Materials Science; Springer International Publishing: Cham, Switzerland, 2015; ISBN 978-3-319-20334-8. [Google Scholar]
- Ruiz-Clavijo, A.; Caballero-Calero, O.; Martín-González, M. Revisiting anodic alumina templates: From fabrication to applications. Nanoscale 2021, 13, 2227–2265. [Google Scholar] [CrossRef] [PubMed]
- Dobosz, I. Influence of the anodization conditions and chemical treatment on the formation of alumina membranes with defined pore diameters. J. Porous Mater. 2021, 28, 1011–1022. [Google Scholar] [CrossRef]
- Zaraska, L.; Jaskuła, M.; Sulka, G.D. Porous anodic alumina layers with modulated pore diameters formed by sequential anodizing in different electrolytes. Mater. Lett. 2016, 171, 315–318. [Google Scholar] [CrossRef]
- Roslyakov, I.V.; Chumakov, A.P.; Eliseev, A.A.; Leontiev, A.P.; Konovalov, O.V.; Napolskii, K.S. Evolution of Pore Ordering during Anodizing of Aluminum Single Crystals: In Situ Small-Angle X-ray Scattering Study. J. Phys. Chem. C 2021, 125, 9287–9295. [Google Scholar] [CrossRef]
- Masuda, H.; Yamada, H.; Satoh, M.; Asoh, H.; Nakao, M.; Tamamura, T. Highly ordered nanochannel-array architecture in anodic alumina. Appl. Phys. Lett. 1997, 71, 2770–2772. [Google Scholar] [CrossRef]
- Masuda, H.; Fukuda, K. Ordered Metal Nanohole Arrays Made by a Two-Step Replication of Honeycomb Structures of Anodic Alumina. Science 1995, 268, 1466–1468. [Google Scholar] [CrossRef] [PubMed]
- Sulka, G.D.; Stroobants, S.; Moshchalkov, V.; Borghs, G.; Celis, J.-P. Synthesis of Well-Ordered Nanopores by Anodizing Aluminum Foils in Sulfuric Acid. J. Electrochem. Soc. 2002, 149, D97. [Google Scholar] [CrossRef]
- Napolskii, K.S.; Noyan, A.A.; Kushnir, S.E. Control of high-order photonic band gaps in one-dimensional anodic alumina photonic crystals. Opt. Mater. 2020, 109, 110317. [Google Scholar] [CrossRef]
- Almasi Kashi, M.; Ramazani, A.; Abbasian, H.; Khayyatian, A. Capacitive humidity sensors based on large diameter porous alumina prepared by high current anodization. Sens. Actuators Phys. 2012, 174, 69–74. [Google Scholar] [CrossRef]
- Inguanta, R.; Sunseri, C.; Piazza, S. Photoelectrochemical Characterization of Cu2O-Nanowire Arrays Electrodeposited into Anodic Alumina Membranes. Electrochem. Solid State Lett. 2007, 10, K63. [Google Scholar] [CrossRef]
- Inguanta, R.; Livreri, P.; Piazza, S.; Sunseri, C. Fabrication and Photoelectrochemical Behavior of Ordered CIGS Nanowire Arrays for Application in Solar Cells. Electrochem. Solid State Lett. 2010, 13, K22. [Google Scholar] [CrossRef][Green Version]
- Zhou, Z.; Nonnenmann, S.S. Progress in Nanoporous Templates: Beyond Anodic Aluminum Oxide and Towards Functional Complex Materials. Materials 2019, 12, 2535. [Google Scholar] [CrossRef]
- Wen, L.; Xu, R.; Mi, Y.; Lei, Y. Multiple nanostructures based on anodized aluminium oxide templates. Nat. Nanotechnol. 2017, 12, 244–250. [Google Scholar] [CrossRef]
- Inguanta, R.; Rinaldo, E.; Piazza, S.; Sunseri, C. Lead Nanowires for Microaccumulators Obtained Through Indirect Electrochemical Template Deposition. Electrochem. Solid State Lett. 2010, 13, K1. [Google Scholar] [CrossRef]
- Battaglia, M.; Inguanta, R.; Piazza, S.; Sunseri, C. Fabrication and characterization of nanostructured Ni-IrO2 electrodes for water electrolysis. Int. J. Hydrogen Energy 2014, 39, 16797–16805. [Google Scholar] [CrossRef]
- Ganci, F.; Cusumano, V.; Livreri, P.; Aiello, G.; Sunseri, C.; Inguanta, R. Nanostructured Ni-Co alloy electrodes for both hydrogen and oxygen evolution reaction in alkaline electrolyzer. Int. J. Hydrogen Energy 2020, 46, 10082–10092. [Google Scholar] [CrossRef]
- Patella, B.; Inguanta, R.; Piazza, S.; Sunseri, C. Nanowire ordered arrays for electrochemical sensing of H2O2. Chem. Eng. Trans. 2016, 47, 19–24. [Google Scholar] [CrossRef]
- Buccheri, B.; Ganci, F.; Patella, B.; Aiello, G.; Mandin, P.; Inguanta, R. Ni-Fe alloy nanostructured electrodes for water splitting in alkaline electrolyser. Electrochim. Acta 2021, 388, 138588. [Google Scholar] [CrossRef]
- Battaglia, M.; Piazza, S.; Sunseri, C.; Inguanta, R. Amorphous silicon nanotubes via galvanic displacement deposition. Electrochem. Commun. 2013, 34, 134–137. [Google Scholar] [CrossRef]
- Ganci, F.; Lombardo, S.; Sunseri, C.; Inguanta, R. Nanostructured electrodes for hydrogen production in alkaline electrolyzer. Renew. Energy 2018, 123, 117–124. [Google Scholar] [CrossRef]
- Patella, B.; Russo, R.R.; O’Riordan, A.; Aiello, G.; Sunseri, C.; Inguanta, R. Copper nanowire array as highly selective electrochemical sensor of nitrate ions in water. Talanta 2021, 221, 121643. [Google Scholar] [CrossRef]
- Inguanta, R.; Ferrara, G.; Piazza, S.; Sunseri, C. Nanostructure fabrication by template deposition into anodic alumina membranes. Chem. Eng. Trans. 2009, 17, 957–962. [Google Scholar] [CrossRef]
- Insinga, M.G.; Oliveri, R.L.; Sunseri, C.; Inguanta, R. Template electrodeposition and characterization of nanostructured Pb as a negative electrode for lead-acid battery. J. Power Sources 2019, 413, 107–116. [Google Scholar] [CrossRef]
- Inguanta, R.; Amodeo, M.; D’Agostino, F.; Volpe, M.; Piazza, S.; Sunseri, C. Developing a procedure to optimize electroless deposition of thin palladium layer on anodic alumina membranes. Desalination 2006, 199, 352–354. [Google Scholar] [CrossRef]
- Ono, S.; Masuko, N. Evaluation of pore diameter of anodic porous films formed on aluminum. Surf. Coat. Technol. 2003, 169, 139–142. [Google Scholar] [CrossRef]
- Feng, S.; Ji, W. Advanced Nanoporous Anodic Alumina-Based Optical Sensors for Biomedical Applications. Front. Nanotechnol. 2021, 3, 678275. [Google Scholar] [CrossRef]
- Inguanta, R.; Butera, M.; Sunseri, C.; Piazza, S. Fabrication of metal nano-structures using anodic alumina membranes grown in phosphoric acid solution: Tailoring template morphology. Appl. Surf. Sci. 2007, 253, 5447–5456. [Google Scholar] [CrossRef]
- Sasikala, N.T.; Boominathasellarajan, S.; Raghupathy, B.P.C. Fabrication of nanostructures using porous anodic alumina template. Int. J. Nanosci. 2011, 10, 783–786. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, L.; Lei, Y. A mini review: Functional nanostructuring with perfectly-ordered anodic aluminum oxide template for energy conversion and storage. Front. Chem. Sci. Eng. 2018, 12, 481–493. [Google Scholar] [CrossRef]
- Lim, H.R.; Eom, N.S.A.; Cho, J.-H.; Choa, Y.-H. Moisture Gettering by Porous Alumina Films on Textured Silicon Wafer. Korean Chem. Eng. Res. 2015, 53, 401–406. [Google Scholar] [CrossRef]
- Wang, G.; Wang, J.; Li, S.-Y.; Zhang, J.-W.; Wang, C.-W. One-dimensional alumina photonic crystals with a narrow band gap and their applications to high-sensitivity concentration sensor and photoluminescence enhancement. Superlattices Microstruct. 2015, 86, 546–551. [Google Scholar] [CrossRef]
- Sunseri, C.; Cocchiara, C.; Ganci, F.; Moncada, A.; Oliveri, R.L.; Patella, B.; Piazza, S.; Inguanta, R. Nanostructured electrochemical devices for sensing, energy conversion and storage. Chem. Eng. Trans. 2016, 47, 43–48. [Google Scholar] [CrossRef]
- Inguanta, R.; Piazza, S.; Sunseri, C.; Cino, A.; Di Dio, V.; Cascia, D.L.; Miceli, R.; Rando, C.; Zizzo, G. An Electrochemical Route towards the Fabrication of Nanostructured Semiconductor Solar Cells. In Proceedings of the SPEEDAM, Pisa, Italy, 14–16 June 2010; IEEE: Pisa, Italy, 2010; pp. 1166–1171. [Google Scholar]
- Genduso, G.; Inguanta, R.; Sunseri, C.; Piazza, S.; Kelch, C.; Sáez-Araoz, R.; Zykov, A.; Fischer, C.-H. Deposition of very thin uniform indium sulfide layers over metallic nano-rods by the Spray-Ion Layer Gas Reaction method. Thin Solid Films 2013, 548, 91–97. [Google Scholar] [CrossRef]
- Volpe, M.; Inguanta, R.; Piazza, S.; Sunseri, C. Optimized bath for electroless deposition of palladium on amorphous alumina membranes. Surf. Coat. Technol. 2006, 200, 5800–5806. [Google Scholar] [CrossRef]
- Zimer, A.M.; Machado, M.M.P.; Júnior, L.J.D.C.; Ike, P.T.L.; Iemma, M.R.C.; Yamamoto, C.F.; Ferreira, C.F.; Souza, D.H.F.; de Oliveira, C.R.; Pereira, E.C. Optimized Porous Anodic Alumina Membranes for Water Ultrafiltration of Pathogenic Bacteria (E. coli). J. Nanosci. Nanotechnol. 2016, 16, 6526–6534. [Google Scholar] [CrossRef]
- Inguanta, R.; Rinaldo, E.; Piazza, S.; Sunseri, C. Formation of lead by reduction of electrodeposited PbO2: Comparison between bulk films and nanowires fabrication. J. Solid State Electrochem. 2012, 16, 3939–3946. [Google Scholar] [CrossRef]
- Ferrara, G.; Inguanta, R.; Piazza, S.; Sunseri, C. Electro-Synthesis of Sn-Co Nanowires in Alumina Membranes. J. Nanosci. Nanotechnol. 2010, 10, 8328–8335. [Google Scholar] [CrossRef] [PubMed]
- Cocchiara, C.; Sunseri, C.; Piazza, S.; Inguanta, R. Pb-PbOHCl Composite Nanowires Synthesized by Galvanic Deposition in Template. J. Nanosci. Nanotechnol. 2019, 19, 4677–4685. [Google Scholar] [CrossRef] [PubMed]
- Inguanta, R.; Vergottini, F.; Ferrara, G.; Piazza, S.; Sunseri, C. Effect of temperature on the growth of α-PbO2 nanostructures. Electrochim. Acta 2010, 55, 8556–8562. [Google Scholar] [CrossRef]
- Fu, J.; Mao, P.; Han, J. Artificial molecular sieves and filters: A new paradigm for biomolecule separation. Trends Biotechnol. 2008, 26, 311–320. [Google Scholar] [CrossRef]
- Shi, W.; Shen, Y.; Ge, D.; Xue, M.; Cao, H.; Huang, S.; Wang, J.; Zhang, G.; Zhang, F. Functionalized anodic aluminum oxide (AAO) membranes for affinity protein separation. J. Membr. Sci. 2008, 325, 801–808. [Google Scholar] [CrossRef]
- Inguanta, R.; Piazza, S.; Sunseri, C. A Route to Grow Oxide Nanostructures Based on Metal Displacement Deposition: Lanthanides Oxy/Hydroxides Characterization. J. Electrochem. Soc. 2012, 159, D493–D500. [Google Scholar] [CrossRef]
- Inguanta, R.; Ferrara, G.; Piazza, S.; Sunseri, C. A new route to grow oxide nanostructures based on metal displacement deposition. Lanthanides oxy/hydroxides growth. Electrochim. Acta 2012, 76, 77–87. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, G.; Ng, K.; Ji, Y.; Gao, B.; Huang, L.; Zhou, J.; Lu, T.J.; Xu, F. Engineering ellipsoidal cap-like hydrogel particles as building blocks or sacrificial templates for three-dimensional cell culture. Biomater. Sci. 2018, 6, 885–892. [Google Scholar] [CrossRef]
- Davoodi, E.; Zhianmanesh, M.; Montazerian, H.; Milani, A.S.; Hoorfar, M. Nano-porous anodic alumina: Fundamentals and applications in tissue engineering. J. Mater. Sci. Mater. Med. 2020, 31, 60. [Google Scholar] [CrossRef]
- Kondo, T.; Nagao, S.; Miyazaki, H.; Yanagishita, T.; Masuda, H. Tuning of the interval in a nanohole array of anodic porous alumina through deformation of polymer templates. RSC Adv. 2017, 7, 44799–44803. [Google Scholar] [CrossRef]
- Ferrara, G.; Inguanta, R.; Piazza, S.; Sunseri, C. Characterization of Sn-Co Nanowires Grown into Alumina Template. Electrochem. Solid State Lett. 2009, 12, K17. [Google Scholar] [CrossRef]
- Ferrara, G.; Arbizzani, C.; Damen, L.; Guidotti, M.; Lazzari, M.; Vergottini, F.G.; Inguanta, R.; Piazza, S.; Sunseri, C.; Mastragostino, M. Toward Tin-Based High-Capacity Anode for Lithium-Ion Battery. ECS Trans. 2014, 48, 153–162. [Google Scholar] [CrossRef]
- Inguanta, R.; Ferrara, G.; Livreri, P.; Piazza, S.; Sunseri, C. Ruthenium Oxide Nanotubes via Template Electrosynthesis. Curr. Nanosci. 2011, 7, 210–218. [Google Scholar] [CrossRef]
- Kohli, P.; Wharton, J.E.; Braide, O.; Martin, C.R. Template Synthesis of Gold Nanotubes in an Anodic Alumina Membrane. J. Nanosci. Nanotechnol. 2004, 4, 605–610. [Google Scholar] [CrossRef]
- Inguanta, R.; Ferrara, G.; Piazza, S.; Sunseri, C. Fabrication and characterization of metal and metal oxide nanostructures grown by metal displacement deposition into anodic alumina membranes. Chem. Eng. Trans. 2011, 24, 199–204. [Google Scholar] [CrossRef]
- Battaglia, M.; Sunseri, C.; Piazza, S.; Inguanta, R. CuZnSnSe Nanotubes and Nanowires by Template Electrosynthesis. Adv. Sci. Technol. 2014, 93, 241–246. [Google Scholar] [CrossRef]
- Şişman, İ. Template-Assisted Electrochemical Synthesis of Semiconductor Nanowires. In Nanowires—Implementations and Applications; Hashim, A., Ed.; InTech: London, UK, 2011; ISBN 978-953-307-318-7. [Google Scholar] [CrossRef]
- Wang, J.; Li, Z.; Gu, Z. A comprehensive review of template-synthesized multi-component nanowires: From interfacial design to sensing and actuation applications. Sens. Actuators Rep. 2021, 3, 100029. [Google Scholar] [CrossRef]
- Viqueira, M.S.; Pozo-López, G.; Urreta, S.E.; Condó, A.M.; Cornejo, D.R.; Fabietti, L.M. Magnetic hysteresis in small-grained Co Pd1-nanowire arrays. J. Magn. Magn. Mater. 2015, 394, 185–194. [Google Scholar] [CrossRef]
- Ferrara, G.; Inguanta, R.; Vergottini, F.; Piazza, S.; Sunseri, C. Nanostructured Material Fabrication for Energy Conversion; ECS Transactions: Brno, Czech Republic, 2011; pp. 55–63. [Google Scholar]
- Du, L.; Feng, D.; Xing, X.; Fu, Y.; Fonseca, L.F.; Yang, D. Palladium/cobalt nanowires with improved hydrogen sensing stability at ultra-low temperatures. Nanoscale 2019, 11, 21074–21080. [Google Scholar] [CrossRef] [PubMed]
- Taşaltın, N.; Öztürk, S.; Kılınç, N.; Yüzer, H.; Öztürk, Z.Z. Fabrication of vertically aligned Pd nanowire array in AAO template by electrodeposition using neutral electrolyte. Nanoscale Res. Lett. 2010, 5, 1137–1143. [Google Scholar] [CrossRef]
- Flaschka, H.A. EDTA Titrations: An Introduction to Theory and Practice; Elsevier Science: Kent, UK, 2013; ISBN 978-1-4832-2175-5. Available online: http://qut.eblib.com.au/patron/FullRecord.aspx?p=1839240 (accessed on 18 September 2021).
- Vogel, A.I.; Jeffery, G.H. Vogel’s Textbook of Quantitative Chemical Analysis, 6th ed.; Prentice Hall: Harlow, UK, 2000; ISBN 978-0-582-22628-9. [Google Scholar]
- Skeldon, P.; Thompson, G.E.; Garcia-Vergara, S.J.; Iglesias-Rubianes, L.; Blanco-Pinzon, C.E. A Tracer Study of Porous Anodic Alumina. Electrochem. Solid-State Lett. 2006, 9, B47. [Google Scholar] [CrossRef]
- Yu, M.; Cui, H.; Li, H.; Zhang, S.; Kong, J.; Zhao, S.; Ai, F.; Song, Y.; Zhu, X. Anodizations of Al and Ti in NH4F or H3PO4 Solutions and Formation of Porous Anodic Alumina with Special Morphology. J. Phys. Chem. C 2018, 122, 549–556. [Google Scholar] [CrossRef]
- Yu, M.; Zhang, W.; Zhang, S.; Zhao, S.; Ai, F.; Zhu, X. Morphology evolution of porous anodic alumina in mixed H3PO4/NH4F electrolytes. Surf. Coat. Technol. 2018, 334, 500–508. [Google Scholar] [CrossRef]
- Inguanta, R.; Piazza, S.; Sunseri, C. Synthesis of self-standing Pd nanowires via galvanic displacement deposition. Electrochem. Commun. 2009, 11, 1385–1388. [Google Scholar] [CrossRef]






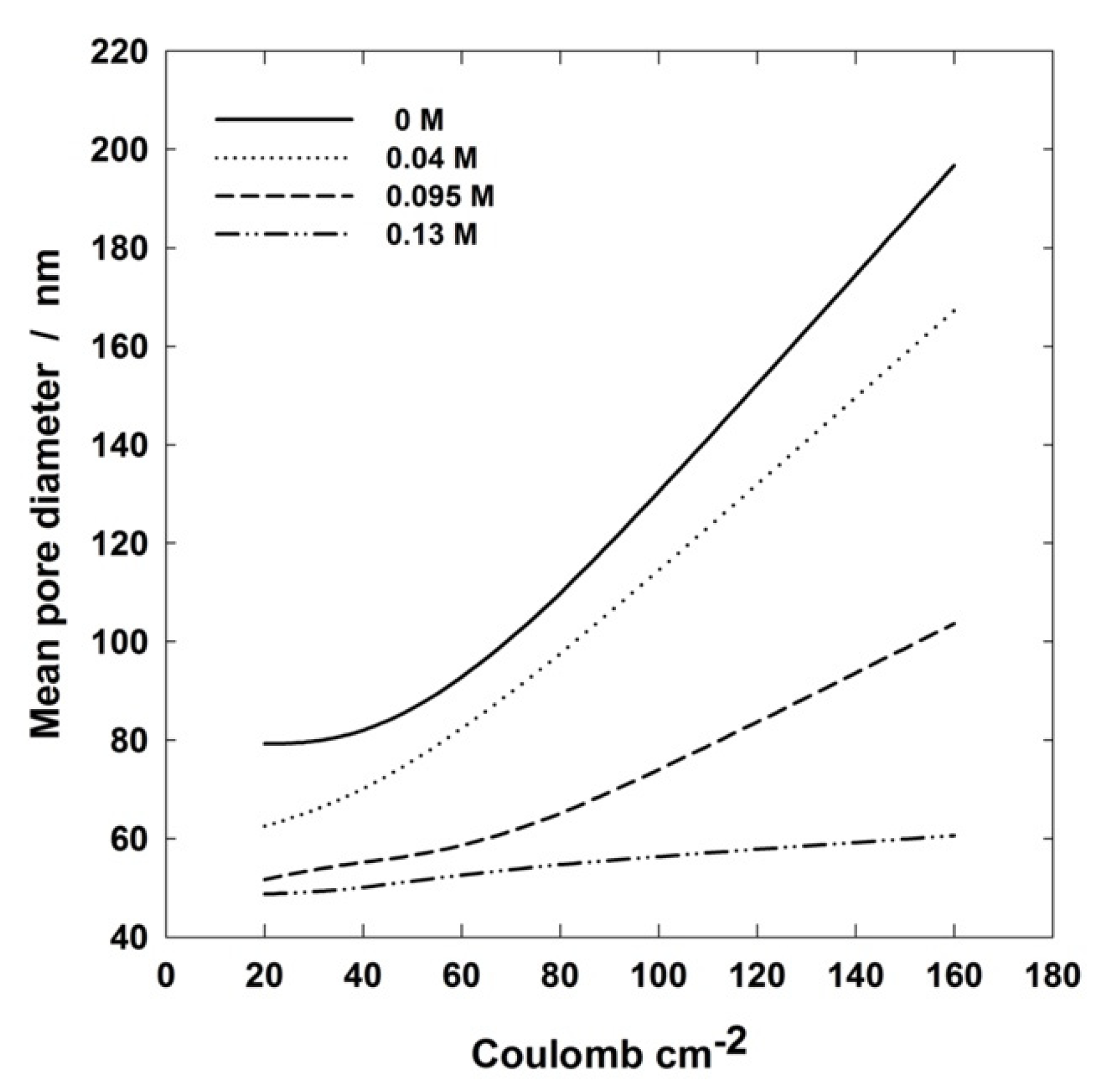
| Electrolyte | 20 C cm−2 | 40 C cm−2 | 80 C cm−2 | 160 C cm−2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pore Mean Size. nm ±5% | Pores m−2 ×1013 ±10% | Cells m−2 ×1012 ±1% | Pore Mean Size. nm ±5% | Pores m−2 ×1013 ±10% | Cells m−2 ×1012 ±1% | Pore Mean Size. nm ±5% | Pores m−2 ×1013 ±10% | Cells m−2 ×1012 ±1% | Pore Mean Size. nm ±5% | Pores m−2 ×1013 ±10% | Cells m−2 ×1012 ±1% | |
| 0.4 M H3PO4 | 79.27 | 1.96 | 6.53 | 81.98 | 1.86 | 6.53 | 109.76 | 1.73 | 6.53 | 196.75 | 0.98 | 6.53 |
| 0.4 M H3PO4 0.04 M Al(OH)3 | 62.5 | 2.48 | 6.53 | 70.12 | 2.12 | 6.53 | 97.56 | 1.84 | 6.53 | 167.25 | 1.21 | 6.53 |
| 0.4 M H3PO4 0.095 M Al(OH)3 | 51.68 | 2.63 | 6.53 | 55.17 | 2.32 | 6.53 | 65.1 | 2.04 | 6.53 | 103.66 | 1.67 | 6.53 |
| 0.4 M H3PO4 0.13 M Al(OH)3 | 48.71 | 1.04 | 6.53 | 50.09 | 1.18 | 6.53 | 54.68 | 1.15 | 6.53 | 60.6 | 1.13 | 6.53 |
| Electrolyte | PAA Layer Thickness, µm ± 5% | |||
|---|---|---|---|---|
| 20 C cm−2 | 40 C cm−2 | 80 C cm−2 | 160 C cm−2 | |
| 0.4 M H3PO4 | 12.15 | 24.7 | 43.3 | 89.3 |
| 0.4 M H3PO4 0.04 M Al(OH)3 | 11.63 | 21.40 | 40.97 | 82.60 |
| 0.4 M H3PO4 0.095 M Al(OH)3 | 9 | 19.3 | 39.4 | 81.2 |
| 0.4 M H3PO4 0.13 M Al(OH)3 | 6.4 | 15.7 | 29.1 | 59.8 |
| Sample | FESEM | EDS | XRD |
|---|---|---|---|
| Pd NWs 3 μm (0.035 M Pd2+) | 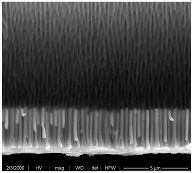 | 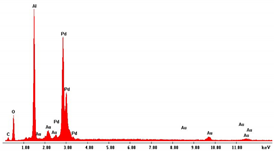 |  |
| Co NWs 4.5 μm (0.035 M Co2+) | 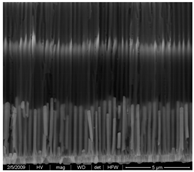 | 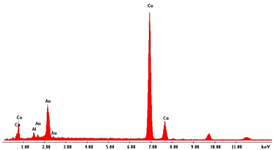 |  |
| PdCo NWs 4.5 μm (0.035 M Pd2+ 0.0069 M Co2+) | 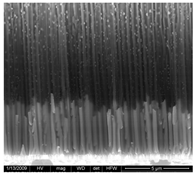 | 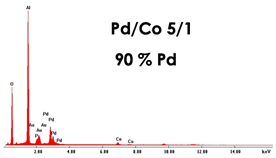 | 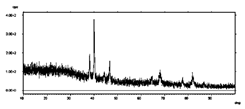 |
| PdCo NWs (0.035 M Pd2+ 0.035 M Co2+) | NWs 4.8 μm | 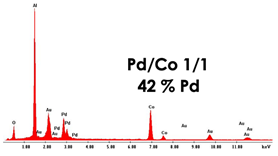 |  |
| PdCo NWs (0.035 M Pd2+ 0.17 M Co2+) | NWs 4.5 μm | 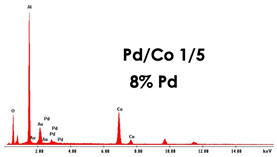 |  |
| PdCo NTs 3.5 μm (0.017 M Pd2+ 0.017 M Co2+) | 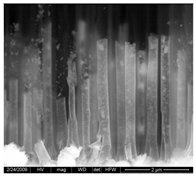 | Pd/Co 1/1 88 % PD | Pd and Au peaks |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patella, B.; Piazza, S.; Sunseri, C.; Inguanta, R. Anodic Alumina Membranes: From Electrochemical Growth to Use as Template for Fabrication of Nanostructured Electrodes. Appl. Sci. 2022, 12, 869. https://doi.org/10.3390/app12020869
Patella B, Piazza S, Sunseri C, Inguanta R. Anodic Alumina Membranes: From Electrochemical Growth to Use as Template for Fabrication of Nanostructured Electrodes. Applied Sciences. 2022; 12(2):869. https://doi.org/10.3390/app12020869
Chicago/Turabian StylePatella, Bernardo, Salvatore Piazza, Carmelo Sunseri, and Rosalinda Inguanta. 2022. "Anodic Alumina Membranes: From Electrochemical Growth to Use as Template for Fabrication of Nanostructured Electrodes" Applied Sciences 12, no. 2: 869. https://doi.org/10.3390/app12020869
APA StylePatella, B., Piazza, S., Sunseri, C., & Inguanta, R. (2022). Anodic Alumina Membranes: From Electrochemical Growth to Use as Template for Fabrication of Nanostructured Electrodes. Applied Sciences, 12(2), 869. https://doi.org/10.3390/app12020869







