Abstract
In the conventional synthesis of biodiesel, not only fatty acid esters (biodiesel) are formed, but also the by-product is the glycerol phase, which amounts to about 10 wt.%. Recently, the studies on the interesterification of oil using carboxylate esters have been launched. In this case, no glycerol is formed, and esters of glycerol and short-chain organic acids soluble in biodiesel are produced. The biodiesel yield is increased, and the biodiesel production process is more economically viable. The process of interesterification with methyl formate yields a mixture of biodiesel and triformylglycerol, which is not inferior in quality to biodiesel, but also has better low-temperature properties. The paper analyzes the application of chemical and enzymatic catalysis methods for the interesterification of triglycerides with methyl formate. The influence of catalyst amount, reagent molar ratio, temperature, and process time on the product yield is presented. The quality indicators of the obtained fuel and their compliance with the requirements of the biodiesel fuel standard are discussed.
1. Introduction
In industry, biodiesel is produced by the process of transesterification of oil (triglycerides) with short-chain alcohols, with the commonly used alcohol being methanol [1,2]. Catalysts, mostly alkaline, are used to accelerate the reaction [3,4]. The transesterification process produces fatty acid methyl esters (biodiesel) and a by-product, the glycerol phase or crude glycerol, which is formed in about 10% [5,6]. The composition of the glycerol phase varies, depending on the biodiesel production technology, but consists of 80–90 wt.% of glycerol and impurities: alcohol, catalyst, esters, and unreacted oil [7]. Crude glycerol can be used for technical purposes: for freeze protection for coal, as a dust suppressant, in grain storage, as an energy source, for the cultivation of microalgae, and the formation of mulching coatings [8,9]. Pure glycerol is more in demand. It can be used in pharmaceuticals, food, feed, fuel and other industry [10,11]; however, the process of purifying the glycerol phase to pure glycerol requires considerable energy and material costs. With increasing biodiesel production, excess glycerol is emerging in the market [12,13]. The absence of glycerol in the production of biodiesel would lead to a higher yield of biodiesel, could solve the problems of glycerol purification and utilization, and, at the same time, make the biodiesel production process more economical. It is known that triacylglycerol or triacetin can be produced from glycerol [14,15]. This product is highly soluble in biodiesel and improves its low-temperature properties [16]. However, the separate production of triacetin and blending with biodiesel to improve the low-temperature properties is uneconomical. The researchers began to study the process of synthesis, which directly produces a mixture of biodiesel and triacetin. No glycerol phase is formed during the process [17,18]. The resulting product is not inferior in quality to biodiesel, and it has better low-temperature properties than biodiesel. The fuel obtained during the interesterification process has an advantage over biofuels, since there is no need to use synthetic depressants, improving the low-temperature properties of biofuels. The prices of industrially obtained depressants are high, and even small quantities increase the cost of biofuels.
Such product is obtained by interesterification of triglycerides with short chain carboxylate esters. The process produces two types of esters: fatty acid esters (conventional biodiesel) and esters of glycerol and carboxylic acids of low molecular mass (Figure 1).
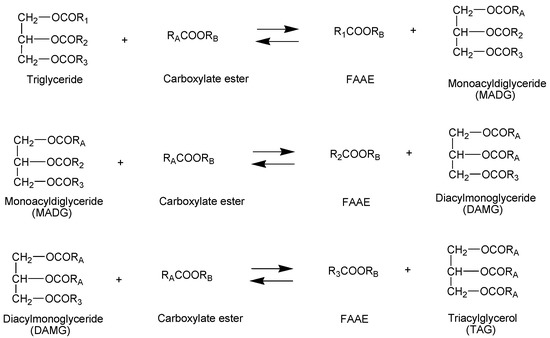
Figure 1.
Triglyceride interesterification reaction with carboxylate esters.
In the production of biofuels, food grade oil is most often used for the interesterification of triglycerides with short chain carboxylate esters. Most studies were conducted using rapeseed oil [19,20]; additionally, the process of interesterification was studied using soybean oil [21,22], olive oil [23,24], and sunflower oil [25,26]. Some studies have also been carried out using oil, which is not used for food because of the harmful compounds contained in it. Scientists have studied the possibilities of using Pongamia pinnata (karanja) [26], cotton oil [27], Jatropha oil [26], palm oil [28], and insect fat [29]. The processes of interestification of the generated oil waste (waste cooking oil [30], waste canola oil [30], and the characteristics of the products obtained were also analyzed.
Most research has been performed, and the best results have been obtained for interesterification using methyl and ethyl acetates [20,28,31,32,33,34,35]. The physical chemical properties of the manufactured fuel and compliance with the requirements of the standard were studied by scientists. Surendhiran et al. found that the fuel produced by interesterification of marine microalga Chlorella sativa oil with methyl acetate met the requirements of the standard (ASTM D675). When compared with jatrofa methyl esters, it was found that there were no differences between the different types of biofuels [36]. Studies of the quality indicators of biofuels made from insects (black soldier fly larvae (BSFL)) oil and methyl acetate have shown that they are not inferior in quality to biodiesel made from rapeseed oil. The basic physico-chemical indicators of biofuels produced from insect fat have been found to meet the requirements of standard EN14214, and many properties meet the requirements of ASTM D6751, with the exception of the flash point and acid count [29].
The use of alkylates of longer alcohol chains (propyl, butyl, propionate, and butyrate) decreases the interesterification efficiency [37,38]. It has been found that the use of longer alcohol chains for interesterification improves the low-temperature properties of products (pour point, flash point, cold filter plugging point, and cloud point). When a longer chain of alcohol is used for oil interesterification, the resulting fuel can be used at in areas of colder climate. However, these fuels have higher viscosity, density, and molecular weight compounds. Such biofuels do not meet the density and viscosity requirements for biodiesel. Studies on the interesterification of oil with acetates under standard conditions using both chemical and enzymatic catalysts or supercritical conditions have been performed, with their results summarized [39,40].
This study is intended to review the process of triglyceride interesterification with methyl formate and analyze the quality of the resulting product. The interesterification of triglycerides with methyl formate yields fatty acid methyl esters and formyl glycerides. In an efficient process, triglycerides react completely with methyl formate to form 84 wt.% fatty acid methyl esters (FAME) and 16 wt.% triformyl glycerol (TFG) [37]. It should be mentioned that the interesterification of oil with methyl fomate has not been studied much, but is pesspective, since the methyl formate is a rather cheap and easily obtained compound in the chemical industry produced from methanol and formic acid.
2. Interesterification of Triglycerides with Methyl Formate
Compared to interesterification studies with methyl and ethyl acetates, the use of methyl formate in the interesterification process has been little studied. Rapeseed oil was used as a raw material in all cases. The process of interesterification is catalytic, and the studies were carried out using alkaline catalysts and biocatalysts. The influence of the four variables on the efficiency of the interesterification (ester yield) was analyzed. As in the process of conventional transesterification with methanol, these variables are the molar ratio of the reagents, amount of catalyst, process temperature, and duration. Table 1 shows the results of the studies obtained.

Table 1.
Summary of studies on using methyl formate for interesterification of rapeseed oil.
2.1. Influence of Catalyst on the Product Yield
The rate of triglyceride transesterification process is increased using homogeneous and heterogeneous catalysts, which can be alkaline and acidic, depending on the quality of the oil. The most commonly used homogeneous catalysts are sodium and potassium hydroxides and methoxides [4,37,44,45]. If a higher acidity oil is used for the synthesis of biodiesel, acid catalysts (sulfuric, sulfitic, and chloric acids) are used as homogeneous catalysts [46,47,48]. Heterogeneous catalysts are both basic [49] and acidic [50], with the advantage of multiple uses [51]. Metal oxides [52], silicates [53], ion exchange resins [54], zeolites [55], and natural rocks [56] are used as heterogeneous catalysts, raw materials, or wastes containing calcium and magnesium (eggshells, crab, mussels, and snail shells) [57,58,59]. Biocatalysts are also used for oil transesterification [60,61].
Similar to the transesterification process, the process of interesterification is catalytic, and catalysts are used to increase its rate. Only a few studies have been performed on the interesterification of oil using methyl formate, most of which were performed using chemical homogeneous catalysts. Sustere et al. used 28.6% sodium methoxide in methanol. A FAME content of 74.2% was obtained using 0.12% of catalyst (molar ratio of methyl formate-to-oil of 18:1, temperature 27 °C, and duration 60 min) [37]. Abelniece et al. [41] used potassium tert-butoxide and 1 M potassium tert-butoxide in tetrahydrofuran for interesterification of rapeseed oil with methyl formate. The process was carried out under the same conditions: catalyst-to-oil molar ratio of 0.08, molar ratio of methyl formate-to-oil of 18:1, temperature 27 °C, and duration 60 min. The amount and yield of the esters were practically the same, both with and without co-solvent tetrahydrofuran. The yield of methyl esters were 71% without co-solvent and 71.7% with co-solvent tetrahydrofuran [41]. Abelniece et al. [42] found that the optimal catalyst-to-oil molar ratio is 0.1, when catalyst tert-butoxide in tert-butanol is used for the interesterification of rapeseed oil. The rapeseed oil methyl ester (RME) yield of 94.8% was obtained at a methyl formate-to-oil molar ratio of 80:1, temperature of 28 °C, and duration of 60 min [42].
Abelniece et al. studied the kinetic parameters of the interesterification process in at room temperature (25 °C), using of potassium tert-butoxide in tetrahydrofuran as a catalyst, and compared interesterification with methyl formate and methyl acetate and transesterification with methanol. Based on the process kinetic parameters, it was found that, although the rate of process of transesterification with methanol is higher, the resulting by-product, glycerol, makes the process more difficult, so it is more promising to perform interesterification with methyl formate or methyl acetate. It has been established that the process of interesterification with methyl fomate is more complicated than using methyl or ethyl acetate. Large yields of methyl are obtained over a longer period of time and by taking a higher ratio of caboxylate ester-to-oil. This can be explained by the kinetic parameters of the process. The rate of interesterification of rapeseed oil using methyl acetate was found to be 1.6 times higher than that of interesterification with the methyl formate. The reaction rate constants corresponded to 6.66 and 4.05 Lmol−1 min−1 [42].
Although the use of biocatalysts is attractive, from an environmental point of view, the biocatalytic process of oil interesterification using carboxylate esters of low molecular weight has been little studied. Makareviciene et al. investigated the use of biocatalysts for the interesterification of rapeseed oil with methyl formate. Fifteen different lipases were tested. Three lipases were more effective: Novozyme 435, lipase with perlite, and Lipozyme RM IM, from which, the most efficient enzyme, Lipozyme RM IM, was selected for further studies. Its optimum content was 13% (by weight of oil). The highest yield of rapeseed oil methyl esters (60.68 ± 0.95%) was obtained at 20 °C, with a molar ratio of methyl formate-to-oil of 32:1 for a process duration of 42 h [43]. Other scientists used the enzymatic preparation Novozym 435 as an interesterification catalyst [19,21,23,31,62]. It has been established that the optimal concentration of the catalyst depends on the type of carboxylete ester used for oil interesterification. The optimum concentration of the catalyst ranged from 8% (by weight of oil) when soy oil with was intereserified with ethyl acetate [62], to 30% (by weight of oil), when with methyl acetate was used for soy oil interesterification [21].
It is difficult to compare the most efficient amount of catalyst because different catalysts were used. It can be stated that the optimal concentration of chemical catalysts is not high (0.12% or catalyst-to-oil molar ratio of 0.08–0.1. On the other hand, biocatalysis require a higher amount of catalyst both using methyl formate and other types of carboxylate esters of low molecular weight.
2.2. Influence of Methyl Formate-To-Oil Molar Ratio on the Product Yield
The interesterification reaction, similar to the reaction of the conventional transesterification used in the production of biodiesel, is reversible. The higher yield of the product is obtained by using a quantity of the transesterification or interesterification agent higher than the equivalent, i.e., by increasing the molar ratio of transesterification or interesterification agent and oil (Figure 1). In the conventional synthesis of biodiesel, the molar ratio of methanol-to-oil most often used is 6:1.
The molar ratio of methyl formate-to-oil is very important for obtaining maximum levels of esters and triformylglycerol. The oil interesterification reaction requires three moles of methyl formate per mole of triglycerides, i.e., a minimum molar ratio of 3:1 (Figure 1). However, an excess of methyl formate is necessary to obtain a higher product yield. In triglyceride interesterification studies, the molar ratio of methyl formate-to-oil ranged from 18: 1 to 80: 1, depending on the used catalyst and process conditions. For interesterification of rapeseed oil, taking a molar ratio of 18:1 methyl formate-to-oil and using chemical catalysts, 71–78.3 wt.% rapeseed oil methyl ester content was obtained [37,41,42]. Abelniece et al. investigated the influence of various molar ratios of methyl formate and oil on the efficiency of the interesterification process and found that increasing the molar ratio also increases the content of esters. At a molar ratio of 18:1, the ester content in reaction product was 78.3 wt.%; when the molar ratio was 42:1, the ester content of 93 wt.% was obtained. At an even higher molar ratio, a slightly higher content of rapeseed oil methyl esters was obtained; at a molar ratio of 80:1, the ester content of 94.8 wt.% was determined. Researchers stated that the optimal molar ratio of methyl formate-to-rapeseed oil is 42:1–51:1 [42]. The results of the studies showed that the high yield of products is obtained only by taking a high molar ratio of methyl formate-to-oil. Similar trends have also been observed in the study of the effectiveness of the chemical interesterification process using other carboxylate esters. At a methyl acetate-to-oil molar ratio of 18:1, triglycerides are fully converted to intermediates, and only when a 50:1 molar ratio is used are the intermediates converted to a final product [63]. Miesiac et al. refers to a slightly lower optimal molar ratio for interesterification using ethyl acetate, which reaches 11:1 [48].
Meanwhile, the biotechnological process of interesterification resulted in high ester yields using a smaller excess of methyl formate. Makareviciene et al. applied a biocatalytic process for the interesterification of rapeseed oil with methyl formate. The influence of the molar on the interesterification efficiency was investigated by changing the molar ratio from 6:1 to 40:1. The optimum molar ratio of methyl formate-to-oil was found to be 32:1 using the enzyme catalyst Lipozyme RM IM to obtain an ester yield of 60.68 ± 0.95%. Increasing the molar ratio by more than 32:1 was not appropriate because the product yield increased insignificantly [43].
Similar trends have been observed in the application of the biotechnological method for oil interesterification with other types of carboxylate esters. The results of numerous studies by scientists who analyzed the biotechnological process of interesterification of oil using methyl acetate coincide that the optimal molar ratio is 12:1 [19,21,36]. The results obtained by Usai et al. (2010) are somewhat different, which showed that a 20:1 molar ratio is required for the interestification of olive oil with methyl acetate [23]. Meanwhile, when using ethyl acetate for the interesterification of triglycerides, a high yield of esters was obtained, at a molar ratio 6:1 to 12.6 [19,26,62].
2.3. Influence of Temperature on the Product Yield
The rate of the reactions increases at higher temperatures, but it is recommended that the transesterification and interesterification processes be performed at temperatures above the boiling point of the acyl receptor. The boiling point of methyl formate is 32 °C; therefore, studies of rapeseed oil interesterification were performed at low temperatures of 20–28 °C [37,41,43]. The researchers performed the interesterification studies with chemical catalysts at 25 °C, aiming to optimize the interesterification process, and obtained 71% rapeseed oil methyl ester yield when the reaction was performed without a co-solvent; a total of 71.7% RME yield was determined with using a co-solvent tetrahydrofuran [41]. Sustere et al. [37] and Abelniece et al. [42] performed interesterification studies of rapeseed oil at similar temperatures of 27 and 28 °C.
It should be noted that the use of other types of carboxylate esters, such as methyl, etyl acetates, and dimethylcarbonate, for interesterification of oil sets a higher optimum temperatures of 50–60 °C, since the boiling point of these caboxylate esters is higher than of methyl formate [64,65]. Sustere et al. (2017) conducted studies of the interesterification of rapeseed oil with methyl-, ethyl, propyl, and isopropyl acetates at 55 °C temperature [37]. Casas et al. suggested an optimal temperature of sunflower oil interesterification with methyl acetate of 50 °C [66]. In this way, Miesieac et al. studied the interesterification of rapeseed oil with ethyl acetate at 78 °C [48]. Even higher optimum temperature levels, interesterification with methyl and ethyl acetate from 210 to 300 °C were determined using heterogeneous catalysts [20,67,68].
When using biocatalysts, it is necessary to carry out processes at 20–70 °C temperatures, since at higher temperatures biocatalysts are denatured and inactivated. As a rule, biotechnological processes are carried out at a temperature not exceeding 70 °C. Given the low boiling point of the methyl formate, it is favorable to carry out interesterification using biocatalysts. The results of the studies showed that, at the lowest temperature of 20 °C, using biocatalysis to interesterification of rapeseed oil with methyl formate methyl ester, a yield of 60.68 ± 0.95% was obtained [43]. Studies on the use of other types of carboxylate esters (methyl and ethyl acetates) using enzymatic catalysis were carried out at a temperature of 40 to 50, at which a yield of more than 90% of the esters was obtained. The highest yield of esters was obtained using the biocatalyst Novozyme 325 for insect fat for interesterification with methyl acetate. It reached 96.97% at a temperature of 39.5 °C [29].
2.4. Influence of Process Duration on the Product Yield
Duration is one of the most important factors influencing the yield of products obtained during the interesterification process. Abelniece et al. investigated the process of interesterification of rapeseed oil with methyl formate using catalyst tert-butoxide in tert-butanol, varying the reaction time from 0 to 90 min [41]. The optimal reaction time was found to be 60 min, and the reaction yield depended mainly on the molar ratio of methyl formate-to-rapeseed oil.
Sustere et al. [37] stated that two layers are formed during the interesterification process of rapeseed oil; the upper one contains more fatty acid methyl esters (FAME), the lower one contains mono- (MFG), di (DFG), and triformylglycerides (TFG) and glycerol (G). The yield of FAME (yield of FAME (%) shows how much oil is converted to FAME) obtained was 88.3 wt.%, and the amount of FAME (FAME amount in the mixture of FAME and formyl glycerides) was 74.2 wt.% (Table 2).

Table 2.
Percentage composition of reaction mixtures.
Scientists have conducted studies of interesterification with other types of carboxylate esters, with reaction times of 20 min to 40 h, depending on the process conditions, using chemical catalysts [20,34,35,36,37]. It was found that 20 min is enough to obtain a 77% yield of ethyl esters during alkaline interesterification with ethyl acetate [48]. Meanwhile, Galia et al., using ethyl acetate, obtained a 90% yield of ethyl esters in 20 h. It can be stated that the interesterification process is greatly influenced by all four factors (molar ratio, amount of catalyst, temperature, and duration), and a change in one of them can lead to changes in other factors, in order to obtain a higher yield of products [20].
A greater influence of duration on the efficiency of the process is observed in the application of the biotechnological synthesis method, the optimal duration of which allows for obtaining a higher yield of esters, compared to the method of chemical catalysis, to be higher. Makareviciene et al. investigated the dependence of the effectiveness of interesterification reaction on the reaction time. The reaction duration was varied from 6 to 48 h, at a molar ratio of methyl formate-to-oil of 32:1, an enzyme content of 13%, and temperature of 20 °C. It has been found that the yield of methyl esters increases with increasing process time. During the 24 h, a yield of 60.68 ± 0.95% of methyl esters was obtained. As the process time was extended, the ester yield increased insignificantly: for a process time of 30 h, the ester yield was 61.47 ± 1.10%. During 42 and 48 h of enzymatic interesterification, RME yields of 65.4 ± 1.11% and 65.3 ± 1.14% were obtained, respectively. It was concluded that the optimal reaction time is 42 h [43]. The results of the majority of studies using other types of carboxylate esters showed that the optimal reaction time is 12–48 h, in order to achieve the maximum yield of the product and using the enzymatic interesterification process [21,28,29].
3. Properties of Products Obtained during the Interesterification Process
The produced fuel must meet the requirements of the standard, in order to be used in diesel engines. Table 3 presents the fuel properties and compares them with the requirements of European standard EN 14214. The density and viscosity of the fuels/samples produced by Abelniece et al. and analyzed exceed the limits of EN 14214 for biodiesel, due to the presence of intermediates in the reaction products. The density and viscosity of the fuel decreases as the molar ratio of methylformate-to-oil used in the production process increases [41]. In the case of a molar ratio of 18:1, the density and viscosity slightly exceeds the requirements of the standard; at higher molar ratios, these parameters meet the requirements of EN 14214 [42]. The flash point of fuel should be above 101 °C. Abelniece et al. reported the flash point of prepared fuel of 95 °C and higher than 101 °C [41]. A flash point above 200 °C was determined for a product synthesized using a molar ratio of methylformate-to-oil of 36:1 and higher [42] The carbon residue of products obtained at this molar ratio achieved products that met the requirements of ASTMD6751, with less than 0.05 wt.%. At a molar ratio of methyl formate-to-oil of 18:1, the carbon residue of the resulting product exceeded the standard limits and was 0.16 wt.%. The CFPP of the reaction products corresponds to the requirements for diesel fuel of C class and is lower than minus 5 °C (EN 590). The pour point increases with increasing molar ratio of methyl formate-to-oil used in the fuel production process.

Table 3.
Properties of products obtained by interesterifying with methyl formate.
Studies by Abelniece et al. [42] prove that results of process optimization a swell as analysis of products properties has huge influence on the selection of optimal process conditions. Based on the process optimization studies, a molar ratio of methyl formate-to-oil of 42:1–51:1 was selected as optimal, and the analysis of the properties showed that the fuels produced using molar ratio of 36:1 were characterized by better physicochemical properties and compliance with the requirements of the standards.
Other researchers have studied the properties of fuel blends, consisting of conventional biodiesel and triacetin, as well as their compliance with the requirements of biofuel standards. Triacetin additive has been found to increase fuel density and viscosity and the lower cetane pour point, cold filter plugging, and flash point [16]. Feng et al. and Nielsem et al. found that some fuel properties—sulfur content, acidity, and viscosity—depend on the production process and raw materials [69,70]. It was determined that a mixture consisting of 80% biodiesel and 20% triacetin can be used as fuel for diesel engines, according to properties such as viscosity, pour point, flash point, cold filter plugging point, and cetane number [16]. The viscosity of this mixture at a temperature of 40 °C is 4.5 mm2/s and meets the requirements of standard EN 14214 (3.5–5.0 mm2/s). This rate was the same as for fuel obtained by interesterification of rapeseed oil with methyl formate using a molar ratio of methyl formate-to-oil of 36:1–51:1 [42]. The density of the mixture of biodiesel and triacetin exceeds the requirements of the European standard for biodiesel and reaches 914–921 kg/m−3. This is explained by the higher density of triacetin (1160 kg/m−3), compared to that of biodiesel (880 kg/m−3) [71]. During direct interesterification of oil both, using methyl formate and other types of carboxylate esters, the density of the products obtained is also higher than required by the European standard [41] Only at the molar ratio of methyl formate-to-rapeseed oil from 36:1 to 51:1, the density of the resulting product complied with the requirements of the standard [31,42]. Only the density of product obtained for the interesterification process using a molar ratio of methyl formate-to-oil between 36:1 and 51:1 met the requirements of standard [42]. As mentioned earlier, the mixture of biodiesel with triacetin has good low-temperature properties; it significantly reduces the pour point and cold filter plugging point. The cold filter plugging point of the fuel mixture containing 20% of triacetin was minus 9 °C [63]. Abelniece et al. also obtained the same cold filter point for fuel produced by interesterification of rapeseed oil with methyl formate [42].
4. Prospects
In the conventional production of biodiesel, the process of transesterification of various types of oil with alcohol is applied [72]. Recently, there has been a growing interest in the possibility of applying the interesterification process for biofuel synthesis. The process of interesterification with methyl formate is catalytic, and chemical and biochemical catalysts were used for research. It was found that a high yield of the product is obtained only with a significant excess of methyl formate and long duration of the process. However, given the low boiling point of the methyl formate, the process can be carried out without additional heating. Some of the physicochemical properties of the product obtained during interesterification were found to meet the requirements of the standard for fuel. However, it is necessary to carry out more detailed studies of physical and chemical properties of innovative biofuel, as well as the compliance of the properties with the requirements of the standards for biofuel; so far, only some of the properties that are presented in the requirements of the standards have been studied. In the future, it is necessary to carry out operational tests of the engine using innovative biofuel and evaluate the concentrations of harmful components in engine emissions. It is not clear what the real effect of the presence of triacetin in fuel could be on the long-term operation of the engine, corrosion, fuel consumption, efficiency of the combustion process, etc., and working and operational characteristics.
More research is needed in the future to improve the process and make it more environmentally friendly by analyzing the use of a wider range of catalysts. It is expedient to study the use of heterogeneous catalysts in the oil interesterification process and evaluate the possibilities of their reusability and regeneration. From an environmental point of view, it is necessary to determine the life cycle indicators and biodegradation in the natural environment of innovative fuel. Equally important is the assessment of the economic indicators of interesterification process, compared to traditional transesterification applied in the production of conventional biodiesel.
5. Conclusions
During the conventional biodiesel production process, about 10% of by-product, glycerol phase, is formed. The application of the oil interesterification process with carboxylate esters prevents the formation of the glycerol and increases the fuel yield, thus providing a mixture of biodiesel and triformylglyceride, which is soluble in biodiesel. Chemical and enzymatic catalysts can be used to catalyze the interesterification process. Using chemical alkaline catalysts, the reaction rate is higher than using enzymatic heterogeneous catalysts. Higher amounts of biocatalysts are required for the effective interesterification of oil, compared to chemical catalysts. The process takes place at low temperatures (20–28 °C), at nearly the boiling point of methyl formate (−32 °C). The molar ratio of methyl formate-to-oil has a significant effect on the yield and quality of the product. A yield of methyl esters greater than 90% was obtained by taking the molar ratio of methyl format-to-oil greater than 36:1 and using homogeneous chemical catalysts. The application of the biocatalysis process provided lower yields of methyl esters.
The resulting interesterification product is equivalent in quality to biodiesel and meets the requirements of the biodiesel standards. The mixture of biodiesel and triformylglyceride has better low-temperature properties than biodiesel and meets the requirements for C-class diesel fuels.
Author Contributions
Conceptualization, V.M. and E.S.; methodology, V.M. and E.S.; software, V.M. and E.S.; validation, V.M. and E.S.; formal analysis, investigation, E.S. and V.M.; resources, E.S.; data curation, V.M. and E.S.; writing—original draft preparation, V.M. and E.S.; writing—review and editing, V.M. and E.S.; visualization, V.M. and E.S.; supervision, V.M. and E.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Research Council of Lithuania (LMT LT), grant number No. MIP-22/59.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Arachchige, U.; Viraj Miyuranga, K.A.; Thilakarathne, D.; Jayasinghe, R.A.; Weerasekara, N.A. Biodiesel-alkaline transesterification process for methyl ester production. Nat. Environ. Pollut. Technol. 2021, 20, 1973–1980. [Google Scholar] [CrossRef]
- Karcauskiene, D.; Sendzikiene, E.; Makareviciene, V.; Zaleckas, E.; Repsiene, R.; Ambrazaitiene, D. False flax (Camelina sativa L.) as an alternative source for biodiesel production. Agriculture 2014, 101, 161–168. [Google Scholar]
- Talavari, R.; Hosseini, S.; GR Moradi, G.R. Low-cost biodiesel production using waste oil and catalyst. Waste Manag. Res. 2021, 39, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Makareviciene, V.; Gumbyte, M.; Yunik, A.; Kalenska, S.; Kalenskii, V.; Rachmetov, D.; Sendzikiene, E. Opportunities for the use of chufa sedge in biodiesel production. Ind. Crops Prod. 2013, 50, 633–637. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Wu, X.; Leung, M.K.H. A review on biodiesel production using catalyzed transesterification. Appl. Energy 2010, 87, 1083–1095. [Google Scholar] [CrossRef]
- Govindaraju, R.; Chen, S.S.; Wang, L.P.; Chang, H.M.; Pasawan, M. Significance of membrane applications for high-quality biodiesel and byproduct (glycerol) in biofuel industries—Review. Curr. Pollut. Rep. 2021, 7, 128–145. [Google Scholar] [CrossRef]
- Ayoub, M.; Ahmad, Z.A. Critical review on the current scenario and significance of crude glycerol resulting from biodiesel industry towards more sustainable renewable energy industry. Renew. Sust. Energ. Rev. 2012, 16, 2671–2686. [Google Scholar] [CrossRef]
- Treinyte, J.; Cesoniene, L.; Bridziuviene, D.; Ostrauskaite, J.; Bucinskas, A.; Rainosalo, E.; Grazuleviciene, V. Applicability of crude glycerol as the multifunctional additive for the preparation of mulching coatings. Waste Biomass Valori. 2018, 9, 1855–1865. [Google Scholar] [CrossRef]
- Sendzikiene, E.; Makareviciene, V. Application of liquid waste from biogas production for microalgae Chlorella sp. cultivation. Cells 2022, 11, 1206. [Google Scholar] [CrossRef]
- Nitayavardhana, S.; Khanal, S. Biodiesel-derived crude glucerol bioconversion to animal feed. A sustainable option for a biodiesel refinery. Bioresour. Technol. 2011, 102, 5808–5814. [Google Scholar] [CrossRef]
- Karinen, R.S.; Krause, A.O.I. New biocomponents from glycerol. Appl. Catal. A General. 2006, 306, 128–133. [Google Scholar] [CrossRef]
- Ardi, M.S.; Aroua, M.K.; Hashim, N.A. Progress, prospect and challenges in glycerol purification process: A review. Renew. Sustain. Energy Rev. 2015, 42, 1164–1173. [Google Scholar] [CrossRef]
- Azita, J.; Hasheminejad, M.; Tahvildari, K.; Tabatabaei, M. High quality potassium phosphate production through step-by step glycerol purification: A strategy to economize biodiesel production. Bioresour. Technol. 2012, 104, 788–790. [Google Scholar]
- Manurung, R.; Anggreawan, M.D.; Siregar, A.G. Triacetin production using SiO2-H3PO4 catalysts derived from bamboo leaf biomass waste for esterification reactions of glycerol and acetic acid. IOP Conf. Ser. Mater. Sci. Eng. 2020, 801, 012052. [Google Scholar] [CrossRef]
- Binhayeeding, N.; Klomklao, S.; Sangkharak, K. Utilization of waste glycerol from biodiesel process as a substrate for mono-, di-, and triacylglycerol production. Energy Procedia 2017, 138, 895–900. [Google Scholar] [CrossRef]
- Casas, A.; Ruiz, J.R.; Ramos, M.J.; Pérez, Á. Effects of triacetin on biodiesel quality. Energy Fuels 2010, 24, 4481–4489. [Google Scholar] [CrossRef]
- Huang, X.; Bi, J.; Wang, J.; Ouyang, J.; Xiao, Y.; Hao, H.; Wang, Y.; Yin, Q. Liquid–liquid equilibrium of binary and ternary systems composed by palm oil or palm oil fractions with methanol/ethanol and water. Fluid Phase Equilibria 2015, 404, 17–25. [Google Scholar] [CrossRef]
- Alavianmehr, M.M.; El-Shaikh, M.; Akbari, F.; Behjatmanesh-ardakani, R. A new equation of state for modeling thermodynamic properties of some fatty acids alkyl esters, methyl ester-based biodiesels and their blends. Fluid Phase Equilibria 2017, 442, 53–61. [Google Scholar] [CrossRef]
- Jeong, G.T.; Park, D.H. Synthesis of rapeseed biodiesel using short-chained alkyl acetates as acyl acceptor. Appl. Biochem. Biotechnol. 2010, 161, 195–208. [Google Scholar] [CrossRef]
- Galia, A.; Centineo, A.; Saracco, G.; Schiavo, B.; Scialdone, O. Interesterification of rapeseed oil catalyzed by tin octoate. Biomass Bioenergy 2014, 67, 193–200. [Google Scholar] [CrossRef]
- Du, W.; Xu, Y.; Liu, D.; Zeng, J. Comparative study on lipase-catalyzed transformation of soybean oil for biodiesel production with different acyl acceptors. J. Mol. Catal. B Enzym. 2004, 30, 125–129. [Google Scholar] [CrossRef]
- Fabbri, D.; Bevoni, V.; Notari, M.; Rivetti, F. Properties of a potential biofuel obtained from soybean oil by transmethylation with dimethyl carbonate. Fuel 2007, 86, 690–697. [Google Scholar] [CrossRef]
- Usai, E.M.; Gualdi, E.; Solinas, V.; Battistel, E. Simultaneous enzymatic synthesis of FAME and triacetyl glycerol from triglycerides and methyl acetate. Bioresour. Technol. 2010, 101, 7707–7712. [Google Scholar] [CrossRef] [PubMed]
- Dorado, M.P.; Ballesteros, E.; Mittelbach, M.; Lopez, F.J. Kinetic parameters affecting the alkali-catalyzed transesterification process of used olive oil. Energy Fuel 2004, 18, 1457–1462. [Google Scholar] [CrossRef]
- Ognjanovic, N.; Bezbradica, D.; Knezevic-Jugovic, Z. Enzymatic conversion of sunflower oil to biodiesel in a solvent-free system: Process optimization and the immobilized system stability. Bioresour. Technol. 2009, 100, 5146–5154. [Google Scholar] [CrossRef]
- Modi, M.K.; Reddy, J.R.C.; Rao, B.; Prasa, R.B.N. Lipase-mediated conversion of vegetable oils into biodiesel using ethyl acetate as acyl acceptor. Bioresour. Technol. 2007, 98, 1260–1264. [Google Scholar] [CrossRef]
- Sentanuhady, J.; Saputro, W.; Muflikhun, M.A. Metals and chemical compounds contaminants in diesel engine lubricant with B20 and B100 biofuels for long term operation. Sustain. Energy Technol. Assess. 2021, 45, 101161. [Google Scholar] [CrossRef]
- Su, E.Z.; Zhang, M.J.; Zhang, J.G.; Gao, J.; Wei, D.Z. Lipase-catalyzed ir reversible transesterification of vegetable oils for fatty acid methyl esters production with dimethyl carbonate as the acyl acceptor. Biochem. Eng. J. 2007, 36, 167–173. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Liang, S.H.; Chen, S.S.; Su, C.H.; Lin, J.H.; Chien, C.C. Enzymatic production of biodiesel from insect fat using methyl acetate as an acyl acceptor: Optimization by using response surface methodology. Energy Convers. Manag. 2018, 158, 168–175. [Google Scholar] [CrossRef]
- Razack, S.A.; Duraiarasan, S. Response surface methodology assisted biodiesel production from waste cooking oil using encapsulated mixed enzyme. Waste Manag. 2016, 47, 98–104. [Google Scholar] [CrossRef]
- Kampars, V.; Abelniece, Z.; Blaua, S. The Unanticipated Catalytic Activity of Lithium tert -Butoxide/THF in the Interesterification of Rapeseed Oil with Methyl Acetate. J. Chem. 2019, 2019, 1509706. [Google Scholar] [CrossRef]
- Kampars, V.; Abelniece, Z.; Lazdovica, K.; Kampare, R. Interesterification of rapeseed oil with methyl acetate in the presence of potassium tert-butoxide solution in tetrahydrofuran. Renew. Energy 2020, 158, 668–674. [Google Scholar] [CrossRef]
- Maddikeri, G.L.; Pandit, A.B.; Gogate, P.R. Ultrasound assisted interesterification of waste cooking oil and methyl acetate for biodiesel and triacetin production. Fuel Process. Technol. 2013, 116, 241–249. [Google Scholar] [CrossRef]
- Interrante, L.; Bensaid, S.; Galletti, C.; Pironeb, R.; Schiavo, B.; Scialdone, O.; Galia, A. Interesterification of rapeseed oil catalysed by a low surface area tin (II) oxide heterogeneous catalyst. Fuel Process. Technol. 2018, 177, 336–344. [Google Scholar] [CrossRef]
- Tian, Y.; Xiang, J.; Verni, C.V.; Soh, L. Fatty acid methyl ester production via ferric sulfate catalyzed interesterification. Biomass Bioenergy 2018, 115, 82–87. [Google Scholar] [CrossRef]
- Surendhiran, D.; Vijay, M.; Sirajunnisa, A.R. Biodiesel production from marine microagla Chlorella salina using whole cell yeast immobilized on sugarcane bagasse. J. Environ. Chem. Eng. 2014, 2, 1294–1300. [Google Scholar] [CrossRef]
- Sustere, Z.; Kampars, V. The influence of acyl and alkohol moieties of carboxylate esters on rapeseed oil chemical interesterification. Int. J. Mater. Methods Technol. 2017, 11, 1–7. [Google Scholar]
- Sustere, Z.; Murnieks, R.; Kampars, V. Chemical interesterification of rapeseed oil with methyl, ethyl, propyl and isopropyl acetates and fuel properties of obtained mixtures. Fuel Process. Technol. 2016, 149, 320–325. [Google Scholar] [CrossRef]
- Sendzikiene, E.; Makareviciene, V. Biodiesel fuel synthesis by interesterification of triglycerides with carboxylate esters of low molecular weight. Rev. Chem. Eng. 2021, 37, 259–276. [Google Scholar] [CrossRef]
- Goembira, F.; Matsuura, K.; Saka, S. Biodiesel production from rapeseed oil by various supercritical carboxylate esters. Fuel 2012, 97, 373–378. [Google Scholar] [CrossRef]
- Abelniece, Z.; Kampars, V. Studying the kinetics of rapeseed oil reactions with methanol, methyl formate, and methyl acetate under mild conditions for biodiesel production. Biofuels 2022, 13, 615–622. [Google Scholar] [CrossRef]
- Abelniece, Z.; Laipniece, L.; Kampars, V. Biodiesel production by interesterification of rapeseed oil with methyl formate in presence of potassium alkoxides. Biomass Convers. Biorefin. 2022, 12, 2881–2889. [Google Scholar] [CrossRef]
- Makareviciene, V.; Sendzikaite, I. Biocatalytic transesterification of rapeseed oil by methyl formate. Agric. Sci. 2019, 26, 47–56. [Google Scholar] [CrossRef]
- López-Yerena, A.; Guerra-Ramírez, D.; Reyes-Trejo, B.; Salgado-Escobar, I.; Cruz-Castillo, J.G. Waste from Persea schiedeana fruits as potential alternative for biodiesel production. Plants 2022, 11, 252. [Google Scholar] [CrossRef]
- Lin, Y.C.; Hsu, K.H.; Lin, J.F. Rapid palm-biodiesel production assisted by a microwave system and sodium methoxide catalyst. Fuel 2014, 115, 306–311. [Google Scholar] [CrossRef]
- Gebremariam, S.N.; Marchetti, J.M. Biodiesel production through sulfuric acid catalyzed transesterification of acidic oil: Techno economic feasibility of different process alternatives. Energy Convers. Manag. 2018, 174, 639–648. [Google Scholar] [CrossRef]
- Su, C.H. Recoverable and reusable hydrochloric acid used as a homogeneous catalyst for biodiesel production. Appl. Energy 2013, 104, 503–509. [Google Scholar] [CrossRef]
- Miesiac, I.; Rogalinski, A.; Jozwiak, P. Transesterification of triglycerides with ethyl acetate. Fuel 2013, 105, 169–175. [Google Scholar] [CrossRef]
- Dai, Y.M.; Wang, Y.F.; Chen, C.C. Synthesis and characterization of magnetic LiFe5O8-LiFeO2 as a solid basic catalyst for biodiesel production. Catal. Commun. 2018, 106, 20–24. [Google Scholar] [CrossRef]
- Alcañiz-Monge, J.; Bakkali, B.E.; Trautwein, G.; Reinoso, S. Zirconia-supported tungstophosphoric heteropolyacid as heterogeneous acid catalyst for biodiesel production. Appl. Catal. B Environ. 2018, 224, 194–203. [Google Scholar] [CrossRef]
- Dantas, J.; Leal, E.; Cornejo, D.R.; Kiminami, R.; Costa, A.C.F.M. Biodiesel production evaluating the use and reuse of magnetic nanocatalysts Ni0. 5Zn0. 5Fe2O4 synthesized in pilot-scale. Arab. J. Chem. 2020, 13, 3026–3042. [Google Scholar] [CrossRef]
- Vasić, K.; Hojnik Podrepšek, G.; Knez, Ž.; Leitgeb, M. Biodiesel production using solid acid catalysts based on metal oxides. Catalysts 2020, 10, 237. [Google Scholar] [CrossRef]
- El Shimi, H.I.; Attia, N.K.; Diwani, G.I.; El Sheltawy, S.T. Investigation of silicates as a catalyst in biodiesel production: A review. Int. J. Energy Res. 2016, 40, 1743–1756. [Google Scholar] [CrossRef]
- Ren, Y.; He, B.; Yan, F.; Wang, H.; Cheng, Y.; Lin, L.; Feng, Y.; Li, Y. Continuous biodiesel production in a fixed bed reactor packed with anion-exchange resin as heterogeneous catalyst. Bioresour. Technol. 2012, 113, 19–22. [Google Scholar] [CrossRef]
- Volli, V.; Purkait, M.K. Selective preparation of zeolite X and A from flyash and its use as catalyst for biodiesel production. J. Hazard. Mater. 2015, 297, 101–111. [Google Scholar] [CrossRef]
- Gaide, I.; Makareviciene, V.; Sendzkiene, E.; Kazancev, K. Natural rocks–heterogeneous catalysts for oil transesterification in biodiesel synthesis. Catalysts 2021, 11, 384. [Google Scholar] [CrossRef]
- Hu, S.; Wang, Y.; Han, H. Utilization of waste freshwater mussel shell as an economic catalyst for biodiesel production. Biomass Bioenergy 2011, 35, 3627–3635. [Google Scholar] [CrossRef]
- Gaide, I.; Makareviciene, V.; Sendzkiene, E. Effectiveness of eggshells as natural heterogeneous catalysts for transesterification of rapeseed oil with methanol. Catalysts 2022, 12, 246. [Google Scholar] [CrossRef]
- Yuliana, M.; Santoso, S.P.; Soetaredjo, F.E.; Ismadji, S.; Ayucitra, A.; Gunarto, C.; Angkawijaya, A.E.; Ju, Y.H.; Truong, C.T. Efficient conversion of leather tanning waste to biodiesel using crab shell-based catalyst: WASTE-TO-ENERGY approach. Biomass Bioenergy 2021, 151, 106155. [Google Scholar] [CrossRef]
- Sendzikiene, E.; Sinkuniene, D.; Kazanceva, I.; Kazancev, K. Optimization of low quality rapeseed oil transesterification with butanol by applying the response surface methodology. Renew. Energy 2016, 87, 266–272. [Google Scholar] [CrossRef]
- Santaraite, M.; Sendzikiene, E.; Makareviciene, V.; Kazancev, K. Biodiesel production by lipase-catalyzed in situ transesterification of rapeseed oil containing a high free fatty acid content with ethanol in diesel fuel media. Energies 2020, 13, 2588. [Google Scholar] [CrossRef]
- Kim, S.J.; Jung, A.M.; Park, Y.C.; Park, K. Lipase catalyzed transesterification of soybean oil using ethyl acetate, an alternative acyl acceptor. Biotechnol. Bioprocess. Eng. 2007, 12, 441–445. [Google Scholar] [CrossRef]
- Casas, A.; Ramos, M.J.; Perez, A. New trends in biodiesel production: Chemical interesterification of sunflower oil with methyl acetate. Biomass Bioenergy 2011, 35, 1702–1709. [Google Scholar] [CrossRef]
- Seong, P.J.; Jeon, B.W.; Lee, M.; Cho, D.H.; Kim, D.K.; Jung, K.S.; Kim, S.W.; Han, S.O.; Kim, Y.H.; Park, C. Enzymatic coproduction of biodiesel and glycerol carbonate from soybean oil and dimethyl carbonate. Enzym. Microb. Technol. 2011, 48, 505–509. [Google Scholar] [CrossRef]
- Dawodu, F.A.; Ayodele, O.O.; Xin, J.; Zhang, S. Dimethyl carbonate mediated production of biodiesel at different reaction temperatures. Renew. Energy 2014, 68, 581–587. [Google Scholar] [CrossRef]
- Casas, A.; Ramos, M.J.; Perez, A. Methanol-enhanced chemical interesterification of sunflower oil with methyl acetate. Fuel 2013, 106, 869–872. [Google Scholar] [CrossRef]
- Ribeiro, J.S.; Celante, D.; Brondani, L.N.; Trojahn, D.O.; Silva, C.; Castilhos, F. Synthesis of methyl esters and triacetin from macaw oil (Acrocomia aculeata) and methyl acetate over γ-alumina. Ind. Crops Prod. 2018, 124, 84–90. [Google Scholar] [CrossRef]
- Tan, K.T.; Lee, K.T.; Mohamed, A.R. A glycerol-free process to produce biodiesel by supercritical methyl acetate technology: An optimization study via response surface methodology. Bioresour. Technol. 2010, 101, 965–969. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, A.; Li, J.; He, B. A continuous process for biodiesel production in a fixed bed reactor packed with cation-exchange resin as heterogeneous catalyst. Bioresour. Technol. 2011, 102, 3607–3609. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, P.M.; Rancke-Madsen, A.; Holm, H.C.; Burton, R. Production of biodiesel using liquid lipase formulations. J. Am. Oil Chem. Soc. 2016, 93, 905–910. [Google Scholar] [CrossRef]
- Ang, G.T.; Tan, K.T.; Lee, K.T. Recent development and economic analysis of glycerol-free processes via supercritical fluid transesterification for biodiesel production. Renew. Sustain. Energy Rev. 2014, 31, 61–70. [Google Scholar] [CrossRef]
- Supriyanto, E.; Sentanuhady, J.; Dwiputra, A.; Permana, A.; Muflikhun, M.A. The Recent Progress of Natural Sources and Ma ufacturing Process of Biodiesel: A Review. Sustainability 2021, 13, 5599. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).