Factors Determining Ticagrelor-Induced Dyspnea in Patients with Acute Coronary Syndrome
Abstract
1. Introduction
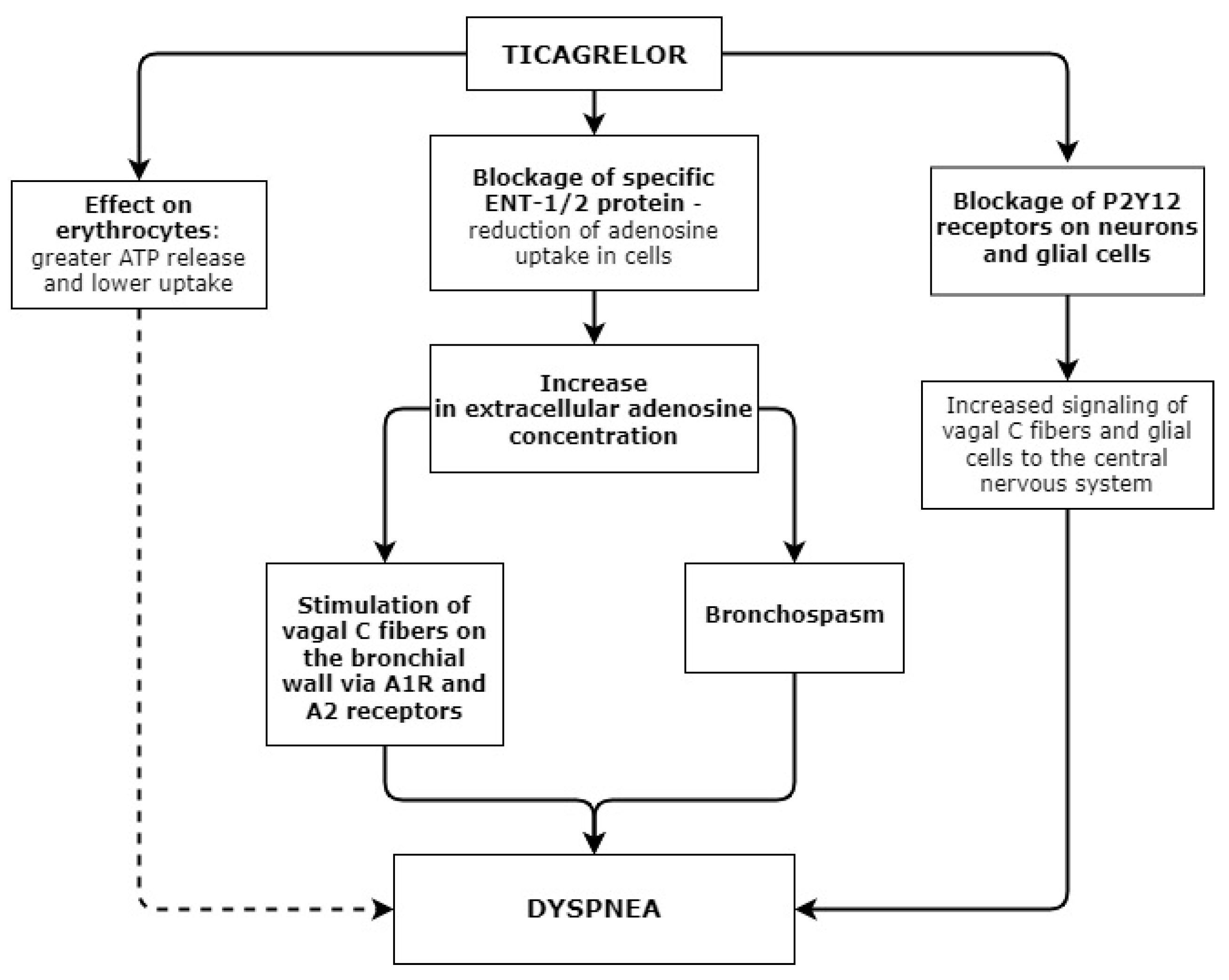
- 1.
- An increase in extracellular adenosine levels.
- 2.
- Dyspnea is caused via P2Y12 receptors.
- 3.
- Dyspnea is associated with ATP release from erythrocytes.
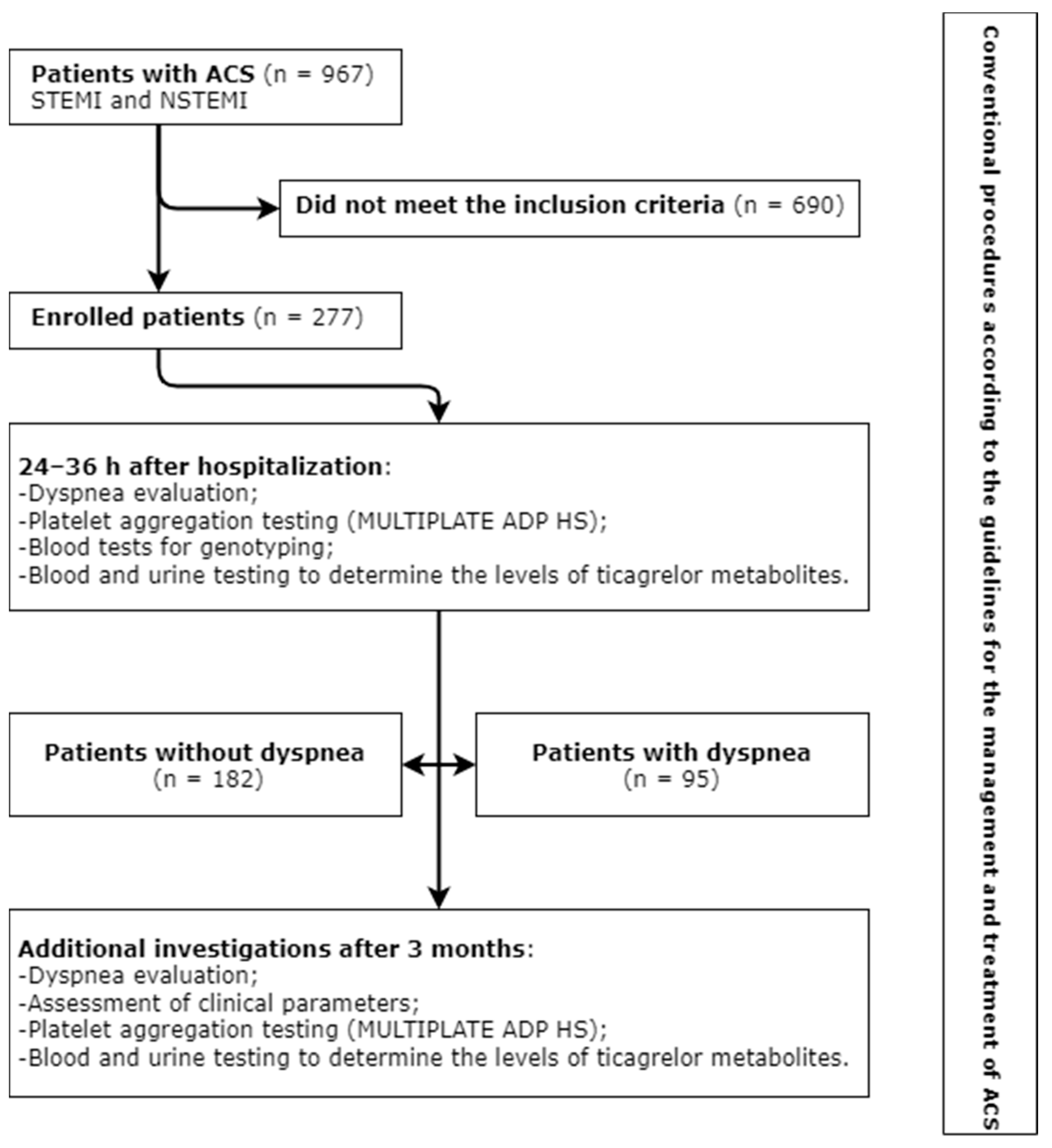
2. Materials and Methods
- Patients treated in the Cardiovascular Centre, Republican Šiauliai Hospital, for ACS (myocardial infarction with and without ST-segment elevation), who underwent coronary angiography and PTCA with stent implantation;
- Treatment with antiplatelet drugs (ticagrelor and aspirin) or a combination of antiplatelet drugs and anticoagulant (ticagrelor, aspirin, and dabigatran etexilate);
- Planned 12-month treatment with ticagrelor.
- The exclusion criteria were as follows:
- Previous dyspnea experienced by a patient;
- Severe comorbid disease (stage IV cancer; significant disease of another organ system, etc.) that could have an influence on the results of the performed investigations;
- Respiratory diseases (bronchial asthma; chronic obstructive pulmonary disease; COVID-19 during ACS);
- Previous or new-onset New York Heart Association (NYHA) class III and IV heart failure;
- Warfarin usage (e.g., due to mechanical heart valve);
- Patients at high risk of bleeding, who were treated with clopidogrel;
- Social indications (e.g., a patient being cared for, no possibility to arrive for follow-up visits);
- Patient’s refusal to take part in this study.
2.1. Investigations and Patients’ Clinical Data
- Venous blood samples were collected for genotyping. Genotyping procedures were carried out at the Laboratory of Molecular Cardiology, Institute of Cardiology, Medical Academy, Lithuanian University of Health Sciences. DNA was extracted from blood using a salting-out method. The determination of gene variants was carried out using TaqMan probes (Thermo Fisher Scientific, Waltham, MA, USA), TaqMan Universal Master Mix (Thermo Fisher Scientific, Waltham, MA, USA), and PCR-grade water. The QuantStudio 5 and 3 Real-Time PCR systems (Thermo Fisher Scientific, Waltham, MA, USA) were employed. A total of 12 gene variants—C148T (rs1800787), CYP4F2 (rs3093135, rs1558139, rs2108622, and rs2074902), CYP2C19 *2 and *17 (rs4244285 and rs12248560), CYP2C9 *15 (rs72558190), ABCB1 (rs1045642), COX-2 (rs689465), PAI-1 (rs5918), CYP1A2*1C (rs2069514)—were investigated;
- Platelet aggregation (induction with high-sensitivity ADP, ADP HS) testing was performed using a MULTIPLATE analyzer and reagents for the determination of P2Y12 receptor activity. Testing was carried out at the Laboratory Diagnostic Unit, Republican Šiauliai Hospital. Platelet aggregation testing with ADP HS was carried out 24–36 h and 3 months after the administration of a loading ticagrelor dose.
2.2. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
3.2. Associations between Clinical and Genetic Characteristics and the Development of Dyspnea
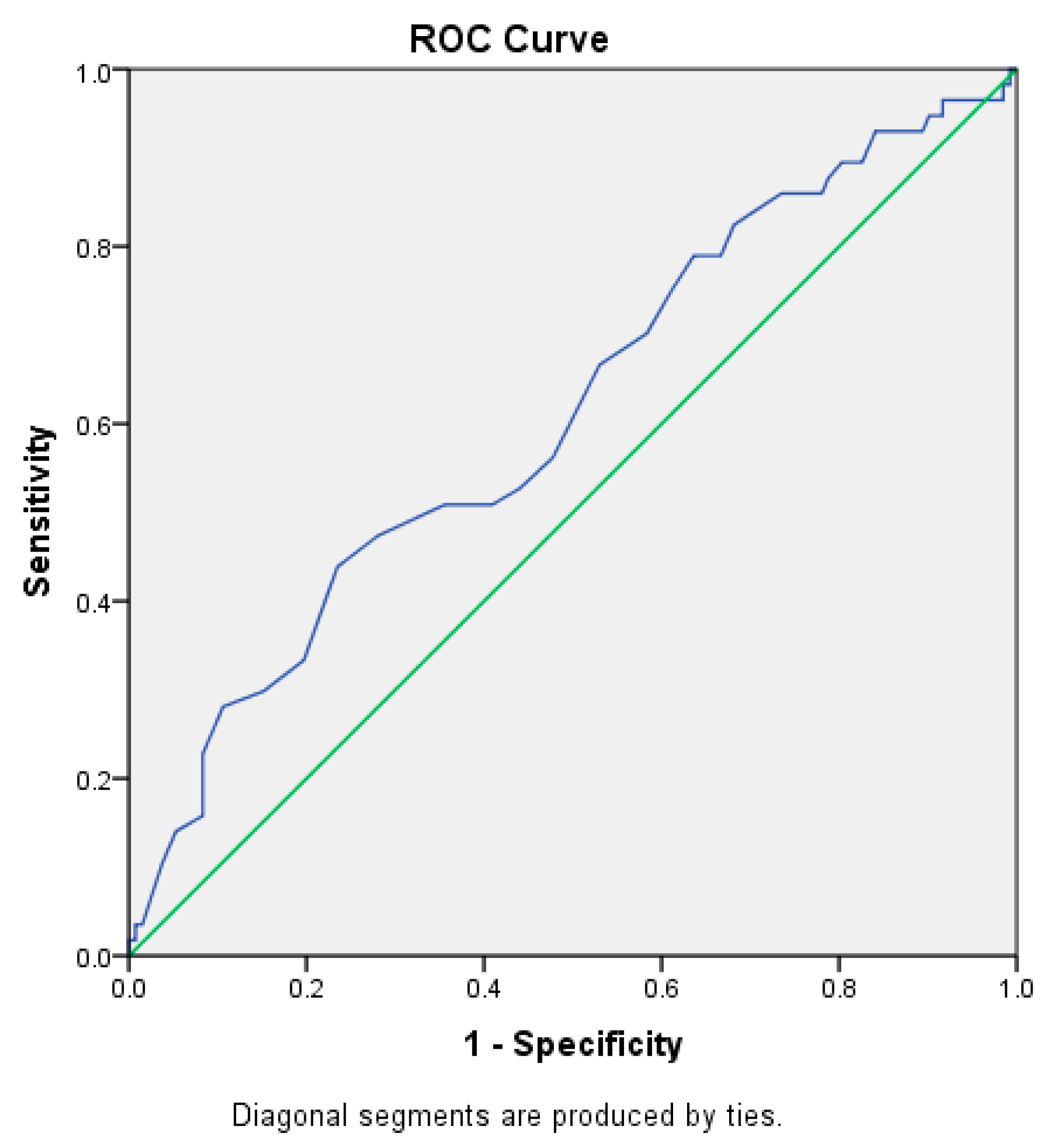
3.3. Logistic Regression Analysis for the Identification of Significant Risk Factors for Dyspnea
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Health for All Database (HFA-DB). Available online: https://cisid.euro.who.int/cisid/ (accessed on 15 May 2022).
- Collet, J.-P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef]
- Adamski, P.; Buszko, K.; Sikora, J.; Niezgoda, P.; Fabiszak, T.; Ostrowska, M.; Barańska, M.; Karczmarska-Wódzka, A.; Navarese, E.P.; Kubica, J. Determinants of high platelet reactivity in patients with acute coronary syndromes treated with ticagrelor. Sci. Rep. 2019, 9, 3924. [Google Scholar] [CrossRef] [PubMed]
- Mehran, R.; Kalkman, D.N.; Angiolillo, D.J. Atrial fibrillation, with ACS and PCI: Walking a tightrope. Eur. Heart J. 2019, 40, 1563–1566. [Google Scholar] [CrossRef] [PubMed]
- Spertus, J.A.; Kettelkamp, R.; Vance, C.; Decker, C.; Jones, P.G.; Rumsfeld, J.S.; Messenger, J.C.; Khanal, S.; Peterson, E.D.; Bach, R.G.; et al. Prevalence, predictors, and outcomes of premature discontinuation of thienopyridine therapy after drug-eluting stent placement: Results from the PREMIER registry. Circulation 2006, 113, 2803–2809. [Google Scholar] [CrossRef]
- Arora, S.; Shemisa, K.; Vaduganathan, M.; Qamar, A.; Gupta, A.; Garg, S.K.; Kumbhani, D.J.; Mayo, H.; Khalili, H.; Pandey, A.; et al. Premature Ticagrelor Discontinuation in Secondary Prevention of Atherosclerotic CVD: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 73, 2454–2464. [Google Scholar] [CrossRef]
- Nawarskas, J.J.; Clark, S.M. Ticagrelor: A novel reversible oral antiplatelet agent. Cardiol. Rev. 2011, 19, 95–100. [Google Scholar] [CrossRef]
- Husted, S.; van Giezen, J.J.J. Ticagrelor: The first reversibly binding oral p2y12 receptor antagonist. Cardiovasc. Ther. 2009, 27, 259–274. [Google Scholar] [CrossRef]
- Teng, R. Ticagrelor: Pharmacokinetic, Pharmacodynamic and Pharmacogenetic Profile: An Update. Clin. Pharmacokinet. 2015, 54, 1125–1138. [Google Scholar] [CrossRef]
- Kalantzi, K.I.; Tsoumani, M.E.; Goudevenos, I.A.; Tselepis, A.D. Pharmacodynamic properties of antiplatelet agents: Current knowledge and future perspectives. Expert Rev. Clin. Pharmacol. 2012, 5, 319–336. [Google Scholar] [CrossRef]
- Rosa, G.M.; Bianco, D.; Valbusa, A.; Massobrio, L.; Chiarella, F.; Brunelli, C. Pharmacokinetics and pharmacodynamics of ticagrelor in the treatment of cardiac ischemia. Expert Opin. Drug Metab. Toxicol. 2016, 12, 1491–1502. [Google Scholar] [CrossRef] [PubMed]
- Schühlen, H. Pre-specified vs. post-hoc subgroup analyses: Are we wiser before or after a trial has been performed? Eur. Heart J. 2014, 35, 2055–2057. [Google Scholar] [CrossRef]
- Husted, S.E.; Stoery, R.F.; Bliden, K.P.; Tantry, U.S. Pharmacokinetics and Pharmacodynamics of Ticagrelor in Patients with Stable Coronary Artery Disease Results from the ONSET-OFFSET and RESPOND Studies. Clin. Pharmacokinet. 2012, 51, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Cannon, C.P.; Husted, S.; Harrington, R.A.; Scirica, B.M.; Emanuelsson, H.; Peters, G.; Storey, R.F.; DISPERSE-2 Investigators. Safety, Tolerability, and Initial Efficacy of AZD6140, the First Reversible Oral Adenosine Diphosphate Receptor Antagonist, Compared With Clopidogrel, in Patients With Non-ST-Segment Elevation Acute Coronary Syndrome. Primary Results of the DISPERSE-2 Trial. J. Am. Coll. Cardiol. 2007, 50, 1844–1851. [Google Scholar] [CrossRef] [PubMed]
- Tatarunas, V.; Kupstyte-Kristapone, N.; Norvilaite, R.; Tamakauskas, V.; Skipskis, V.; Audrone, V.; Jurgaityte, J.; Stuoka, M.; Lesauskaite, V. The impact of CYP2C19 and CYP4F2 variants and clinical factors on treatment outcomes during antiplatelet therapy. Pharmacogenomics 2019, 20, 483–492. [Google Scholar] [CrossRef]
- Wallentin, L.; James, S.; Storey, R.F.; Armstrong, M.; Barratt, B.J.; Horrow, J.; Husted, S.; Katus, H.; Steg, P.G.; Shah, S.H.; et al. Effect of CYP2C19 and ABCB1 single nucleotide polymorphisms on outcomes of treatment with ticagrelor versus clopidogrel for acute coronary syndromes: A genetic substudy of the PLATO trial. Lancet 2010, 376, 1320–1348. [Google Scholar] [CrossRef]
- Nie, S.; Chen, K.; Guo, C.; Pei, Q.; Zou, C.; Yao, L.; Yuan, H.; Zhao, X.; Xie, R.; He, X.; et al. Effect of CYP4F2 Polymorphisms on Ticagrelor Pharmacokinetics in Healthy Chinese Volunteers. Front. Pharmacol. 2022, 12, 797278. [Google Scholar] [CrossRef]
- Tatarunas, V.; Aldujeli, A.; Kurnickaite, Z.; Maciulevicius, L.; Burkanas, M.; Venius, J.; Ciapiene, I.; Skipskis, V.; Norvilaite, R.; Giedraitiene, A.; et al. Blood direct PCR: Impact of CYP2C19 and CYP4F2 variants for bleeding prediction in ST-elevation myocardial infarction patients with ticagrelor. Pers. Med. 2022, 19, 207–217. [Google Scholar] [CrossRef]
- Tatarunas, V.; Kupstyte-Kristapone, N.; Zvikas, V.; Jakstas, V.; Zaliunas, R.; Lesauskaite, V. Factors associated with platelet reactivity during dual antiplatelet therapy in patients with diabetes after acute coronary syndrome. Sci. Rep. 2020, 10, 3175. [Google Scholar] [CrossRef]
- Nanhwan, M.K.; Ling, S.; Kodakandla, M.; Nylander, S.; Ye, Y.; Birnbaum, Y. Chronic treatment with ticagrelor limits myocardial infarct size: An adenosine and cyclooxygenase-2-dependent effect. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2078–2085. [Google Scholar] [CrossRef]
- Reiner, M.F.; Breitenstein, A.; Holy, E.W.; Glanzmann, M.; Amstalden, H.; Stämpfli, S.F.; Bonetti, N.R.; Falk, V.; Keller, S.; Savarese, G.; et al. Ticagrelor, but not clopidogrel active metabolite, displays antithrombotic properties in the left atrial endocardium. Eur. Heart J. 2017, 38, 916–919. [Google Scholar] [CrossRef] [PubMed]
- Gibson, J.S. Mechanism of O2-sensitive red cell properties. Blood 2016, 128, 2593–2595. [Google Scholar] [CrossRef] [PubMed]
- Gasecka, A.; Nieuwland, R.; Budnik, M.; Dignat-George, F.; Eyileten, C.; Harrison, P.; Lacroix, R.; Leroyer, A.; Opolski, G.; Pluta, K.; et al. Ticagrelor attenuates the increase of extracellular vesicle concentrations in plasma after acute myocardial infarction compared to clopidogrel. J. Thromb. Haemost. 2020, 18, 609–623. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhao, W.B.; Chen, Y.; Hu, H.Y. Higher Plasma Concentrations of Platelet Microparticles in Patients With Acute Coronary Syndrome: A Systematic Review and Meta-analysis. Can. J. Cardiol. 2016, 32, 1325.e1. [Google Scholar] [CrossRef]
- Zhang, N.; Xu, W.; Li, O.; Zhang, B. The risk of dyspnea in patients treated with third-generation P2Y12 inhibitors compared with clopidogrel: A meta-analysis of randomized controlled trials. BMC Cardiovasc. Disord. 2020, 20, 140. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Paz, L.; Brugaletta, S.; Ariotti, S.; Akkerhuis, K.M.; Karagiannis, A.; Windecker, S.; Valgimigli, M. Adenosine and ticagrelor plasma levels in patients with and without ticagrelor- related dyspnea. Circulation 2018, 138, 646–648. [Google Scholar] [CrossRef]
- Hai-Ling, L.I. Risk factors of ticagrelor-associated dyspnea in patients with acute coronary syndrome. Acad. J. Second. Mil. Med. Univ. 2020, 12, 11–17. [Google Scholar]
- Parshall, M.B.; Schwartzstein, R.M.; Adams, L.; Banzett, R.B.; Manning, H.L.; Bourbeau, J.; Calverley, P.M.; Gift, A.G.; Harver, A.; Lareau, S.C.; et al. An official American thoracic society statement: Update on the mechanisms, assessment, and management of dyspnea. Am. J. Respir. Crit. Care Med. 2012, 185, 435–452. [Google Scholar] [CrossRef]
- Butler, K.; Maya, J.; Teng, R. Effect of ticagrelor on pulmonary function in healthy elderly volunteers and asthma or chronic obstructive pulmonary disease patients. Curr. Med. Res. Opin. 2013, 29, 569–577. [Google Scholar] [CrossRef]
- Storey, R.F.; Bliden, K.P.; Patil, S.B.; Karunakaran, A.; Ecob, R.; Butler, K.; Teng, R.; Wei, C.; Tantry, U.S.; Gurbel, P.A. Incidence of dyspnea and assessment of cardiac and pulmonary function in patients with stable coronary artery disease receiving ticagrelor, clopidogrel, or placebo in the ONSET/OFFSET study. J. Am. Coll. Cardiol. 2010, 56, 185–193. [Google Scholar] [CrossRef]
- Krakowiak, A.; Kuleta, J.; Plech, I.; Zarębiński, M.; Wojciechowska, M.; Wretowski, D.; Cudnoch-Jędrzejewska, A. Ticagrelor-Related Severe Dyspnoea: Mechanisms, Characteristic Features, Differential Diagnosis and Treatment. Clin. Med. Insights Case Rep. 2020, 13, 1179547620956634. [Google Scholar] [CrossRef] [PubMed]
- Arif, A.S. Salicylic Acid (Aspirin); Updated 15 July 2021; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK519032/ (accessed on 3 May 2022).
- Giannoni, A.; Borrelli, C.; Gentile, F.; Mirizzi, G.; Coceani, M.; Paradossi, U.; Vergaro, G.; Bramanti, F.; Iudice, G.; Emdin, M.; et al. Central Apnoeas and Ticagrelor Related Dyspnoea in Patients with Acute Coronary Syndrome Running Title: Ticagrelor and Central Apnoeas. Eur. Heart J.-Cardiovasc. Pharmacother. 2020, 7, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Wittfeldt, A.; Emanuelsson, H.; Brandrup-Wognsen, G.; van Giezen, J.; Jonasson, J.; Nylander, S.; Gan, L.-M. Ticagrelor enhances adenosine-induced coronary vasodilatory responses in humans. J. Am. Coll. Cardiol. 2013, 61, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Teng, R.; Mitchell, P.D.; Butler, K.A. Pharmacokinetic interaction studies of co-administration of ticagrelor and atorvastatin or simvastatin in healthy volunteers. Eur. J. Clin. Pharmacol. 2013, 69, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Hou, L.; Li, C.; Zhao, Y.; Yao, X.; Zhang, X.; Tian, X. Contributions of UDP-Glucuronosyltransferases to Human Hepatic and Intestinal Metabolism of Ticagrelor and Inhibition of UGTs and Cytochrome P450 Enzymes by Ticagrelor and its Glucuronidated Metabolite. Front. Pharmacol. 2021, 12, 761814. [Google Scholar] [CrossRef]
- Feidt, D.M.; Klein, K.; Hofmann, U.; Riedmaier, S.; Knobeloch, D.; Thasler, W.E.; Weiss, T.S.; Schwab, M.; Zanger, U.M. Profiling induction of cytochrome P450 enzyme activity by statins using a new liquid chromatography-tandem mass spectrometry cocktail assay in human hepatocytes. Drug Metab. Dispos. 2010, 38, 1589–1597. [Google Scholar] [CrossRef] [PubMed]
- Wypasek, E.; Stepien, E.; Kot, M.; Plicner, D.; Kapelak, B.; Sadowski, J.; Undas, A. Fibrinogen beta-chain -C148T polymorphism is associated with increased fibrinogen, C-reactive protein, and interleukin-6 in patients undergoing coronary artery bypass grafting. Inflammation 2012, 35, 429–435. [Google Scholar] [CrossRef]
- de Maatab, M.P.; Pietersma, A.; Kofflardd, M.; Sluiter, W.; Kluftb, C. Association of plasma fibrinogen levels with coronary artery disease, smoking and inflammatory markers. Atherosclerosis 1996, 121, 185–191. [Google Scholar] [CrossRef]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef]
- Arman, M.; Payne, H.; Ponomaryov, T.; Brill, A. Role of Platelets in Inflammation. In The Non-Thrombotic Role of Platelets in Health and Disease; InTech: Vienna, Austria, 2015. [Google Scholar] [CrossRef]
- Xie, Q.; Xiang, Q.; Liu, Z.; Mu, G.; Zhou, S.; Zhang, Z.; Ma, L.; Gong, Y.; Jiang, J.; Cui, Y. Effect of CYP2C19 genetic polymorphism on the pharmacodynamics and clinical outcomes for patients treated with ticagrelor: A systematic review with qualitative and quantitative meta-analysis. BMC Cardiovasc. Disord. 2022, 22, 1–11. [Google Scholar] [CrossRef]
- Tantry, U.S.; Bliden, K.P.; Wei, C.; Storey, R.F.; Armstrong, M.; Butler, K.; Gurbel, P.A. First analysis of the relation between CYP2C19 genotype and pharmacodynamics in patients treated with ticagrelor versus clopidogrel: The ONSET/OFFSET and RESPOND genotype studies. Circ. Cardiovasc. Genet. 2010, 3, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Tatarunas, V.; Kupstyte, N.; Zaliunas, R.; Giedraitiene, A.; Lesauskaite, V. The impact of clinical and genetic factors on ticαelor and clopidogrel antiplatelet therapy. Pharmacogenomics 2017, 18, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Bonaca, M.P.; Bhatt, D.L.; Cohen, M.; Steg, P.G.; Storey, R.F.; Jensen, E.C.; Magnani, G.; Bansilal, S.; Fish, M.P.; Im, K.; et al. Long-Term Use of Ticagrelor in Patients with Prior Myocardial Infarction. N. Eng. J. Med. 2015, 372, 1791–1800. [Google Scholar] [CrossRef] [PubMed]
| Investigation | Timing |
|---|---|
| Complete blood count and blood biochemistry (e.g., potassium and creatinine concentration, lipid panel, troponin I, CRB, glycemia) | On hospitalization, during treatment based on the need, and after 3 months |
| Troponin I dynamics | 6 h after hospitalization |
| Coronary angiography and PTCA with stent implantation | Up to 60 min after hospitalization |
| Additional blood and urine testing following the study protocol | 24–36 h after hospitalization and 3 months |
| Dyspnea evaluation | 24–36 h from hospitalization and after 3 months |
| 2D echocardiography and BNP measurement | 36–48 h from hospitalization and after 3 months |
| Other instrumental investigations (e.g., ECG) | During treatment based on the need and after 3 months |
| Degree | Symptoms |
|---|---|
| 0 | No symptoms of dyspnea |
| 1 | Mild dyspnea |
| 2 | Moderate dyspnea |
| 3 | Severe dyspnea |
| 4 | Very severe dyspnea followed by forced sitting position and panic attack |
| Characteristic | Dyspnea | p | |
|---|---|---|---|
| No (n = 182) | Yes (n = 95) | ||
| Gender, n (%) | 0.696 | ||
| Male | 138 (75.8) | 70 (73.7) | |
| Female | 44 (24.2) | 25 (26.3) | |
| Age, years | 64 (42–88) | 66 (32–90) | 0.066 |
| BMI, kg/m2 | 29 (21–50) | 28 (21–45) | 0.859 |
| MI, n (%) | 0.720 | ||
| NSTEMI | 84 (46.2) | 46 (48.4) | |
| STEMI | 98 (53.8) | 49 (51.6) | |
| CAD, n (%) | |||
| One-vessel | 62 (31.1) | 27 (28.4) | 0.162 |
| Two-vessel | 57 (31.3) | 42 (44.2) | |
| Three-vessel | 63 (34.6) | 26 (27.4) | 0.034 |
| DM, n (%) | 23 (12.6) | 11 (11.6) | 0.799 |
| Smoking, n (%) | 50 (27.5) | 29 (30.5) | 0.068 |
| Hypothyroidism, n (%) | 2 (1.1) | 5 (5.3) | 0.049 |
| AF, n (%) | 24 (13.2) | 16 (16.8) | 0.411 |
| Troponin I, ng/mL | 495 (20–89977) | 496 (20–85725) | 0.697 |
| Troponin I after 6 h, ng/mL | 7816 (20–635352) | 4324 (20–147527) | 0.146 |
| BNP, pmol/L | 131 (11–1858) | 140 (6–2000) | 0.89 |
| BNP after 3 months, pmol/l | 70 (9–368) | 51 (5–271) | 0.708 |
| ADP HS, U | 25 (9–147) | 19 (2–133) | <0.001 |
| ADP HS after 3 months, U | 23 (9–58) | 20 (1–32) | 0.009 |
| Creatinine, µmol/L | 80 (42–480) | 85 (41–182) | 0.019 |
| CRP, mg/L | 8.25 (0.3–145) | 5.8 (0.3–152.5) | 0.615 |
| LDL-C, mmol/L | 3.5 (1.4–6.7) | 3.3 (1.4–7.1) | 0.348 |
| HGB, g/L | 144 (93–387) | 142 (85–345) | 0.726 |
| LVEF, % | 50 (20–55) | 45 (20–55) | 0.429 |
| Medications, n (%) | |||
| Beta blockers | 177 (97.8) | 92 (97.9) | 0.894 |
| ACE inhibitors | 177 (97.8) | 93 (98.9) | 0.974 |
| Statins (irrespective of dose), n (%) | |||
| Atorvastatin | 95 (52.5) | 40 (42.6) | 0.027 |
| Rosuvastatin | 86 (47.5) | 54 (57.4) | 0.255 |
| Anticoagulants | 21 (11.5) | 15 (15.8) | 0.318 |
| Genes | Dyspnea | χ2 | p | |
|---|---|---|---|---|
| No | Yes | |||
| CYP4F2 (rs2108622) | ||||
| CC | 83 (62.9) | 31 (54.4) | 1.307 | 0.520 |
| CT | 41 (31.1) | 21 (36.8) | ||
| TT | 8 (6.1) | 5 (8.8) | ||
| C alleleT allele | 207 (78.4)57 (21.6) | 83 (72.8)31 (27.2) | 1.399 | 0.236 |
| CYP4F2 (rs1558139) | ||||
| AA | 23 (17.4) | 14 (24.6) | 1.606 | 0.448 |
| AG | 76 (57.6) | 32 (56.1) | ||
| GG | 33 (25.0) | 11 (19.3) | ||
| A allele | 122 (46.2) | 60 (52.6) | 1.314 | 0.251 |
| G allele | 142 (53.8) | 54 (47.4) | ||
| CYP4F2 (rs3093135) | ||||
| AA | 18 (13.6) | 10 (17.5) | 1.867 | 0.393 |
| AT | 79 (59.8) | 28 (49.1) | ||
| TT | 35 (26.5) | 19 (33.3) | ||
| A allele | 115 (43.6) | 48 (42.1) | 0.068 | 0.793 |
| T allele | 149 (56.4) | 66 (57.9) | ||
| CYP4F2 (rs2074902) | ||||
| CC | 3 (2.3) | 3 (5.3) | 1.566 | 0.474 |
| CT | 36 (27.3) | 17 (29.8) | ||
| TT | 93 (70.5) | 37 (64.9) | ||
| C allele | 42 (15.9) | 23 (20.1) | 1.017 | 0.313 |
| T allele | 222 (84.1) | 91 (79.8) | ||
| CYP2C19 (rs4244285) (*2) | ||||
| AA | 1 (0.8) | 1 (1.8) | 0.777 | 0.851 |
| AG | 27 (20.5) | 11 (19.3) | ||
| GG | 104 (78.8) | 45 (78.9) | ||
| A allele | 29 (11.0) | 13 (11.4) | 0.014 | 0.905 |
| G allele | 235 (89.0) | 101 (88.6) | ||
| ABCB1 (rs1045642) | ||||
| CC | 37 (28.0) | 14 (24.6) | 0.252 | 0.882 |
| CT | 70 (53.0) | 32 (56.1) | ||
| TT | 25 (18.9) | 11 (19.3) | ||
| C allele | 144 (54.6) | 60 (52.6) | 0.117 | 0.731 |
| T allele | 120 (45.4) | 54 (47.4) | ||
| FBG-C148T (rs1800787) | ||||
| CC | 60 (45.5) 1 | 37 (64.9) 1 | 9.489 | 0.009 |
| CT | 59 (44.7) 2 | 12 (21.1) 2 | ||
| TT | 13 (9.8) | 8 (14.0) | ||
| C allele | 179 (67.8) | 86 (75.4) | 2.214 | 0.136 |
| T allele | 85 (32.2) | 28 (24.6) | ||
| COX-2 (rs689465) | ||||
| CC | 2 (1.5) | 0 (0.0) | 0.532 | 1.000 |
| CT | 22 (16.8) | 9 (15.8) | ||
| TT | 107 (81.7) | 48 (84.2) | ||
| C allele | 26 (9.9) | 9 (7.9) | 0.387 | 0.533 |
| T allele | 236 (90.1) | 105 (92.1) | ||
| PAI-1 (rs5918) | ||||
| CC | 5 (3.8) | 2 (3.5) | 0.619 | 0.840 |
| CT | 49 (37.1) | 18 (31.6) | ||
| TT | 78 (59.1) | 37 (64.9) | ||
| C allele | 59 (22.4) | 22 (19.3) | 0.44 | 0.507 |
| T allele | 205 (77.6) | 92 (80.7) | ||
| CYP2C19 (rs12248560) (*17) | ||||
| CC | 63 (47.7) | 36 (63.2) | 3.871 | 0.138 |
| CT | 61 (46.2) | 18 (31.6) | ||
| TT | 8 (6.1) | 3 (5.3) | ||
| C allele | 187 (70.8) | 90 (79.0) | 2.677 | 0.101 |
| T allele | 77 (29.2) | 24 (21.0) | ||
| Timing | ADP HS, U | p | |
|---|---|---|---|
| Without Dyspnea | With Dyspnea | ||
| Patients with diabetes mellitus (n = 34) | |||
| On enrolment | 25 (16–71) | 15 (10–41) | 0.002 |
| After 3 months | 23 (12–51) | 22 (20–32) | 0.836 |
| Patients without diabetes mellitus (n = 243) | |||
| On enrolment | 25 (9–147) | 19 (2–133) | <0.001 |
| After 3 months | 23 (9–58) | 20 (1–79) | 0.004 |
| One-vessel CAD (n = 89) | |||
| On enrolment | 25 (12–71) | 22 (12–47) | 0.048 |
| After 3 months | 21 (9–43) | 19 (12–28) | 0.018 |
| Two- and three-vessel CAD (n = 188) | |||
| On enrolment | 25 (9–147) | 18 (2–133) | <0.001 |
| After 3 months | 24 (11–58) | 21 (1–79) | 0.022 |
| Variable | OR (95% CI) | p |
|---|---|---|
| ADP HS > 19.5 | 1 | |
| ADP HS ≤ 19.5 | 4.07 (2.37–6.99) | ≤0.001 |
| Maximal atorvastatin dose (80 mg) | 1 | |
| Any atorvastatin dose (or no atorvastatin) | 2.18 (1.18–4.01) | 0.012 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamakauskas, V.; Žaliūnas, R.; Lesauskaitė, V.; Kupstytė-Krištaponė, N.; Šakalytė, G.; Jurgaitytė, J.; Čiapienė, I.; Tatarūnas, V. Factors Determining Ticagrelor-Induced Dyspnea in Patients with Acute Coronary Syndrome. Appl. Sci. 2022, 12, 10021. https://doi.org/10.3390/app121910021
Tamakauskas V, Žaliūnas R, Lesauskaitė V, Kupstytė-Krištaponė N, Šakalytė G, Jurgaitytė J, Čiapienė I, Tatarūnas V. Factors Determining Ticagrelor-Induced Dyspnea in Patients with Acute Coronary Syndrome. Applied Sciences. 2022; 12(19):10021. https://doi.org/10.3390/app121910021
Chicago/Turabian StyleTamakauskas, Vytenis, Remigijus Žaliūnas, Vaiva Lesauskaitė, Nora Kupstytė-Krištaponė, Gintarė Šakalytė, Julija Jurgaitytė, Ieva Čiapienė, and Vacis Tatarūnas. 2022. "Factors Determining Ticagrelor-Induced Dyspnea in Patients with Acute Coronary Syndrome" Applied Sciences 12, no. 19: 10021. https://doi.org/10.3390/app121910021
APA StyleTamakauskas, V., Žaliūnas, R., Lesauskaitė, V., Kupstytė-Krištaponė, N., Šakalytė, G., Jurgaitytė, J., Čiapienė, I., & Tatarūnas, V. (2022). Factors Determining Ticagrelor-Induced Dyspnea in Patients with Acute Coronary Syndrome. Applied Sciences, 12(19), 10021. https://doi.org/10.3390/app121910021





