Optimizing the Ultrasound Image Quality of Carotid Artery Stenosis Patients via Taguchi’s Dynamic Analysis and an Indigenous Water Phantom
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
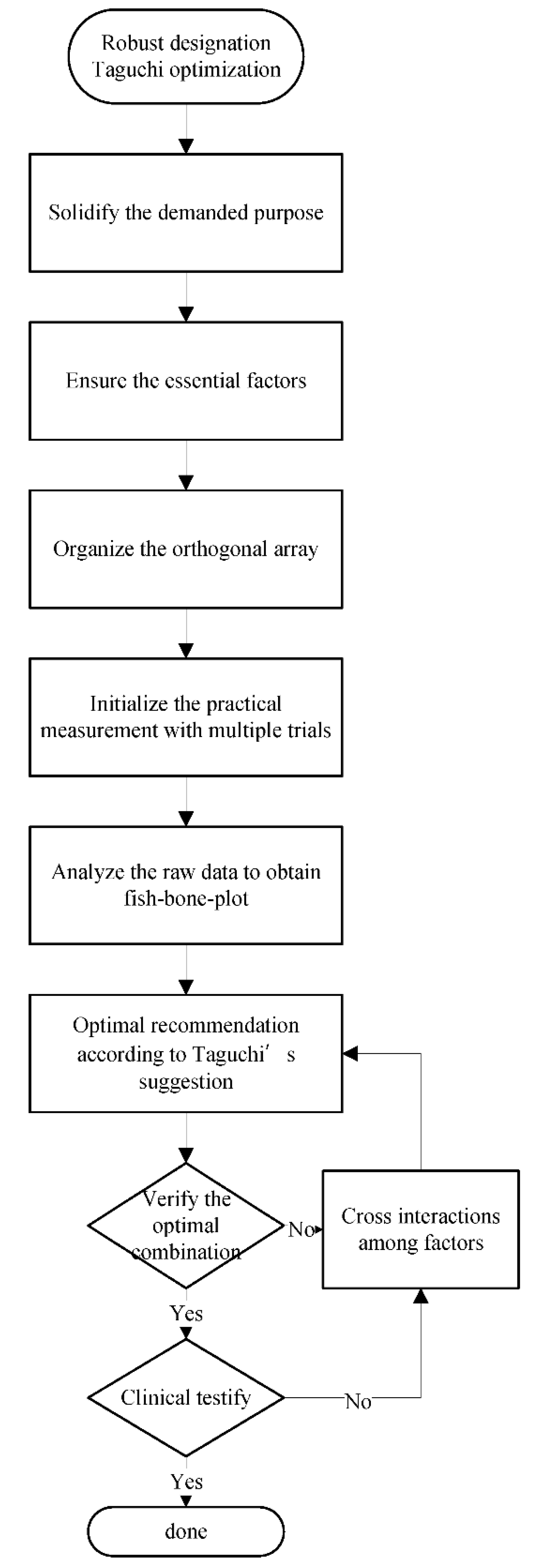
2.2. Dynamic Taguchi’s Analysis
2.3. Orthogonal Arrays
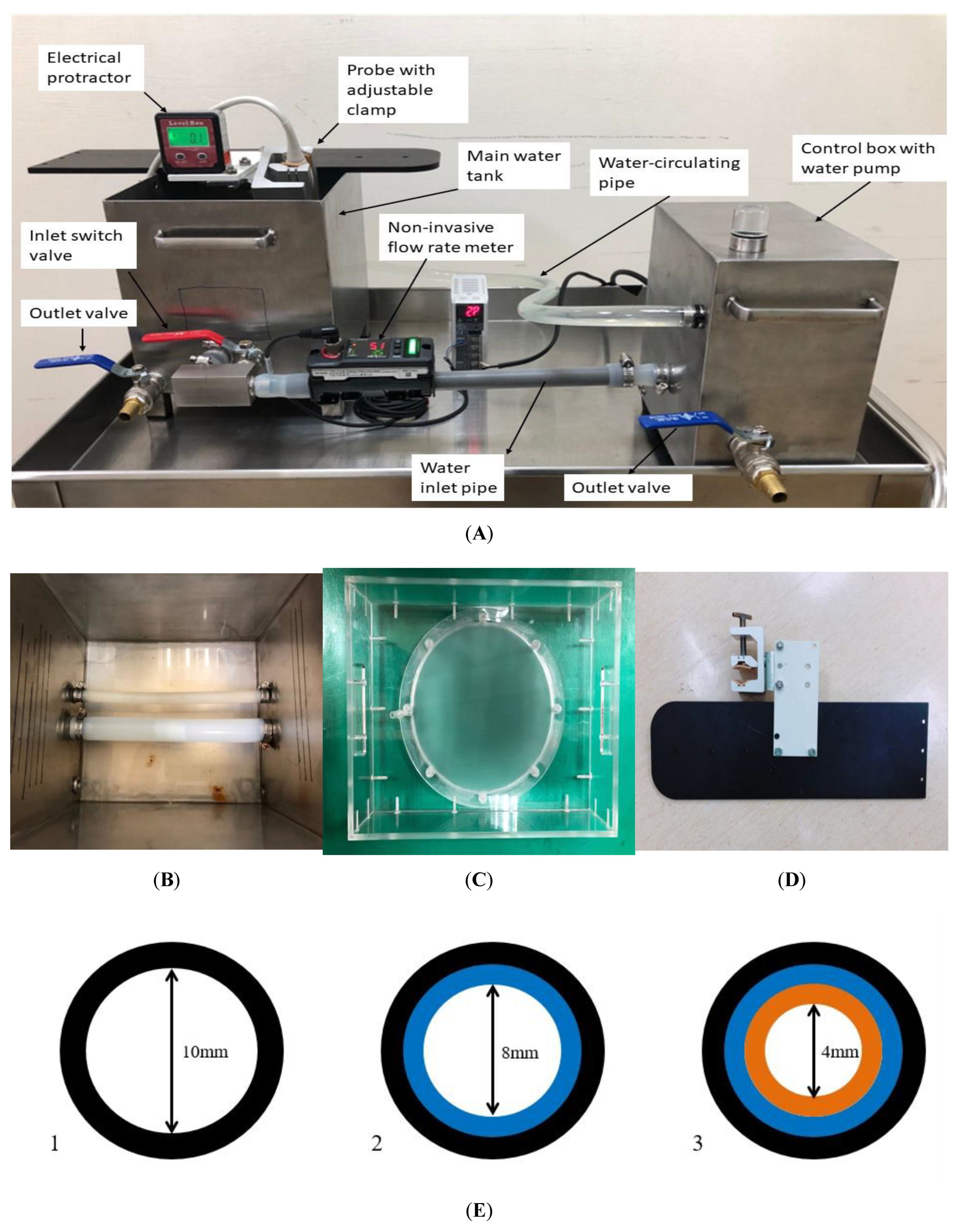
2.4. The Customized Water Phantom
2.5. Quality Characteristics in Dynamic Taguchi Analysis
2.6. Quantifying the Ultrasound Scanned Images
3. Results
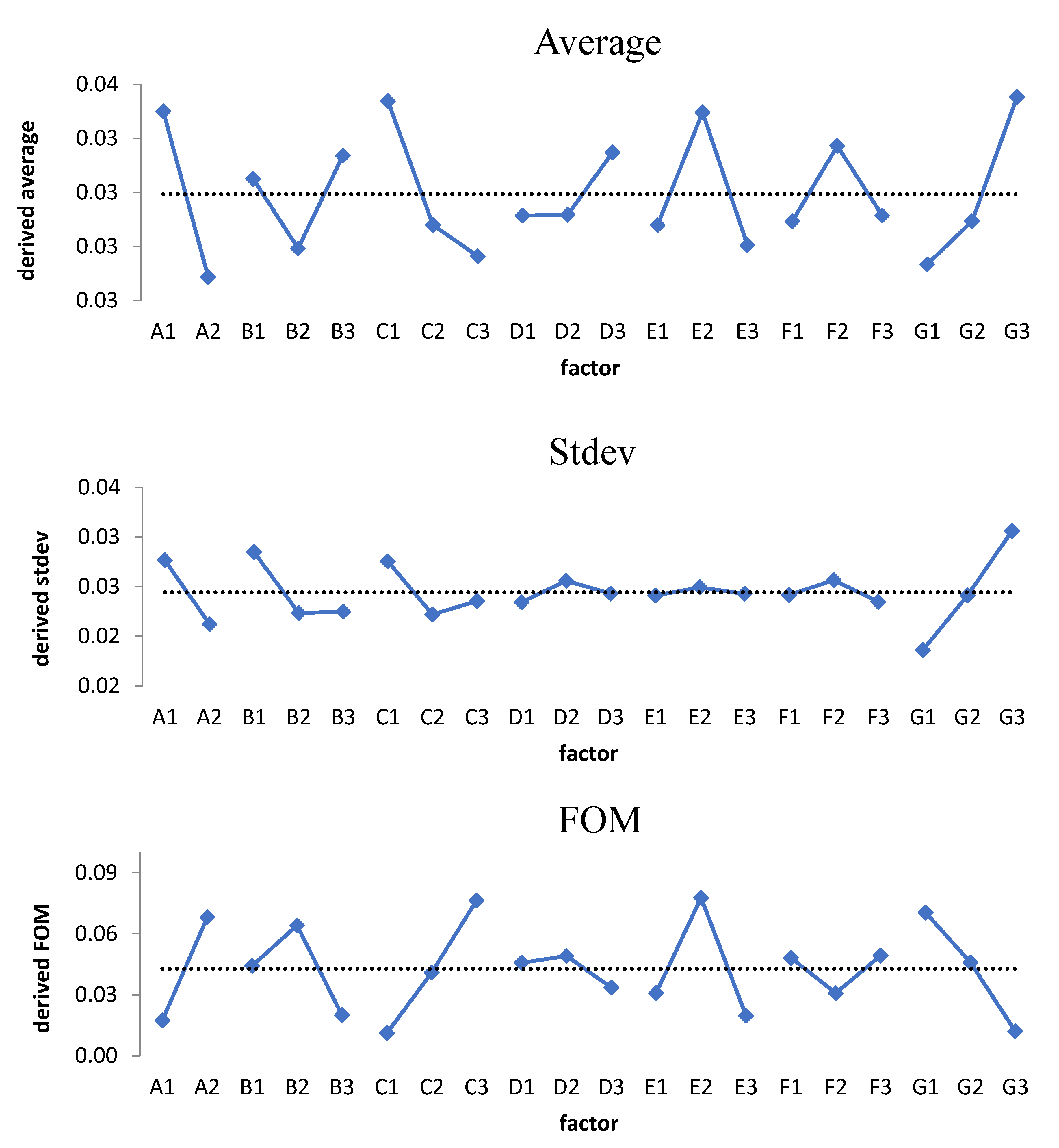
3.1. Raw Data Analysis
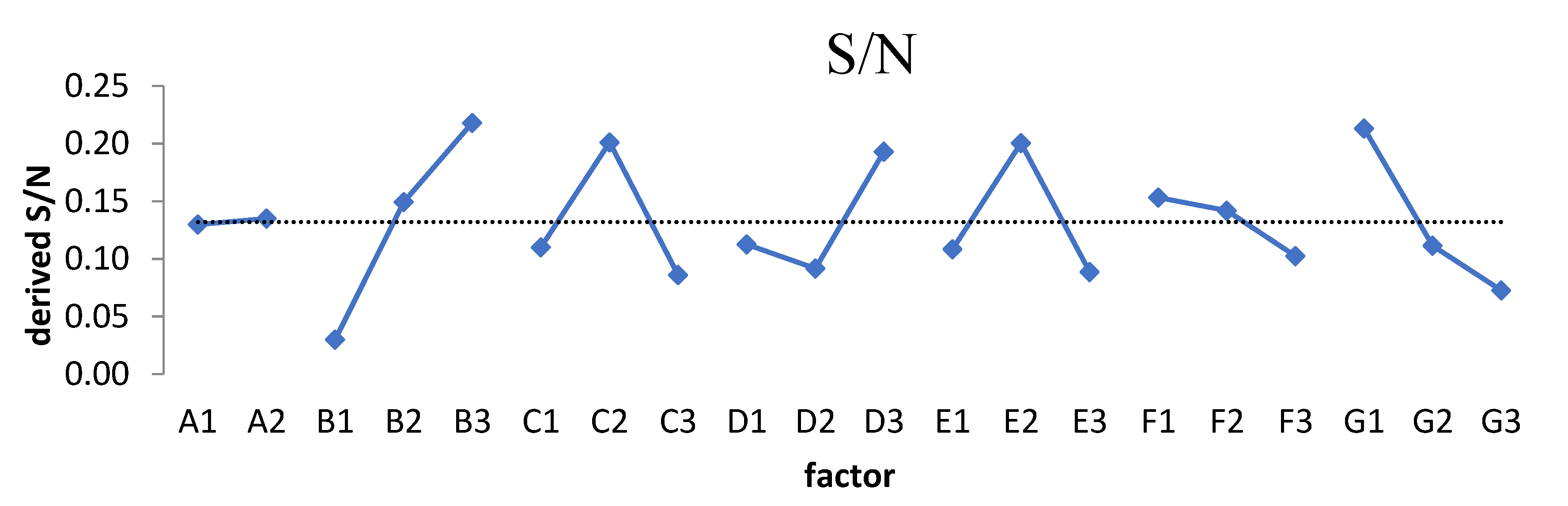
3.2. Inspecting the Domination of Factor
4. Discussion
4.1. Taguchi’s Analysis Verification
4.2. The FOM Focused on the Difference in Area or Diameter
4.3. The FOM Advantages over the S/N Ratio
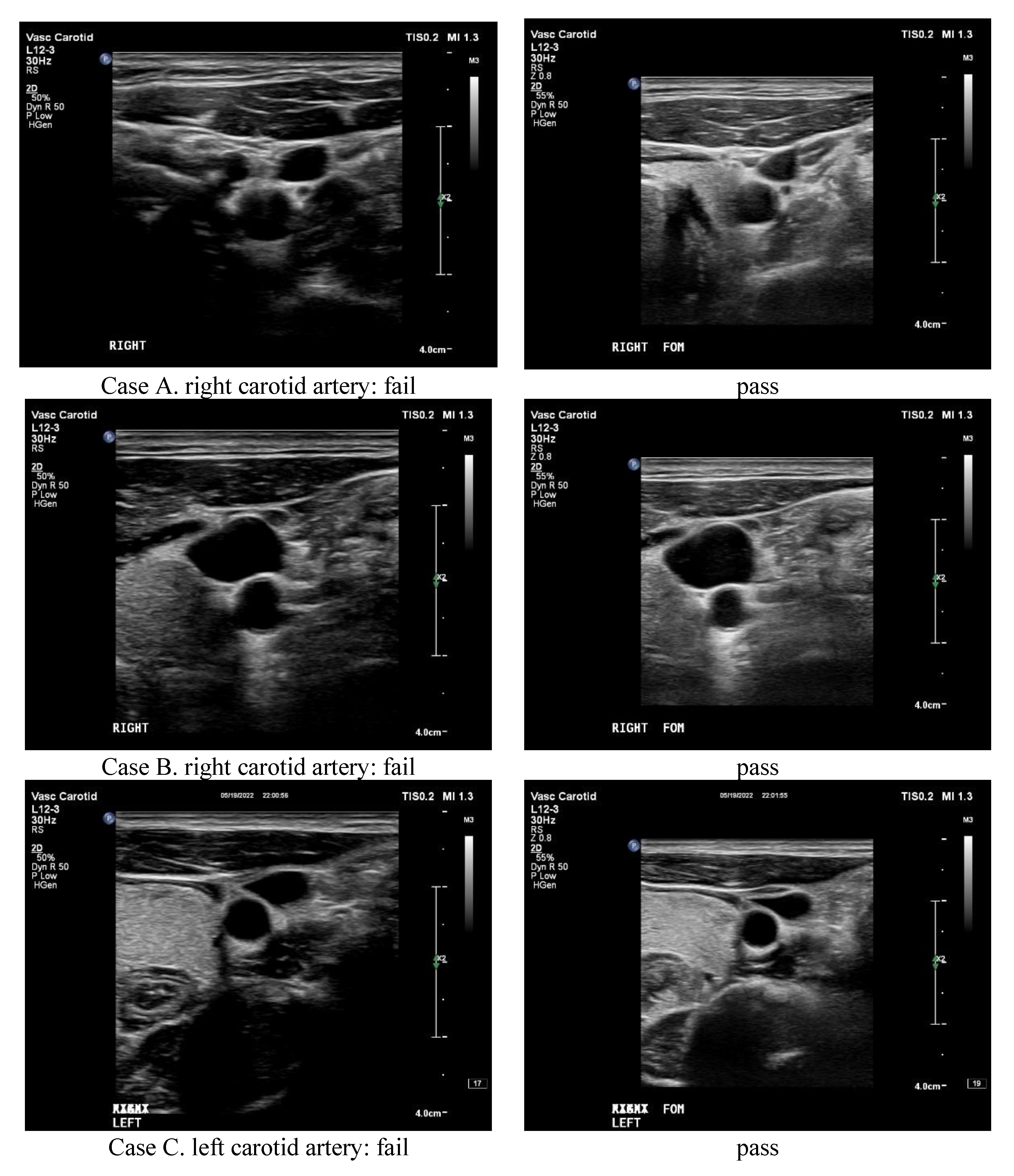
4.4. Clinical Testification
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Mitchell, C.; Korcarz, C.E.; Gepner, A.D.; Kaufman, J.D.; Post, W.; Tracy, R.; Gassett, A.J.; Ma, N.; McClelland, R.L.; Stein, J.H. Ultrasound carotid plaque features, cardiovascular disease risk factors and events: The Multi-Ethnic Study of Atherosclerosis. Atherosclerosis 2018, 276, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Lee, W. General principles of carotid Doppler ultrasonography. Ultrasonography 2013, 33, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Rafailidis, V.; Chryssogonidis, I.; Xerras, C.; Nikolaou, I.; Tegos, T.; Kouskouras, K.; Rafailidis, D.; Charitanti-Kouridou, A. A comparative study of color Doppler imaging and contrast-enhanced ultrasound for the detection of ulceration in patients with carotid atherosclerotic disease. Eur. Radiol. 2019, 29, 2137–2145. [Google Scholar] [CrossRef] [PubMed]
- Demattè, S.; Di Sarra, D.; Schiavi, F.; Casadei, A.; Opocher, G. Role of ultrasound and color Doppler imaging in the detection of carotid paragangliomas. J. Ultrasound 2012, 15, 158–163. [Google Scholar] [CrossRef]
- Lal, B.K.; Hobson, R.W.; Pappas, P.J.; Kubicka, R.; Hameed, M.; Chakhtura, E.Y.; Jamil, Z.; Padberg, F.T.; Haser, P.B.; Durán, W.N. Pixel distribution analysis of B-mode ultrasound scan images predicts histologic features of atherosclerotic carotid plaques. J. Vasc. Surg. 2002, 35, 1210–1217. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.M.F.; dos Santos, R.M.; Castro, P.M.A.; Azevedo, E.; Sousa, L.; Tavares, J.M.R. A novel automatic algorithm for the segmentation of the lumen of the carotid artery in ultrasound B-mode images. Expert Syst. Appl. 2013, 40, 6570–6579. [Google Scholar] [CrossRef]
- Araki, T.; Ikeda, N.; Shukla, D.; Londhe, N.D.; Shrivastava, V.K.; Banchhor, S.K.; Saba, L.; Nicolaides, A.; Shafique, S.; Laird, J.R.; et al. A new method for IVUS-based coronary artery disease risk stratification: A link between coronary & carotid ultrasound plaque burdens. Comput. Methods Programs Biomed. 2015, 124, 161–179. [Google Scholar] [CrossRef] [PubMed]
- Yau, O.; Hétu, M.-F.; Herr, J.E.; Adams, M.A.; Johri, A.M. Development of a Carotid Vulnerable Plaque Phantom Model Evaluated by Pixel Distribution Analysis. Ultrasound Med. Biol. 2018, 44, 2768–2779. [Google Scholar] [CrossRef] [PubMed]
- Ponnle AHasegawa, H.; Kanai, H. Multi element diverging beam from a linear array transducer for transverse cross sectional imaging of carotid artery: Simulations and phantom vessel validation. Jpn. J. Appl. Phys. 2011, 50, 07HF05. [Google Scholar] [CrossRef]
- Poree, J.; Chayer, B.; Soulez, G.; Ohayon, J.; Cloutier, G. Noninvasive Vascular Modulography Method for Imaging the Local Elasticity of Atherosclerotic Plaques: Simulation and In Vitro Vessel Phantom Study. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2017, 64, 1805–1817. [Google Scholar] [CrossRef] [PubMed]
- Kingstone, L.L.; Castonguay, M.; Torres, C.; Currie, G. Carotid Artery Disease Imaging: A Home-Produced, Easily Made Phantom for Two- and Three-Dimensional Ultrasound Simulation. J. Vasc. Ultrasound 2013, 37, 76–80. [Google Scholar] [CrossRef]
- Wu, K.Y.; Liang, C.C.; Chuang, C.H.; Pan, L.F.; Pan, L.K. Optimization of common iliac artery sonography image via an ingenious water phantom and Taguchi’s analysis: A feasibility study. Appl. Sci. 2022, 12, 8197. [Google Scholar] [CrossRef]
- Pan, L.-F.; Wu, K.-Y.; Chen, K.-L.; Kittipayak, S. Taguchi method-based optimization of the minimum detectable difference of a cardiac X-ray imaging system using a precise line pair gauge. J. Mech. Med. Biol. 2019, 19, 1940030. [Google Scholar] [CrossRef]
- Pan, L.F.; Chen, Y.H.; Wang, C.C.; Peng, B.R.; Kittipayak, S.; Pan, L.K. Optimizing cardiac CT angiography minimum detectable difference via Taguchi’s dynamic algorithm, a V-shaped line gauge, and three PMMA phantoms. THC 2022, 30, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.-F.; Chu, K.H.; Sher, H.-F. Optimizing left anterior oblique (LAO) caudal imaging in coronary angiography using the Taguchi method: A phantom study with clinical verification. Int. J. Cardiovasc. Imaging 2017, 130, 185–1295. [Google Scholar] [CrossRef] [PubMed]
- Hsun-Nan, K.; Juei-En, Y.; Chia-Hui, C.; Lung-Fa, P.; Lung-Kwang, P. Taguchi dynamic analysis application to computer tomography number-mass density linear dependence optimization. Comput. Assist. Surg. 2017, 22, 45–53. [Google Scholar] [CrossRef]
- Taguchi, G. Taguchi on Robust Technology Development: Bringing Quality Engineering Upstream; ASME Press: New York, NY, USA, 1992; ISBN 9780791861523. [Google Scholar]
- Meuwly, J.Y.; Thiran, J.P.; Gudinchet, F. Application of adaptive image processing technique to real-time spatial compound ultrasound imaging improves image quality. Investig. Radiol. 2003, 38, 257–262. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, C.Y.; Liu, K.C.; Chen, H.H.; Pan, L.K. Optimizing the TLD-100 readout system for various radiotherapy beam doses using the Taguchi methodology. Appl. Radiat. Isot. 2010, 68, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.F.; Le, Y.; Yen, Y.C.; Weng, J.H.; Liang, C.C.; Chen, C.Y.; Pan, L.K. Optimizing the TLD-100H readout system under various radioactive I-131 doses via the revised Taguchi dynamic quality loss function. JMMB 2020, 20, 2040024. [Google Scholar] [CrossRef]
- Yeh, D.M.; Wang, T.H.; Pan, L.K. Evaluating the quality characteristics of TLD-100T and TLD-100H exposed to diagnostic X-rays and 64 multislice CT using Taguchi’s quality loss function. Radiat. Meas. 2015, 80, 17–22. [Google Scholar] [CrossRef]
- Pan, L.K.; Wang, C.C.; Shih, Y.C.; Sher, H.F. Optimizing multiple qualities of Nd: YAG laser welding onto magnesium alloy via grey relational analysis. Sci. Technol. Weld. Join. 2005, 10, 503–510. [Google Scholar] [CrossRef]
- Pan, L.K.; Wang, C.C.; Wei, S.L.; Sher, H.F. Optimizing multiple quality characteristics via Taguchi method-based Grey analysis. J. Mater. Process. Technol. 2007, 182, 107–116. [Google Scholar] [CrossRef]
- Peng, B.R.; Kittipayak, S.; Pan, L.F.; Pan, L.K. Optimizing the minimum detectable difference of computed tomography scanned images via the Taguchi analysis: A feasibility study with an indigenous hepatic phantom and a line group gauge. JMMB 2019, 19, 1940048. [Google Scholar] [CrossRef]

| Group | Factor | ||||||
|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | |
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 2 | 1 | 1 | 2 | 2 | 2 | 2 | 2 |
| 3 | 1 | 1 | 3 | 3 | 3 | 3 | 3 |
| 4 | 1 | 2 | 1 | 1 | 2 | 2 | 3 |
| 5 | 1 | 2 | 2 | 2 | 3 | 3 | 1 |
| 6 | 1 | 2 | 3 | 3 | 1 | 1 | 2 |
| 7 | 1 | 3 | 1 | 2 | 1 | 3 | 2 |
| 8 | 1 | 3 | 2 | 3 | 2 | 1 | 3 |
| 9 | 1 | 3 | 3 | 1 | 3 | 2 | 1 |
| 10 | 2 | 1 | 1 | 3 | 3 | 2 | 2 |
| 11 | 2 | 1 | 2 | 1 | 1 | 3 | 3 |
| 12 | 2 | 1 | 3 | 2 | 2 | 1 | 1 |
| 13 | 2 | 2 | 1 | 2 | 3 | 1 | 3 |
| 14 | 2 | 2 | 2 | 3 | 1 | 2 | 1 |
| 15 | 2 | 2 | 3 | 1 | 2 | 3 | 2 |
| 16 | 2 | 3 | 1 | 3 | 2 | 3 | 1 |
| 17 | 2 | 3 | 2 | 1 | 3 | 1 | 2 |
| 18 | 2 | 3 | 3 | 2 | 1 | 2 | 3 |
| Factor | Levels | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| (A) angle of probe (degree) | 30 | 0 | |
| (B) signal amplification gain (dB/cm) | 50 | 55 | 60 |
| (C) resolution vs. speed | R1 | RS | S1 |
| (D) dynamic range (dB) | 40 | 50 | 60 |
| (E) XRES (scenario) | 1 | 2 | 3 |
| (F) zoom | 0.8 | 1.0 | 1.2 |
| (G) time gain compensation | −1 | 0 | +1 |
| Group | 78.5 mm2 | 50.2 mm2 | 12.6 mm2 | Avg. | Stdev | β | r2 | FOM | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| #1 | #2 | #3 | #1 | #2 | #3 | #1 | #2 | #3 | ||||||
| 1 | 1.50 | 10.50 | 3.50 | 3.800 | 1.200 | 5.800 | 0.400 | 3.400 | 0.600 | 3.411 | 3.194 | 6.0 | 0.990 | 0.015 |
| 2 | 13.50 | 10.50 | 7.50 | 0.200 | 0.800 | 4.800 | 1.400 | 4.400 | 0.400 | 4.833 | 4.792 | 12.0 | 0.558 | 0.002 |
| 3 | 8.50 | 7.50 | 12.50 | 0.200 | 2.200 | 0.200 | 1.400 | 0.400 | 1.600 | 3.833 | 4.501 | 11.9 | 0.544 | 0.003 |
| 4 | 7.50 | 3.50 | 12.50 | 0.800 | 6.200 | 5.800 | 2.400 | 2.400 | 0.600 | 4.633 | 3.810 | 9.5 | 0.916 | 0.005 |
| 5 | 4.50 | 1.50 | 4.50 | 1.800 | 1.200 | 3.800 | 1.600 | 0.400 | 0.600 | 2.211 | 1.618 | 4.3 | 0.977 | 0.064 |
| 6 | 2.50 | 8.50 | 8.50 | 4.200 | 1.200 | 0.800 | 3.400 | 2.400 | 0.400 | 3.544 | 3.059 | 6.3 | 0.571 | 0.008 |
| 7 | 2.50 | 1.50 | 7.50 | 2.200 | 3.200 | 0.200 | 0.400 | 2.400 | 1.600 | 2.389 | 2.150 | 3.5 | 0.722 | 0.040 |
| 8 | 2.50 | 1.50 | 5.50 | 4.200 | 5.200 | 1.200 | 2.400 | 4.400 | 0.400 | 3.033 | 1.849 | 1.5 | 0.628 | 0.075 |
| 9 | 1.50 | 6.50 | 4.50 | 2.200 | 2.200 | 3.800 | 0.600 | 0.400 | 0.600 | 2.478 | 2.077 | 6.0 | 0.995 | 0.032 |
| 10 | 1.50 | 4.50 | 0.50 | 6.200 | 2.200 | 1.800 | 0.400 | 2.400 | 0.600 | 2.233 | 1.958 | 2.3 | 0.379 | 0.038 |
| 11 | 1.50 | 1.50 | 8.50 | 1.200 | 2.800 | 5.800 | 1.400 | 0.400 | 0.600 | 2.633 | 2.733 | 5.2 | 0.976 | 0.026 |
| 12 | 0.50 | 0.50 | 3.50 | 2.200 | 0.200 | 2.800 | 1.400 | 0.400 | 1.400 | 1.433 | 1.176 | 0.9 | 0.600 | 0.413 |
| 13 | 2.50 | 3.50 | 0.50 | 4.200 | 11.200 | 0.800 | 2.400 | 1.400 | 0.600 | 3.011 | 3.334 | 2.4 | 0.123 | 0.005 |
| 14 | 1.50 | 3.50 | 1.50 | 0.800 | 3.200 | 2.200 | 0.600 | 0.600 | 0.600 | 1.611 | 1.129 | 2.8 | 0.923 | 0.184 |
| 15 | 0.50 | 4.50 | 0.50 | 1.800 | 1.200 | 0.800 | 0.400 | 1.600 | 1.600 | 1.433 | 1.268 | 0.9 | 0.664 | 0.393 |
| 16 | 10.50 | 6.50 | 7.50 | 7.200 | 4.200 | 4.200 | 2.600 | 0.400 | 0.600 | 4.856 | 3.372 | 11.4 | 0.989 | 0.005 |
| 17 | 5.50 | 6.50 | 1.50 | 2.200 | 4.200 | 0.800 | 0.400 | 2.400 | 1.400 | 2.767 | 2.147 | 4.8 | 0.854 | 0.030 |
| 18 | 5.50 | 5.50 | 2.50 | 1.200 | 10.200 | 3.200 | 0.400 | 2.400 | 0.600 | 3.500 | 3.132 | 6.1 | 0.832 | 0.012 |
| Group A (Original G12) | Group B (Conventional) | Group C (Highest FOM of Each Factor) | Group D (Considering Cross-Interaction I) | Group E (Considering Cross-Interaction II) | |
|---|---|---|---|---|---|
| Factor: | |||||
| (A) angle of probe (degree) | 0 | 0 | 0 | 0 | 30 |
| (B) signal gain (dB/cm) | 50 | 50 | 55 | 55 | 55 |
| (C) resolution vs. speed | S1 | RS | S1 | RS | RS |
| (D) dynamic range (dB) | 50 | 50 | 50 | 40 | 50 |
| (E) XRES (scenario) | 2 | 2 | 2 | 2 | 2 |
| (F) zoom | 0.8 | 1.0 | 0.8 | 0.8 | 0.8 |
| (G) time gain compensation | −1 | 0 | −1 | −1 | −1 |
| Area: | |||||
| Difference in 78.5 mm2 | 1.50 | 8.5 | 3.50 | 1.50 | 3.50 |
| Difference in 50.2 mm2 | 1.73 | 2.8 | 1.20 | 0.20 | 1.20 |
| Difference in 12.6 mm2 | 1.07 | 0.6 | 6.40 | 4.40 | 5.40 |
| Avg. | 1.43 | 3.97 | 3.70 | 2.03 | 3.37 |
| Stdev | 1.18 | 4.08 | 2.61 | 2.15 | 2.10 |
| β | 0.86 | 12.07 | 6.00 | 5.64 | 4.21 |
| r2 | 0.60 | 0.82 | 0.50 | 0.64 | 0.38 |
| FOM | 0.413 | 0.004 | 0.012 | 0.031 | 0.013 |
| Diameter: | |||||
| Difference in 10 mm | 0.50 | 0.70 | 0.003 | 0.30 | 0.30 |
| Difference in 8 mm2 | 0.20 | 0.02 | 0.002 | 0.00 | 0.38 |
| Difference in 4 mm2 | 0.11 | 0.47 | 0.001 | 0.15 | 0.00 |
| Avg. | 0.27 | 0.40 | 0.002 | 0.15 | 0.23 |
| Stdev | 0.33 | 0.35 | 0.001 | 0.15 | 0.20 |
| β | 0.662 | 0.168 | 0.003 | 0.16 | 0.56 |
| r2 | 0.844 | 0.022 | 0.964 | 0.108 | 0.740 |
| FOM | 14.24 | 0.95 | 1.61E8 | 29.81 | 29.00 |
| Pair Numbers | Conventional | Optimal |
|---|---|---|
| 17 | Pass | Pass |
| 13 | Fail | Pass |
| 2 | Pass | Fail |
| Total: 32 | 30 Pass | 66 Pass |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsiao, K.-Y.; Lin, C.-S.; Li, W.-M.; Huang, S.-H.; Cho, Y.-T.; Peng, B.-R.; Pan, L.-K.; Pan, L.-F. Optimizing the Ultrasound Image Quality of Carotid Artery Stenosis Patients via Taguchi’s Dynamic Analysis and an Indigenous Water Phantom. Appl. Sci. 2022, 12, 9751. https://doi.org/10.3390/app12199751
Hsiao K-Y, Lin C-S, Li W-M, Huang S-H, Cho Y-T, Peng B-R, Pan L-K, Pan L-F. Optimizing the Ultrasound Image Quality of Carotid Artery Stenosis Patients via Taguchi’s Dynamic Analysis and an Indigenous Water Phantom. Applied Sciences. 2022; 12(19):9751. https://doi.org/10.3390/app12199751
Chicago/Turabian StyleHsiao, Kai-Yu, Chih-Sheng Lin, Wan-Ming Li, Shih-Hsun Huang, Yu-Ting Cho, Bing-Ru Peng, Lung-Kwang Pan, and Lung-Fa Pan. 2022. "Optimizing the Ultrasound Image Quality of Carotid Artery Stenosis Patients via Taguchi’s Dynamic Analysis and an Indigenous Water Phantom" Applied Sciences 12, no. 19: 9751. https://doi.org/10.3390/app12199751
APA StyleHsiao, K.-Y., Lin, C.-S., Li, W.-M., Huang, S.-H., Cho, Y.-T., Peng, B.-R., Pan, L.-K., & Pan, L.-F. (2022). Optimizing the Ultrasound Image Quality of Carotid Artery Stenosis Patients via Taguchi’s Dynamic Analysis and an Indigenous Water Phantom. Applied Sciences, 12(19), 9751. https://doi.org/10.3390/app12199751






