Abstract
Chemical warfare agents (CWAs) are highly toxic and fast-acting and are easy to cause large-scale poisoning to humans and livestock after being released. The activated carbon used for CWAs adsorption has disadvantages of limited adsorption capacity, easy aging and deactivation. Metal oxides have environmental stability, and they are characterized by long lasting and broad spectrum when used for thermal catalytic decomposition. Therefore, in this study, the supported copper–cerium catalyst CuO-CeO2/γ-Al2O3 was prepared using an equal volume impregnation method. The thermal catalytic decomposition performance was studied using sarin CWAs simulant dimethyl methyl phosphonate (DMMP) as the target compound. The results show that the CuO-CeO2/γ-Al2O3 catalyst with a CeO2 loading of 5% exhibited better thermal catalytic decomposition performance of DMMP. The catalyst provided protection against DMMP for 237 min at 350 °C; CuO was highly dispersed on CuO-5% CeO2/γ-Al2O3, and there was a strong interaction between Cu and Ce on CuO-5% CeO2/γ-Al2O3, which promoted the generation of surface-adsorbed oxygen, leading to a better thermal catalytic decomposition performance of DMMP. This study is expected to provide a reference for the study of catalysts for the thermal catalytic decomposition of CWAs.
1. Introduction
Chromium-free impregnated carbon adsorption technology is the preferred method for protection of chemical warfare agents (CWAs). However, it has disadvantages, such as short protection time, narrow protection spectrum, and easy aging and inactivation. To this end, further research on and development of alternative technologies is required. Thermal catalytic purification is one of the most commonly used technologies for the treatment of volatile organic pollutants [1,2]; it can also be used for the removal of CWAs. The technology can be used to convert poisonous molecules into non-toxic or low-toxicity small molecules through catalysis, and has a broad spectrum, long-lasting effects, and broad application prospects for chemical protection.
Long-term research has been conducted on catalysts for the thermal catalytic decomposition of CWAs. Pt catalysts are the most widely studied catalysts. Numerous reports have shown that Pt catalysts have the advantages of high catalytic oxidation activity and long protection time [3,4,5,6]; however, they are prone to chemical poisoning and are difficult to regenerate after deactivation. In addition, Pt catalysts have limitations, such as high prices and scarcity of reserves [7]; therefore, the discovery of other catalysts that can achieve long-term protection against poisonous vapors is necessary. Recently, research on supported metal oxide catalysts for the thermal catalytic decomposition of CWAs has progressed [8,9,10,11]. Cu catalysts have considerable potential in such cases. For example, Wang et al. [12] reported the thermal catalytic decomposition performance of HCN CWAs by metal oxide catalysts (Mn, Fe, Co, Ni, and Cu) supported by activated carbon, and activated carbon-supported CuO had superior performance, with a conversion of HCN exceeding 96% between 200 and 350 °C. Cao et al. [6] demonstrated that CuO/γ-Al2O3 can achieve complete conversion of dimethyl methyl phosphonate (DMMP) at 400 °C with a protection time as long as 7.5 h. However, the supported single-component CuO catalysts reported to date do not meet the requirements for protective applications, and their thermal catalytic decomposition performance requires further improvement. Recently, Chen et al. [13] reported the complete decomposition of DMMP is achieved based on crystalline ceria films under vacuum heating. Furthermore, CeO2 can influence the charge transfer between catalyst components through the conversion of Ce3+/Ce4+ and is often used as a catalyst promoter or carrier to improve the oxygen storage capacity of catalysts [14,15,16]. Therefore, it is worthwhile to prepare CuO-CeO2/γ-Al2O3 catalyst and investigate the effect of CeO2 loadings on thermal catalytic decomposition of CWAs.
Herein, CuO/γ-Al2O3 and CuO-CeO2/γ-Al2O3 with different CeO2 loadings were prepared to investigate whether the addition of CeO2 could improve the thermal catalytic decomposition performance of the catalyst, and the mechanism of the catalyst was studied. The sarin simulant DMMP was selected as the target for catalytic decomposition. CuO/γ-Al2O3 and CuO-CeO2/γ-Al2O3 with different CeO2 loadings were prepared using an equal volume impregnation method, and their thermal catalytic decomposition of DMMP was investigated. The crystal structure, pore distribution, chemical state, and interaction of the active components were studied using X-ray powder diffraction (XRD), N2 adsorption, X-ray photoelectron spectroscopy (XPS), and hydrogen temperature-programmed reduction (H2-TPR). This study provides a reference for the development of high-performance catalysts for the thermal catalytic decomposition of CWAs. Moreover, this study is of great significance for the decontamination of pollutants in the fields of national defense security and environmental protection, and the as-prepared materials have potential application value in decontamination of CWAs.
2. Materials and Methods
2.1. Materials
The active component precursors used in catalyst preparation were analytically pure cerium nitrate hexahydrate (Ce(NO3)3·6H2O, Aladdin Reagent Co., Ltd., Shanghai, China), analytically pure copper nitrate trihydrate (Cu(NO3)2·3H2O, Beijing Chemical Factory, Beijing, China), and analytically pure γ-alumina powder (γ-Al2O3, Aladdin Reagent Co., Ltd., particle diameter = ~20 nm).
2.2. Synthesis of Catalysts
The CuO-CeO2/γ-Al2O3 catalyst was prepared using the equal volume impregnation method. First, the water absorption rate of γ-Al2O3 was measured, and the concentrations of copper nitrate and cerium nitrate in the mixed impregnation solution were calculated according to the water absorption rate of γ-Al2O3 and the CuO and CeO2 content to be supported. A mixed solution of the corresponding concentration was prepared, and a certain volume of the mixed solution was immersed in the corresponding mass of γ-Al2O3 powder, stirred rapidly for 15 min, and then aged for 24 h in air. After drying at 110 °C for 10 h, the obtained block material was ground into powder and calcined at 550 °C for 5 h. The obtained catalyst was denoted CuO-X CeO2/γ-Al2O3, where X represents the mass percentage of CeO2.
In addition, CuO/γ-Al2O3 was prepared using the same impregnation method for comparison. The concentrations of copper nitrate in the mixed impregnation solution were calculated according to the water absorption rate of γ-Al2O3 and the CuO content to be supported. A certain volume of copper nitrate solution was immersed in the corresponding mass of γ-Al2O3 powder, stirred rapidly for 15 min, and then aged for 24 h in air. After drying at 110 °C for 10 h, the obtained block material was ground into powder and calcined at 550 °C for 5 h. The obtained catalyst was denoted CuO/γ-Al2O3. Preliminary experimental studies demonstrated that when the CuO loading is 5%, the thermal catalytic decomposition of DMMP by CuO/γ-Al2O3 is enhanced [17]. Therefore, the loading of both CuO in CuO/γ-Al2O3 and CuO-CeO2/γ-Al2O3 was 5%.
2.3. Catalyst Characterization Methods
The phase structure of the catalyst was characterized using a SmartLab X-ray powder diffractometer from Rigaku Corporation, Saitama, Japan (Cu target, Kα ray, λ = 0.15418 nm, tube flow 40 mA). The scanning speed was set to 6°/min and the scanning range (2θ) was between 5° and 80° with a scan step size of 0.02°.
The N2 adsorption isotherm was measured using a NOVA 4200e automatic specific surface analyzer (Quantachrome Instruments, Boynton Beach, FL, USA) at 77 K. The specific surface area of the material was obtained using the the Brunauer–Emmett–Teller (BET) model, and the mesopore distribution of the material was obtained using the Barret–Joyner–Halend (BJH) method.
The surface elements and corresponding chemical valence states of the samples were analyzed using an AXIS Ultra DLD X-ray photoelectron spectrometer (XPS) from (Shimadzu Corporation, Kyoto, Japan). The incident light was an Al Kα ray, and the spectrum was corrected using the binding energy of C1s (284.8 eV) as a reference. The XPS data was analyzed using the Avantage software. All peaks of the corrected spectra were fitted with a Gaussian–Lorentzian shape function to fit the data.
The temperature-programmed reduction of H2 was carried out using a CATLAB catalytic microreactor (Hiden Company, Warrington, UK). A 100 mg sample was weighed and pretreated in an He atmosphere at 300 °C for 3 h to remove absorbed water from the catalyst surface. After the temperature dropped to 50 °C, 5 vol% H2/Ar mixed gas was introduced, the gas flow rate was maintained at 30 mL/min, the heating rate was 10 °C/min, and the temperature range was 50–650 °C. The H2 signal was measured using a HPR-20 mass spectrometer (HIDEN Company, Warrington, UK).
2.4. Evaluation of Catalyst Performance
In this study, the thermal catalytic decomposition of DMMP was evaluated using protection time as a criterion. Protection time usually refers to the duration required for the complete conversion of a target object [8]. To mitigate potential errors, protection time is considered to be the duration from the beginning of the reaction to the conversion below 99%. The performance of the catalyst was evaluated using a thermocatalytic fixed-bed reactor, and the experimental setup is shown in Figure 1. Prior to testing, the catalyst powder was tableted, crushed, and sieved. The catalyst particles (0.3 g of 20–40 mesh) were weighed, loaded into the reactor, and fixed with quartz wool. The reactor temperature was set to 350 °C. A dried-air carrier gas of 100 mL·min−1 was bubbled through a flask of DMMP at 30 °C to generate a DMMP vapor of 4 g·m−3. The concentration of DMMP in the exhaust gas was determined using gas chromatography (Agilent 6890N) with a DB-1701 column and an FID detector. The conversion is calculated using the following formula:
where Cout represents the concentration of DMMP in the exhaust, Cin represents the concentration of DMMP created by bubbling.
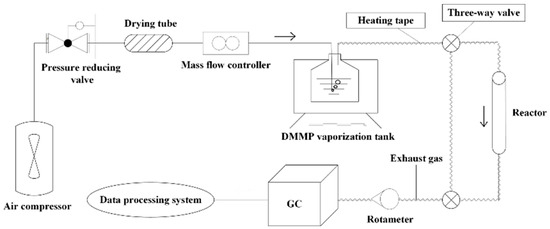
Figure 1.
Schematic diagram of the device for thermocatalytic decomposition.
3. Results and Discussion
3.1. Thermal Catalytic Decomposition of DMMP by CuO-CeO2/γ-Al2O3
Figure 2 shows the DMMP conversion of CuO/γ-Al2O3 and CuO-CeO2/γ-Al2O3 as a function of the reaction time. The protection time decreased in the following order: CuO-5% CeO2/γ-Al2O3 (237 min) > CuO-1% CeO2/γ-Al2O3 (127 min) > CuO-10% CeO2/γ-Al2O3 (112 min) > CuO/γ-Al2O3 (110 min). Only the catalytic performance of CuO-5% CeO2/γ-Al2O3 was significantly improved relative to that of CuO/γ-Al2O3, indicating that the thermal catalytic decomposition performance of the catalyst can be manipulated using the CeO2 loading. Therefore, good catalytic performance of CuO-5% CeO2/γ-Al2O3 is attributable to the interactions between the components. CeO2 affects the chemical state and redox activity of the Cu component and improves the catalytic performance for the thermal decomposition of DMMP. A comparison of protection time on various catalysts reported in literatures is shown in Table 1.
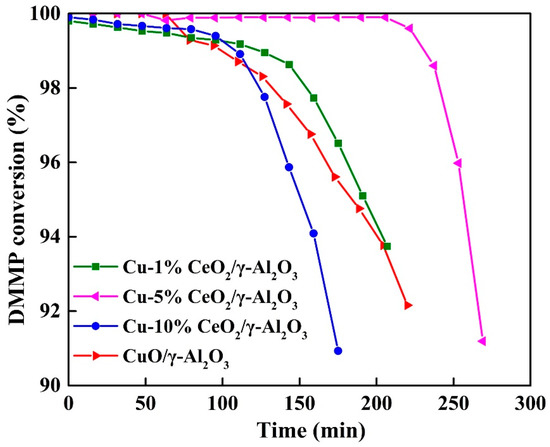
Figure 2.
Effects of different Cu/Ce ratios of the CuO/CeO2/γ-Al2O3 catalysts on protection time.

Table 1.
A comparison of protection time on various catalysts.
3.2. Phase Analysis of CuO-CeO2/γ-Al2O3
Figure 3 shows the XRD results for the catalyst. The CeO2 and CuO diffraction patterns match the characteristic peaks of the fluorite structure of CeO2 (PDF# 75-0151) and the monoclinic structure of CuO (PDF# 80-1916), respectively. There were no diffraction peaks for the CuO on the CuO/γ-Al2O3 and CuO-CeO2/γ-Al2O3 catalysts. A study by Xia et al. [20] demonstrated that the capacity of CuO dispersed in the monolayer on the surface of γ-Al2O3 is 0.75 mmol/100 m2 Al2O3; in the highly dispersed state, Cu2+ occupies octahedral and tetrahedral vacancies on γ-Al2O3. Related studies by Wan et al. [21] reveal that when the Cu loading is less than 0.6 mmol/100 m2 Al2O3, CuO is highly dispersed on the surface of γ-Al2O3 and no crystalline CuO are formed. For the CuO/γ-Al2O3 and CuO-CeO2/γ-Al2O3 prepared in this experiment, the Cu loading is 0.35 mmol/100 m2 Al2O3, which is within the range described in the literature. Therefore, the high dispersion of CuO components on the surface of γ-Al2O3 explains the absence of CuO diffraction peaks in the three catalysts spectra.
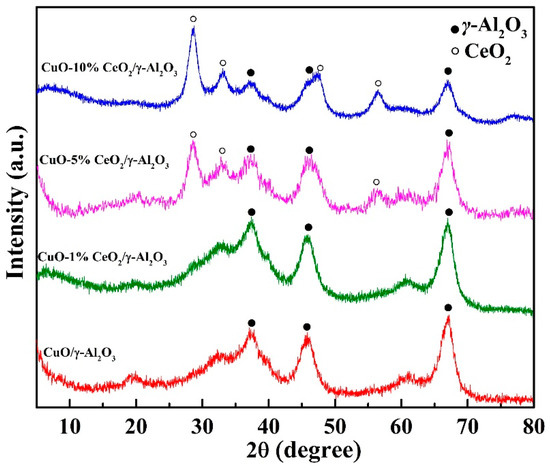
Figure 3.
XRD patterns of CuO/γ-Al2O3, CuO-1% CeO2/γ-Al2O3, CuO-5% CeO2/γ-Al2O3, and CuO-10% CeO2/γ-Al2O3.
There were no diffraction signals for the Ce component of CuO-1% CeO2/γ-Al2O3. CuO-5% CeO2/γ-Al2O3 shows characteristic diffraction peaks of CeO2 crystals at 28.5°, 33.1°, and 56.3° (PDF# 75-0151). The CeO2 diffraction peaks for CuO-10% CeO2/γ-Al2O3 have greater intensity, and the peak widths at half height are smaller, indicating the formation of larger CeO2 grains. There is no diffraction signal for the Ce component of CuO-1% CeO2/γ-Al2O3, because the Ce component is also a highly dispersed amorphous species, whereas there is a CeO2 crystal on the CuO-5% CeO2/γ-Al2O3 grain. When considered in combination with the performance evaluation results, CuO-1% CeO2/γ-Al2O3 did not demonstrate significantly better thermal catalytic decomposition of DMMP than CuO/γ-Al2O3, whereas CuO-5% CeO2/γ-Al2O3 performed significantly better. These results indicate that the key to improving the catalyst performance maybe the size of the CeO2 grains, rather than the highly dispersed Ce species. This may be due to the catalysis of the specific crystal plane of CeO2 or the Cu component entering the crystal lattice of CeO2 as a dopant atom, resulting in an increase in the number of oxygen defects [22,23]. In addition, the grain size of CeO2 in the CuO-10% CeO2/γ-Al2O3 catalyst was larger than that of CeO2 on CuO-5% CeO2/γ-Al2O3. However, the thermal catalytic decomposition performance of CuO-10% CeO2/γ-Al2O3 was relatively limited. Therefore, the size of the CeO2 grains is one of the essential considerations for the successful development of catalysts for thermal catalytic decomposition [24].
3.3. Pore Structure Analysis of CuO-CeO2/γ-Al2O3
Figure 4 shows the N2 adsorption and desorption isotherms of the catalysts. The adsorption and desorption isotherms of the four catalysts were all type III [25]. When the relative pressure (P/P0) was low, the adsorption and desorption curves of the catalysts overlapped. When the relative pressure (P/P0) was high, the adsorption–desorption curves had a hysteresis loop. This indicates that the pore structure in each catalyst results from the slit-like pores formed by the accumulation of loose flaky particles. In addition, when the relative pressure (P/P0) was close to 1, the adsorption isotherm did not reach equilibrium, confirming the existence of a slit-like pore structure in the catalyst.
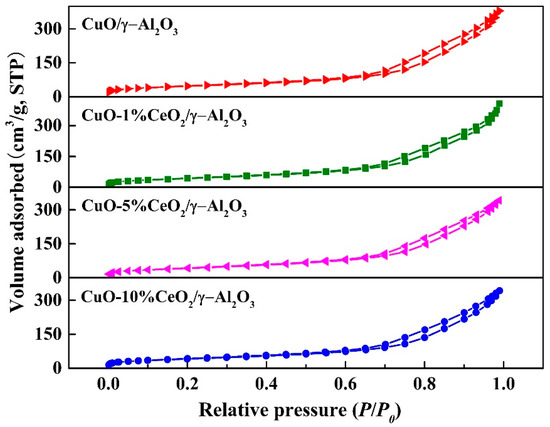
Figure 4.
N2 adsorption isotherm of CuO/γ-Al2O3, CuO-1% CeO2/γ-Al2O3, CuO-5% CeO2/γ-Al2O3, and CuO-10% CeO2/γ-Al2O3.
Table 2 shows the specific surface area, pore diameter, and pore volume of CuO/γ-Al2O3 and CuO-CeO2/γ-Al2O3. The specific surface area and pore volume of the catalysts decreased with increasing Ce loading. In addition, the mean pore size of CuO-CeO2/γ-Al2O3 was similar to that of CuO/γ-Al2O3. The experimental results show that the CeO2 loading does not have a significant effect on the pore size of the catalyst.

Table 2.
Specific surface area and pore structure data of CuO/γ-Al2O3 and CuO-CeO2/γ-Al2O3.
3.4. H2-TPR Analysis of CuO-CeO2/γ-Al2O3
Figure 5 shows the H2-TPR spectra of CuO/γ-Al2O3 and CuO-CeO2/γ-Al2O3. As the reduction temperature of CeO2 is higher than 400 °C [14], the reduction peaks shown in the figure are all reduction signals of the Cu component. The reduction peak temperatures of CuO/γ-Al2O3, CuO-1% CeO2/γ-Al2O3, and CuO-5% CeO2/γ-Al2O3 were 217, 255, 262, and 270 °C, respectively, which correspond to the reduction of Cu2+. According to the literature [26,27], when Cu2+ is doped into the lattice of CeO2, a relatively stable system can be formed, resulting in an increase in the reduction temperature. The same phenomenon was observed in this experiment: the reduction temperature of CuO in CuO-CeO2/γ-Al2O3 was higher than that in CuO/γ-Al2O3, and the reduction temperature of CuO increased with increasing CeO2 content. The CuO-CeO2/γ-Al2O3 catalysts show a strong reduction peak at approximately 220 °C. This peak was attributed to CuO, which was highly dispersed and weakly interacted with CeO2. In addition, it is speculated that the reduction peak appears at 160 °C for the CuO-5% CeO2/γ-Al2O3 and CuO-10% CeO2/γ-Al2O3 catalysts is due to the electron transfer between the Cu and Ce components, and the Cu component that receives electrons can be reduced at a lower temperature. The new reduction peak appears at 160 °C for the CuO-5% CeO2/γ-Al2O3 and CuO-10% CeO2/γ-Al2O3 catalysts, suggesting that the loaded catalyst had more active oxygen. Evidently, the addition of CeO2 improved the oxidation activity of CuO/γ-Al2O3 catalyst.
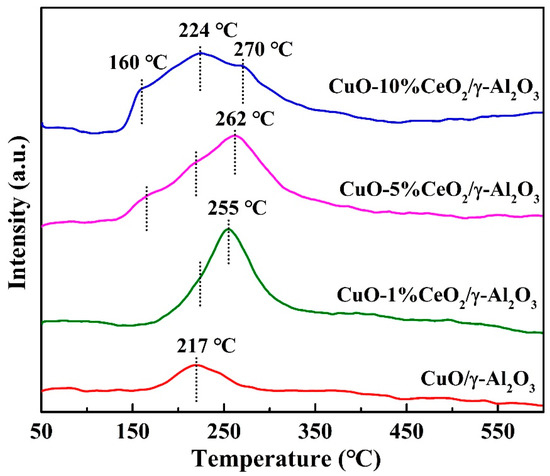
Figure 5.
H2-TPR profiles of CuO/γ-Al2O3, CuO-1% CeO2/γ-Al2O3, CuO-5% CeO2/γ-Al2O3, and CuO-10% CeO2/γ-Al2O3.
3.5. XPS Analysis of CuO-CeO2/γ-Al2O3
Figure 6 shows the Cu 2p XPS spectra of CuO/γ-Al2O3 and CuO-CeO2/γ-Al2O3. CuO/γ-Al2O3 shows signals of Cu2+ at approximately 934.9 eV and Cu+ and/or Cu0 932.6 eV, respectively [28,29]. The XPS spectra of CuO-1% CuO/γ-Al2O3 were similar to those of CuO/γ-Al2O3, indicating that the chemical states and electronic structures of the two catalysts were similar. When the CeO2 content increased to 5% (CuO-5% CeO2/γ-Al2O3) and 10% (CuO-10% CeO2/γ-Al2O3), the XPS spectra revealed a significant change. This was a result of the doping of Cu into the CeO2 lattice, which strengthened the interactions between Cu and Ce and promoted electron transfer between Cu and Ce. The reversible reactions Ce3+ + Cu2+ ↔ Ce4+ + Cu+ and 2Ce3+ + Cu2+ ↔ 2Ce4+ + Cu0 can occur on the surface of the CuO/CeO2 catalyst [30,31,32]. As Zeng et al. reported [28], Cu+ is bonded on oxygen vacancy (Ov) of CeO2, forming a Cu+–Ov–Ce3+ structure, which further enhanced the performance of the CuO/CeO2 catalyst.
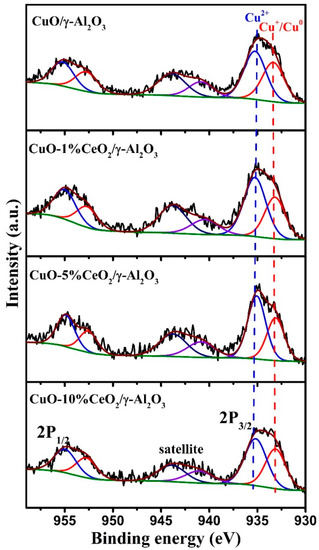
Figure 6.
Cu 2p spectra of CuO/γ-Al2O3, CuO-1% CeO2/γ-Al2O3, CuO-5% CeO2/γ-Al2O3, and CuO-10% CeO2/γ-Al2O3.
Figure 7 shows the O1s XPS spectra of CuO/γ-Al2O3 and CuO-CeO2/γ-Al2O3. The oxygen species on the catalyst surface can be divided into two categories: (1) lattice oxygen (Olat), which typically produces a binding energy peak at 529.0–531.0 eV; (2) surface adsorbed oxygen (Oads), which includes chemically adsorbed oxygen, hydroxyl oxygen, and oxygen in adsorbed water, and produces a binding energy peak at 531.0–534.0 eV. Compared with lattice oxygen, surface-adsorbed oxygen has greater mobility and higher reactivity [14,33].
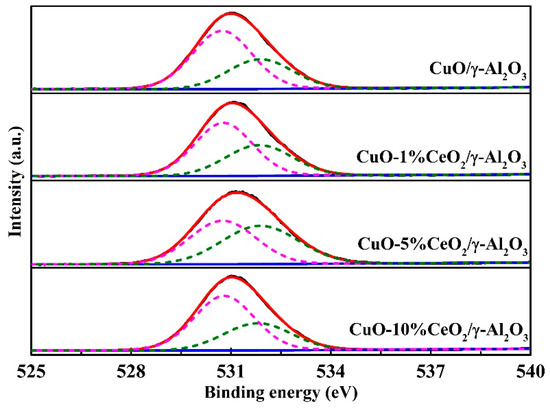
Figure 7.
O1s XPS spectra of CuO/γ-Al2O3, CuO-1% CeO2/γ-Al2O3, CuO-5% CeO2/γ-Al2O3, and CuO-10% CeO2/γ-Al2O3.
The O1s XPS spectra of the catalyst are fitted, and the ratio of surface-adsorbed oxygen and lattice oxygen to total surface oxygen species (Oads/[Oads + Olat], OLat/[Oads + Olat]) were calculated in Table 3. The results show that the ratio of surface-adsorbed oxygen on CuO-5% CeO2/γ-Al2O3 is the highest, at 0.50; CuO-1% CeO2/γ-Al2O3 has the second highest, at 0.39, and the surface adsorption oxygen ratios of CuO-10% CeO2/γ-Al2O3 and CuO/γ-Al2O3 are both 0.35. Considering these results together with the protection time, the thermal catalytic decomposition performances of the catalysts were consistent with the amount of surface-adsorbed oxygen.

Table 3.
The various oxygen ratios of CuO/γ-Al2O3 and CuO-CeO2/γ-Al2O3.
4. Conclusions
In this study, CuO-CeO2/γ-Al2O3 was prepared using an equal volume impregnation method. The CuO was highly dispersed on CuO/γ-Al2O3 and CuO-CeO2/γ-Al2O3. The CeO2 loading had minimal effect on the pore structure of the catalyst. The thermal catalytic decomposition performance of DMMP was studied by measuring the protection time. Compared with the other CuO/γ-Al2O3 and CuO-CeO2/γ-Al2O3 catalysts tested, CuO-5% CeO2/γ-Al2O3 exhibited the longest protection time (237 min at 350 °C). A strong interaction between Cu and Ce was evident in the case of the CuO-CeO2/γ-Al2O3 catalysts with 5% CeO2. This interaction promotes the generation of more surface-adsorbed oxygen, which is favorable for the thermal catalytic decomposition of DMMP. This research provides a reference for the design and preparation of efficient CWAs removal catalysts.
Author Contributions
H.G. and W.K. made the main contribution to the experimental works and managed the experimental and writing process as co-first author; X.W. and Q.H. assisted in accomplishing a part of the experimental works and characterizations; S.Z. and Y.D. provided the concept of this research and participated in the guidance of this work and gave some advice on the synthesis of catalyst and catalyst characterization as the corresponding author. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (No. 21701186) and the Fundamental Research Funds from State Key Laboratory of NBC Protection for Civilian (SKLNBC 2019-04), China.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lin, F.; Zhang, Z.; Li, N.; Yan, B.; He, C.; Hao, Z.; Chen, G. How to achieve complete elimination of Cl-VOCs: A critical review on byproducts formation and inhibition strategies during catalytic oxidation. Chem. Eng. J. 2021, 404, 126534. [Google Scholar] [CrossRef]
- Yang, C.; Miao, G.; Pi, Y.; Xia, Q.; Wu, J.; Li, Z.; Xiao, J. Abatement of various types of VOCs by adsorption/catalytic oxidation: A review. Chem. Eng. J. 2019, 370, 1128–1153. [Google Scholar] [CrossRef]
- Monji, M.; Ciora, R.; Liu, P.K.; Parsley, D.; Egolfopoulos, F.N.; Tsotsis, T.T. Thermocatalytic decomposition of dimethyl methylphosphonate (DMMP) in a multi-tubular, flow-through catalytic membrane reactor. J. Membr. Sci. 2015, 482, 42–48. [Google Scholar] [CrossRef]
- Motamedhashemi, M.M.Y.; Egolfopoulos, F.; Tsotsis, T. Application of a flow-through catalytic membrane reactor (FTCMR) for the destruction of a chemical warfare simulant. J. Membr. Sci. 2011, 376, 119–131. [Google Scholar] [CrossRef]
- Patil, L.A.; Bari, A.R.; Shinde, M.D.; Deo, V.; Kaushik, M.P. Detection of dimethyl methyl phosphonate—A simulant of sarin: The highly toxic chemical warfare—Using platinum activated nanocrystalline ZnO thick films. Sens. Actuators B Chem. 2012, 161, 372–380. [Google Scholar] [CrossRef]
- Ratliff, J.S.; Tenney, S.A.; Hu, X.; Conner, S.F.; Ma, S.; Chen, D.A. Decomposition of Dimethyl Methylphosphonate on Pt, Au, and Au-Pt Clusters Supported on TiO2(110). Langmuir 2009, 25, 216–225. [Google Scholar] [CrossRef]
- Sushma Kumari, M.; Saroha, A.K. Performance of various catalysts on treatment of refractory pollutants in industrial wastewater by catalytic wet air oxidation: A review. J. Environ. Manag. 2018, 228, 169–188. [Google Scholar] [CrossRef]
- Cao, L.; Segal, S.R.; Suib, S.L.; Tang, X.; Satyapal, S. Thermocatalytic Oxidation of Dimethyl Methylphosphonate on Supported Metal Oxides. J. Catal. 2000, 194, 61–70. [Google Scholar] [CrossRef]
- Tesfai, T.M.; Sheinker, V.N.; Mitchell, M.B. Decomposition of Dimethyl Methylphosphonate (DMMP) on Alumina-Supported Iron Oxide. J. Phys. Chem. B 1998, 102, 7299–7302. [Google Scholar] [CrossRef]
- Segal, S.R.; Cao, L.; Suib, S.L.; Tang, X.; Satyapal, S. Thermal Decomposition of Dimethyl Methylphosphonate over Manganese Oxide Catalysts. J. Catal. 2001, 198, 66–76. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Schoenitz, M.; Dreizin, E.L. Vapor-phase decomposition of dimethyl methylphosphonate (DMMP), a sarin surrogate, in presence of metal oxides. Def. Technol. 2020, 17, 1095–1114. [Google Scholar] [CrossRef]
- Wang, X.; Jing, X.; Wang, F.; Ma, Y.; Cheng, J.; Wang, L.; Xu, K.; Cheng, C.; Ning, P. Coupling catalytic hydrolysis and oxidation on metal-modified activated carbon for HCN removal. RSC Adv. 2016, 6, 57108–57116. [Google Scholar] [CrossRef]
- Chen, D.A.; Ratliff, J.S.; Hu, X.; Gordon, W.O.; Senanayake, S.D.; Mullins, D.R. Dimethyl methylphosphonate decomposition on fully oxidized and partially reduced ceria thin films. Surf. Sci. 2010, 604, 574–587. [Google Scholar] [CrossRef]
- López, J.M.; Gilbank, A.L.; García, T.; Solsona, B.; Agouram, S.; Torrente-Murciano, L. The prevalence of surface oxygen vacancies over the mobility of bulk oxygen in nanostructured ceria for the total toluene oxidation. Appl. Catal. B Environ. 2015, 174–175, 403–412. [Google Scholar] [CrossRef]
- Montini, T.; Melchionna, M.; Monai, M.; Fornasiero, P. Fundamentals and Catalytic Applications of CeO2-Based Materials. Chem. Rev. 2016, 116, 5987–6041. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Grinter, D.C.; Liu, Z.; Palomino, R.M.; Senanayake, S.D. Ceria-based model catalysts: Fundamental studies on the importance of the metal-ceria interface in CO oxidation, the water-gas shift, CO2 hydrogenation, and methane and alcohol reforming. Chem. Soc. Rev. 2017, 46, 1824–1841. [Google Scholar] [CrossRef]
- Gao, H.; Dong, Y.; Zhou, S. Thermocatalytic decomposition of a sarin simulating agent by metal oxides supported on γ-Al2O3. Chin. J. Environ. Eng. 2019, 13, 1148–1156. [Google Scholar]
- Graven, W.M.; Weller, S.W.; Peters, D.L. Catalytic conversion of a organophosphate vapor over platinum-alumina. Ind. Eng. Chem. Process Des. Dev. 1966, 5, 183. [Google Scholar] [CrossRef]
- Lee, K.Y.; Houalla, M.; Hercules, D.M.; Hall, W.K. Catalytic Oxidative Decomposition of Dimethyl Methylphosphonate over Cu-Substituted Hydroxyapatite. J. Catal. 1994, 145, 223–231. [Google Scholar] [CrossRef]
- Xia, W.S.; Wan, H.L.; Chen, Y. Cluster model study on the surface interactions of γ-alumina-supported metal oxides. J. Mol. Catal. A Chem. 1999, 138, 185–195. [Google Scholar] [CrossRef]
- Wan, H.; Wang, Z.; Zhu, J.; Li, X.; Liu, B.; Gao, F.; Dong, L.; Chen, Y. Influence of CO pretreatment on the activities of CuO/γ-Al2O3 catalysts in CO + O2 reaction. Appl. Catal. B Environ. 2008, 79, 254–261. [Google Scholar] [CrossRef]
- Bera, P.; Priolkar, K.R.; Sarode, P.R.; Hegde, M.S.; Emura, S.; Kumashiro, R.; Lalla, N.P. Structural Investigation of Combustion Synthesized Cu/CeO2 Catalysts by EXAFS and Other Physical Techniques: Formation of a Ce1-xCuxO2-δ Solid Solution. Chem. Mater. 2002, 14, 3591–3601. [Google Scholar] [CrossRef]
- Guo, X.; Zhou, R. Identification of the nano/micro structure of CeO2(rod) and the essential role of interfacial copper-ceria interaction in CuCe(rod) for selective oxidation of CO in H-2-rich streams. J. Power Sources 2017, 361, 39–53. [Google Scholar] [CrossRef]
- Mitchell, M.B.; Sheinker, V.N.; Cox, W.W.; Gatimu, E.N.; Tesfamichael, A.B. The Room Temperature Decomposition Mechanism of Dimethyl Methylphosphonate (DMMP) on Alumina-Supported Cerium Oxide—Participation of Nano-Sized Cerium Oxide Domains. J. Phys. Chem. B 2004, 108, 1634–1645. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Qi, L.; Yu, Q.; Dai, Y.; Tang, C.; Liu, L.; Zhang, H.; Gao, F.; Dong, L.; Chen, Y. Influence of cerium precursors on the structure and reducibility of mesoporous CuO-CeO2 catalysts for CO oxidation. Appl. Catal. B Environ. 2012, 119–120, 308–320. [Google Scholar] [CrossRef]
- Jia, A.-P.; Hu, G.S.; Meng, L.; Xie, Y.L.; Lu, J.Q.; Luo, M.F. CO oxidation over CuO/Ce1−xCuxO2−δ and Ce1−xCuxO2−δ catalysts: Synergetic effects and kinetic study. J. Catal. 2012, 289, 199–209. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Q.; Wu, J.; Zhang, H.; Liu, Y.; Zhang, T.; Tian, H.; Zeng, S. Active Sites and Interfacial Reducibility of CuxO/CeO2 Catalysts Induced by Reducing Media and O2/H2 Activation. ACS Appl. Mater. Interfaces 2021, 13, 35804–35817. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, Q.; Wang, X.; Ma, K.; Bai, X.; Tan, S.; Tian, Y.; Ding, T.; Zheng, L.; Zhang, J.; et al. Enhanced catalytic performance for CO preferential oxidation over CuO catalysts supported on highly defective CeO2 nanocrystals. Appl. Surf. Sci. 2017, 422, 932–943. [Google Scholar] [CrossRef]
- Wang, F.; Buchel, R.; Savitsky, A.; Zalibera, M.; Widmann, D.; Pratsinis, S.E.; Lubitz, W.; Schuth, F. In Situ EPR Study of the Redox Properties of CuO–CeO2 Catalysts for Preferential CO Oxidation (PROX). ACS Catal. 2016, 6, 3520–3530. [Google Scholar] [CrossRef]
- Polster, C.S.; Nair, H.; Baertsch, C.D. Study of active sites and mechanism responsible for highly selective CO oxidation in H2 rich atmospheres on a mixed Cu and Ce oxide catalyst. J. Catal. 2009, 266, 308–319. [Google Scholar] [CrossRef]
- Sedmak, G.; Hočevar, S.; Levec, J. Kinetics of selective CO oxidation in excess of H2 over the nanostructured Cu0.1Ce0.9O2—catalyst. J. Catal. 2003, 213, 135–150. [Google Scholar] [CrossRef]
- Liao, Y.; Fu, M.; Chen, L.; Wu, J.; Huang, B.; Ye, D. Catalytic oxidation of toluene over nanorod-structured Mn–Ce mixed oxides. Catal. Today 2013, 216, 220–228. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).