Spectroscopic and Molecular Docking Analysis of π-Acceptor Complexes with the Drug Barbital
Abstract
1. Introduction
2. Materials and Methods
2.1. Compounds
2.2. Preparation of the CT Complexes
2.3. Molecular Docking
2.4. Molecular Dynamics (MD) Investigation
2.5. Binding Free Energy Calculation Using MM-PBSA
2.6. Density Functional Theory (DFT)
3. Results and Discussion
3.1. Spectroscopic Analysis on Charge-Transfer Complexes of Bar
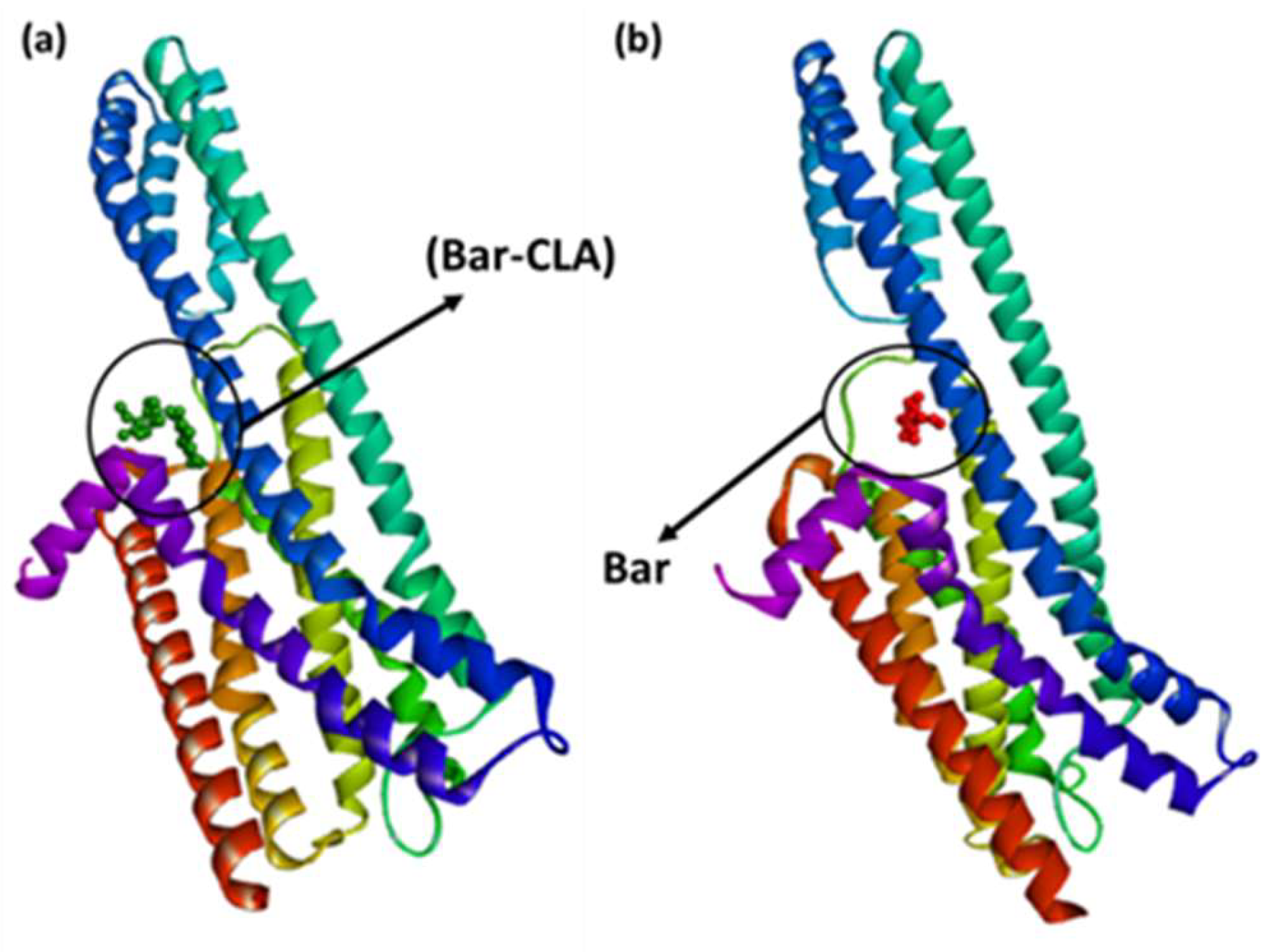
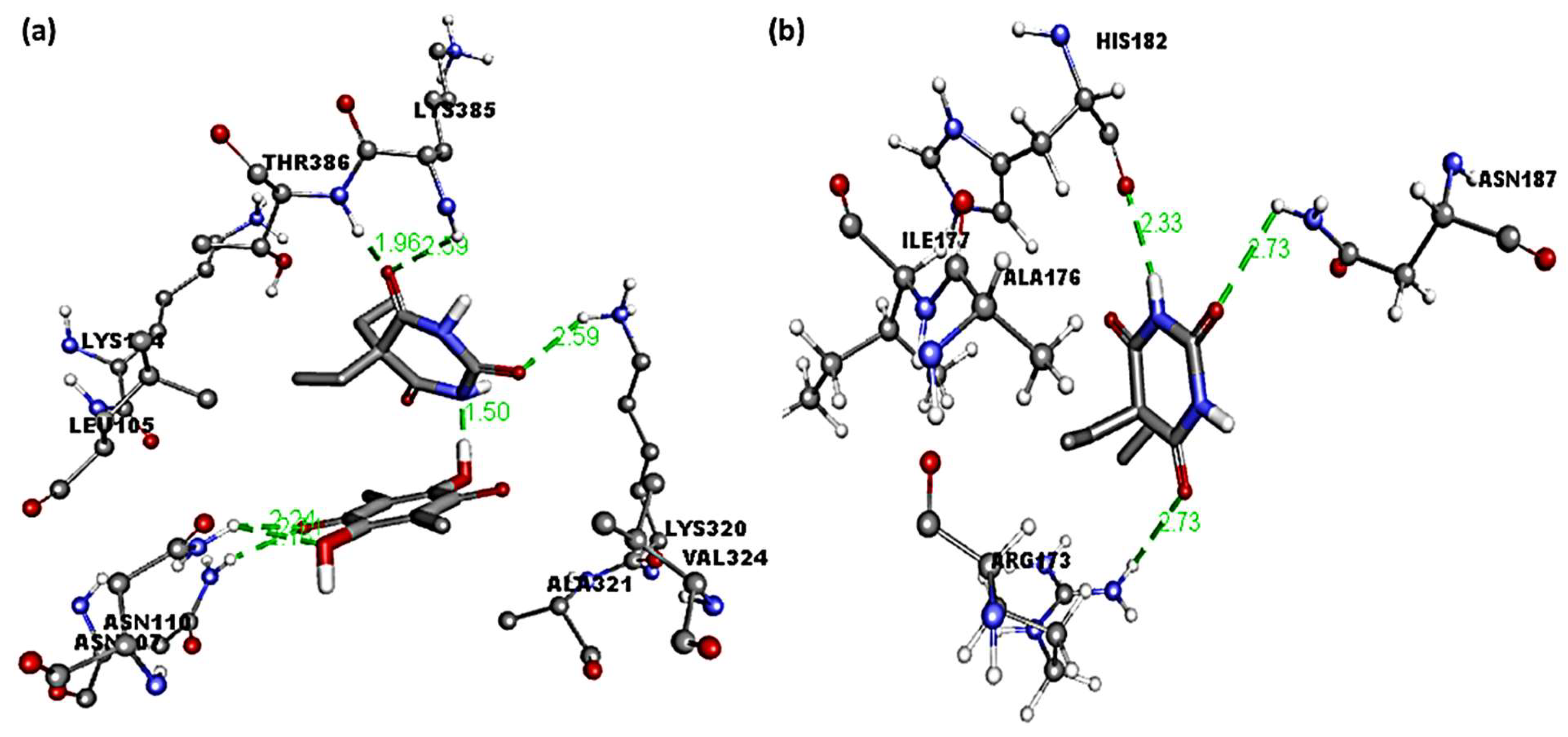
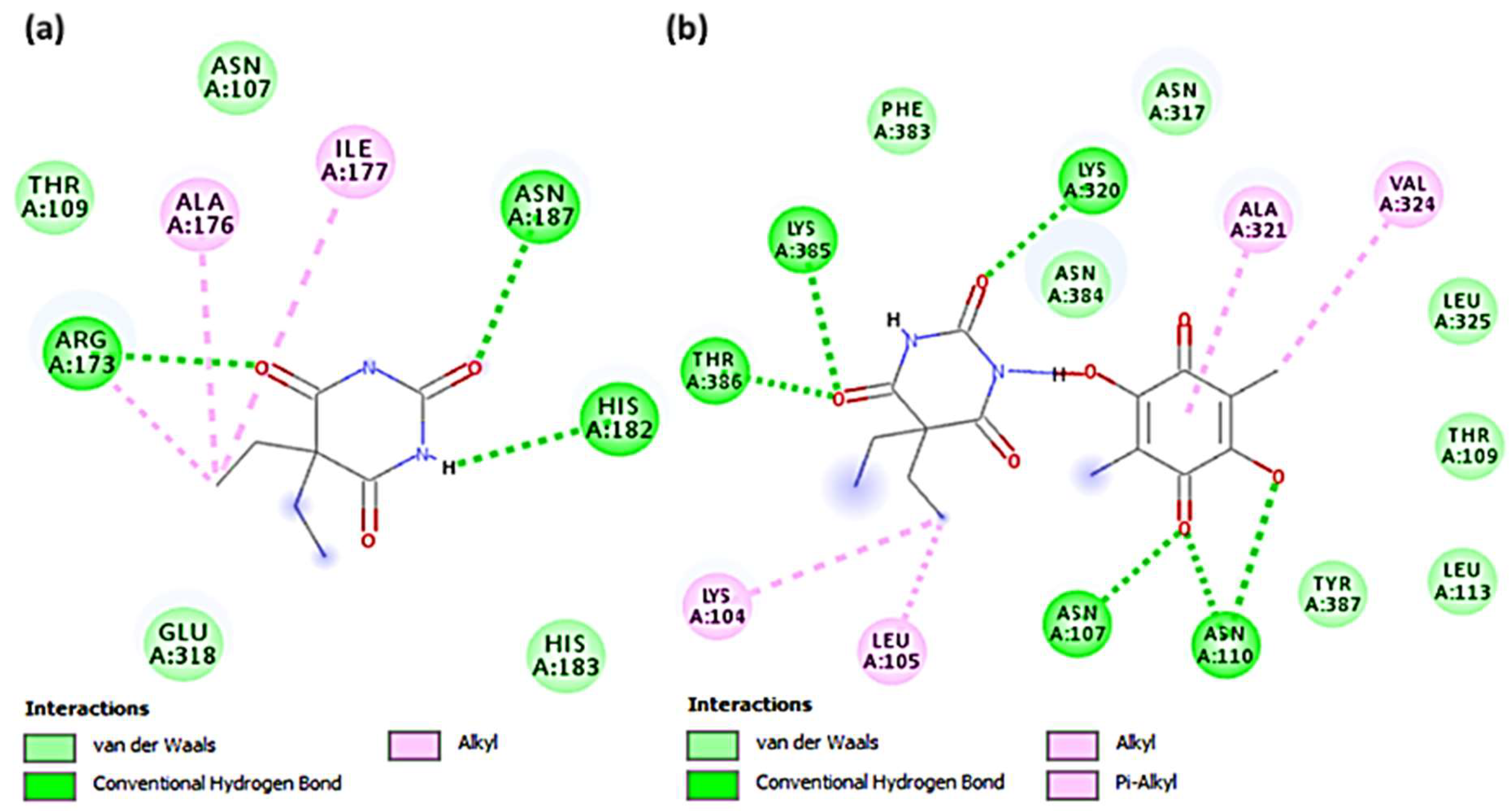
3.2. Molecular Docking Studies
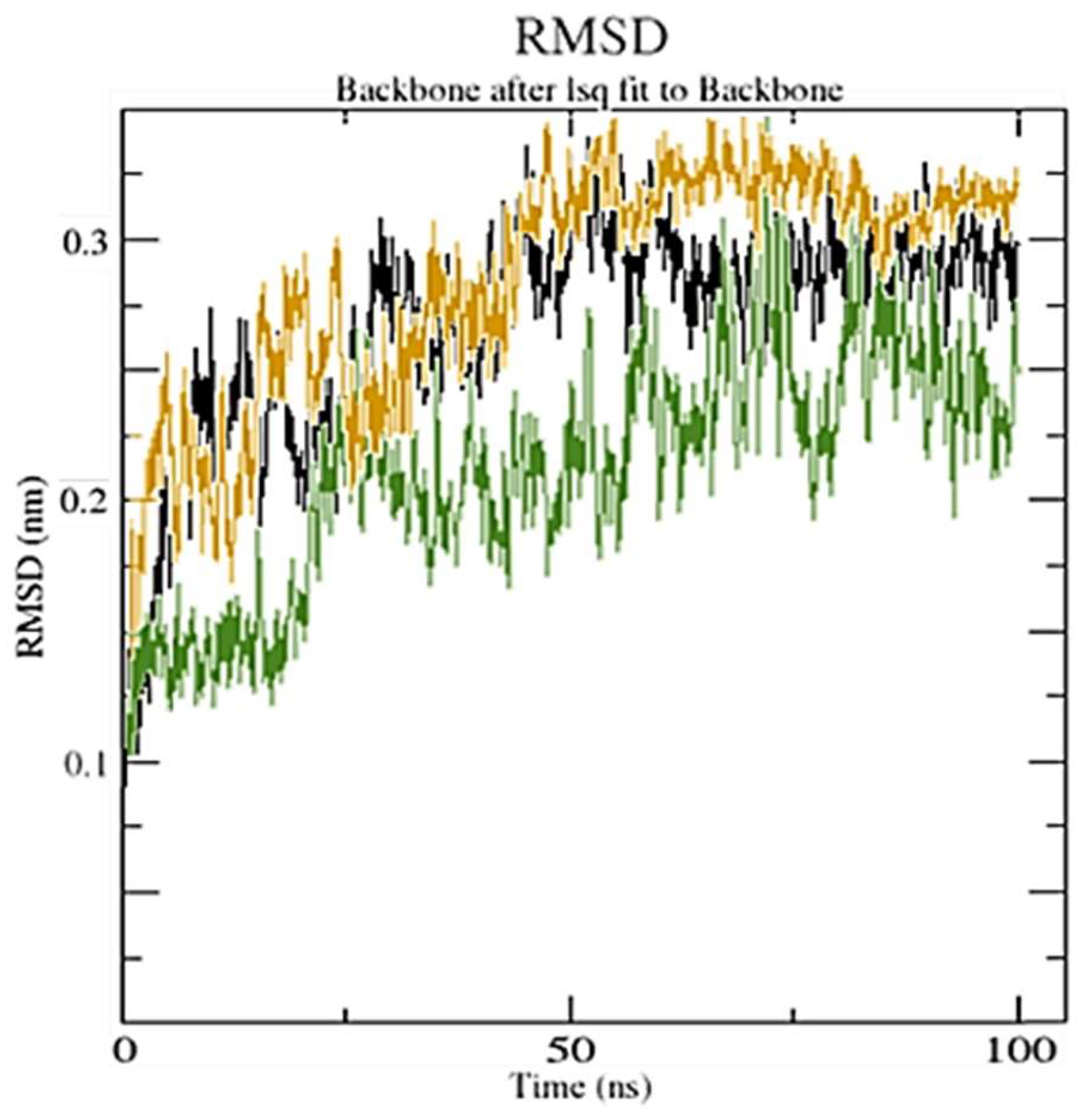
3.3. MD Simulation
3.3.1. Hydrogen Bond Analysis
3.3.2. Solvent-Accessible Surface Area Analysis
3.4. Binding Free Energy Calculation Using MM-PBSA
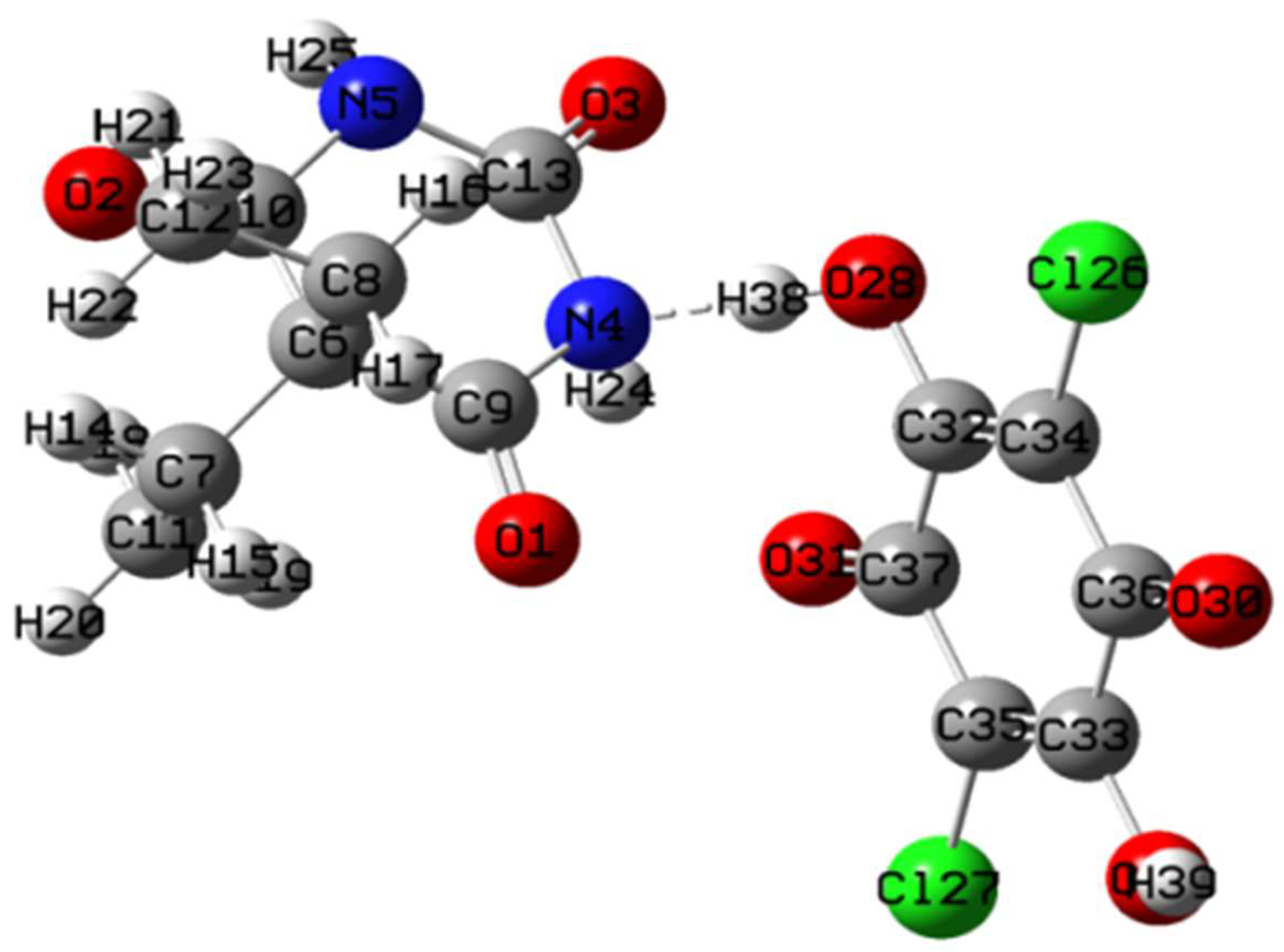
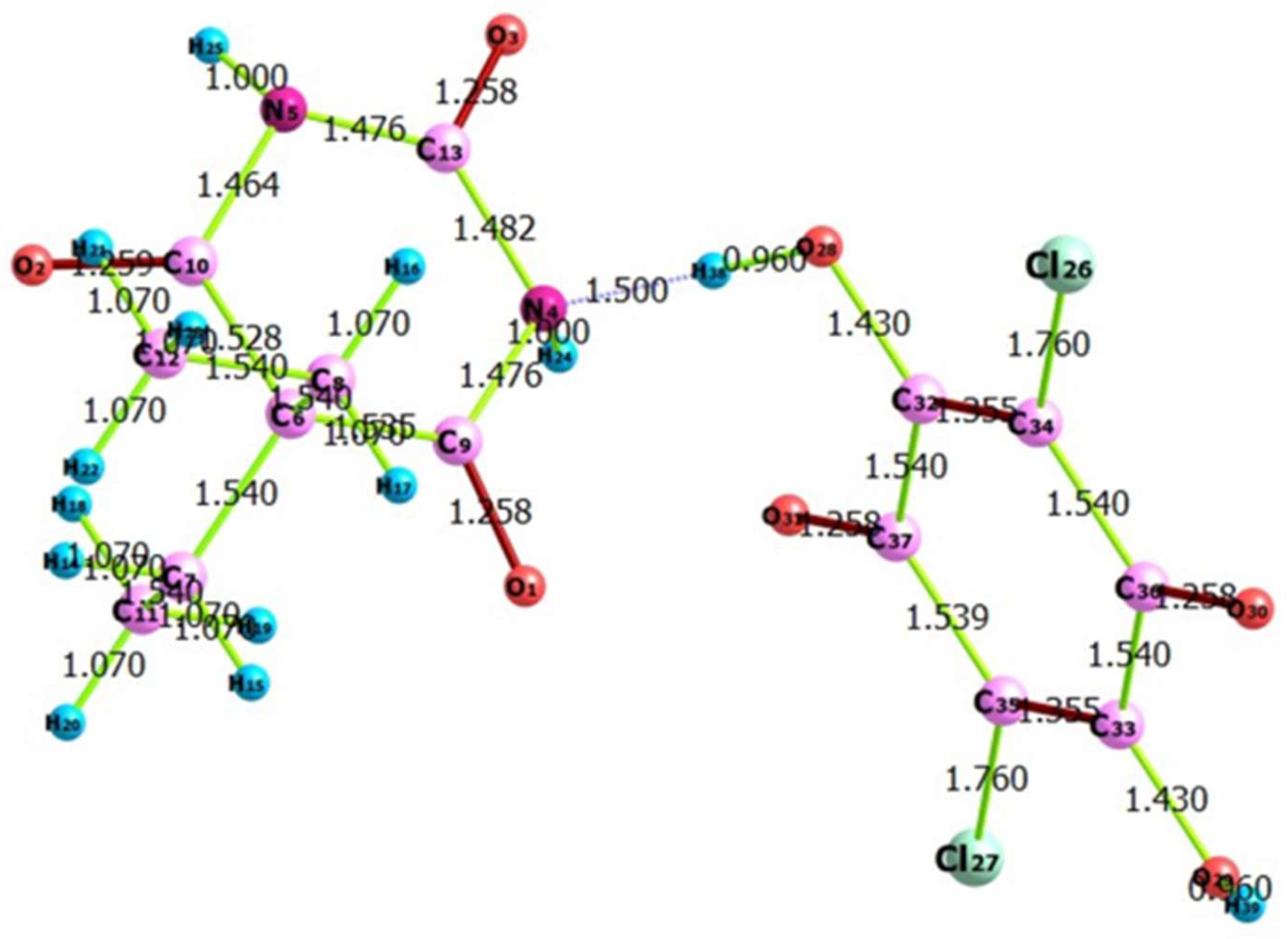
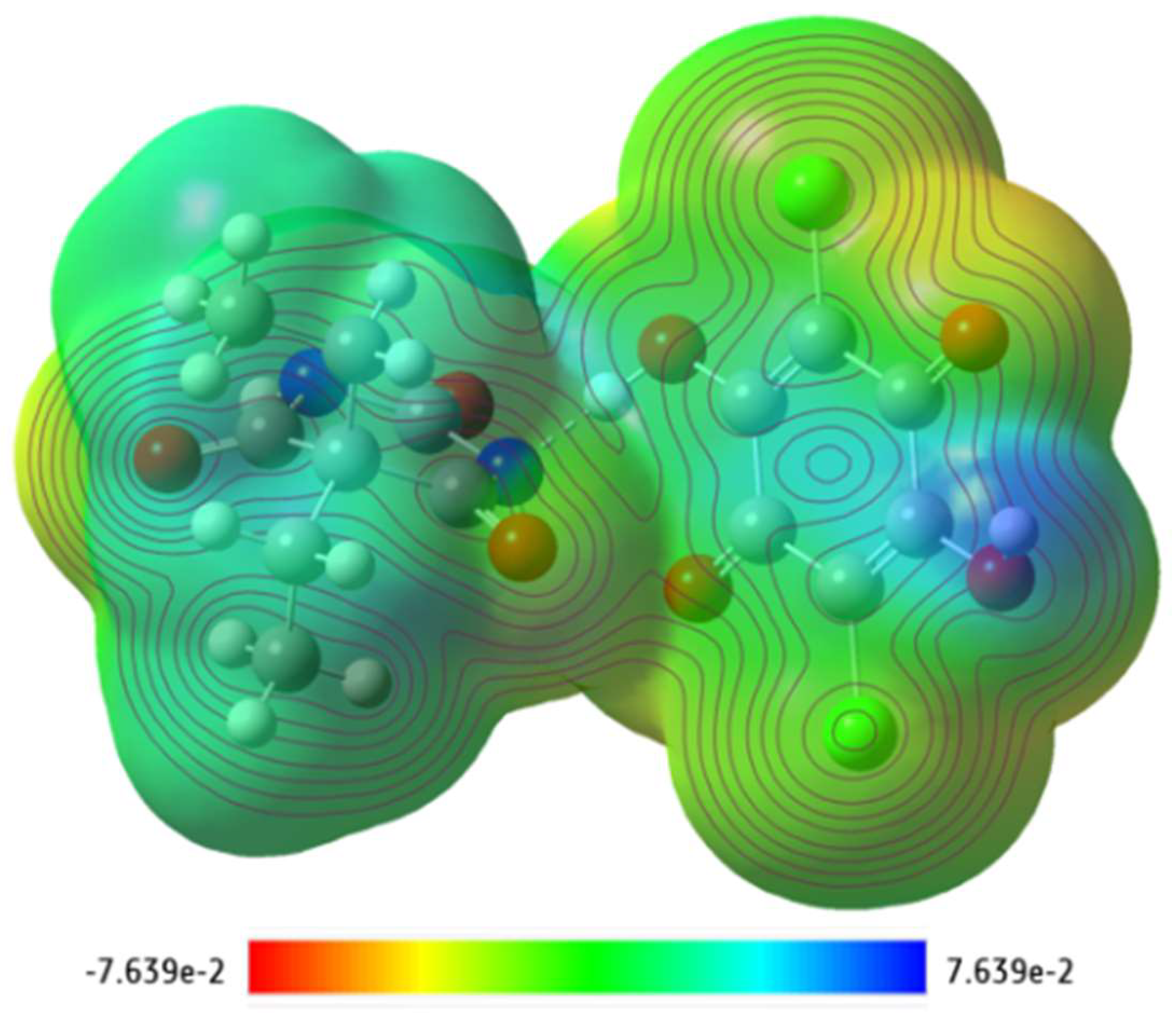
3.5. Density Functional Theory (DFT) Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patrick, G.L. An Introduction to Medicinal Chemistry, 4th ed.; Oxford University Press: Oxford, UK, 2009; p. 752. [Google Scholar]
- Muthiah, P.T.; Hemamalini, M.; Bocelli, G.; Cantoni, A. Hydrogen-bonded supramolecular motifs in crystal structures of trimethoprim barbiturate monohydrate (I) and 2-amino-4,6-dimethylpyrimidinium barbiturate trihydrate (II). Struct. Chem. 2007, 18, 171. [Google Scholar] [CrossRef]
- Habibi, A.; Tarameshloo, Z. A new and convenient method for synthesis of barbituric acid derivatives. Iran J. Chem. Soc. 2011, 8, 287. [Google Scholar] [CrossRef]
- Vieira, A.; Gomes, N.; Matheus, M.; Fernandes, P.; Figueroa-Villar, J.; Braz, J. Synthesis and In Vivo Evaluation of 5-Chloro-5-benzobarbiturates as New Central Nervous System Depressants. Chem. Soc. 2011, 22, 364. [Google Scholar] [CrossRef]
- Mallah, E.; Sweidan, K.; Engelmann, J.; Steimann, M.; Kuhn, N.; Maier, M.E. Nucleophilic substitution approach towards 1,3-dimethylbarbituric acid derivatives—New synthetic routes and crystal structures. Tetrahedron 2012, 68, 1005. [Google Scholar] [CrossRef]
- Mohamed, M.E.; Frag, E.Y.Z.; Hathoot, A.A.; Shalaby, E.A. Spectrophotometric determination of fenoprofen calcium drug in pure and pharmaceutical preparations. Spectroscopic characterization of the charge transfer solid complexes. Spectrochim. Acta A 2018, 189, 357. [Google Scholar] [CrossRef]
- Shehab, O.R.; AlRabiah, H.; Abdel-Aziz, H.A.; Mostafa, G.A.E. Charge-transfer complexes of cefpodoxime proxetil with chloranilic acid and 23-dichloro-56-dicyano-14-benzoquinone: Experimental and theoretical studies. J. Mol. Liq. 2018, 257, 42. [Google Scholar] [CrossRef]
- Mohamed, G.G.; Hamed, M.M.; Zaki, N.G.; Abdou, M.M.; Mohamed, M.E.; Abdallah, A.M. Melatonin charge transfer complex with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone: Molecular structure, DFT studies, thermal analyses, evaluation of biological activity and utility for determination of melatonin in pure and dosage forms. Spectrochim. Acta A 2017, 182, 143. [Google Scholar] [CrossRef]
- Adegoke, O.A.; Babalola, C.P.; Kotila, O.A.; Obuebhor, O. Simultaneous spectrophotometric determination of trimethoprim and sulphamethoxazole following charge-transfer complexation with chloranilic acid. Arab. J. Chem. 2017, 10 (Suppl. 2), S3848. [Google Scholar] [CrossRef]
- Sawsan, A.A.; Nahla, S.N.; Manal, F.M.; Shimaa, A.; Naglaa, E. Spectrophotometric determination and thermodynamic studies of the charge transfer complexation of emedastine difumarate with some π-acceptors. Arab. J. Chem. 2017, 10 (Suppl. 2), S1855. [Google Scholar] [CrossRef]
- Ismail, N.B.S.; Narayana, B. Spectrophotometric and spectroscopic studies on charge transfer complexes of the antifungal drug clotrimazole. JTUSCI11 2017, 5, 710. [Google Scholar] [CrossRef]
- Adegoke, O.A.; Thomas, O.E.; Emmanuel, S.N. Colorimetric determination of olanzapine via charge-transfer complexation with chloranilic acid. JTUSCI 2016, 10, 651. [Google Scholar]
- Abdulrahman, S.A.M.; Devi, O.Z.; Basavaiah, K.; Vinay, K.B. Use of picric acid and iodine as electron acceptors for spectrophotometric determination of lansoprazole through a charge-transfer complexation reaction. JTUSCI 2016, 10, 80. [Google Scholar] [CrossRef]
- Rahman, N.; Sameen, S.; Kashif, M. Spectroscopic study of charge transfer complexation between doxepin and π–acceptors and its application in quantitative analysis. J. Mol. Liq. 2016, 222, 944. [Google Scholar] [CrossRef]
- Belal, T.S.; El-Kafrawy, D.S.; Mahrous, M.S.; Abdel-Khalek, M.M.; Abo-Gharam, A.H. Validated spectrophotometric methods for determination of sodium valproate based on charge transfer complexation reactions. Spectrochim. Acta A 2016, 155, 47. [Google Scholar]
- Gouda, A.A.; Kasssem, M. Novel spectrophotometric methods for determination of desloratidine in pharmaceutical formulations based on charge transfer reaction. Arab. J. Chem. 2016, 9 (Suppl. 2), S1712. [Google Scholar] [CrossRef]
- Alzoman, N.Z.; Alshehri, J.M.; Darwish, I.A.; Khalil, N.Y.; Abdel-Rahman, H.M. Charge-transfer reaction of 2,3-dichloro-1,4-naphthoquinone with crizotinib: Spectrophotometric study, computational molecular modeling and use in development of microwell assay for crizotinib. Saudi Pharm. J. 2015, 23, 75. [Google Scholar]
- Elqudaby, H.M.; Mohamed, G.G.; El-Din, G.M.G. Analytical studies on the charge transfer complexes of loperamide hydrochloride and trimebutine drugs. Spectroscopic and thermal characterization of CT complexes. Spectrochim. Acta A 2014, 129, 84. [Google Scholar] [CrossRef]
- Soltani, S.; Magri, P.; Rogalski, M.; Kadri, M. Charge-transfer complexes of hypoglycemic sulfonamide with π-acceptors: Experimental and DFT-TDDFT studies. J. Mol. Struct. 2019, 1175, 105. [Google Scholar]
- Fathima, K.S.; Sathiyendran, M.; Anitha, K. Structure elucidation, biological evaluation and molecular docking studies of 3-aminoquinolinium 2-carboxy benzoate- A proton transferred molecular complex. J. Mol. Struct. 2019, 1176, 238. [Google Scholar] [CrossRef]
- Man, L.; Li, T.; Wu, X.; Lu, K.; Yang, L.; Liu, X.; Yang, Z.; Zhou, J.; Ni, C. Synthesis, crystal structure, vibrational spectra, nonlinear optical property of an organic charge-transfer compound―4-nitrobenzyl isoquinolinium picrate based on DFT calculations. J. Mol. Struct. 2019, 1175, 971. [Google Scholar] [CrossRef]
- Afroz, Z.; Faizan, M.; Alam, M.J.; Rodrigues, V.H.N.; Ahmad, S.; Ahmad, A. Synthesis, structural, hirshfeld surface, spectroscopic studies and quantum chemical calculation of the proton transfer complex between 2-amino-4-hydroxy-6-methylpyrimidine and salicylic acid. J. Mol. Struct. 2018, 1171, 438. [Google Scholar] [CrossRef]
- Alghanmi, R.M.; Soliman, S.M.; Basha, M.T.; Habeeb, M.M. Electronic spectral studies and DFT computational analysis of hydrogen bonded charge transfer complexes between chloranilic acid and 2,5-dihydroxy-p-benzoquinone with 2-amino-4-methylbenzothiazole in methanol. J. Mol. Liq. 2018, 256, 433. [Google Scholar] [CrossRef]
- Al-Ahmary, K.M.; Habeeb, M.M.; Al-Obidan, A.H. Charge transfer complex between 2,3-diaminopyridine with chloranilic acid. Synthesis, characterization and DFT, TD-DFT computational studies. Spectrochim. Acta A 2018, 196, 247. [Google Scholar] [CrossRef] [PubMed]
- Alhomrani, M.; Alsanie, W.F.; Alamri, A.S.; Alyami, H.; Habeeballah, H.; Alkhatabi, H.A.; Felimban, R.I.; Haynes, J.M.; Shakya, S.; Raafat, B.M.; et al. Enhancing the Antipsychotic Effect of Risperidone by Increasing Its Binding Affinity to Serotonin Receptor via Picric Acid: A Molecular Dynamics Simulation. Pharmaceuticals 2022, 15, 285. [Google Scholar] [CrossRef] [PubMed]
- Alsanie, W.F.; Alamri, A.S.; Alyami, H.; Alhomrani, M.; Shakya, S.; Habeeballah, H.; Alkhatabi, H.A.; Felimban, R.I.; Alzahrani, A.S.; Alhabeeb, A.A.; et al. Increasing the Efficacy of Seproxetine as an Antidepressant Using Charge-Transfer Complexes. Molecules 2022, 27, 3290. [Google Scholar] [CrossRef] [PubMed]
- Alamri, A.S.; Alhomrani, M.; Alsanie, W.F.; Alyami, H.; Shakya, S.; Habeeballah, H.; Alamri, A.; Alzahrani, O.; Alzahrani, A.S.; Alkhatabi, H.A.; et al. Enhancement of Haloperidol Binding Affinity to Dopamine Receptor via Forming a Charge-Transfer Complex with Picric Acid and 7,7,8,8-Tetracyanoquinodimethane for Improvement of the Antipsychotic Efficacy. Molecules 2022, 27, 3295. [Google Scholar] [CrossRef] [PubMed]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An open chemical toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef]
- Dallakyan, S. PyRx-Python Prescription v. 0.8; The Scripps Research Institute: San Diego, CA, USA, 2008. [Google Scholar]
- Chu, C.-H.; Li, K.-M.; Lin, S.-W.; Chang, M.D.-T.; Jiang, T.-Y.; Sun, Y.-J. Crystal structures of starch binding domain from Rhizopusoryzaeglucoamylase in complex with isomaltooligosaccharide: Insights into polysaccharide binding mechanism of CBM21 family. Proteins Struct. Funct. Bioinform. 2014, 82, 1079–1085. [Google Scholar] [CrossRef]
- Rehman, M.T.; AlAjmi, M.F.; Hussain, A. Natural compounds as inhibitors of SARS-CoV-2 main protease (3CLpro): A molecular docking and simulation approach to combat COVID-19. Curr. Pharm. Des. 2021, 27, 3577–3589. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDockVina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar]
- Bekker, H.; Berendsen, H.J.C.; Dijkstra, E.J.; Achterop, S.; Vondrumen, R.; Vanderspoel, D.; Sijbers, A.; Keegstra, H.; Renardus, M.K.R. Gromacs—A parallel computer for molecular-dynamics simulations. In Proceedings of the 4th International Conference on Computational Physics (PC 92), Prague, Czech Republic, 24–28 August 1992; World Scientific Publishing: Singapore, 1993; pp. 252–256. [Google Scholar]
- Yu, W.; He, X.; Vanommeslaeghe, K.; MacKerell, A.D., Jr. Extension of the CHARMM General Force Field to sulfonylcontaining compounds and its utility in biomolecular simulations. J. Comput. Chem. 2012, 33, 2451–2468. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1, 19–25. [Google Scholar] [CrossRef]
- Allen, M.P.; Tildesley, D.J. Computer Simulations of Liquids; Clarendon Press: Oxford, UK, 1987. [Google Scholar]
- Oostenbrink, C.; Villa, A.; Mark, A.E.; Van Gunsteren, W.F. A biomolecular force field based on the free enthalpy of hydration and solvation: The GROMOS force-field parameter sets 53A5 and 53A6. J. Comput. Chem. 2004, 25, 1656–1676. [Google Scholar] [CrossRef]
- Steinbach, P.J.; Brooks, B.R. New Spherical-Cutoff Methods for Long-Range Forces in Macromolecular Simulation. J. Comput. Chem. 1994, 15, 667–683. [Google Scholar] [CrossRef]
- DeLano, W.L. PyMOL; DeLano Scientific: Palo Alto, CA, USA, 2002. [Google Scholar]
- Lee, H.K.; Zhang, L.; Smith, M.D.; Walewska, A.; Vellore, N.A.; Baron, R.; McIntosh, J.M.; White, H.S.; Olivera, B.M.; Bulaj, G. A marine analgesic peptide, Contulakin-G, and neurotensin are distinct agonists for neurotensin receptors: Uncovering structural determinants of desensitization properties. Front. Pharmacol. 2015, 6, 11. [Google Scholar] [CrossRef]
- Frisch, M.J.; Clemente, F.R. Gaussian 09, Revision A. 01; Gaussian, Inc.: Wallingford, CT, USA, 2009; pp. 20–44. [Google Scholar]
- Hariharan, P.C.; Pople, J.A. The effect of d-functions on molecular orbital energies for hydrocarbons. Chem. Phys. Lett. 1972, 16, 217–219. [Google Scholar] [CrossRef]
- Zhurko, G.A.; Zhurko, D.A. Chemcraft—Graphical Program for Visualization of Quantum Chemistry Computations, Academic Version 1.5; Grigoriy Andrienko: Ivanovo, Russia, 2004; Available online: https://www.chemcraftprog.com/ (accessed on 5 September 2022).
- Shakya, B.; Shakya, S.; Hasan Siddique, Y. Effect of geraniol against arecoline induced toxicity in the third instar larvae of transgenic Drosophila melanogaster (hsp70-lacZ) Bg9. Toxicol. Mech. Methods 2019, 29, 187–202. [Google Scholar] [CrossRef]
- Skoog, D.A. Principle of Instrumental Analysis, 3rd ed.; Saunders: New York, NY, USA, 1985; Chapter 7. [Google Scholar]
- Ding, X.; Li, Y.; Wang, S.; Li, X.; Huang, W. Proton-transfer supramolecular salts based on proton sponge 2,2′-dipyridylamin. J. Mol. Struct. 2013, 1051, 124. [Google Scholar] [CrossRef]
- Ding, X.; Li, Y.; Wang, S.; Li, X.; Huang, W. Proton-transfer supramolecular salts of d-/l-tartaric acid and 1-(2-Pyrimidyl)piperazine. J. Mol. Struct. 2014, 1062, 61. [Google Scholar] [CrossRef]
- Akram, M.; Lal, H.; Shakya, S.; Kabir-ud-Din. Multispectroscopic and Computational Analysis Insight into the Interaction of Cationic Diester-Bonded Gemini Surfactants with Serine Protease α-Chymotrypsin. ACS Omega 2020, 5, 3624–3637. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.M.; Shakya, S.; Islam, M.; Khan, S.; Najnin, H. Synthesis and spectrophotometric studies of CT complex between 1, 2-dimethylimidazole and picric acid in different polar solvents: Exploring antimicrobial activities and molecular (DNA) docking. Phys. Chem. Liq. 2021, 59, 753–769. [Google Scholar] [CrossRef]
- Krivák, R.; Jendele, L.; Hoksza, D. Peptide-Binding Site Prediction from Protein Structure via points on the Solvent Accessible Surface. In Proceedings of the 2018 ACM International Conference on Bioinformatics, Computational Biology, and Health Informatics, Washington, DC, USA, 29 August–1 September 2018; p. 645. [Google Scholar]
- Ranjbar, A.; Jamshidi, M.; Torabi, S. Molecular modelling of the antiviral action of Resveratrol derivatives against the activity of two novel SARS-CoV-2 and 2019-nCoV receptors. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 7834–7844. [Google Scholar] [PubMed]
- Kufareva, I.; Abagyan, R. Methods of protein structure comparison. Methods Mol. Biol. 2012, 857, 231–257. [Google Scholar]
- Wu, S.; Zhang, Y. A comprehensive assessment of sequence-based and template-based methods for protein contact prediction. Bioinformatics 2008, 24, 924–931. [Google Scholar] [CrossRef]
- Shakya, S.; Khan, M.I.; Ahmad, M. Charge transfer complex based real-time colorimetric chemosensor for rapid recognition of dinitrobenzene and discriminative detection of Fe2+ ions in aqueous media and human hemoglobin. J. Photochem. Photobiol. A Chem. 2020, 392, 112402. [Google Scholar] [CrossRef]
- Islam, M.R.; Shakya, S.; Selim, A.; Alam, M.S.; Ali, M. Solvatochromic Absorbance and Fluorescence Probe Behavior within Ionic Liquid+ γ-Butyrolactone Mixture. J. Chem. Eng. Data 2019, 64, 4169–4180. [Google Scholar] [CrossRef]
- Al-Saif, F.A.; El-Habeeb, A.A.; Refat, M.S.; Adam, A.M.A.; Saad, H.A.; El-Shenawy, A.I.; Fetooh, H. Characterization of charge transfer products obtained from the reaction of the sedative-hypnotic drug barbital with chloranilic acid, chloranil, TCNQ and DBQ organic acceptors. J. Mol. Liq. 2019, 287, 110981. [Google Scholar] [CrossRef]
- Murugavel, S.; Ravikumar, C.; Jaabil, G.; Alagusundaram, P. Synthesis, crystal structure analysis, spectral investigations (NMR, FT-IR, UV), DFT calculations, ADMET studies, molecular docking and anticancer activity of 2-(1-benzyl-5-methyl-1H-1, 2, 3-triazol-4-yl)-4-(2-chlorophenyl)-6-methoxypyridine—A novel potent human topoisomerase IIα inhibitor. J. Mol. Struct. 2019, 1176, 729–742. [Google Scholar]
- Khan, I.M.; Shakya, S. Exploring Colorimetric Real-Time Sensing Behavior of a Newly Designed CT Complex toward Nitrobenzene and Co2+: Spectrophotometric, DFT/BarCS-DFT, and Mechanistic Insights. ACS Omega 2019, 4, 9983–9995. [Google Scholar] [CrossRef]
- Foresman, J.B. Exploring Chemistry with Electronic Structure Methods: A Guide to Using Gaussian; Frisch, E., Ed.; Gaussian: Pittsburg, PA, USA, 1996. [Google Scholar]

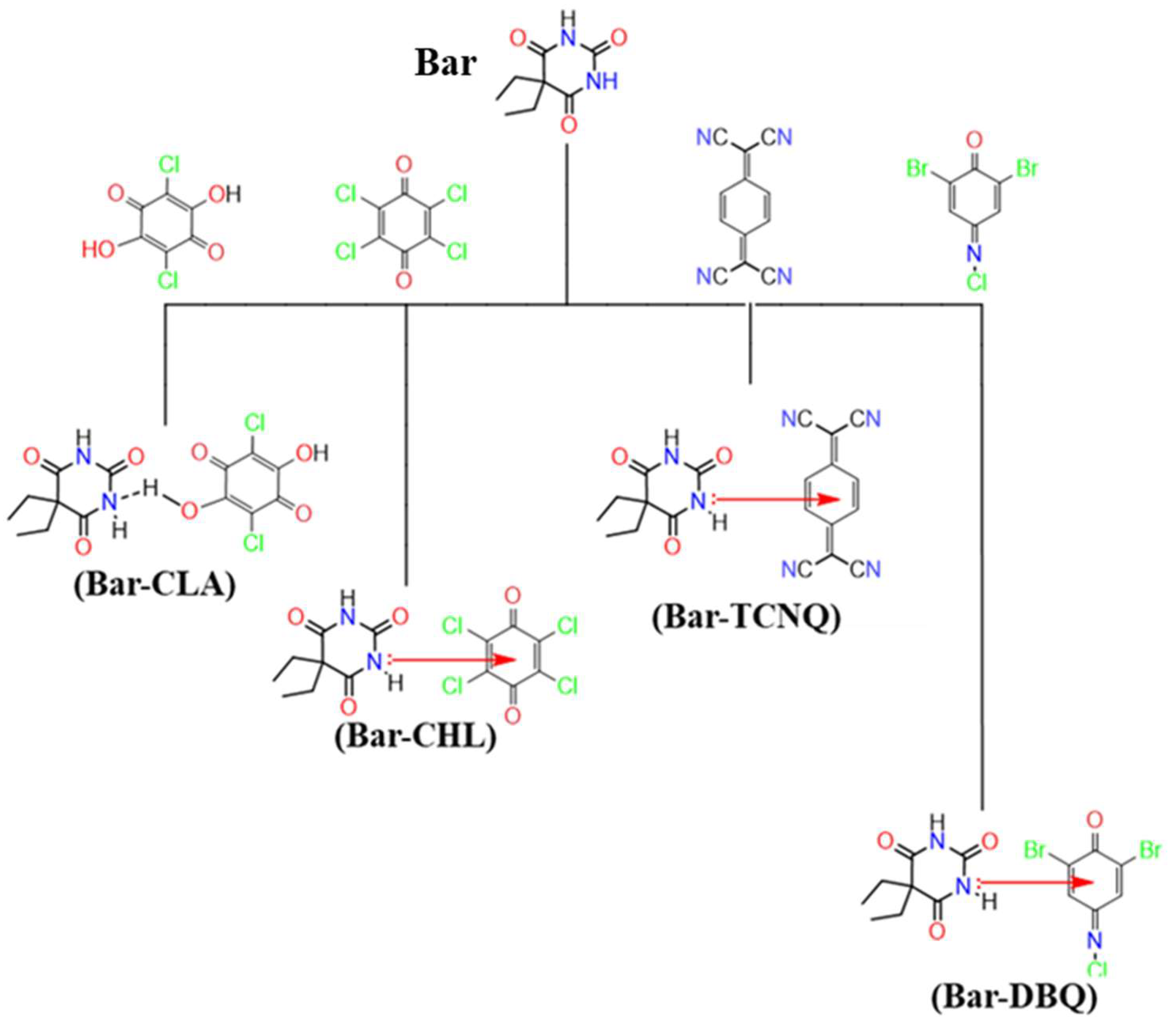




| CT Complex | M.Wt g/mol | F.Wt | Elemental Analysis % (Calcd.) | Infrared Assignments cm−1 | 1HNMR Assignments δ (ppm) |
|---|---|---|---|---|---|
| (Bar–CLA) | 393.17 | C14H14Cl2N2O7 | N; 7.07 (7.12) H; 3.50 (3.56) C; 42.66 (42.73) | 1174; νs(C–N) 1270; νas(C–N) 1368 and 1456; δ(C–H) 1544; ν(C=C) 1625; δ(N–H) 1674 and 1712; νs(C=O) 1771; νas(C=O) 3220 and 3170; ν(N–H) | 11.37; (s, 2H, 2NH) 10.05; (s, 2H, 2OH) 1.89; (q, 4H, 2CH2) 0.81; (t, 6H, 2CH3) |
| (Bar–CHL) | 430.05 | C14H12Cl4N2O5 | N; 6.43 (6.51) H; 2.84 (2.79) C; 39.13 (39.07) | 1107; νs(C–N) 1320; νas(C–N) 1371 and 1439; δ(C–H) 1460; ν(C=C) 1574; δ(N–H) 1684; νs(C=O) 1752; νas(C=O) 3160 and 3242; ν(N–H) | 11.17; (s, 2H, 2NH) 1.92; (q, 4H, 2CH2) 0.83; (t, 6H, 2CH3) |
| (Bar–TCNQ) | 388.38 | C20H16N6O3 | N; 21.70 (21.63) H; 4.08 (4.12) C; 61.75 (61.80) | 1138; νs(C–N) 1321; νas(C–N) 1380 and 1468; δ(C–H) 1521; ν(C=C) 1675; δ(N–H) 1714; νs(C=O) 1763; νas(C=O) 2223; ν(C≡N) 3171 and 3209; ν(N–H) | 11.33; (s, 2H, 2NH) 7.72; (s, 4H, ArH) 1.90; (q, 4H, 2CH2) 0.82; (t, 6H, 2CH3) |
| (Bar–DBQ) | 483.54 | C14H14Br2ClN3O4 | N; 8.64 (8.69) H; 2.96 (2.90) C; 34.70 (34.74) | 1157; νs(C–N) 1241; νas(C–N) 1380 and 1409; δ(C–H) 1473; ν(C=C) 1568; ν(C=N) 1669; δ(N–H) 1718; νs(C=O) 1767; νas(C=O) 3168 and 3256; ν(N–H) | 11.31; (s, 2H, 2NH) 7.27; (s, 2H, ArH) 1.91; (q, 4H, 2CH2) 0.80; (t, 6H, 2CH3) |
| Docking Score (kcal/mol) | ||
|---|---|---|
| PDB:6BQH | PDB:6CM4 | |
| (Bar–CHL) | −7.7 | −6.9 |
| (Bar–TCNQ) | −8.1 | −7.1 |
| (Bar–DBQ) | −7.5 | −7.2 |
| (Bar–CLA) | −8.2 | −7.8 |
| Bar | −5.3 | −5.1 |
| Receptor | Binding Free Energy (kcal/mol) | Interactions | |
|---|---|---|---|
| HBond | Others | ||
| (Bar–CLA) | −8.2 | Thr386, Lys385, Lys320, Asn110, and Asn107 | Ala321, Val324, Leu105, and Lys104 (π–alkyl) |
| Bar | −5.3 | Arg173, Asn187, and His182 | Ala176 and Ile177 (π–alkyl) |
| Energies in (kJ/mol) | ||
|---|---|---|
| BarCS Complex | BarS Complex | |
| Binding energy (Total) | −40.642 4.324 | −31.089 9.997 |
| Van der Waals energy (EvdW) | −83.954 7.623 | −39.276 11.001 |
| Electrostatic energy (Eelec) | −186.670 4.302 | −288.981 8.653 |
| Nonpolar solvation energy (Gnonpolar) | −58.527 3.121 | −31.801 9.741 |
| Polar solvation energy (Gpolar) | 288.509 2.652 | 328.969 3.955 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alamri, A.S.; Alhomrani, M.; Alsanie, W.F.; Alyami, H.; Shakya, S.; Habeeballah, H.; Abdulaziz, O.; Alamri, A.; Alkhatabi, H.A.; Felimban, R.I.; et al. Spectroscopic and Molecular Docking Analysis of π-Acceptor Complexes with the Drug Barbital. Appl. Sci. 2022, 12, 10130. https://doi.org/10.3390/app121910130
Alamri AS, Alhomrani M, Alsanie WF, Alyami H, Shakya S, Habeeballah H, Abdulaziz O, Alamri A, Alkhatabi HA, Felimban RI, et al. Spectroscopic and Molecular Docking Analysis of π-Acceptor Complexes with the Drug Barbital. Applied Sciences. 2022; 12(19):10130. https://doi.org/10.3390/app121910130
Chicago/Turabian StyleAlamri, Abdulhakeem S., Majid Alhomrani, Walaa F. Alsanie, Hussain Alyami, Sonam Shakya, Hamza Habeeballah, Osama Abdulaziz, Abdulwahab Alamri, Heba A. Alkhatabi, Raed I. Felimban, and et al. 2022. "Spectroscopic and Molecular Docking Analysis of π-Acceptor Complexes with the Drug Barbital" Applied Sciences 12, no. 19: 10130. https://doi.org/10.3390/app121910130
APA StyleAlamri, A. S., Alhomrani, M., Alsanie, W. F., Alyami, H., Shakya, S., Habeeballah, H., Abdulaziz, O., Alamri, A., Alkhatabi, H. A., Felimban, R. I., Alhabeeb, A. A., Refat, M. S., & Gaber, A. (2022). Spectroscopic and Molecular Docking Analysis of π-Acceptor Complexes with the Drug Barbital. Applied Sciences, 12(19), 10130. https://doi.org/10.3390/app121910130









