Distribution of Diatom Resting Stages in Sediment near Artificial Reefs Deployed in the Dysphotic Zone: Exploration of New Artificial Reef Function
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Hydrographic Observation
2.3. Particle Size Distribution and Organic Matter Measurements
2.4. Diatom Community and Population in Sediment
2.4.1. Sampling
2.4.2. Direct Microscopic Observation
2.4.3. MPN Method
3. Results
3.1. Environmental Conditions
3.2. Diatom Resting Stages
3.2.1. Abundances and Composition Based on Direct Microscopic Observation
3.2.2. Abundance of Viable Cells Estimated by MPN
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baine, M. Artificial reefs: A review of their design, application, management and performance. Ocean Coast. Manag. 2001, 44, 241–259. [Google Scholar] [CrossRef]
- Becker, A.; Taylor, M.D.; Folpp, H.; Lowry, M.B. Managing the development of artificial reef systems: The need for quantitative goals. Fish Fish. 2018, 19, 740–752. [Google Scholar] [CrossRef]
- Lee, M.O.; Otake, S.; Kim, J.K. Transition of artificial reefs (ARs) research and its prospects. Ocean Coast. Manag. 2018, 154, 55–65. [Google Scholar] [CrossRef]
- London Convention and Protocol/UNEP. London Convention and Protocol/UNEP Guidelines for the Placement of Artificial Reefs; IMO (The International Marine Organization): London, UK, 2009; pp. 1–100. [Google Scholar]
- Paxton, A.B.; Shertzer, K.W.; Bacheler, N.M.; Kellison, G.T.; Riley, K.L.; Taylor, J.C. Meta-analysis reveals artificial reefs can be effective tools for fish community enhancement but are not one-size-fits-all. Front. Mar. Sci. 2020, 7, 282. [Google Scholar] [CrossRef]
- Simon, T.; Pinheiro, H.T.; Joyeux, J. Target fishes on artificial reefs: Evidences of impacts over nearby natural environments. Sci. Total Environ. 2011, 409, 4579–4584. [Google Scholar] [CrossRef]
- Layman, C.A.; Allgeier, J.E. An ecosystem ecology perspective on artificial reef production. J. Appl. Ecol. 2020, 57, 2139–2148. [Google Scholar] [CrossRef]
- Layman, C.A.; Allgeier, J.E.; Montaña, C.G. Mechanistic evidence of enhanced production on artificial reefs: A case study in a Bahamian seagrass ecosystem. Ecol. Eng. 2016, 95, 574–579. [Google Scholar] [CrossRef]
- Jeong, Y.K.; Lee, H.N.; Park, C.; Kim, D.S.; Kim, M.C. Variation of phytoplankton and zooplankton communities in a sea area, with the building of an artificial upwelling structure. Anim. Cells Syst. 2013, 17, 63–72. [Google Scholar] [CrossRef]
- Yu, J.; Chen, P.; Tang, D.; Qin, C. Ecological effects of artificial reefs in Daya Bay of China observed from satellite and in situ measurements. Adv. Space Res. 2015, 55, 2315–2324. [Google Scholar] [CrossRef]
- Field, C.B.; Behrenfeld, M.J.; Randerson, J.T.; Falkowski, P. Primary production of the biosphere: Integrating terrestrial and oceanic components. Science 1998, 281, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Mann, D.G. The species concept in diatoms. Phycologia 1999, 38, 437–495. [Google Scholar] [CrossRef]
- McQuoid, M.R.; Hobson, L.A. Diatom resting stages. J. Phycol. 1996, 32, 889–902. [Google Scholar] [CrossRef]
- Belmonte, G.; Rubino, F. Resting cysts from coastal marine plankton. In Oceanography and Marine Biology: An Annual Review; Hawkins, S.J., Allcock, A.L., Bates, A.E., Firth, L.B., Smith, I.P., Swearer, S.E., Todd, P.A., Eds.; CRC Press: Boca Raton, FL, USA, 2019; Volume 57, pp. 1–88. [Google Scholar]
- Hargraves, P.E.; French, F.W. Diatom resting spores: Significance and strategies. In Survival Strategies of the Algae; Fryxell, G.A., Ed.; Cambridge University Press: Cambridge, UK, 1983; pp. 49–67. [Google Scholar]
- Pitcher, G.C. Phytoplankton seed populations of the Cape Peninsula upwelling plume, with particular reference to resting spores of Chaetoceros (Bacillariophyceae) and their role in seeding upwelling waters. Estuar. Coast. Shelf Sci. 1990, 31, 283–301. [Google Scholar] [CrossRef]
- Ishikawa, A.; Furuya, K. The role of diatom resting stages in the onset of the spring bloom in the East China Sea. Mar. Biol. 2004, 145, 633–639. [Google Scholar] [CrossRef]
- Itakura, S.; Imai, I.; Itoh, K. “Seed bank” of coastal planktonic diatoms in bottom sediments of Hiroshima Bay, Seto Inland Sea, Japan. Mar. Biol. 1997, 128, 497–508. [Google Scholar] [CrossRef]
- Oh, T.G.; Otake, S.; Lee, M.O. Estimating the effective wake region (current shadow) of artificial reefs. In Artificial Reefs in Fisheries Management; Bortone, S.A., Brandini, F.P., Fabi, G., Otake, S., Eds.; CRC Press LLC: Boca Raton, FL, USA, 2011; pp. 279–295. [Google Scholar]
- Sheng, P.Y. Physical characteristics and engineering at reef sites. In Artificial Reef Evaluation: With Application to Natural Marine Habitats; Seaman, W.J., Ed.; CRC Press LLC: Boca Raton, FL, USA, 2000; pp. 51–94. [Google Scholar]
- Ishii, K.-I.; Imai, I.; Natsuike, M.; Sawayama, S.; Ishino, R.; Liu, W.; Fukusaki, K.; Ishikawa, A. A simple technique for establishing axenic cultures of centric diatoms from resting stage cells in bottom sediments. Phycologia 2018, 57, 674–679. [Google Scholar] [CrossRef]
- Rines, J.E.B.; Hargraves, P.E. The Chaetoceros Ehrenberg (Bacillariophyceae) Flora of Narragansett Bay, Rhode Island, U.S.A.; Lubrecht & Cramer: New York, NY, USA, 1988; pp. 1–196. [Google Scholar]
- Hasle, G.R.; Syvertsen, E.E. Marine Diatoms. In Identifying Marine Phytoplankton; Tomas, C.R., Ed.; Academic Press: London, UK, 1997; pp. 5–385. [Google Scholar]
- Jensen, K.G.; Moestrup, Ø. The Genus Chaetoceros (Bacillariophyceae) in inner Danish coastal waters. Opera Bot. 1998, 133, 5–68. [Google Scholar] [CrossRef]
- Ishii, K.-I.; Iwataki, M.; Matsuoka, K.; Imai, I. Proposal of identification criteria for resting spores of Chaetoceros species (Bacillariophyceae) from a temperate coastal sea. Phycologia 2011, 50, 351–362. [Google Scholar] [CrossRef]
- Imai, I.; Itoh, K.; Anraku, M. Extinction dilution method for enumeration of dormant cells of res tide organisms in marine sediments. Bull. Plankton Soc. Jpn. 1984, 31, 123–124. [Google Scholar]
- Anderson, D.M.; Fukuyo, Y.; Matsuoka, K. Cyst methodologies. In Manual on Harmful Marine Microalgae; Hallegraeff, G.M., Anderson, D.M., Cembella, A.D., Eds.; IOC-UNESCO: Paris, France, 1995; pp. 229–249. [Google Scholar]
- Throndsen, J. The dilution-culture method. In Phytoplankton Manual; Sournia, A., Ed.; UNESCO: Paris, France, 1978; pp. 218–224. [Google Scholar]
- Okano, T.; Takeda, M.; Nakagawa, Y.; Hirata, K.; Mitsuhashi, K.; Kawaguchi, S.; Ito, J. Artificial reefs to induce upwelling to increase fishery resources. In Artificial Reefs in Fisheries Management; Bortone, S.A., Brandini, F.P., Fabi, G., Otake, S., Eds.; CRC Press LLC: Boca Raton, FL, USA, 2011; pp. 265–278. [Google Scholar]
- Leitão, F. Artificial reefs: From ecological processes to fishing enhancement tools. Braz. J. Oceanogr. 2013, 61, 77–81. [Google Scholar] [CrossRef]
- Armbrust, E.V. The life of diatoms in the world’s oceans. Nature 2009, 459, 185–192. [Google Scholar] [CrossRef]
- Jewson, D.H.; Lowry, S.F.; Bowen, R. Co-existence and survival of diatoms on sand grains. Eur. J. Phycol. 2006, 41, 131–146. [Google Scholar] [CrossRef]
- Sugie, K.; Kuma, K. Resting spore formation in the marine diatom Thalassiosira nordenskioeldii under iron- and nitrogen-limited conditions. J. Plankton Res. 2008, 30, 1245–1255. [Google Scholar] [CrossRef]
- Lebranc, K.; Arístegui, J.; Armand, L.; Assmy, P.; Beker, B.; Bode, A.; Breton, E.; Cornet, V.; Gibson, J.; Gosselin, M.-P.; et al. A global diatom database—Abundance, biovolume and biomass in the world ocean. Earth Syst. Sci. Data 2012, 4, 149–165. [Google Scholar] [CrossRef]
- Higaki, N.; Isoda, Y.; Honda, S. Mode waters observed around Musashi Rise west of Hokkaido. Oceanogr. Jpn. 2009, 18, 335–350. [Google Scholar]
- United States Department of Commerce, National Oceanic and Atmospheric Administration. National Artificial Reef Plan (As Amended): Guidelines for Siting, Construction, Development, and Assessment of Artificial Reefs; NOAA: Silver Spring, MD, USA, 2007; pp. 1–54.
- Shinada, A.; Shiga, N.; Ban, S. Origin of Thalassiosira diatoms that cause the spring phytoplankton bloom in Funka Bay, southwestern Hokkaido, Japan. Plankton Biol. Ecol. 1999, 46, 89–93. [Google Scholar]
- Kuribayashi, T.; Abe, T.; Montani, S. Historical δ15N records of Saccharina specimens from oligotrophic waters of Japan Sea (Hokkaido). PLoS ONE 2017, 12, e0180760. [Google Scholar] [CrossRef]
- Fukui, D.; Kitatsuji, S.; Ikeda, T.; Shiga, N.; Yamaguchi, A. Long-term changes in the abundance and community structure of net-phytoplankton in Oshoro Bay, Hokkaido. Bull. Plankton. Soc. Jpn. 2010, 57, 30–40. [Google Scholar]
- Matsumoto, T.; Matsuno, K.; Katakura, S.; Kasai, H.; Yamaguchi, A. Seasonal variability of the protist community and production in the southern Okhotsk Sea revealed by weekly monitoring. Reg. Stud. Mar. Sci. 2021, 43, 101683. [Google Scholar] [CrossRef]
- Hollibaugh, J.T.; Seibert, D.L.R.; Thomas, W.H. Observations on the survival and germination of resting spores of three Chaetoceros (Bacillariophyceae) species. J. Phycol. 1981, 17, 1–9. [Google Scholar] [CrossRef]
- Sanyal, A.; Larsson, J.; van Widum, F.; Andrén, T.; Moros, M.; Lönn, M.; Andrén, F. Not dead yet: Diatom resting spores can survive in nature for several millennia. Am. J. Bot. 2022, 109, 67–82. [Google Scholar] [CrossRef] [PubMed]
- McGee, D.; Laws, R.A.; Cahoon, L.B. Live benthic diatoms from the upper continental slope: Extending the limits of marine primary production. Mar. Ecol. Prog. Ser. 2008, 356, 103–112. [Google Scholar] [CrossRef]
- Tsukazaki, C.; Ishii, K.-I.; Saito, R.; Matsuno, K.; Yamaguchi, A.; Imai, I. Distribution of viable diatom resting stage cells in bottom sediments of the eastern Bering Sea shelf. Deep-Sea Res. II 2013, 94, 22–30. [Google Scholar] [CrossRef]
- Fukai, Y.; Matsuno, K.; Fujiwara, A.; Suzuki, K.; Richlen, M.L.; Fachon, E.; Anderson, D.M. Impact of sea-ice dynamics on the spatial distribution of diatom resting stages in sediments of the Pacific Arctic Region. J. Geophys. Res. Oceans 2021, 126, e2021JC017223. [Google Scholar] [CrossRef]
- Cahoon, L.B.; Laws, R.A.; Savidge, T.W. Characteristics of benthic microalgae from the North Carolina outer continental shelf and slope: Preliminary results. In Diving for Science. Proceedings of the 12th Annual Scientific Diving Symposium, Wilmington, NC, USA, 24–27 September 1992; Cahoon, L.B., Ed.; American Academy of Underwater Sciences: Costa Mesa, CA, USA, 1992; pp. 61–68. [Google Scholar]
- Marella, T.K.; Bhattacharjya, R.; Tiwari, A. Impact of organic carbon acquisition on diatom regrowth and functional biomolecule production in diatoms. Microb. Cell Fact. 2021, 20, 135. [Google Scholar] [CrossRef]
- Anderson, R.O. Respiration and photosynthesis during resting cell formation in Amphora coffeaeformis (Ag.) Kütz. Limnol. Oceanogr. 1976, 21, 452–456. [Google Scholar] [CrossRef]
- von Stosch, H.A.; Fecher, K. “Internal thecae” of Eunotia soleirolii (Bacillariophyceae): Development, structure, and function as resting spores. J. Phycol. 1979, 15, 233–243. [Google Scholar] [CrossRef]
- Ishii, K.-I.; Matsuoka, K.; Imai, I.; Ishikawa, A. Life cycle strategies of the centric diatoms in a shallow embayment revealed by the plankton emergence trap/chamber (PET chamber) experiments. Front. Mar. Sci. 2022, 9, 889633. [Google Scholar] [CrossRef]
- Sudo, K.; Inaba, N.; Matsumoto, T.; Matono, H.; Ishii, K. Growth characteristics of diatom resting stage cells in sediments around offshore artificial reefs. In Abstracts for the 2021 Joint Meeting of The Japanese Association of Benthology and The Plankton Society of Japan; Joint Meeting of The Japanese Association of Benthology and The Plankton Society of Japan: Kagoshima, Japan, 2021; p. 91. [Google Scholar]
- Cahoon, L.B. The role of benthic microalgae in neritic ecosystems. Oceanogr. Mar. Biol. Annu. Rev. 1999, 37, 47–86. [Google Scholar]
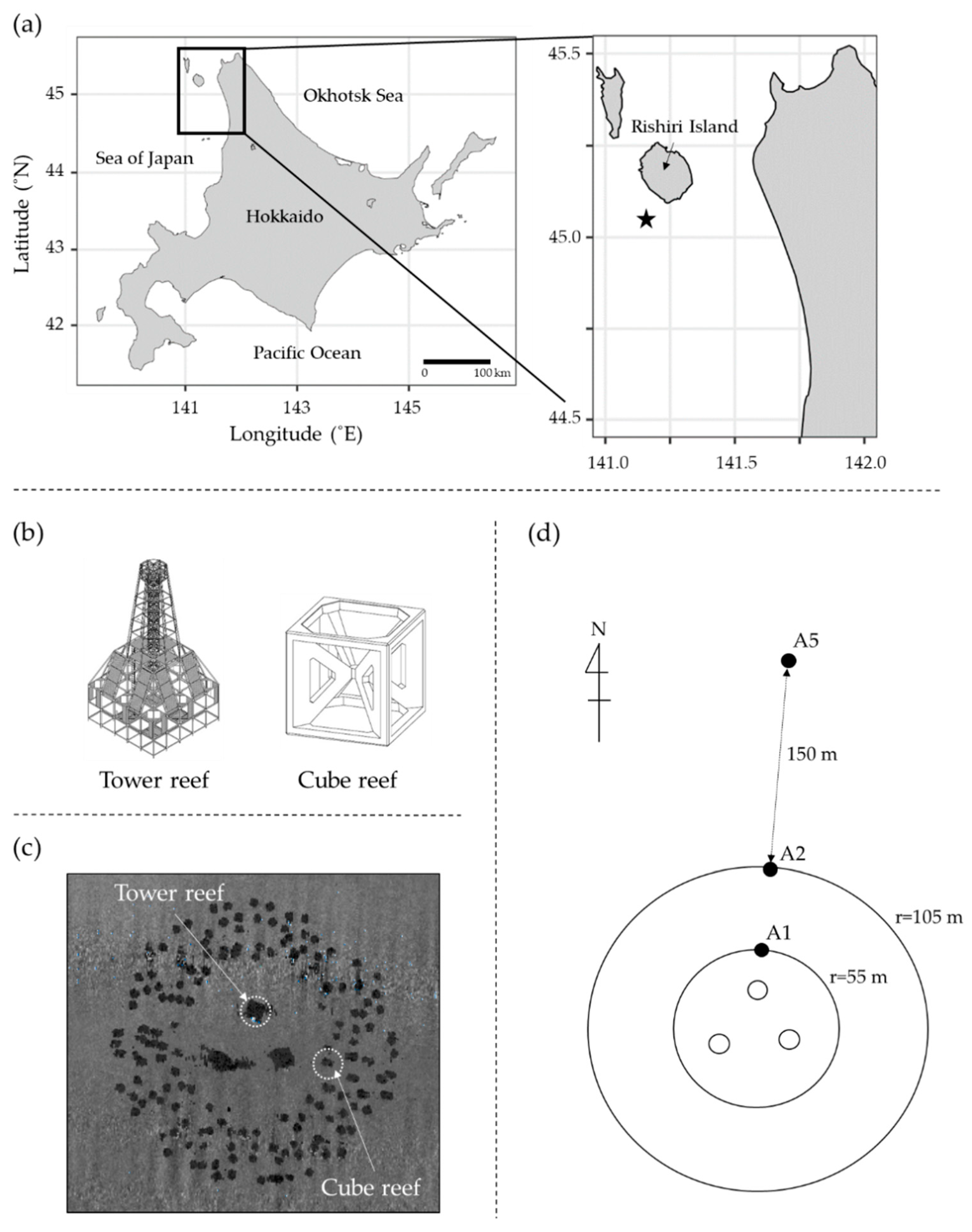

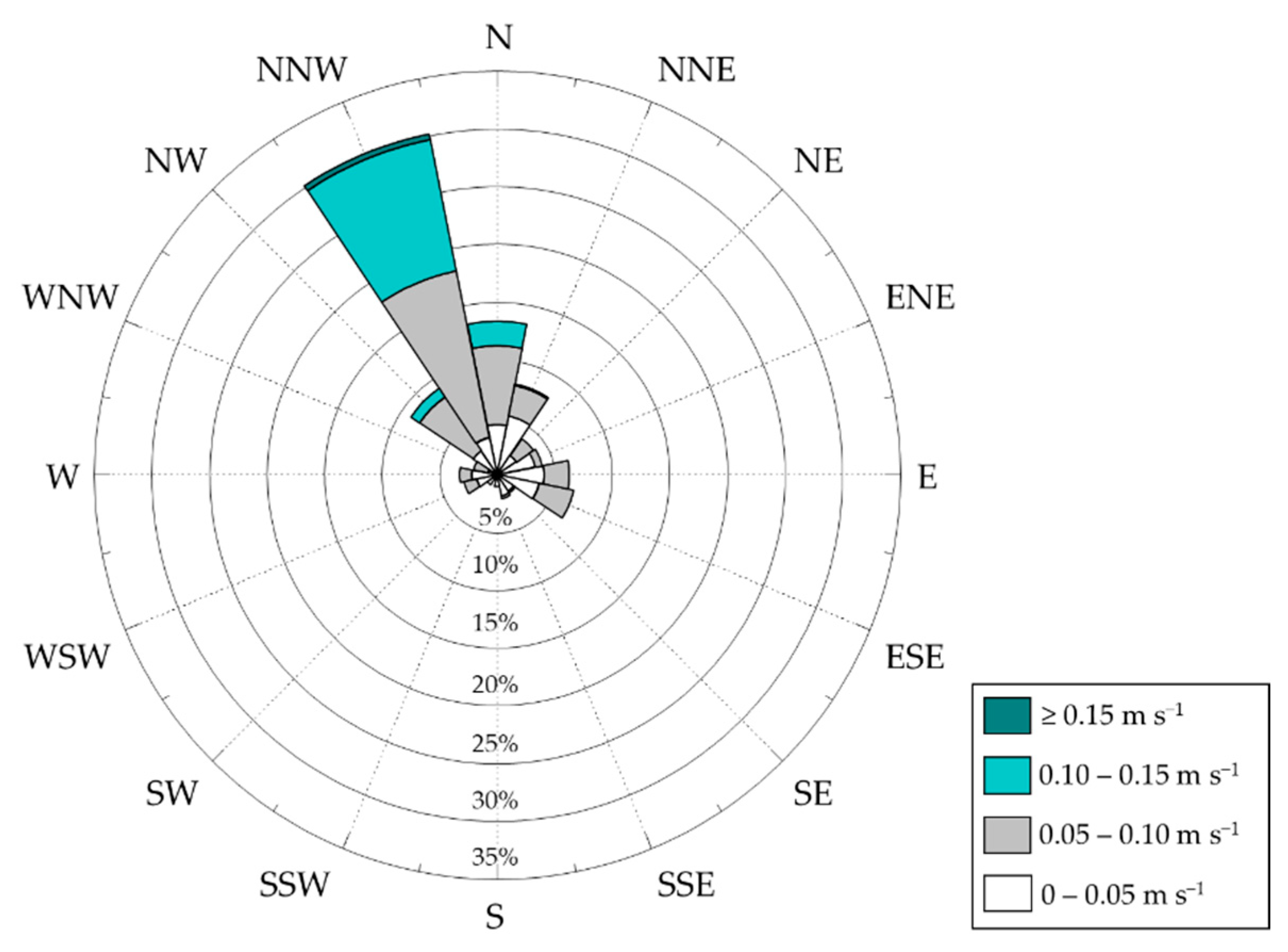



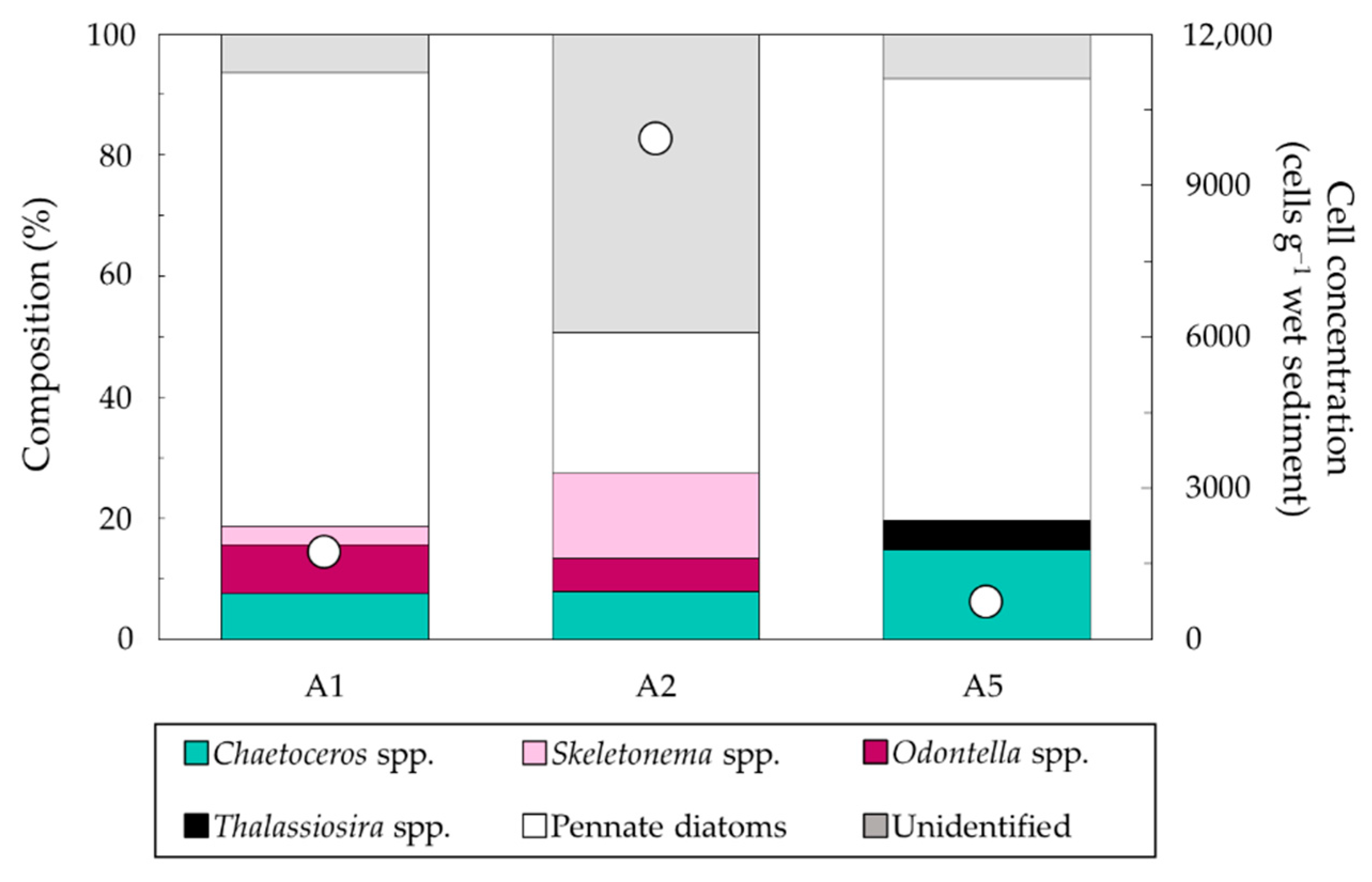
| Station | Latitude (N) | Longitude (E) | Water Depth (m) |
|---|---|---|---|
| A1 | 45°02′32.600″ | 141°07′54.368″ | 90.9 |
| A2 | 45°02′34.220″ | 141°07′55.028″ | 91.7 |
| A5 | 45°02′39.079″ | 141°07′54.848″ | 93.7 |
| Station | Particle Size Distribution (%) | D50 (mm) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Clay | Silt | Sand | Gravel | ||||||
| Fine | Medium | Coarse | Fine | Medium | Coarse | ||||
| <0.005 mm | 0.005–0.075 mm | 0.075–0.25 mm | 0.25–0.85 mm | 0.85–2.0 mm | 2.0–4.75 mm | 4.75–19 mm | 19–75 mm | ||
| A1 | 10.5 | 9.9 | 7.1 | 8.3 | 5.8 | 14.0 | 44.4 | 0.0 | 3.70 |
| A2 | 16.6 | 11.9 | 8.8 | 12.4 | 5.9 | 14.9 | 29.5 | 0.0 | 0.89 |
| A5 | 28.3 | 18.1 | 13.8 | 20.9 | 3.8 | 5.7 | 9.4 | 0.0 | 0.12 |
| Species | Cell Concentration (Cells g−1 Wet Sediment) | ||
|---|---|---|---|
| A1 | A2 | A5 | |
| Actinocyclus spp. | 0 | 0 | 40 |
| Actinoptychus senarius | 60 | 80 | 20 |
| Chaetoceros affinis or vanheurckii | 20 | 840 | 20 |
| Chaetoceros contortus | 90 | 2560 | 150 |
| Chaetoceros coronatus | 0 | 20 | 0 |
| Chaetoceros debilis | 10 | 1000 | 10 |
| Chaetoceros diadema | 60 | 360 | 80 |
| Chaetoceros didymus | 10 | 180 | 10 |
| Chaetoceros radicans | 80 | 2480 | 190 |
| Coscinodiscus spp. | 0 | 20 | 0 |
| Leptocylindrus danicus | 0 | 40 | 0 |
| Odontella aurita | 30 | 0 | 0 |
| Thalassiosira spp. | 60 | 180 | 30 |
| Pennate diatoms | 50 | 260 | 130 |
| Unidentified Chaetoceros spp. | 30 | 800 | 20 |
| Unidentified diatoms | 60 | 0 | 140 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsumoto, T.; Sudo, K.; Ishii, K.-I.; Imura, A.; Inaba, N. Distribution of Diatom Resting Stages in Sediment near Artificial Reefs Deployed in the Dysphotic Zone: Exploration of New Artificial Reef Function. Appl. Sci. 2022, 12, 9972. https://doi.org/10.3390/app12199972
Matsumoto T, Sudo K, Ishii K-I, Imura A, Inaba N. Distribution of Diatom Resting Stages in Sediment near Artificial Reefs Deployed in the Dysphotic Zone: Exploration of New Artificial Reef Function. Applied Sciences. 2022; 12(19):9972. https://doi.org/10.3390/app12199972
Chicago/Turabian StyleMatsumoto, Takuma, Kenya Sudo, Ken-Ichiro Ishii, Ayako Imura, and Nobuharu Inaba. 2022. "Distribution of Diatom Resting Stages in Sediment near Artificial Reefs Deployed in the Dysphotic Zone: Exploration of New Artificial Reef Function" Applied Sciences 12, no. 19: 9972. https://doi.org/10.3390/app12199972
APA StyleMatsumoto, T., Sudo, K., Ishii, K.-I., Imura, A., & Inaba, N. (2022). Distribution of Diatom Resting Stages in Sediment near Artificial Reefs Deployed in the Dysphotic Zone: Exploration of New Artificial Reef Function. Applied Sciences, 12(19), 9972. https://doi.org/10.3390/app12199972






