Abstract
This study aimed to compare the color stability of monolithic zirconia (MLZ) after immersion in various staining liquids. Fifty MLZ samples (n = 50) were color infiltrated by brushing/painting the samples pre-sintering. All zirconia samples were then brushed inside a toothbrushing simulator machine to create abrasion. Ten samples each were immersed in artificial saliva (gp-1), coffee (gp-2), protein shake (gp-3), chlorhexidine mouthwash (gp-4), and a soft drink (gp-5). Besides chlorhexidine mouthwash (immersion time: 14 days), the samples were immersed in the liquids for 28 days. A spectrophotometer was utilized to observe the color differences (∆E) at the baseline vs. 28 days post-immersion. The means and standard deviations were compared using analysis of variance (ANOVA) and a multiple comparisons test. A p-value < 0.05 was considered significant. The greatest ∆E (4.57) was observed after immersion in the coffee, followed by a soft drink (∆E: 4.03). Chlorhexidine mouthwash immersion of MLZ samples also significantly changed their color stability (∆E: 3.60). The inter-group comparisons revealed statistically significant differences (p < 0.05) when ∆E values of gp-1 (artificial saliva) were compared with all other groups. Significant differences (p < 0.05) were also seen when ∆E values of gp-2 (coffee) were compared with gp-3 (protein shake) and gp-4 (chlorhexidine). All other inter-group comparisons revealed non-significant results (p > 0.05). The results of the present study demonstrate that coffee immersion of MLZ samples causes more significant discoloration (increased ∆E values) than other liquids. Further in vitro and clinical studies are warranted to corroborate the present study’s findings.
Keywords:
dentistry; prosthodontics; monolithic zirconia; color; staining liquids; spectrophotometer 1. Introduction
Zirconia (an oxide of zirconium) is used in dentistry for numerous clinical applications due to its admirable aesthetics, biocompatibility, and mechanical properties [1]. Zirconia has been used to develop crowns, bridges, posts, and implants due to its high biocompatibility, increased toughness, and improved fracture resistance [2]. Among all its clinical applications, the use of zirconia in implant dentistry stands out [3]. Like titanium, zirconia is a material that does not inhibit osteoblasts, and hence, promotes the osseointegration of dental implants [4]. One disadvantage associated with zirconia in the past was its lack of translucency; to rectify this, veneering with porcelain was introduced [5]. However, failure of conventional zirconia restorations was reported due to debonding and adhesion failure between these materials resulting in the chipping of veneering ceramic [6,7]. This problem was resolved by introducing monolithic zirconias (MLZs) as they have high flexural strength, cause minimal wear of antagonists (if properly polished), and are more in line with minimally invasive dentistry (MID) as they require more conservative tissue preparation that helps avoid chipping [8].
Conventionally, MLZs were not used for anterior restorations due to their low translucency [9]. The literature has reported that the translucency of MLZs can be increased by adding colors to facilitate their usage in the anterior region of the mouth [10]. In this technique, staining solutions are smeared on the milled restoration using a brush, which helps the color to infiltrate the ceramic in this permeable, pre-sintered stage [11]. However, this addition of color can significantly reduce its strength [12], which affects its clinical success. Plus, under in vivo conditions, exposure to various colored consumable drinks can further deteriorate the color stability [13]. In an in vitro environment, the discoloration produced by different drinks in vivo can be reproduced by immersing the material in a consumable drink, which is considered a good test to evaluate its potential to discolor by measuring the staining levels [14].
Color stability is of utmost importance among various optical properties of dental restoration [15]. In an earlier in vitro study, the color stability of different zirconia types was studied after exposure to chlorhexidine, coffee, and green tea [16]. The study’s results demonstrated that MLZ samples significantly discolored after coffee immersion [16]. Another previous study also revealed that coffee produced more noticeable color changes in zirconia samples after immersion than all the other beverages [17]. These former studies have verified that while zirconia has commendable mechanical properties and aesthetics, its color stability can still be compromised due to the exposure to various beverages. Hence, it is necessary to test this material further with different testing conditions and multiple beverages to provide a more robust challenge to test its color stability.
In that context, the present study aimed at coloring the zirconia samples and testing their color stability after exposure to different staining liquids including coffee, protein shake, chlorhexidine mouthwash, and soft drink (artificial saliva was used as a control). It was hypothesized that the immersion of zirconia samples in various liquids would affect its color stability differently.
2. Materials and Methods
Ethical approval was obtained from the Institutional Review Board (IRB) of the College of Dentistry, Prince Sattam bin Abdulaziz University, Saudi Arabia, and all the protocols were strictly followed.
2.1. Sample Preparation
A total of fifty specimens (n = 50) of white zirconia blanks were fabricated (Cercon® ht high translucent, Dentsply Sinora, Milford, DE, USA) with a Cercon® brain expert (Dentsply Sirona, York, PA, USA) milling system. All the samples were burnished manually with 600 grit SiC abrasive paper for a uniform surface. The zirconia samples were prepared and stained to the target color VITA A2. A minimum of ten samples was considered for each group based on the observations and sample size from a previous study [18].
2.2. Coloring of Zirconia Samples
The specimen coloring was accomplished by color infiltration utilizing a color solution from Zirkonzahn (Color Liquid Prettau Watercolor, A2). The color was applied by brushing/painting the samples pre-sintering. A single application of paint was achieved with three brush strokes. For reference, a VITA A2 shade tab was measured with a spectrophotometer (SR, SpectraScan PR-650, MS-75 lens, Photo Research Inc., Chatsworth, CA, USA). To achieve the target color A2, six applications were made with ∼32 mg/cm2 of the coloring solution. The target color was assessed using a spectrophotometer to match the shade tab A2. After evaluating the color difference (ΔE) values with the shade tab color, the amount of color solution providing the slightest difference to the reference color was adopted as the staining methodology. Post-staining, all the samples were dried in the oven at 130 °C for 20 min. The final sintering process was completed in a Cercon® heat furnace (DeguDent GmbH, Hanau, Germany), following the manufacturer’s instructions. The specimens were entrenched in polymethylmethacrylate (PMMA) resin to guarantee consistent placement for color assessment and stored in normal saline for 24 h.
All zirconia samples were brushed inside a toothbrushing simulator machine (ZM-3, SD Mechatronik, GMBH, Berlin, Germany) to create abrasion. The toothpaste (Colgate®, Total Clean Mint, Colgate Palmolive Arabia Ltd., Riyadh, Saudi Arabia) with a relative dentin abrasivity (RDA) value of 70 was mixed with deionized water (ratio 1:1), then this slurry was applied to each zirconia sample. During the procedure, the container was monitored for any refill of the slurry. Soft conventional toothbrushes (0.6 × 2.2 × 8.7 inches) (Oral-B®, Sensi-Soft Toothbrush, Gillette India Ltd., India) were used to brush the samples. Each sample was exposed to 43,800 cycles, corresponding to approximately three years of brushing by a healthy individual [19].
2.3. Immersion in Staining Liquids
Ten zirconia samples each were immersed in artificial saliva, coffee (Nestlé Middle East Manufacturing LLC, Dubai Nescafe Gold, Ajman, United Arab Emirates), protein shake (Hydrolyzed Whey Protein Shake Isolate, Dymatize Nutrition, Kings Mountain, NC, USA), chlorhexidine mouthwash (Chlorhexidine Gluconate 0.2% Mouthwash, Avalon Pharma, Saudi Arabia), and a soft drink (Coca Cola®, Saudi Arabia). The samples were immersed for 28 days in all these liquids other than chlorhexidine mouthwash, in which they were immersed for 14 days. All the liquids were kept at 37 °C [20]. The artificial saliva was prepared following the recommendations of an earlier study [21]. The coffee was freshly prepared daily using 15 gm of ground beans and 250 mL of hot water using the filter technique to make the coffee clear without any residues. Similarly, the protein shake was also prepared daily using 34 g of protein powder mixed with 180 mL of water. Zirconia samples were immersed in 50 mL of these solutions, which were replenished daily.
2.4. Color Measurements
The color measurements were performed via a spectrophotometer (Hunterlab, Reston, VA, USA). These measurements were taken at the baseline (before immersion experiments) and 28 days post-immersion. Before taking color measurements at each time point, the samples were cleaned with water and a soft-bristled toothbrush (Oral B®, Procter and Gamble Co., Cincinnati, OH, USA). These measurements were performed using the CIELAB formula [18]. Three color measurements were taken at each time point, then mean values were calculated for L (lightness of the color), a* (chromaticity of red-green), and b* (chromaticity of yellow-blue). The formula used for the measurement of color difference (∆E*) was as follows:
∆E*ab = [(∆L*)2 + (∆a*)2 + (∆b*)2]1/2
2.5. Conversion of ∆E Values to National Bureau of Standards (NBS) Units
The ∆E values were converted to NBS units by utilizing the following formula:
NBS units = ΔE* × 0.92
The NBS units, color change remarks, and clinical interpretation are shown below (Table 1).

Table 1.
NBS interpretation of color changes.
2.6. Statistical Analysis
The results were analyzed using statistical software (SPSS, version 22; SPSS Inc., Chicago, IL, USA). The means and standard deviations were compared using analysis of variance (ANOVA) and a multiple comparisons test. A p-value < 0.05 was considered significant.
3. Results
The ∆E values for zirconia samples are presented in Table 2 and Figure 1. The greatest ∆E was observed for coffee (4.57), followed by a soft drink (4.03). The lowest ∆E was observed for the samples immersed in saliva (1.80). The mean and standard deviation (SD) of ∆L, ∆a, and ∆b values among the study groups are shown in Figure 2.

Table 2.
∆E of zirconia samples in different mediums; values are expressed as the mean (SD).
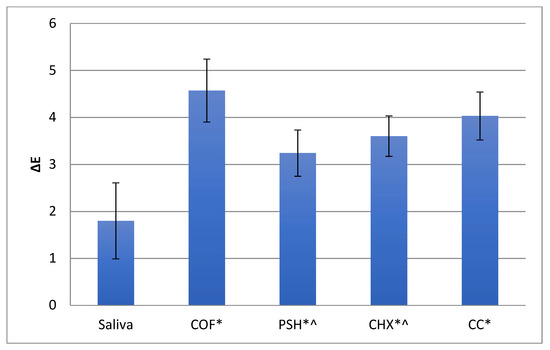
Figure 1.
Mean and SD of ∆E values among the study groups. * Shows significantly different ∆E values compared to saliva; ^ shows significantly different ∆E values compared to coffee (COF).
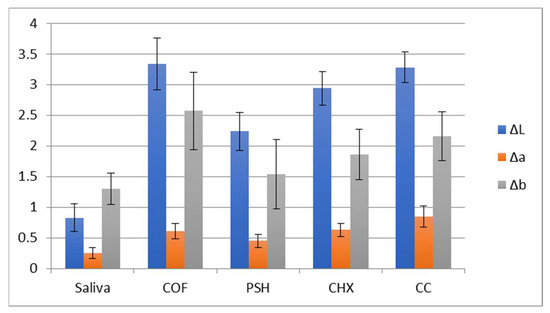
Figure 2.
Mean and SD of ∆L, ∆a, and ∆b values among study groups.
Concerning statistical analysis, the inter-group comparisons revealed statistically significant differences when ∆E values of gp-1 (artificial saliva) were compared with all other groups (Table 3). Significant differences were also seen when ∆E values of gp-2 (coffee) were compared with gp-3 (protein shake) and gp-4 (chlorhexidine). All other inter-group comparisons revealed non-significant results (p > 0.05) (Table 3).

Table 3.
Statistical comparison of ∆E values among study groups (Tukey’s post hoc test).
4. Discussion
This study’s findings facilitated the acceptance of the hypothesis as our results revealed that the ∆E of MLZ samples was affected after immersion in different liquids. Traditionally, a visual color guide has been used to assess color changes in dental materials, but the accuracy of this method is questionable and dependent on the observer’s perception of different colors [22]. A spectrophotometer is a device utilized in dentistry to analyze a material’s color and changes in the color. This device is beneficial for the color assessment of various dental restorations and their shade verification [23]. To utilize the benefits of a spectrophotometer for detecting color changes, the technique was applied in this study to analyze the ∆E of MLZ samples post-immersion in different staining liquids. To analyze the color differences, we used the CIELAB formula. Although CIEDE 2000 has been introduced, we preferred to stick with the CIELAB formula as numerous previous studies in the literature also used it [16,24].
Previously established standards in this field have indicated that an ∆E of between 1 and 3.3 units signifies an important but clinically acceptable difference; however, ∆E > 3.3 is considered unacceptable and perceivable even by an inexperienced observer [24,25]. In this study, the greatest ∆E was observed when the samples were immersed in the coffee (∆E > 3.3). Former studies have shown that coffee can induce color changes in natural teeth, adhesive resin cement, and dental ceramics [26,27,28]. In an earlier study, the immersion of zirconia ceramics in coffee resulted in significant changes in their ∆E values [24]. Another study revealed zirconia samples immersed in coffee expressed increased color differences as opposed to those immersed in other beverages [17]. The current study’s findings corroborate with the results of these previous studies as increased ∆E values were observed post-immersion in the coffee for MLZ samples. Coffee is a popular drink globally, even though it is known to cause teeth staining and discoloration of materials [29]. Coffee comprises tannin and chlorogenic acids, which are thought to be responsible for its discoloration potential [17]. The marked color changes seen in MLZ samples after immersion in the coffee could be attributed to these two ingredients. It should also be noted that coffee has an acidic pH, which can augment its discoloration potential [17]. However, in this study, the pH of liquids used for immersion experiments was not analyzed.
The immersion of MLZ samples in the soft drink also brought marked color changes post-immersion (∆E > 3.3). The discoloring potential of soft drinks is well-known as they contain artificial colorants and citric acid that facilitate discoloration of teeth and restorations [30]. Moreover, the acidic pH of these soft drinks causes demineralization of teeth and discolors dental restorations [30]. The increased ∆E after the samples were immersed in the soft drink could be attributed to these two reasons.
In the present study, the immersion of MLZ samples in protein shake also discolored the specimens (∆E > 3.3). There is a scarcity of similar studies in the literature that have tested the discoloration impact of protein shakes on zirconia samples. The findings of the present study, therefore, report unique results. The young population commonly uses protein shakes to fulfill their protein requirements and stay healthy. Regrettably, protein shakes may contain significant quantities of heavy metals, including lead, arsenic, mercury, and cadmium [31]. These ingredients are not always mentioned on the packaging of the protein powder; therefore, the consumer remains unaware of their presence and potentially harmful effects. It is anticipated that the presence of these heavy metals in the protein shake might have played an essential role in the discoloration of MLZ samples in this study, although more conclusive evidence is required.
Chlorhexidine immersion of the MLZ samples also discolored these specimens (∆E < 3.3). Chlorhexidine mouthwash is used in dentistry to restrict bacterial growth and decrease the incidence of periodontal diseases and dental caries [32]. Yet, its extended use has been shown to cause discoloration of natural teeth and dental restorations [16]. Chlorhexidine mouthwash is usually recommended to patients for a period of 7–14 days [20,33]. Although it can be recommended for more than 14–28 days [34], a large increase in extrinsic tooth staining in participants using chlorhexidine mouth rinse for 28–42 days (4–6 weeks) has been reported previously [35]. Therefore, it was decided in our study to immerse the samples in CHX mouthwash for 14 days instead of 28 days. According to a former study, zirconia-based samples are prone to discoloration after they are exposed to chlorhexidine gluconate [32]. Another study revealed that chlorhexidine could affect the color stability of nanoceramic CAD/CAM restorative material within clinically acceptable limits [36]. The findings of the present study agree with these earlier studies as immersion of MLZ samples in chlorhexidine brought changes in their color stability, but the ∆E was <3.3 (clinically acceptable level). The precise mechanism via which chlorhexidine induces discoloration in teeth remains unknown. Nevertheless, it is speculated that the chlorhexidine molecule breaks inside the oral cavity and forms parachloranilin, which results in the denaturation of proteins and formation of metal sulfides that cause discoloration of teeth and restorations [37,38]. This could be the reason for the discoloration of MLZ samples in the current study.
The results of the present study should be cautiously interpreted. The present study utilized a color difference formula for color stability assessment; however, a visual judgment method is more frequently used in clinical dentistry. In other words, a significant difference in ∆E values may not cross the clinically perceptible threshold where it comes to be noticed by the observer [39]. Therefore, along with numerical assessment using the ∆E values, the clinical perceptibility threshold and acceptability threshold must also be observed for color stability assessment to conduct a holistic investigation in future studies. Another limitation of the current study was its in vitro design. It should be noted that restorations could act differently inside the oral cavity due to the presence of saliva. Plus, under clinical conditions, as the restorations are bonded to the tooth, they are exposed to the staining liquids unequally (more on one side than the other). In our experiments, they were immersed completely in the liquids; hence, our study could have presented inflated discoloration values. Additionally, oral hygiene maintenance habits (toothbrushing, use of mouthwash) could also affect the color stability of restorations in vivo. Furthermore, only a single type of MLZ was used in this study and combined with a color solution from a particular manufacturer. To improve on that, additional combinations should be explored in future studies. It should also be noted that in the present study, only one type of each staining liquid was used for the immersion experiments. It is anticipated that using other types/brands of the same beverages will affect the color stability of zirconia differently. Additionally, the present report evaluated color stability, but in the future, other variables should be evaluated such as flexural strength and hardness [40,41,42,43]. Hence, further investigations are warranted to validate the current study’s findings.
5. Conclusions
The present study verified that the immersion of MLZ samples in coffee caused a more significant discoloration (∆E values > 3.3, clinically unacceptable) than any other staining liquid. The soft drink and protein shake immersion also induced significant discoloration in MLZ samples (∆E values > 3.3, clinically unacceptable). The immersion of MLZ samples in chlorhexidine, meanwhile, caused discoloration, but it was within clinically acceptable levels (∆E values < 3.3). Future clinical studies are warranted to corroborate the current study’s findings.
Funding
Author would like to thank the College of Dentistry Research Center and Deanship of Scientific Research at King Saud University, Saudi Arabia for their support.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data from the study are available through contact with the corresponding author.
Conflicts of Interest
The author declares no conflict of interest.
References
- Bona, A.D.; Pecho, O.E.; Alessandretti, R. Zirconia as a Dental Biomaterial. Materials 2015, 8, 4978–4991. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, F.; Jalali, H.; Mostafavi, A.S.; Zeighami, S.; Memarian, M. Retention and Clinical Performance of Zirconia Crowns: A Comprehensive Review. Int. J. Dent. 2020, 15, 8846534. [Google Scholar] [CrossRef] [PubMed]
- Parmigiani-Izquierdo, J.M.; Cabana-Munoz, M.E.; Merino, J.J.; Sanchez-Perez, A. Zirconia implants and peek restorations for the replacement of upper molars. Int. J. Implant. Dent. 2017, 3, 5. [Google Scholar] [CrossRef]
- Kligman, S.; Ren, Z.; Chung, C.H.; Perillo, M.A.; Chang, Y.C.; Koo, H.; Zheng, Z.; Li, C. The Impact of Dental Implant Surface Modifications on Osseointegration and Biofilm Formation. J. Clin. Med. 2021, 10, 1641. [Google Scholar] [CrossRef]
- Malkondu, O.; Tinastepe, N.; Ender, A.; Kazazoglu, E. An overview of monolithic zirconia in dentistry. Biotechnol. Biotechnol. Equip. 2016, 30, 644–652. [Google Scholar] [CrossRef]
- Al-Amleh, B.; Lyons, K.; Swain, M. Clinical trials in zirconia: A systematic review. J. Oral Rehabil. 2010, 37, 641–652. [Google Scholar] [CrossRef]
- Guess, P.C.; Kulis, A.; Witkowski, S.; Wolkewitz, M.; Zhang, Y.; Strub, J.R. Shear bond strengths between different zirconia cores and veneering ceramics and their susceptibility to thermocycling. Dent. Mater. 2008, 24, 1556–1567. [Google Scholar] [CrossRef]
- Kontonasaki, E.; Rigos, A.E.; Ilia, C.; Istantsos, T. Monolithic Zirconia: An Update to Current Knowledge. Optical Properties, Wear, and Clinical Performance. Dent. J. 2019, 7, 90. [Google Scholar] [CrossRef]
- Tabatabaian, F. Color Aspect of Monolithic Zirconia Restorations: A Review of the Literature. J. Prosthodont. 2019, 28, 276–287. [Google Scholar] [CrossRef]
- Kim, H.K.; Kim, S.H.; Lee, J.B.; Ha, S.R. Effects of surface treatments on the translucency, opalescence, and surface texture of dental monolithic zirconia ceramics. J. Prosthet. Dent. 2016, 115, 773–779. [Google Scholar] [CrossRef]
- Kim, H.K.; Kim, S.H. Effect of the number of coloring liquid applications on the optical properties of monolithic zirconia. Dent. Mater. 2014, 30, e229–e237. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y. Making yttria-stabilized tetragonal zirconia translucent. Dent. Mater. 2014, 30, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Adusumilli, H.; Avula, J.S.; Kakarla, P.; Bandi, S.; Mallela, G.M.; Vallabhaneni, K. Color stability of esthetic restorative materials used in pediatric dentistry: An in vitro study. J. Indian Soc. Pedod. Prev. Dent. 2016, 34, 233–237. [Google Scholar] [PubMed]
- Kim, J.H.; Lee, Y.K.; Powers, J.M. Influence of a series of organic and chemical substances on the translucency of resin composites. J. Biomed. Mater. Res. B Appl. Biomater. 2006, 77, 21–27. [Google Scholar] [CrossRef]
- Nistor, L.G.M.; Rîcă, R.; Mărășescu, P.; Stan, M.; Manolea, H.; Ionescu, A.; Moraru, I. Zirconia Use in Dentistry—Manufacturing and Properties. Curr. Health Sci. J. 2019, 45, 28–35. [Google Scholar]
- Haralur, S.B.; Raqe, S.A.N.; Alhassan, M.F. Effect of Hydrothermal Aging and Beverages on Color Stability of Lithium Disilicate and Zirconia Based Ceramics. Materials (Kaunas) 2019, 55, 749. [Google Scholar] [CrossRef]
- Alaqeel, S. Effect of Grit-blasting on the Color Stability of Zirconia Ceramics Following Exposure to Beverages. Cureus 2020, 12, e7170. [Google Scholar] [CrossRef]
- Alkhudhairy, F.; Vohra, F.; Naseem, M.; Owais, M.M.; Amer, A.H.; Almutairi, K.B. Color stability and degree of conversion of a novel dibenzoyl germanium derivative containing photo-polymerized resin luting cement. J. Appl. Biomater. & Funct. Mater. 2020, 18, 2280800020917326. [Google Scholar]
- Wiegand, A.; Kuhn, M.; Sener, B.; Roos, M.; Attin, T. Abrasion of eroded dentin caused by toothpaste slurries of different abrasivity and toothbrushes of different filament diameter. J. Dent. 2009, 37, 480–484. [Google Scholar] [CrossRef][Green Version]
- Francetti, L.; Fabbro, M.D.; Basso, M.; Testori, T.; Taschieri, S.; Weinstein, R. Chlorhexidine spray versus mouthwash in the control of dental plaque after implant surgery. J. Clin. Periodontol. 2004, 31, 857–862. [Google Scholar] [CrossRef]
- Farooq, I.M.A.; AlShwaimi, E.; Almas, K. Efficacy of a novel fluoride containing bioactive glass based dentifrice in remineralizing artificially induced demineralization in human enamel. Fluoride 2019, 52, 447–455. [Google Scholar]
- Ibrahim, A.F.E.; Badih, R. A comparison of color stability between hybrid ceramic and veneers: An in vitro study. Int. Arab. J. Dent. 2019, 10, 25–30. [Google Scholar] [CrossRef]
- Chu, S.J.; Trushkowsky, R.D.; Paravina, R.D. Dental color matching instruments and systems. Review of clinical and research aspects. J. Dent. 2010, 38 (Suppl. S2), e2–e16. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Cavallo, M.; Miegge, M.; Dagna, A.; Beltrami, R.; Chiesa, M.; Poggio, C. Color stability of CAD/CAM Zirconia ceramics following exposure to acidic and staining drinks. J. Clin. Exp. Dent. 2017, 9, e1297–e1303. [Google Scholar] [CrossRef] [PubMed]
- AlHamdan, E.M.; Bashiri, A.; Alnashmi, F.; Al-Saleh, S.; Al-shahrani, K.; Al-shahrani, S.; Alsharani, A.; Alzahrani, K.M.; Alqarawi, F.K.; Vohra, F.; et al. Evaluation of Smart Chromatic Technology for a Single-Shade Dental Polymer Resin: An In Vitro Study. Appl. Sci. 2021, 11, 10108. [Google Scholar] [CrossRef]
- Barutcugil, C.; Bilgili, D.; Barutcigil, K.; Dundar, A.; Buyukkaplan, U.S.; Yilmaz, B. Discoloration and translucency changes of CAD-CAM materials after exposure to beverages. J. Prosthet. Dent. 2019, 122, 325–331. [Google Scholar] [CrossRef]
- Shiozawa, M.; Takahashi, H.; Asakawa, Y.; Iwasaki, N. Color stability of adhesive resin cements after immersion in coffee. Clin. Oral Investig. 2015, 19, 309–317. [Google Scholar] [CrossRef]
- Nascimento Oliveira, A.L.; Elias, C.N.; Salomao Dos Santos, H.E.; Dos Santos, C.; de Biasi, R.S. Physical Properties and Color Stainability by Coffee and Red Wine of Opaque and High Translucency Zirconia Dental Ceramics after Hydrothermal Degradation. Int. J. Biomater. 2022, 2022, 1571729. [Google Scholar] [CrossRef]
- Pratomo, A.; Triaminingsih, S.; Indrani, D.J. Effect on tooth discoloration from the coffee drink at various smoke disposal during coffee bean roasting. J. Phys. Conf. Ser. 2018, 1073, 032031. [Google Scholar] [CrossRef]
- Şişmanoğlu, S.; Sengez, G. Effects of acidic beverages on color stability of bulk-fill composites with different viscosities. Int. J. Dent. Sci. 2021, 24, 90–99. [Google Scholar] [CrossRef]
- Bandara, S.B.; Towle, K.M.; Monnot, A.D. A human health risk assessment of heavy metal ingestion among consumers of protein powder supplements. Toxicol. Rep. 2020, 7, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Derafshi, R.; Khorshidi, H.; Kalantari, M.; Ghaffarlou, I. Effect of mouthrinses on color stability of monolithic zirconia and feldspathic ceramic: An in vitro study. BMC Oral Health 2017, 17, 129. [Google Scholar] [CrossRef] [PubMed]
- Heitz-Mayfield, L.J.; Mombelli, A. The therapy of peri-implantitis: A systematic review. Int. J. Oral Maxillofac. Implants 2014, 29, 325–345. [Google Scholar] [CrossRef] [PubMed]
- Brookes, Z.L.S.; Bescos, R.; Belfield, L.A.; Ali, K.; Roberts, A. Current uses of chlorhexidine for management of oral disease: A narrative review. J. Dent. 2020, 103, 103497. [Google Scholar] [CrossRef] [PubMed]
- James, P.; Worthington, H.V.; Parnell, C.; Harding, M.; Lamont, T.; Cheung, A.; Whelton, H.; Riley, P. Chlorhexidine mouthrinse as an adjunctive treatment for gingival health. Cochrane Database Syst. Rev. 2017, 3, CD008676. [Google Scholar]
- Saglam, G.; Cengiz, G. Effect of mouthrinses and repolishing on color stability of hybrid nanoceramic computer-aided design/computer-aided manufacturing material. Tanta Dent. J. 2021, 18, 45–48. [Google Scholar] [CrossRef]
- Carey, C.M.; Yagudayev, A.; Font, K. Effect of Temperature on Tooth Staining by 0.12% Chlorhexidine Gluconate. Front. Dent. Med. 2021, 2, 779852. [Google Scholar] [CrossRef]
- Zanatta, F.B.; Antoniazzi, R.P.; Rosing, C.K. Staining and calculus formation after 0.12% chlorhexidine rinses in plaque-free and plaque covered surfaces: A randomized trial. J. Appl. Oral Sci. 2010, 18, 515–521. [Google Scholar] [CrossRef]
- Paravina, R.D.; Pérez, M.M.; Ghinea, R. Acceptability and perceptibility thresholds in dentistry: A comprehensive review of clinical and research applications. J. Esthet. Restor. Dent. 2019, 31, 103–112. [Google Scholar] [CrossRef]
- Cacciafesta, V.; Sfondrini, M.F.; Lena, A.; Scribante, A.; Vallittu, P.K.; Lassila, L.V. Flexural strengths of fiber-reinforced composites polymerized with conventional light-curing and additional postcuring. Am. J. Orthod Dentofacial. Orthop. 2007, 132, 524–527. [Google Scholar] [CrossRef]
- Pieniak, D.; Walczak, A.; Walczak, M.; Przystupa, K.; Niewczas, A.M. Hardness and wear resistance of dental biomedical nanomaterials in a humid environment with non-stationary temperatures. Materials 2020, 13, 1255. [Google Scholar] [CrossRef] [PubMed]
- Binhasan, M.; Solimanie, A.H.; Almuammar, K.S.; Alnajres, A.R.; Alhamdan, M.M.; Al Ahdal, K.; Alfaawaz, Y.F.; Ali, K.; Vohra, F.; Abduljabbar, T. The Effect of Dentifrice on Micro-Hardness, Surface Gloss, and Micro-Roughness of Nano Filled Conventional and Bulk-Fill Polymer Composite—A Micro Indentation and Profilometric Study. Materials 2022, 15, 4347. [Google Scholar] [CrossRef] [PubMed]
- Almohareb, T.; Alayed, A.A.; Alzahrani, K.M.; Maawadh, A.M.; Almutairi, B.; Alhamdan, R.S.; Bahkali, A.; Abduljabbar, T.; Vohra, F. Influence of curing duration and mixing techniques of bulk fill resin composites on bi-axial flexural strength and degree of conversion. J. Appl. Biomater. Funct. Mater. 2020, 18, 2280800020975721. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).