Abstract
This study describes the effect of adding carrot on the nutritional and biological value of canned goat meat. Four batches of canned goat meat were produced: control (without carrot), and three experimental treatments T10, T20, and T30 that was canned goat meat with the addition of 10, 20, and 30% carrot, respectively. Canned goat meat with 30% added carrot had the lowest fat (5.76%) and protein (17.08%) content. The mass fraction of fiber was significantly increased, up to 1.96% in T10, 2.33% in T20, and 2.71% in T30. The same trend was observed for β-carotene content (from 0.78 mg/100 g in the control sample to 1.91 mg/100 g in T30). Among the amino acids, histidine was significantly increased in T30 (p < 0.05), and lysine in T20 and T30 (p < 0.05). There was also evidence of increased vitamin A, B6 (pyridoxine), B9 (folic acid), and B5 (pantothenic acid) content in experimental samples than in the control sample. Among the minerals, the most significant increase was in potassium, magnesium, and phosphorus levels in samples with carrot. Organoleptic evaluation showed differences in color, taste, consistency, and odor. Thus, the addition of carrots increases the nutritive value and improves the palatability of canned goat meat.
1. Introduction
Meat products are considered one of the most important components of the human diet. The global production of meat products is increasing, which is not only associated with an increasing world population, but also with an increasing level of consumption of meat products in developed and developing countries. In Asia, for example, meat consumption has increased 15 times over the last 50 years, reaching almost half of the global meat consumption [1]. Experts predict that if dietary habits in developing countries continue to evolve as in richer countries, global meat consumption will increase dramatically in this century [1,2].
Meat products are rich in nutrients and vitamins, which are essential for the human body. Despite the popular concepts of healthy eating and veganism, meat is still the basis of nutrition. It contains a high concentration of essential minerals, trace elements, and amino acids [3,4]. Among the variety of types of meat products, canned meat is in high demand. Canned meat is a meat product that has been hermetically sealed in tin or glass jars. It is exposed to high temperatures for destruction of pathogenic microflora to be made storage-friendly. Canned meat is characterized by its high nutritional value, long shelf life, and easy transportation [5,6]. In addition to traditional types of meat (beef, pork) used in the production of canned meat products, horse meat, mutton, and goat meat are widely used.
Goat breeding is widespread in Central Asian countries. Goats were domesticated by humans a long time ago; their milk, hide, and meat have been used since ancient times. Goats are unevenly distributed in Kazakhstan. Their highest numbers are in the southern regions of Kazakhstan, where a large part of the year has warm temperatures, allowing year-round maintenance of animals on pastures [7,8].
Goat meat has a positive perception compared with other meats in terms of health. The low cholesterol content of goat meat is one of its many health benefits. This makes it an ideal food for dietary or therapeutic purposes. In addition, goat meat contains iron and various antioxidants [9]. Consumption of this product helps to reduce cholesterol and supplies the body with important nutrients [10]. Goat meat is easily digestible and absorbed by the body. One of the main advantages of goat meat is hypoallergenicity; it can even be used in the production of baby food [11].
Goat meat is considered a delicacy in most countries. The chemical composition of goat meat is as follows: 74–76% moisture, 20–22% protein, 0.6–2.6% fat, and 1% ash [12]. Goat meat is traditionally considered a lean red meat with dietary properties, characterized by a slightly darker red color, a coarser texture, and a different taste and aroma from mutton [13,14]. The results of sensory studies show that goat meat is different, but certainly not inferior to mutton [15]. Goat meat is a valuable source of essential amino acids and minerals (potassium, zinc, iron, etc.). Goat meat and its meat products also tend to be less succulent than lamb, mainly due to their lower fat content [16,17].
These days, an issue of technology improvement and expansion of the assortment of meat products receives special urgency. Developing meat product recipes that involves replacing a part of the raw animal material with vegetable additives is a promising way to solve the problem of increasing the availability of meat products, expanding their assortment, and increasing their nutritional value.
It is known that one of the most effective methods of normalizing the nutritional status of human beings is the use of protein from raw vegetable material in the diet, which makes it possible to enrich food with necessary micronutrients and compensate for a lack of animal protein. Enrichment of meat products with useful nutrients from raw plant material helps to compensate for the deficit of vitamins, minerals, dietary fibers, and other useful substances in the human body. This could reduce the presence of nutrient deficiencies in the diet of the population [18,19].
Carrot (Daucus carota subsp. Sativus) is a vegetable whose root is widely used in the human diet due to its global distribution, nutritional value, health benefits, and technological properties. It is a rich source of vitamin A (beta-carotene) and dietary fiber [20]. Beta-carotene is correlated with improved cognitive health because of its antioxidant effects. Beta-carotene is also potentially useful for treating neurodegenerative diseases such as Alzheimer’s disease [21].
Carrots help reduce cholesterol level and risk of cancer and increase immune function. Carrots also support the immune system with antiseptic and antibacterial properties that help the body’s immune reactions to infections and disease [22]. In addition, vitamin A works together with vitamin C, another powerful antioxidant, to boost immunity and prevent cell damage caused by free radicals. Carrots are rich in potassium. Potassium helps lower blood pressure through its interaction with kidney and sodium [23]. Regular consumption of carrots may significantly reduce the risk of cardiovascular disease. It contains the antioxidant luteolin, which prevents arterial inflammation caused by cholesterol clots [24].
Therefore, the combination of raw animal and plant material makes it possible to supplement them with biologically active substances and give the final food products functional, therapeutic, and prophylactic properties [25,26]. The aim of this study is to evaluate the effect that adding carrot has on the nutritional and biological value of canned goat meat.
2. Materials and Methods
2.1. Materials
Raw meat: the butchering of 35 goats of the Zaanen breed (age: 8 months, weight of carcass: 11.58 ± 1.24 kg) was carried out in a slaughter shop of FE called “Aigerim” (South Kazakhstan region, the Republic of Kazakhstan). Carcasses were kept for 24 h at −18 °C, subjected to deboning, roasting, and subsequent technological processing. Cuts of meat samples were from the loin side. The back part of the goat carcass was cut lengthwise along the vertebrae into two loin parts (ham). Three basic techniques were used for deboning the hind leg: the pelvic bone was separated, then the tibia and femur bones. The flesh of the loin mass was cut into small pieces for grinding in the meat grinder.
Raw vegetable material: carrots, onions, beans, salt, and spices (black pepper) were purchased from the supermarket network called “Magnum” (Republic of Kazakhstan, Almaty).
2.2. Preparation of Control and Experimental Canned Meat
Four batches of canned meat were prepared: one control and three experimental batches, with the addition of carrots from 10 to 30% instead of goat meat (Table 1). Each batch consisted of 12 cans (Figure 1). The weight of one can with product before autoclaving was 350 g.

Table 1.
Composition of canned meat with different carrot content (g/kg).

Figure 1.
Canned goat meat.
The process of canned meat production was carried out according to the patent [27]. To produce canned meat, the goat meat was cut using Cutter ZB-40 (Hualian Machinery, China); the size of the pieces after cutting was 10–15 mm. All raw vegetable material were cleaned and washed. Defective or rotten roots were removed. Onions were cut by a cutter into pieces 5 mm in size, carrots were sliced by a Torgmash OM-350-01P vegetable slicer (Torgmash Plant, Belarus) into pieces 10–12 mm in size. Sliced vegetables were sauteed at 105 °C in vegetable oil. The beans were half-cooked.
For canning, a manual canning machine MZ.04 (Forcom LLC, Moscow, Russia) was used. The canned food was sterilized using a Malysh Nerzh autoclave (Forcom LLC, Moscow, Russia) under the following regime: heating for 20 min, sterilization for 60 min at a pressure of 0.25 MPa and sterilization temperature of 115 °C, and gradually decreasing the pressure and temperature down to 35 °C for 30 min. All ingredients were weighed according to the recipe presented in Table 1.
2.3. Determination of the Chemical Composition
The moisture content was determined on an MX-50 moisture meter (A&D Co., LTD, Tokyo, Japan). All samples for moisture content determination were weighed at 5 g each and evenly distributed inside the device cup.
Moisture measurement was based on the method described in [28]. After determining moisture content, each dried sample was used for fat determination according to [29]. The samples were calcined in a muffle furnace (500–600 °C) to measure the ash content [28]. The protein content was analyzed according to [30].
2.4. Calculation of Energy Value of Canned Food
The energy value of canned food was determined by a formula based on the values of protein, fat, and carbohydrates and their caloric index in the finished product according to the method described in [4]:
where CV—caloric value, kCal; P—protein content, g; F—fat content, g; C—carbohydrate content, g; 4—caloric index for protein and carbohydrate; and 9—caloric index for fat.
CV = 4(P + C) + 9F
2.5. Determination of β-Carotene Content
Our method was based on the extraction of carotenoids from precipitate, previously obtained by treating the sample with Carrez I and Carrez II solutions, followed by purification of the isolated substance with petroleum ether and spectrophotometric determination of the mass concentration of β-carotene [31].
The obtained extract was used for spectrometric determination of total carotenoids (spectrophotometer SF-56 (OKB Spectr, Sankt-Peterburg, Russia), 450 nm, optical glass cuvettes, optical path length 1 cm). Petroleum ether was used as a comparative solution.
The mass concentration of carotenoids C, mg/dm3, is calculated by Formula (2):
where 4.0—the optical density conversion factor;
A—measured optical density value;
F—dilution factor (ratio of extract volume in petroleum ether, or volumes of different fractions to the volume of sample taken for analysis).
2.6. Determination of Fiber Content
Mass fraction of dietary fiber in the product was determined by the gravimetric method, according to GOST R 54014-2010. The method was based on the enzymatic hydrolysis of starch and non-starch compounds using α-amylase, protease, and amyloglucosidase to mono-, di-, and oligosaccharides and peptides. Dietary fibers were precipitated with ethyl alcohol, dried, and gravimetrically determined. The tests were performed on the Velp FIWE-3 fiber analyzer (“VELP”, Usmate, Italy). The total mass fraction of dietary fiber is expressed as a percentage (g/100 g) [32].
2.7. Determination of Salt Content
The amount of salt in the canned food was determined by GOST 9957-2015 [33]. Samples from each batch were ground, then mixed for sample homogeneity. An amount of 5 g of the sample was placed in a beaker and 100 cm3 of distilled water was added. After 40 min of infusion (with periodic stirring with a glass rod), the aqueous extract was filtered through a filter paper.
After cooling to room temperature, 5 cm3 of the filtrate was titrated with 0.05 mol/dm3 silver nitrate solution in the presence of 0.5 cm3 potassium chromate solution until the color changed to orange.
The mass fraction of sodium chloride X, %, was calculated by Formula (3), where:
0.00292—amount of sodium chloride equivalent to 1 cm of 0.05 mol/dm3 silver nitrate solution, g;
K—correction for the titer of 0.05 mol/dm3 silver nitrate solution;
v—amount of 0.05 mol/dm3 silver nitrate solution used for the titration of the test solution, cm3
v1—amount of aqueous extract taken for titration, cm3;
m—weight of the sample, g;
100—volume to which the analyzed sample is diluted, cm3;
100—conversion coefficient to percent.
2.8. Determination of Amino Acid Composition
The determination of amino acid composition was performed on a SHIMADZU LC-20 Prominence HPLC instrument (Kyoto, Japan) with fluorimetric and spectrophotometric detectors. We used a 25 cm by 4.6 mm SUPELCO C18 chromatographic column, 5 in front of the column to protect the main column from impurities. Chromatographic analysis was performed in eluent gradient mode at a flow rate of 1.2 mL min−1 and a column thermostat temperature of 40 °C in reversed phase with fluorimetric and spectrophotometric detectors at 246 and 260 nm, using acid hydrolysis and amino acid modification with phenylisothiocyanate solution in isopropanol to obtain phenylthiohydantoins. A mixture of 6.0 mm of CH3 SONA solution at pH 5.5 (component A), 1% isopropanol in acetonitrile solution (component B), and 6.0 mm of CH3 SONA solution at pH 4.05 (component C) was used as the mobile phase. We used standard amino acid samples produced by Sigma Aldrich (Burghausen, Germany) [4].
2.9. Determination of Mineral Content
The mineral content of the samples was determined according to the method of AOAC (2000) [34]. All samples for determining the mineral content weighed 5 g each, being placed on a container and incinerated in a microwave muffle for 12 h to a final temperature of 600 °C. After microwave splitting, the samples were diluted with a 10 ml solution of hydrochloric acid (HCl) in distilled water (1:1), mixed with a glass rod, and passed through a paper filter. Mineral elements were determined on an atomic emission spectrometer ICP-OES (Spectro, Boschstr, Burghausen, Germany).
2.10. Determination of Vitamin Composition
Vitamin content was determined according to GOST 55482-2013 [35] for water-soluble vitamins using a single-substituted potassium phosphate buffer solution and GOST 32307-2013 for fat-soluble vitamins [36]. The analysis was performed on a Dionex Ultimate 3000 chromotograph (“Dionex”, Sunnyvale, CA, USA) with a Supelco SUPELCOSIL LC-PAH 5 µm 4.6 × 150 mm column. The eluent composition was a mixture of methanol and distilled water (92:8).
2.11. Organoleptic Evaluation
Organoleptic evaluation of canned meat was carried out according to the requirements of state standard GOST 33741-2015 [37]. Organoleptic characteristics of canned meat were evaluated by pre-trained and instructed tasters consisting of 7 people from the Kazakh Research Institute of Processing and Food Industry. The appearance of cans was evaluated by external signs of the container, the presence or absence of leakage, deformation and rust, etc. Canned food was heated before tasting; the can was placed in a water bath for 20 min. Tasters compared opinions on the appearance, color, odor, consistency, and taste of each product. Organoleptic indicators of canned products were determined in the following sequence: appearance, color, odor, consistency, and taste. When assessing the appearance of canned products, tasters assessed the degree of chopping, whether shape was maintained by chopped or molded ingredients, the state of the broth, and the presence of foreign impurities. When assessing color, various deviations from the color typical for a given type of canned food were determined. When assessing odor, tasters determined whether the aroma was typical, harmonious, and whether there was a presence of an extraneous odor. When assessing the consistency, tasters looked at tenderness, fibrousness, coarseness, crumbliness, uniformity, chewability, presence of rigid structural components, etc.. When evaluating taste, tasters determined the characteristic taste for this type of canned food and established the presence of specific unfavorable flavors and other extraneous flavors.
2.12. Statistical Analysis
The experiments were carried out in triplicate. Standard deviation values are given for all measurements. Differences between the experimental and control groups were calculated using a one-way ANOVA with Tukey test. p ˂ 0.05 was considered as significant.
3. Results and Discussion
3.1. Influence of Carrots on the Chemical Composition of Canned Food
The results of the study of the chemical composition of canned meat under the standard recipe (control) and with the replacement of goat meat with carrots at 10% (T10), 20% (T20), and 30% (T30) are presented in Table 2. The experimental samples significantly differed from the control (p ˂ 0.05) in moisture, carbohydrates, ash, carotenoids, and fiber content.

Table 2.
Chemical composition of canned goat meat containing different amounts of carrot.
The highest mass fraction of fat was found in the control samples and gradually decreased as the proportion of carrots in the product increased. This confirms the findings of Roccetti et al. who showed a decrease in fat when substituting vegetable ingredients—carrots (Daucus Carota L.)—for turkey meat in sausages in amounts of 10 to 30% [38], as well as Corvalho et al., who added spinach to chicken burgers [39]. Roccetti et al. attributed this effect to the low fat content of vegetable ingredients [38].
The protein content of the experimental samples slightly decreased (p ˃ 0.05), which in turn agrees with the experimental data of other studies [40,41]. The carbohydrate content significantly increased in the experimental samples with the addition of carrots (p < 0.05). Thus, in sample T30, the carbohydrate content was the highest and amounted to 14.36%. The proportion of carbohydrates in samples T20 and T10 increased to 13.77 and 13.03% compared with the control (11.78%). The increase in the proportion of carbohydrates was expected, as carrot composition contain up to 9% of carbohydrates [42]. The salt content in all samples was 1.63%. By lowering the fat content in the experimental samples, the energy value of canned meat slightly decreased. The salt content was the same in all samples (1.63%).
In terms of the mass fraction of fiber and content of β-carotene, the experimental samples significantly exceeded the control samples (p ˂ 0.05), with maximum values obtained when 30% of goat meat was replaced with carrots(Figure 2 and Figure 3). It is known that β-carotene has diverse functions, such as protecting against cancer, increasing immune response, possessing antioxidant properties, and other health benefits including anti-ulcer and anti-aging activities [43,44]. Carrots contain dietary fiber, which activates the intestines. Fibers activate the cleansing process of the body and help recover intestinal functions. Due to this, the metabolism is normalized, and the skin, hair, and nails become healthier [45,46].
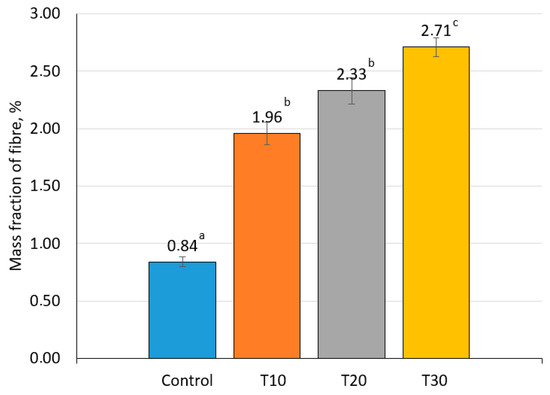
Figure 2.
Fiber content in canned goat meat samples (the values for bars sharing different letters (a–c) are significantly different (p < 0.05)).
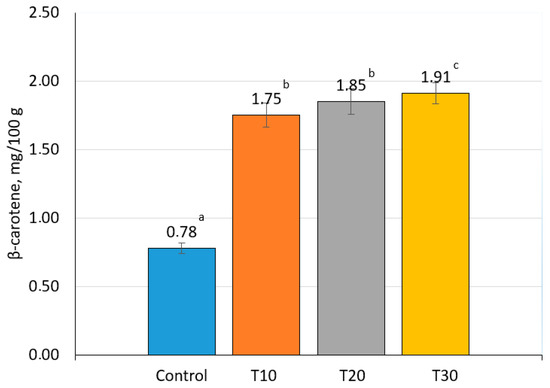
Figure 3.
β-carotene content in canned goat meat samples (the values for bars sharing different letters (a–c) are significantly different (p < 0.05)).
3.2. Amino Acid Composition of Canned Goat Meat
Analysis of amino acid composition of the control and experimental canned samples showed that replacement of 20 and 30% of goat meat with carrots significantly increased the histidine and lysine content in the T30 sample (Table 3). Also, in T30 samples, there was a significant increase in levels of leucine + isoleucine from 1737 (control) to 1822 mg/100 g (T30). However, levels of other amino acids were decreased, which is expected due to a decrease in protein content. These data are consistent with past experimental findings [38,40,41]. Among the samples with added carrot, the highest total amino acid content (8508 mg/100 g) was identified in the T20 sample, but T30 showed the highest total content for essential amino acids (5956 mg/100 g), which is important for complete nutrition.

Table 3.
Amino acid composition of canned goat meat, (mg/100 g).
3.3. Vitamin and Mineral Content of Canned Goat Meat
A study of the vitamin composition of canned meat products showed a trend towards an increase in the content of both fat-soluble and water-soluble groups of vitamins. As carrots are rich in vitamin A, the levels of vitamin A increased to 212.08 and 193.77 µg/100 g in samples T20 and T30, respectively, compared with 73.46 µg/100 g in the control.
It should be noted that 100 g of the product with 20 and 30% carrot substitution for meat ingredients satisfied 23.6 and 21.53% of the daily recommended vitamin A intake (by the WHO) [47], whereas control samples showed only 8.16% of the daily recommended intake. There was also an increase in vitamins B6 (pyridoxine), B9 (folic acid), and B5 (pantothenic acid) (Table 4).

Table 4.
Vitamin content of canned goat meat, mg/100 g.
The analysis of mineral composition in canned products showed that the increase in ash content in the experimental samples corresponded to an increase in the content of almost all the studied mineral elements. The most significant increases were in potassium, magnesium, and phosphorus content (Table 5). It should be noted that in samples T20 and T30, the iron content satisfies up to 32 and 42% of the daily requirement in men and up to 17 and 22% of the daily requirement in women. The control sample satisfies 28 (for men) and 14.9% (for women) [47]. It should also be noted that excessive intake of a number of elements can lead to serious diseases in humans [48,49].

Table 5.
Mineral content of canned goat meat (mg/100 g).
3.4. Organoleptic Characteristics
Organoleptic characteristics of food products are one of the main criteria of choice for consumers. We evaluated the appearance, appearance of the product packaging, appearance on the cut, color, recipe composition, etc. (Figure 4).
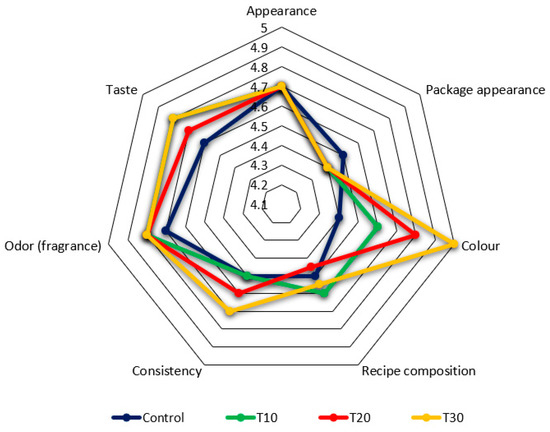
Figure 4.
Organoleptic characteristics of the control and experimental samples of canned meat.
According to the results of organoleptic evaluation (Figure 4) all experimental and control samples had no critical differences in the type of packaging and labels, odor, formulation composition, and appearance of canned food. Differences were noticeable in color assessment: the control sample (4.4 points) and sample T10 (4.6 points) had a pale color, samples T30 (4.8 points) and T20 (5.0 points) had a richer color due to carrot inclusions. The color scores were higher in the experimental samples compared with the control. The highest score was in the T-30 sample with 30% carrot inclusion. This effect may be associated with the presence of orange pigment and carotenoids affecting color in carrot. Similar findings were also reported by [50] in chicken nuggets and by [51] in turkey meat sausages.
Canned samples T30 (4.6 points) and T20 (4.7 points) had a more tender and juicy consistency. In evaluating the taste of the experimental samples, T30 and T20 also received the highest score (4.7 and 4.8 points, respectively) and were described as typical of canned meat, without specific flavors. Organoleptic evaluation showed improved consistency in samples T-20 (4.6 points) and T-30 (4.7 points). Similar results were obtained by [51] in a study of turkey meat sausages with the inclusion of carrot paste. Improving juiciness and tenderness of the meat product by increasing the proportion of carrots is due to the presence of natural polysaccharides and dietary fiber, which join with the water–protein–fat matrix to form a more gel-like consistency [52,53].
Any change in the chemical composition of the finished product due to the partial replacement of one ingredient by another, consequently, can affect the sensory characteristics and consumer qualities of the product. As supposed by [54], the amount and type of amino acids in the composition of finished meat and meat products can affect its flavor characteristics and sensorimetric score. For example, alanine, glycine, proline, serine, and threonine can contribute to a sweet taste; histidine, allo-isoleucine, leucine, methionine, pheninalanine, tryptophan, and valine can add a bitter taste to products [55]. The maximum content of amino acids responsible for sweet taste was found in the control and T-20 sample. The highest content of the amino acids contributing to bitter taste corresponded to the control and T-30 sample. The highest grades of taste in organoleptic evaluation were given to the T-10 and T-30 samples. These findings differ from those of [56], on the reduction of taste characteristics in meat bread made of buffalo liver and vegetables with the inclusion of carrot paste, and with the results of [52], in a study on beef frankfurters with carrot paste (3,5, and 10%). This difference in results may be explained by differences in the type of finished products, the raw meat, and the formulation.
4. Conclusions
To summarize the results of the study, we can conclude that replacing part of the goat meat with carrot had a significant effect on the chemical, amino acid, vitamin, and mineral composition of canned food. In particular, some parameters significantly increased (p ˂ 0.05), depending on the amount of carrot addition: the mass content of moisture, ash, carotenoids, and fiber, with a simultaneous decrease in fat content. The vitamin and mineral content were significantly increased (p ˂ 0.05), according to our tests. Organoleptic evaluation showed differences in color, taste, consistency, and odor. Thus, the addition of carrots could increase the biological value and improve the palatability of canned goat meat.
Author Contributions
Conceptualization, U.C. and G.K.; data curation, A.T. and N.T.; investigation, A.T. and N.T.; methodology, T.Z.; project administration, U.C.; resources, T.Z.; software, T.Z.; visualization, A.S.; writing—review and editing, G.K. and A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Agriculture of Kazakhstan (grant number IRN BR10764970) and the Ministry of Education and Science of the Republic of Kazakhstan (grant number IRN AP09058083).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors wish to thank laboratory staff of Kazakh Research Institute of Processing and Food Industry for meat sample analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bonnet, C.; Bouamra-Mechemache, Z.; Réquillart, V.; Treich, N. Regulating meat consumption to improve health, the environment and animal welfare. Food Policy 2020, 10, 101847. [Google Scholar] [CrossRef]
- FAO. Food Outlook—Biannual Report on Global Food Markets; Food Outlook: Rome, Italy, 2021. [Google Scholar] [CrossRef]
- Toldrá, F.; Reig, M. Innovations for healthier processed meats. Trends Food Sci. Technol. 2011, 22, 517–522. [Google Scholar] [CrossRef]
- Yessimbekov, Z.; Kakimov, A.; Caporaso, N.; Suychinov, A.; Kabdylzhar, B.; Shariati, M.A.; Baikadamova, A.; Domínguez, R.; Lorenzo, J.M. Use of meat-bone paste to develop calcium-enriched liver pâté. Foods 2021, 10, 2042. [Google Scholar] [CrossRef] [PubMed]
- Słowiński, M.; Miazek, J.; Chmiel, M. Influence of the dose and length of wheat fiber on the quality of model sterilized canned meat products. Foods 2020, 9, 1001. [Google Scholar] [CrossRef]
- Ebeed, A.; Elsayed, E.; Nabil, M.; Yasser, G.; Safaa, H. Quality assurance of imported canned meat. Glob. Vet. 2015, 14, 511–516. [Google Scholar]
- Kakimov, M.M.; Tokysheva, G.M.; Makangali, K.K. Perspectives for goat meat processing development in the Republic of Kazakhstan. Sci. Technol. Kazakhstan 2021, 2, 95–100. [Google Scholar]
- Kazhybayeva, G.; Agibayeva, A.; Kuderinova, N.; Harlap, S.; Fedoseeva, N.; Usov, V.; Bakirova, L. Development of technology and assessment of nutritional value of a delicacy goat meat product. Int. J. Innov. Technol. Explor. Eng. 2019, 8, 239–242. [Google Scholar]
- Nassu, R.T.; Gonçalves, L.A.G.; da Silva, M.A.A.P.; Beserra, F.J. Oxidative stability of fermented goat meat sausage with different levels of natural antioxidant. Meat Sci. 2003, 63, 43–49. [Google Scholar] [CrossRef]
- Ali, M.; Park, J.Y.; Lee, S.Y.; Choi, Y.S.; Nam, K.C. Physicochemical and microbial characteristics of longissimus lumborum and biceps femoris muscles in Korean native black goat with wet-aging time. J. Anim. Sci. Technol. 2021, 63, 149. [Google Scholar] [CrossRef]
- Tokysheva, G.; Makangali, K.; Uzakov, Y.; Kakimov, M.; Vostrikova, N.; Baiysbayeva, M.; Mashanova, N. The potential of goat meat as a nutrition source for schoolchildren. Potravin. Slovak J. Food Sci. 2022, 16, 398–410. [Google Scholar] [CrossRef]
- Malekian, F.; Khachaturyan, M.; Gebrelul, S.; Henson, J. Nutritional characteristics and consumer acceptability of sausages with different combinations of goat and beef meats. Funct. Foods Health Dis. 2016, 6, 42–58. [Google Scholar] [CrossRef]
- Teixeira, A.; Almeida, S.; Pereira, E.; Mangachaia, F.; Rodrigues, S. Physicochemical characteristics of sheep and goat pâtés. differences between fat sources and proportions. Heliyon 2019, 5, e02119. [Google Scholar] [CrossRef]
- Babiker, S.A.; El Khider, I.A.; Shafie, S.A. Chemical composition and quality attributes of goat meat and lamb. Meat Sci. 1990, 28, 273–277. [Google Scholar] [CrossRef]
- Rababah, T.M.; Ereifej, K.I.; Alhamad, M.N.; Al-Qudah, K.M.; Rousan, L.M.; Al-Mahasneh, M.A.; Al-u’datt, M.H.; Yang, W. Effects of green tea and grape seed and TBHQ on physicochemical properties of Baladi goat meats. Int. J. Food Prop. 2011, 14, 1208–1216. [Google Scholar] [CrossRef]
- Madruga, M.S.; Bressan, M.C. Goat meats: Description, rational use, certification, processing and technological developments. Small Rumin. Res. 2011, 98, 39–45. [Google Scholar] [CrossRef]
- Oliveira, A.N.D.; Selaive-Villarroel, A.B.; Monte, A.L.S.; Costa, R.G.; Costa, L.B.A. Evaluation of carcass characteristics of crossbred Anglo-Nubian, Boer and undefined breed goats. Ciência Rural. 2008, 38, 1073–1077. [Google Scholar] [CrossRef]
- Assenova, B.; Okuskhanova, E.; Rebezov, M.; Zinina, O.; Baryshnikova, N.; Vaiscrobova, E.; Kasatkina, E.; Shariati, M.A.; Khan, M.U.; Ntsefong, G.N. Effect of germinated wheat (Triticum Aestivum) on chemical, amino acid and organoleptic properties of meat pate. Potravin. Slovak J. Food Sci. 2020, 14, 580–586. [Google Scholar] [CrossRef]
- Zinina, O.; Merenkova, S.; Tazeddinova, D.; Rebezov, M.; Stuart, M.; Okuskhanova, E.; Yessimbekov, Z.; Baryshnikova, N. Enrichment of meat products with dietary fibers: A review. Agron. Res. 2019, 17, 1808–1822. [Google Scholar]
- Sharma, K.D.; Karki, S.; Thakur, N.S.; Attri, S. Chemical composition, functional properties and processing of carrot—A review. J. Food Sci. Technol. 2012, 49, 22–32. [Google Scholar] [CrossRef]
- Hira, S.; Saleem, U.; Anwar, F.; Sohail, M.F.; Raza, Z.; Ahmad, B. β-carotene: A natural compound improves cognitive impairment and oxidative stress in a mouse model of streptozotocin-induced Alzheimer’s disease. Biomolecules 2019, 9, 441. [Google Scholar] [CrossRef]
- Alvarado-Ramírez, M.; Santana-Gálvez, J.; Santacruz, A.; Carranza-Montealvo, L.D.; Ortega-Hernández, E.; Tirado-Escobosa, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Using a functional carrot powder ingredient to produce sausages with high levels of nutraceuticals. J. Food Sci. 2018, 83, 2351–2361. [Google Scholar] [CrossRef] [PubMed]
- Średnicka-Tober, D.; Kopczyńska, K.; Góralska-Walczak, R.; Hallmann, E.; Barański, M.; Marszałek, K.; Kazimierczak, R. Are organic certified carrots richer in health-promoting phenolics and carotenoids than the conventionally grown ones? Molecules 2022, 27, 4184. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.; Costa, M.; Velasco, C.; Cunha, L.M.; Lima, R.C.; Baião, L.F.; Batista, S.; Marques, A.; Sá, T.; Campos, D.A.; et al. Comparative analysis between synthetic vitamin E and natural antioxidant sources from tomato, carrot and coriander in diets for market-sized Dicentrarchus labrax. Antioxidants 2022, 11, 636. [Google Scholar] [CrossRef] [PubMed]
- Kamani, M.H.; Meera, M.S.; Bhaskar, N.; Modi, V.K. Partial and total replacement of meat by plant-based proteins in chicken sausage: Evaluation of mechanical, physico-chemical and sensory characteristics. J. Food Sci. Technol. 2019, 56, 2660–2669. [Google Scholar] [CrossRef] [PubMed]
- Zheleuova, Z.S.; Uzakov, Y.M.; Shingisov, A.U.; Alibekov, R.S.; Khamitova, B.M. Development of halal cooked smoked beef and turkey sausage using a combined plant extracts. J. Food Process. Preserv. 2021, 45, 1. [Google Scholar] [CrossRef]
- Kvasenkov, O.I. Method of Making Canned Goat Roast Spiked. Patent No. 2358573, 28 January 2008. [Google Scholar]
- Antipova, L.V.; Glotova, I.A.; Rogov, I.A. Meat and Meat Products Research Methods; “KoloS” Publishing House: Moscow, Russia, 2001. [Google Scholar]
- GOST 23042-86; Interstate Standard. Meat and Meat Products. Methods of Determination of Fat. Standartinfrom: Moscow, Russia, 2010.
- GOST 25011-81; Interstate Standard. Meat and Meat Products. Methods of Determination of Protein. Standartinfrom: Moscow, Russia, 2010.
- GOST R 54058-2010; Functional Food Products. Method for Determination of Carotenoids. Standardinform: Moscow, Russia, 2011.
- GOST R 54014-2010; Functional Food. Determination of Soluble and Unsoluble Dietary Fiber by Enzymatic-Gravimetric Method. Standardinform: Moscow, Russia, 2011.
- GOST 9957-2015; Meat and Meat Products. Methods of Determination of Sodium Chloride Content. Standardinform: Moscow, Russia, 2016.
- AOAC. Official Methods of Analysis, 17th ed.; Official Methods of Analysis of AOAC International; CAB Publisher: Gaithersburg, MD, USA, 2000. [Google Scholar]
- GOST R 55482-2013; Meat and Meat Products. Method for Determination of Water-Soluble Vitamins Content. Standardinform: Moscow, Russia, 2014.
- GOST 32307-2013; Meat and Meat Products. Determination of Fat-Soluble Vitamins by High Performance Liquid Chromatography. Standardinform: Moscow, Russia, 2014.
- GOST-33741-2015; Canned Meat and Meat-Containing Products. Methods for Determining Organoleptic Characteristics, Net Weight and Mass Portion of Constituent Parts. Standartinfrom: Moscow, Russia, 2016.
- Rocchetti, G.; Pateiro, M.; Campagnol, P.C.; Barba, F.J.; Tomasevic, I.; Montesano, D.; Lucini, L.; Lorenzo, J.M. Effect of partial replacement of meat by carrot on physicochemical properties and fatty acid profile of fresh turkey sausages: A chemometric approach. J. Sci. Food Agric. 2020, 100, 4968–4977. [Google Scholar] [CrossRef]
- Carvalho, F.A.L.; Pateiro, M.; Domínguez, R.; Barba-Orellana, S.; Mattar, J.; Rimac Brnčić, S.; Barba, F.J.; Lorenzo, J.M. Replacement of meat by spinach on physicochemical and nutritional properties of chicken burgers. J. Food Process. Preserv. 2019, 43, e13935. [Google Scholar] [CrossRef]
- Zargar, F.A.; Kumar, S.; Bhat, Z.F.; Kumar, P. Effect of incorporation of carrot on the quality characteristics of chicken sausages. Indian J. Poult. Sci. 2017, 52, 91–95. [Google Scholar] [CrossRef]
- Yadav, S.; Pathera, A.K.; Islam, R.U.; Malik, A.K.; Sharma, D.P. Effect of wheat bran and dried carrot pomace addition on quality characteristics of chicken sausage. Asian-Australas. J. Anim. Sci. 2018, 31, 729–737. [Google Scholar] [CrossRef]
- Aleksashina, S.A.; Makarova, N.V. Study of the chemical composition and antioxidant activity of carrots, beets and pumpkins. Storage Process. Farm Prod. 2016, 6, 29–32. [Google Scholar]
- Rao, A.V.; Rao, L.G. Carotenoids and human health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Zaini, R.; Clench, M.R.; Le Maitre, C.L. Bioactive chemicals from carrot (Daucus carota) juice extracts for the treatment of leukemia. J. Med. Food 2011, 14, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.-W.; Yu, E.-Z.; Feng, Q. Soluble dietary fiber, one of the most important nutrients for the gut microbiota. Molecules 2021, 26, 6802. [Google Scholar] [CrossRef]
- Yang, X.; Dai, J.; Zhong, Y.; Wei, X.; Wu, M.; Zhang, Y.; Huang, A.; Wang, L.; Huang, Y.; Zhang, C.; et al. Characterization of insoluble dietary fiber from three food sources and their potential hypoglycemic and hypolipidemic effects. Food Funct. 2021, 12, 6576–6587. [Google Scholar] [CrossRef] [PubMed]
- Human Vitamin and Mineral Requirements. Report of a Joint FAO/WHO Expert Consultation Bangkok, Thailand; World Health Organization: Geneva, Switzerland; Food and Agriculture Organization of the United Nations: Rome, Italy, 2002. [Google Scholar]
- Sitalakshmi, R.; Kumar, S.P. Trace Elements in Health and Disease: A Review. Res. J. Pharm. Biol. Chem. Sci. 2014, 5, 450–455. [Google Scholar]
- Khanvilkar, D.G.; Nagarjee, S.; Noah, A.A.; Adedeji, M.A.; Jabeen, N.M.; Kumar, G.P.; Devanna, N.; Manjunath, S.S.; Jithendran, L.; Kalpana, C.A. Trace element status and human endocrine health: A perspective. Int. J. Food Nutr. Sci. 2021, 10, 1–5. [Google Scholar] [CrossRef]
- Bhosale, S.S.; Biswas, A.K.; Sahoo, J.; Chatli, M.K.; Sharma, D.K.; Sikka, S.S. Quality evaluation of functional chicken nuggets incorporated with ground carrot and mashed sweet potato. FSTI 2011, 17, 233–239. [Google Scholar] [CrossRef]
- Reddy, M.N.K.; Kumar, M.S.; Reddy, G.V.B.; Reddy, N.A.; Rao, V.K. Quality evaluation of turkey meat sausages incorporated with ground carrot. Pharm. Innov. J. 2018, 7, 773–777. [Google Scholar]
- Sam, F.E.; Ma, T.Z.; Atuna, R.A.; Salifu, R.; Nubalanaan, B.A.; Amagloh, F.K.; Han, S.Y. Physicochemical, Oxidative Stability and Sensory Properties of Frankfurter-Type Sausage as Influenced by the Addition of Carrot (Daucus carota) Paste. Foods 2021, 10, 3032. [Google Scholar] [CrossRef]
- Slima, S.B.; Ktari, N.; Triki, M.; Trabelsi, I.; Moussa, H.; Makni, S.; Abdeslam, A.; Herrero, A.M.; Jiménez-Colmenero, F.; Ruiz-Capillas, C.; et al. Effects of two fibers used separately and in combination on physico-chemical, textural, nutritional and sensory properties of beef fresh sausage. Br. Food J. 2019, 121, 1428–1440. [Google Scholar] [CrossRef]
- Zamuz, S.; Purriños, L.; Galvez, F.; Zdolec, N.; Muchenje, V.; Barba, F.J.; Lorenzo, J.M. Influence of the addition of different origin sources of protein on meat products sensory acceptance. J. Food Process. Preserv. 2019, 43, e13940. [Google Scholar] [CrossRef]
- Food Ingredients Brazil. Os Aminoácidos e o Sabor. Food Ingredients Brazil, 2017, 31. Available online: www.revista-fi.com (accessed on 24 August 2022).
- Devatkal, S.; Mendiratta, S.K.; Kondaiah, N. Quality Characteristics of Loaves from Buffalo Meat, Liver and Vegetables. Meat Sci. 2004, 67, 377–383. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).