Digestate Management and Processing Practices: A Review
Abstract
:1. Introduction
2. Regulations and Standards for Manure Management in the EU
3. Anaerobic Digestion
4. Digestate
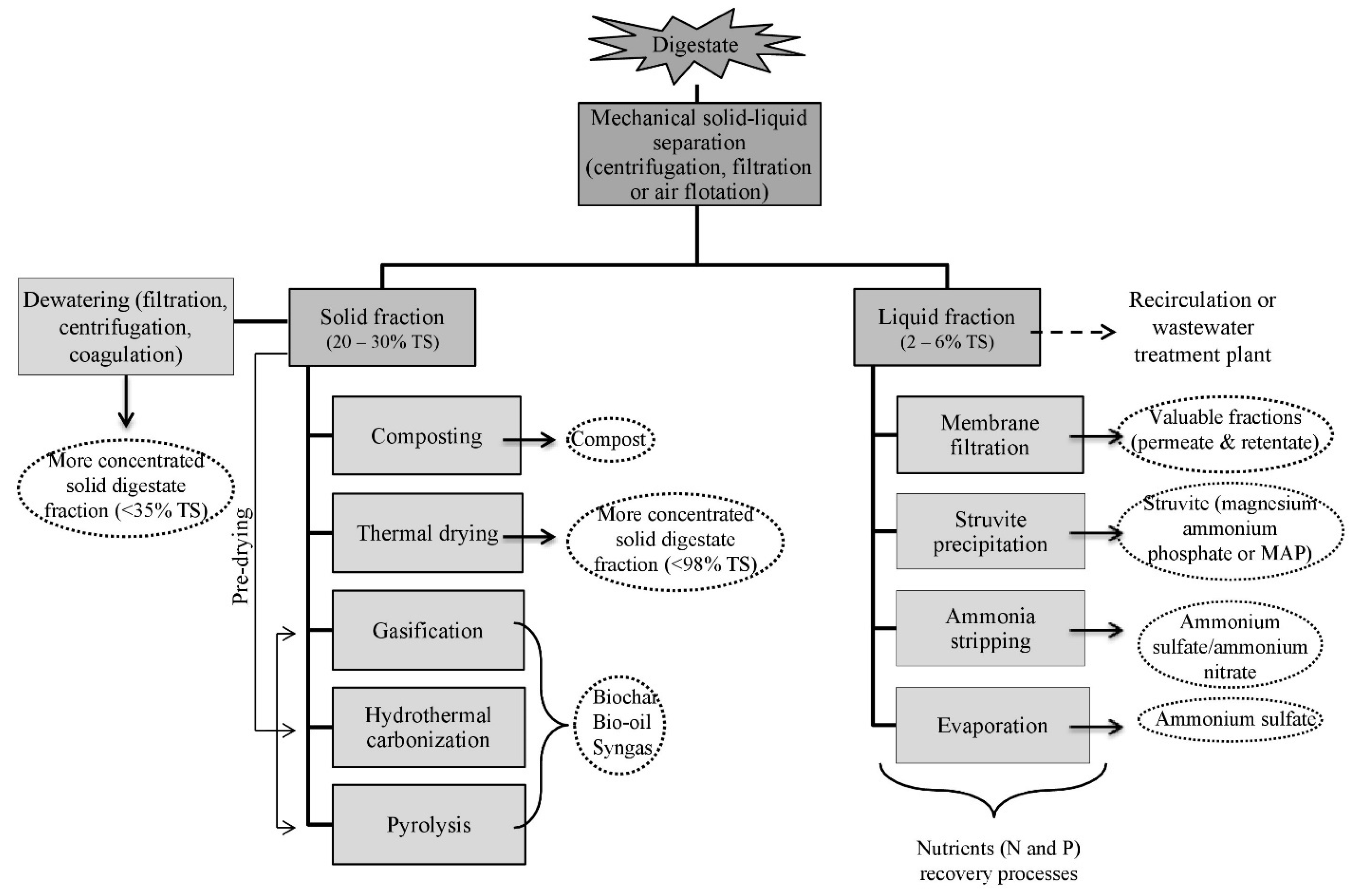
5. Digestate Processing
5.1. Solid-Liquid Separation
5.2. Treatments of the Solid Fraction
5.2.1. Composting
5.2.2. Thermal Drying
5.2.3. Thermochemical Treatment
5.3. Treatments of the Liquid Fraction
5.3.1. Membrane Technology
5.3.2. Struvite Precipitation
5.3.3. Ammonia Stripping
5.3.4. Vacuum Evaporation
6. Regulations and Standards for Digestate Management
7. Fertilization Value of the Digestate
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| ACI | antioxidant capacity index |
| AD | anaerobic digestion |
| AIR | agro-industrial residues |
| APW | aloe peel waste |
| ASP | almond shell powder |
| AW | agricultural wastes |
| BA | bulking agent |
| BMP | biochemical methane potential |
| BOD | biological oxygen demands |
| BS | biogas slurry |
| C | carbon |
| CBS | concentrated biogas slurry |
| CH4 | methane |
| CM | cattle manure |
| CMm | conventional management |
| COM | cow manure |
| CO2 | carbon dioxide |
| COD | chemical oxygen demand |
| CODt | total chemical oxygen demand |
| CT | blank control |
| DG | digestate |
| DM | dairy manure |
| DOC | dissolved organic carbon |
| DSC | differential scanning calorimetry |
| DWC | dry wood chips |
| EC | electric conductivity |
| ECs | energy crops |
| EGM | exhausted grape marc |
| EPS | extracellular polymeric substance |
| FVW | fruit and vegetable waste |
| GI | germination index |
| GHG | greenhouse gasses |
| GW | green wastes |
| H | hydrogen |
| HHV | higher heating value |
| HM | heavy metal |
| HRT | hydraulic retention time |
| HTC | hydrothermal carbonization |
| K | potassium |
| LBA | lignocellulosic bulking agent |
| MAR | macroalgal residue |
| MS | mudstones |
| MSW | municipal solid wastes |
| N | nitrogen |
| NH3 | ammonium |
| OC | organic carbon |
| OFMSW | organic fraction of municipal solid waste |
| OLR | organic loading rate |
| OM | organic matter |
| OM0 | organic matter of digestate |
| OS | oyster shells |
| P | phosphorus |
| PAO | potential ammonia oxidation rate |
| PDRI | potential dynamic respiration index |
| PL | poultry litter |
| PM | poultry manure |
| PPP | pepper plant pruning |
| PS | pig slurry |
| S | sulfur |
| SD | sewage sludge digestate |
| SGI | seedling growth index |
| SS | sewage sludge |
| SW | solid wastes |
| T | temperature |
| TC | total carbon |
| TG | thermogravimetry |
| TK | total potassium |
| TKN | total Kjeldahl nitrogen |
| TN | total nitrogen |
| TOC | total organic carbon |
| TP | total phosphorus |
| TPt | total proteins |
| TS | total solids |
| TWAS | thickened waste activated sludge |
| VFA | volatile fatty acids |
| VS | volatile solids |
| VSP | vine shoot pruning |
| WS | wheat straw |
| WSC | water-soluble carbon |
| WSD | wood sawdust |
| WTP | wastewater treatment plant |
References
- Kamilaris, A.; Engelbrecht, A.; Pitsillides, A.; Prenafeta-Boldú, F.X. Transfer of manure as fertilizer from livestock farms to crop fields: The case of Catalonia. Comput. Electron. Agric. 2020, 175, 105550. [Google Scholar] [CrossRef]
- UN. Available online: https://www.un.org/development/desa/en/news/population/world-population-prospects-2019.html (accessed on 26 April 2022).
- Nkoa, R. Agricultural benefits and environmental risks of soil fertilization with anaerobic digestates: A review. Agron. Sustain. Dev. 2014, 34, 473–492. [Google Scholar] [CrossRef]
- Galic, M.; Mesic, M.; Zgorelec, Z. Influence of Organic and Mineral Fertilization on Soil Greenhouse Gas Emissions. A Review. Agric. Conspec. Sci. 2019, 85, 1–8. [Google Scholar]
- Rabès, A.; Seconda, L.; Langevin, B.; Allès, B.; Touvier, M.; Hercberg, S.; Lairon, D.; Baudry, J.; Pointereau, P.; Kesse-Guyot, E. Greenhouse gas emissions, energy demand and land use associated with omnivorous, pesco-vegetarian, vegetarian, and vegan diets accounting for farming practices. Sustain. Prod. Consum. 2020, 22, 138–146. [Google Scholar] [CrossRef]
- Adegbeye, M.J.; Reddy, P.R.K.; Obaisi, A.I.; Elghandour, M.M.M.Y.; Oyebamiji, K.J.; Salem, A.Z.M.; Morakinyo-Fasipe, O.T.; Cipriano-Salazar, M.; Camacho-Díaz, L.M. Sustainable agriculture options for production, greenhouse gasses, and pollution alleviation, and nutrient recycling in emerging and transitional nations—An overview. J. Clean. Prod. 2020, 242, 118319. [Google Scholar] [CrossRef]
- Wongsaroj, L.; Chanabun, R.; Tunsakul, N.; Prombutara, P.; Panha, S.; Somboonna, N. First reported quantitative microbiota in different livestock manures used as organic fertilizers in the Northeast of Thailand. Sci. Rep. 2021, 11, 102. [Google Scholar] [CrossRef]
- Ramirez, J.; McCabe, B.; Jensen, P.D.; Speight, R.; Harrison, M.; Van den Berg, L.; O’Hara, I. Wastes to profit: A circular economy approach to value-addition in livestock industries. Anim. Prod. Sci. 2021, 61, 541–550. [Google Scholar] [CrossRef]
- Panuccio, M.R.; Romeo, F.; Mallamaci, C.; Muscolo, A. Digestate Application on Two Different Soils: Agricultural Benefit and Risk. Waste Biomass Valor. 2021, 12, 4341–4353. [Google Scholar] [CrossRef]
- Vitti, A.; Elshafie, H.S.; Logozzo, G.; Marzario, S.; Scopa, A.; Camele, I.; Nuzzaci, M. Physico-Chemical Characterization and Biological Activities of a Digestate and a More Stabilized Digestate-Derived Compost from Agro-Waste. Plants 2021, 10, 386. [Google Scholar] [CrossRef]
- Courtney, R.G.; Mullen, G.J. Soil quality and barley growth as influenced by the land application of two compost types. Bioresour. Technol. 2008, 99, 2913–2918. [Google Scholar] [CrossRef]
- Gondek, M.; Weindorf, D.C.; Thiel, C.; Kleinheinz, G. Soluble Salts in Compost and Their Effects on Soil and Plants: A Review. Compos. Sci. Util. 2020, 28, 59–75. [Google Scholar] [CrossRef]
- Soobhany, N.; Mohee, R.; Garg, V.K. Inactivation of bacterial pathogenic load in compost against vermicompost of organic solid waste aiming to achieve sanitation goals: A review. Waste Manag. 2017, 64, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, Y.; Yang, X.; Li, C.; Wang, L.; Feng, J.; Chen, S.; Li, X.; Yang, Y. Effects of integrated biocontrol on bacterial wilt and rhizosphere bacterial community of tobacco. Sci. Rep. 2021, 11, 2653. [Google Scholar] [CrossRef]
- Longhurst, P.J.; Tompkins, D.; Pollard, S.J.T.; Hough, R.L.; Chambers, B.; Gale, P.; Tyrrel, S.; Villa, R.; Taylor, M.; Wu, S.; et al. Risk assessments for quality-assured, source-segregated composts and anaerobic digestates for a circular bioeconomy in the UK. Environ. Int. 2019, 127, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Eip-agri-fg, Nutrient Recycling. Available online: https://ec.europa.eu/eip/agriculture/sites/agri-eip/files/eip-agri_fg_nutrients_recycling_final_report_2017_en.pdf (accessed on 26 April 2022).
- Vaneeckhaute, C.; Darveau, O.; Meers, E. Fate of micronutrients and heavy metals in digestate processing using vibrating reversed osmosis as resource recovery technology. Sep. Purif. Technol. 2019, 223, 81–87. [Google Scholar] [CrossRef]
- Köninger, J.; Lugato, E.; Panagos, P.; Kochupillai, M.; Orgiazzi, A.; Briones, M.J.I. Manure management and soil biodiversity: Towards more sustainable food systems in the EU. Agric. Syst. 2021, 194, 103251. [Google Scholar] [CrossRef]
- Loyon, L. Overview of Animal Manure Management for Beef, Pig, and Poultry Farms in France. Front. Sustain. Food Syst. 2018, 2, 36. [Google Scholar] [CrossRef]
- Cervelli, E.; di Perta, E.S.; Mautone, A.; Pindozzi, S. The landscape approach as support to the livestock manure management. The buffalo herds case-study in Sele plain, Campania region. In Proceedings of the IEEE International Workshop on Metrology for Agriculture and Forestry (MetroAgriFor), Trento-Bolzano, Italy, 3–5 November 2021; pp. 151–156. [Google Scholar] [CrossRef]
- Mussachio, A.; Re, V.; Mas-Pla, J.; Sacchi, E. EU Nitrates Directive, from theory to practice: Environmental effectiveness and influence of regional governance on its performance. Ambio 2020, 49, 504–516. [Google Scholar] [CrossRef]
- Serebrennikov, D.; Thorne, F.; Kallas, Z.; McCarthy, S.N. Factors influencing adoption of sustainable farming practices in Europe: A systemic review of empirical literature. Sustainability 2020, 12, 9719. [Google Scholar] [CrossRef]
- EC EUROPA EU Eurostat Statistics Explained. Available online: https://ec.europa.eu/eurostat/statisticsexplained/index.php?title=Glossary:Integrated_pollution_prevention_and_control_(IPPC) (accessed on 25 April 2022).
- Ofosu, B.O.; Addeh, G.Y.A.; Kodua, L.T.; Korankye, E. Environmental Issues of Livestock Production in Developing Countries: Need for Government Intervention Using the Truck Based Approach. J. Biol. Agric. Healthc. 2020, 10, 32–48. [Google Scholar]
- Dumont, É. Impact of the treatment of NH3 emissions from pig farms on greenhouse gas emissions. Quantitative assessment from the literature data. N. Biotechnol. 2018, 46, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Wiering, M.; Boezeman, D.; Crabbé, A. The Water Framework Directive and Agricultural Diffuse Pollution: Fighting a Running Battle? Water 2020, 12, 1447. [Google Scholar] [CrossRef]
- Náthia-Neves, G.; Berni, M.; Dragone, G.; Mussatto, S.I.; Forster-Carneiro, T. Anaerobic digestion process: Technological aspects and recent developments. Int. J. Environ. Sci. Technol. 2018, 15, 2033–2046. [Google Scholar] [CrossRef]
- Khoshnevisan, B.; Duan, N.; Tsapekos, P.; Awasthi, M.K.; Liu, Z.; Mohammadi, A.; Angelidaki, I.; Tsang, D.C.W.; Zhang, Z.; Pan, J.; et al. A critical review on livestock manure biorefinery technologies: Sustainability, challanges, and future perspectives. Renew. Sust. Energ. Rev. 2021, 135, 110033. [Google Scholar] [CrossRef]
- Löw, P.; Karatay, Y.N.; Osterburg, B. Erratum: Nitrogen use efficiency on dairy farms with different grazing sys-tems in northwestern Germany (2020 Environ. Res. Commun. 2 105002). Environ. Res. Commun. 2020, 2, 119601. [Google Scholar] [CrossRef]
- Kuhn, T.; Kokemohr, L.; Holm-Müller, K. A life cycle assessment of liquid pig manure transport in line with EU regulations: A case study from Germany. J. Environ. Manag. 2018, 217, 456–467. [Google Scholar] [CrossRef]
- Haupt, R.; Heinemann, C.; Schmid, S.M.; Steinhoff-Wagner, J. Survey on storage, application and incorporation practices for organic fertilizers in Germany. J. Environ. Manag. 2021, 296, 113380. [Google Scholar] [CrossRef]
- Van Grinsven, H.; Bleeker, A. Evaluation of the Manure and Fertilisers Act 2016: Synthesis Report; PBL Netherlands Environmental Assessment Agency: The Hague, The Netherlands, 2017. [Google Scholar]
- Nilsson, A.K. Regulating Zero Eutrophication. In Swedish Law on Controlling Emissions of Nutirients to the Baltic Sea; Stockholm University: Stockholm, Sweden, 2013. [Google Scholar]
- Kiran, E.U.; Stamatelatou, K.; Antonopoulou, G.; Lyberatos, G. Production of biogas via anaerobic digestion. In Handbook of Biofuels Production; Luque, R., Lin, C.S.K., Wilson, K., Clark, J., Eds.; Woodhead Publishing: Sawston, UK, 2016; p. 259. [Google Scholar] [CrossRef]
- Pasalari, H.; Gholami, M.; Rezaee, A.; Esrafili, A.; Farzadkia, M. Perspectives on microbial community in anaerobic digestion with emphasis on environmental parameters: A systematic review. Chemosphere 2020, 270, 128618. [Google Scholar] [CrossRef]
- Kovačić, Đ.; Kralik, D.; Jovičić, D.; Rupčić, S.; Popović, B.; Tišma, M. Thermal Pretreatment of Harvest Residues and Their Use in Anaerobic Co-digestion with Dairy Cow Manure. Appl. Biochem. Biotech. 2018, 184, 471–483. [Google Scholar] [CrossRef]
- Kovačić, Đ.; Kralik, D.; Jovičić, D.; Spajić, R. An assessment of anaerobic thermophilic co-digestion of dairy cattle manure and separated tomato greenhouse waste in lab-scale reactors. Acta Technol. Agric. 2019, 22, 41–45. [Google Scholar] [CrossRef] [Green Version]
- Kovačić, Đ.; Kralik, D.; Rupčić, S.; Jovičić, D.; Spajić, R.; Tišma, M. Electroporation of harvest residues for enhanced biogas production in anaerobic co- digestion with dairy cow manure. Bioresour. Technol. 2019, 274, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Deže, D.; Mihaljević, M.; Kovačić, Đ.; Jovičić, D.; Kralik, D. Natural Communities of Microalgae and Cyanobacteria from Eutrophicated Waters as Potential Co-substrates for Small-scale Biogas Production. Appl. Biochem. Biotechnol. 2020, 192, 1016–1028. [Google Scholar] [CrossRef] [PubMed]
- Uzinger, N.; Szécsy, O.; Szűcs-Vásárhelyi, N.; Padra, I.; Sándor, D.B.; Lončarić, Z.; Draskovits, E.; Rékási, M. Short-Term Decomposition and Nutrient-Supplying Ability of Sewage Sludge Digestate, Digestate Compost, and Vermicompost on Acidic Sandy and Calcareous Loamy Soils. Agronomy 2021, 11, 2249. [Google Scholar] [CrossRef]
- Tišma, M.; Planinić, M.; Bucić-Kojić, A.; Panjičko, M.; Zupančič, G.D.; Zelić, B. Corn silage fungal-based solid-state pretreatment for enhanced biogas production in anaerobic co-digestion with cow manure. Bioresour. Technol. 2018, 253, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Panjičko, M.; Zupančić, G.D.; Fanedl, L.; Marinšek Logar, R.; Tišma, M.; Zelić, B. Biogas production from brewery spent grain as a mono-substrate in a two-stage process composed of solid-state anaerobic digestion and granular biomass reactors. J. Clean. Prod. 2017, 166, 519–529. [Google Scholar] [CrossRef]
- Kovačić, Đ.; Kralik, D.; Rupčić, S.; Jovičić, D.; Spajić, R.; Tišma, M. Soybean Straw, Corn Stover and Sunflower Stalk as Possible Substrates for Biogas Production in Croatia: A Review. Chem. Biochem. Eng. Q. 2017, 31, 187–198. [Google Scholar] [CrossRef]
- Issah, A.-A.; Kabera, T.; Kemausuor, F. Biogas optimisation processes and effluent quality: A review. Biomass Bioenerg. 2020, 133, 105449. [Google Scholar] [CrossRef]
- Ryue, J.; Lin, L.; Kakar, F.L.; Elbeshbishy, E.; Al-Mamun, A. A critical review of conventional and emerging methods for improving process stability in thermophilic anaerobic digestion. Energy Sustain. Dev. 2020, 54, 72–84. [Google Scholar] [CrossRef]
- Schnürer, A. Biogas Production: Microbiology and Technology. In Anaerobes in Biotechnology; Hatti-Kaul, R., Mamo, G., Mattiasson, B., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 195–234. [Google Scholar] [CrossRef]
- Stronach, S.M.; Rudd, T.; Lester, J.N. Anaerobic Digestion Processes in Industrial Wastewater Treatment (Biotechnology Monographs); Springer: Berlin/Heidelberg, Germany, 1986; p. 26. [Google Scholar] [CrossRef]
- Koszel, M.; Lorencowicz, E. Agricultural use of biogas digestate as a replacement fertilizers. Agric. Agric. Sci. Procedia 2015, 7, 119–124. [Google Scholar] [CrossRef]
- Peng, W.; Lü, F.; Hao, L.; Zhang, H.; Shao, L.; He, P. Digestate management for high-solid anaerobic digestion of organic wastes: A review. Bioresour. Technol. 2019, 297, 122485. [Google Scholar] [CrossRef]
- Tambone, F.; Scaglia, B.; D’Imporzano, G.; Schievano, A.; Orzi, V.; Salati, S.; Adani, F. Assessing amendment and fertilizing properties of digestates from anaerobic digestion through a comparative study with digested sludge and compost. Chemosphere 2010, 81, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Luo, W.; Lu, J.; Zhang, X.; Li, S.; Wu, Y.; Li, G. Effects of digestion time in anaerobic digestion on subsequent digestate composting. Bioresour. Technol. 2018, 267, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Muscolo, A.; Settineri, G.; Papalia, T.; Attinà, E.; Basile, C.; Panuccio, M.R. Anaerobic co-digestion of recalcitrant agricultural wastes: Characterizing of biochemical parameters of digestate and its impacts on soil ecosystem. Sci. Total Environ. 2017, 586, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Bres, P.; Beily, M.E.; Young, B.J.; Gasulla, J.; Butti, M.; Crespo, D.; Candal, R.; Komilis, D. Performance of semi-continuous anaerobic co-digestion of poultry manure with fruit and vegetable waste and analysis of digestate quality: A bench scale study. Waste Manag. 2018, 82, 276–284. [Google Scholar] [CrossRef]
- Elalami, D.; Monlau, F.; Carrere, H.; Abdelouahdi, K.; Charbonnel, C.; Oukarroum, A.; Zeroual, Y.; Barakat, A. Evaluation of agronomic properties of digestate from macroalgal residues anaerobic digestion: Impact of pretreatment and co-digestion with waste activated sludge. Waste Manag. 2020, 108, 127–136. [Google Scholar] [CrossRef]
- Bonetta, S.; Bonetta, S.; Ferretti, E.; Fezia, G.; Gilli, G.; Carraro, E. Agricultural Reuse of the Digestate from Anaerobic Co-Digestion of Organic Waste: Microbiological Contamination, Metal Hazards and Fertilizing Performance. Water Air Soil Pollut. 2014, 225, 2046. [Google Scholar] [CrossRef]
- Demirel, B.; Göl, N.P.; Onay, T.T. Evaluation of heavy metal content in digestate from batch anaerobic co-digestion of sunflower hulls and poultry manure. J. Mater. Cycles Waste Manag. 2013, 15, 242–246. [Google Scholar] [CrossRef]
- Govasmark, E.; Stäb, J.; Holen, B.; Hoornstra, D.; Nesbakk, T.; Salkinoja-Salonen, M. Chemical and microbiological hazards associated with recycling of anaerobic digested residue intended for agricultural use. Waste Manag. 2011, 31, 2577–2583. [Google Scholar] [CrossRef]
- Iocoli, G.A.; Zabaloy, M.C.; Pasdevicelli, G.; Gómez, M.A. Use of biogas digestates obtained by anaerobic digestion and co-digestion as fertilizers: Characterization, soil biological activity and growth dynamic of Lactuca sativa L. Sci. Total Environ. 2019, 647, 11–19. [Google Scholar] [CrossRef]
- Risberg, K.; Cederlund, H.; Pell, M.; Arthurson, V.; Schnürer, A. Comparative characterization of digestate versus pig slurry and cow manure—Chemical composition and effects on soil microbial activity. Waste Manag. 2017, 61, 529–538. [Google Scholar] [CrossRef]
- Huang, X.; Yun, S.; Zhu, J.; Du, T.; Zhang, C.; Li, X. Mesophilic anaerobic co-digestion of aloe peel waste with dairy manure in the batch digester: Focusing on mixing ratios and digestate stability. Bioresour. Technol. 2016, 218, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Beggio, G.; Schievano, A.; Bonato, T.; Hennebert, P.; Pivato, A. Statistical analysis for the quality assessment of digestates from separately collected organic fraction of municipal solid waste (OFMSW) and agro-industrial feedstock. Should input feedstock to anaerobic digestion determine the legal status of digestate? Waste Manag. 2019, 87, 546–558. [Google Scholar] [CrossRef] [PubMed]
- Alburquerque, J.A.; de la Fuente, C.; Campoy, M.; Carrasco, L.; Nájera, I.; Baixauli, C.; Caravaca, F.; Roldán, A.; Cegarra, J.; Bernal, M.P. Agricultural use of digestate for horticultural crop production and improvement of soil properties. Eur. J. Agron. 2012, 43, 119–128. [Google Scholar] [CrossRef]
- Alburquerque, J.A.; de la Fuente, C.; Ferrer-Costa, A.; Carrasco, L.; Cegarra, J.; Abad, M.; Bernal, M.P. Assessment of the fertiliser potential of digestates from farm and agroindustrial residues. Biomass Bioenerg. 2012, 40, 181–189. [Google Scholar] [CrossRef]
- Walsh, J.J.; Jones, D.L.; Edwards-Jones, G.; Williams, A.P. Replacing inorganic fertilizer with anaerobic digestate may maintain agricultural productivity at less environmental cost. J. Plant Nutr. Soil Sci. 2012, 175, 840–845. [Google Scholar] [CrossRef]
- Głowacka, A.; Szostak, B.; Klebaniuk, R. Effect of Biogas Digestate and Mineral Fertilisation on the Soil Properties and Yield and Nutritional Value of Switchgrass Forage. Agronomy 2020, 10, 490. [Google Scholar] [CrossRef]
- Makádi, M.; Tomócsik, A.; Orosz, V. Digestate: A New Nutrient Source—Review. In Biogas; Kumar, S., Ed.; InTech: London, UK, 2012. [Google Scholar] [CrossRef]
- Tambone, F.; Genevini, P.; Adani, F. The Effects of Short-Term Compost Application On Soil Chemical Properties and on Nutritional Status of Maize Plant. Compos. Sci. Util. 2007, 15, 176–183. [Google Scholar] [CrossRef]
- Möller, K.; Müller, T. Effects of anaerobic digestion on digestate nutrient availability and crop growth: A review. Eng. Life Sci. 2012, 12, 242–257. [Google Scholar] [CrossRef]
- Goberna, M.; Podmirseg, S.M.; Waldhuber, S.; Knapp, B.A.; García, C.; Insam, H. Pathogenic bacteria and mineral N in soils following the land spreading of biogas digestates and fresh manure. Appl. Soil Ecol. 2011, 49, 18–25. [Google Scholar] [CrossRef]
- Al Seadi, T.; Drosg, B.; Fuchs, W.; Rutz, D.; Janssen, R. Biogas digestate quality and utilization. In The Biogas Handbook—Science, Production and Applications; Wellinger, A., Murphy, J., Baxter, D., Eds.; Woodhead Publishing: Sawston, UK, 2013; pp. 269–274. [Google Scholar] [CrossRef]
- Hutchison, M.L.; Walters, L.D.; Avery, S.M.; Synge, B.A.; Moore, A. Levels of zoonotic agents in British livestock manures. Lett. Appl. Microbiol. 2004, 39, 207–214. [Google Scholar] [CrossRef]
- Smith, S.R.; Lang, N.L.; Cheung, K.H.M.; Spanoudaki, K. Factors controlling pathogen destruction during anaerobic digestion of biowastes. Waste Manag. 2005, 25, 417–425. [Google Scholar] [CrossRef]
- Erickson, M.C.; Ortega, Y.R. Inactivation of protozoan parasites in food, water, and environmental systems. J. Food Prot. 2006, 69, 2786–2808. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lendormi, T.; Lanoisellé, J.-L. Conventional and Innovative Hygienization of Feedstock for Biogas Production: Resistance of Indicator Bacteria to Thermal Pasteurization Pulsed Electric Field Treatment, and Anaerobic Digestion. Energies 2021, 14, 1938. [Google Scholar] [CrossRef]
- Seruga, P.; Krzywonos, M.; Paluszak, Z.; Urbanowska, A.; Pawlak-Kruczek, H.; Niedźwiecki, Ł.; Pińkowska, H. Pathogen Reduction Potential in Anaerobic Digestion of Organic Fraction of Municipal Solid Waste and Food Waste. Molecules 2020, 25, 275. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lendormi, T.; Lanoisellé, J.-L. Overview of hygienization pretreatment for pasteurization and methane potential enhancement of biowaste: Challenges, state of the art and alternative technologies. J. Clean. Prod. 2019, 236, 117525. [Google Scholar] [CrossRef]
- Fröschle, B.; Heiermann, M.; Lebuhn, M.; Messelhäusser, U.; Plöchl, M. Hygiene and Sanitation in Biogas Plants. Adv. Biochem. Eng. Biotechnol. 2015, 151, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Golovko, O.; Ahrens, L.; Schelin, J.; Sörengård, M.; Bergstrand, K.-J.; Asp, H.; Hultberg, M.; Wiberg, K. Organic micropollutants, heavy metals and pathogens in anaerobic digestate based on food waste. J. Environ. Manag. 2022, 313, 114997. [Google Scholar] [CrossRef]
- Bagge, E. Hygiene Aspects of the Biogas Process with Emphasis on Spore-Forming Bacteria. Ph.D. Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2009. [Google Scholar]
- Le Maréchal, C.; Druilhe, C.; Repérant, E.; Boscher, E.; Rouxel, S.; Le Roux, S.; Poёzévara, T.; Ziebal, C.; Houdayer, C.; Nagard, B.; et al. Evaluation of the occurrence of sporulating and nonsporulating pathogenic bacteria in manure and in digestate of five agricultural biogas plants. Microbiol. Open 2019, 8, e872. [Google Scholar] [CrossRef]
- Pulvirenti, A.; Ronga, D.; Zaghi, M.; Tomasselli, A.R.; Mannella, L.; Pecchioni, N. Pelleting is a successful method to eliminate the presence of Clostridium spp. from the digestate of biogas plants. Biomass Bioenerg. 2015, 81, 479–482. [Google Scholar] [CrossRef]
- Kupper, T.; Bürge, D.; Bachmann, H.J.; Güsewell, S.; Mayer, J. Heavy metals in source-separated compost and digestates. Waste Manag. 2014, 34, 867–874. [Google Scholar] [CrossRef]
- Yekta, S.S.; Hedenström, M.; Svensson, B.H.; Sundgren, I.; Dario, M.; Enrich-Prast, A.; Hertkorn, N.; Björn, A. Molecular characterization of particulate organic matter in full scale anaerobic digesters: An NMR spectroscopy study. Sci. Total. Environ. 2019, 685, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Fierro, J.; Martinez, E.J.; Rosas, J.G.; Fernández, R.A.; López, R.; Gomez, X. Co-Digestion of Swine Manure and Crude Glycerine: Increasing Glycerine Ratio Results in Preferential Degradation of Labile Compounds. Water Air Soil Pollut. 2016, 227, 78. [Google Scholar] [CrossRef]
- Tambone, F.; Genevini, P.; D’Imporzano, G.; Adani, F. Assessing amendment properties of digestate by studying the organic matter composition and the degree of biological stability during the anaerobic digestion of the organic fraction of MSW. Bioresour. Technol. 2009, 100, 3140–3142. [Google Scholar] [CrossRef] [PubMed]
- Schievano, A.; Adani, F.; Tambone, F.; D’Imporzano, G.; Scaglia, B.; Genevini, P.L. What is the Digestate? In Anaerobic Digestion: Opportunities for Agriculture and Environment; Regione Lombardia: Milano, Italy, 2009. [Google Scholar]
- Gómez, X.; Blanco, D.; Lobato, A.; Calleja, A.; Martínez-Núñez Martin-Villacorta, J. Digestion of cattle manure under mesophilic conditions: Characterization of organic matter applying thermal analysis and 1H NMR. Biodegradation 2011, 22, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Plana, P.V.; Noche, B. A review of the current digestate distribution models: Storage and transport. In WIT Transactions on Ecology and the Environment; Brebbia, C.A., Itoh, H., Eds.; WIT Press: Southampton, UK, 2016; p. 346. [Google Scholar] [CrossRef]
- Di Maria, F.; Sisani, F.; El-Hoz, M.; Mersky, R.L. How collection efficiency and legal constraints on digestate management can affect the effectiveness of anaerobic digestion of bio-waste: An analysis of the Italian context in a life cycle perspective. Sci. Total Environ. 2020, 726, 138555. [Google Scholar] [CrossRef]
- Fuchs, W.; Drosg, B. Assessment of the state of the art of technologies for the processing of digestate residue from anaerobic digesters. Water Sci. Technol. 2013, 67, 1984–1993. [Google Scholar] [CrossRef]
- Monfet, E.; Aubry, G.; Ramirez, A.A. Nutrient removal and recovery from digestate: A review of the technology. Biofuels 2018, 9, 247–262. [Google Scholar] [CrossRef]
- AgriKomp. Available online: https://agrikomp.com/plants/agrifer-plus/ (accessed on 26 April 2022).
- Tambone, F.; Orzi, V.; D’Imporzano, G.; Adani, F. Solid and liquid fractionation of digestate: Mass balance, chemical characterization, and agronomic and environmental value. Bioresour. Technol. 2017, 243, 1251–1256. [Google Scholar] [CrossRef]
- Zeng, Y.; De Guardia, A.; Dabert, P. Improving composting as a post-treatment of anaerobic digestate. Bioresour. Technol. 2016, 201, 293–303. [Google Scholar] [CrossRef]
- Wang, X.; Wang, W.; Zhou, B.; Xu, M.; Wu, Z.; Liang, J.; Zhou, L. Improving solid-liquid separation performance of anaerobic digestate from food waste by thermally activated persulfate oxidation. J. Hazard. Mater. 2020, 398, 122989. [Google Scholar] [CrossRef]
- Herbes, C.; Roth, U.; Wulf, S.; Dahlin, J. Economic assessment of different biogas digestate processing technologies: A scenario-based analysis. J. Clean. Prod. 2020, 255, 120282. [Google Scholar] [CrossRef]
- Vondra, M.; Máša, V.; Bobák, P. The Energy Performance of Vacuum Evaporators for Liquid Digestate Treatment in Biogas Plants. Energy 2017, 146, 141–155. [Google Scholar] [CrossRef]
- Logan, M.; Visvanathan, C. Management strategies for anaerobic digestate of organic fraction of municipal solid waste: Current status and future prospects. Waste Manag. Res. 2019, 37, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Tuomela, M.; Vikman, M.; Hatakka, A.; Itävaara, M. Biodegradation of lignin in a compost environment: A review. Bioresour. Technol. 2000, 72, 169–183. [Google Scholar] [CrossRef]
- Jędrczak, A. Composting and fermentation of biowaste—Advantages and disadvantages of processes. Civ. Environ. Eng. Rep. 2018, 28, 71–87. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.; Xu, F.; Ge, X.; Li, Y. Biological treatment of organic materials for energy and nutrients production—Anaerobic digestion and composting. Adv. Bioenergy 2019, 4, 121–181. [Google Scholar] [CrossRef]
- Li, Y.; Han, Y.; Zhang, Y.; Fang, Y.; Li, S.; Li, G.; Luo, W. Factors affecting gaseous emissions, maturity, and energy efficiency in composting of livestock manure digestate. Sci. Total. Environ. 2020, 731, 139157. [Google Scholar] [CrossRef]
- Torres-Climent, A.; Martin-Mata, J.; Marhuenda-Egea, F.; Moral, R.; Barber, X.; Perez-Murcia, M.D.; Paredes, C. Composting of the solid phase of digestate from biogas production: Optimization of the moisture, C/N ratio, and pH conditions. Commun. Soil Sci. Plant Anal. 2015, 46, 197–207. [Google Scholar] [CrossRef]
- Lu, M.-Y.; Shi, X.-S.; Li, X.; Lian, S.-J.; Xu, D.-Y.; Guo, R.-B. Addition of oyster shell to enhance organic matter degradation and nitrogen conservation during anaerobic digestate composting. Environ. Sci. Pollut. Res. 2020, 27, 33732–33742. [Google Scholar] [CrossRef]
- Meng, X.; Yan, J.; Zuo, B.; Wang, Y.; Yuan, X.; Cui, Z. Full-scale of composting process of biogas residues from corn stover anaerobic digestion: Physical-chemical, biology parameters and maturity indexes during whole process. Bioresour. Technol. 2020, 302, 122742. [Google Scholar] [CrossRef]
- Czekała, W.; Dach, J.; Dong, R.; Janczak, D.; Malińska, K.; Jóźwiakowski, K.; Smurzyńska, A.; Cieślik, M. Composting potential of the solid fraction of digested pulp produced by a biogas plant. Biosyst. Eng. 2017, 160, 25–29. [Google Scholar] [CrossRef]
- Knoop, C.; Dornack, C.; Raab, T. Effect of drying, composting and subsequent impurity removal by sieving on the properties of digestates from municipal organic waste. Waste Manag. 2017, 72, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Tambone, F.; Terruzzi, L.; Scaglia, B.; Adani, F. Composting of the solid fraction of digestate derived from pig slurry: Biological processes and compost properties. Waste Manag. 2015, 35, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, M.A.; Restrepo, A.P.; Alburquerque, J.A.; Pérez-Murcia, M.D.; Paredes, C.; Moral, R.; Bernal, M.P. Recycling of anaerobic digestates by composting: Effect of the bulking agent used. J. Clean. Prod. 2013, 47, 61–69. [Google Scholar] [CrossRef]
- Ince, O.; Ozbayram, E.G.; Akyol, Ç.; Erdem, E.I.; Gunel, G.; Ince, B. Bacterial Succession in the Thermophilic Phase of Composting of Anaerobic Digestates. Waste Biomass Valori. 2020, 11, 841–849. [Google Scholar] [CrossRef]
- Rincón, C.A.; De Guardia, A.; Couvert, A.; Le Roux, S.; Soutrel, I.; Daumoin, M.; Benoist, J.C. Chemical and odor characterization of gas emissions released during composting of solid wastes and digestates. J. Environ. Manag. 2019, 233, 39–53. [Google Scholar] [CrossRef]
- Digestate and Compost Use in Agriculture; WRAP: Banbury, UK, 2016.
- Eunomia. Economic Analysis of Options for Managing Biodegradable Municipal Waste; Final Report of Eunomia Research and Consulting to the European Commission; Eunomia Research & Consulting Ltd.: Bristol, UK, 2009. [Google Scholar]
- Thomson, A.; Price, G.W.; Arnold, P.; Dixon, M.; Graham, T. Review of the potential for recycling CO2 from organic waste composting into plant production under controlled environment agriculture. J. Clean. Prod. 2022, 333, 130051. [Google Scholar] [CrossRef]
- Pantelopoulos, A.; Magid, J.; Jensen, L.S. Thermal drying of the solid fraction from biogas digestate: Effects of acidification, temperature and ventilation on nitrogen content. Waste Manag. 2015, 48, 218–226. [Google Scholar] [CrossRef]
- Barampouti, E.M.; Mai, S.; Malamis, D.; Moustakas, K.; Loizidou, M. Exploring technological alternatives of nutrient recovery from digestate as a secondary resource. Renew. Sust. Energ. Rev. 2020, 134, 110379. [Google Scholar] [CrossRef]
- Awiszus, S.; Meissner, K.; Reyer, S.; Müller, J. Ammonia and methane emissions during drying of dewatered biogas digestate in a two-belt conveyor dryer. Bioresour. Technol. 2018, 247, 419–425. [Google Scholar] [CrossRef]
- Chen, G.; Guo, X.; Cheng, Z.; Yan, B.; Dan, Z.; Ma, W. Air gasification of biogas-derived digestate in a downdraft fixed bed gasifier. Waste Manag. 2017, 69, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Rezaee, M.; Gitipour, S.; Sarrafzadeh, M.-H. Different Pathways to Integrate Anaerobic Digestion and Thermochemical Processes: Moving Toward the Circular Economy Concept. Environ. Energy Econ. Res. 2020, 4, 57–67. [Google Scholar] [CrossRef]
- Karaeva, J.V.; Timofeeva, S.S.; Bashkirov, V.N.; Bulygina, K.S. Thermochemical processing of digestate from biogas plant for recycling dairy manure and biomass. Biomass Convers. Biorefin. 2021. [Google Scholar] [CrossRef]
- Giuliano, A.; Catizzone, E.; Freda, C.; Cornacchia, G. Valorization of OFMSW Digestate-Derived Syngas toward Methanol, Hydrogen, or Electricity: Process Simulation and Carbon Footprint Calculation. Processes 2020, 8, 526. [Google Scholar] [CrossRef]
- Nizamuddin, S.; Baloch, H.A.; Griffin, G.J.; Mubarak, N.M.; Bhutto, A.W.; Abro, R.; Mazari, S.A.; Ali, B.S. An overview of effect of process parameters on hydrothermal carbonization of biomass. Renew. Sustain. Energy Rev. 2017, 73, 1289–1299. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F. Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuel Bioprod. Biorefin. 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Balagurumurthy, B.; Bhaskar, T. Hydropyrolysis of lignocellulosic biomass: State of the art review. Biomass Convers. Biorefin. 2014, 4, 67–75. [Google Scholar] [CrossRef]
- Xiang, W.; Zhang, X.; Chen, J.; Zou, W.; He, F.; Hu, X.; Tsang, D.C.W.; Ok, Y.S.; Gao, B. Biochar technology in wastewater treatment: A critical review. Chemosphere 2020, 252, 126539. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Preparation, modification and environmental application of biochar: A review. J. Clean Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Joseph, S.; Pow, D.; Dawson, K.; Rust, J.; Munroe, P.; Taherymoosavi, S.; Mitchell, D.R.G.; Robb, S.; Solaiman, Z.M. Biochar increases soil organic carbon, avocado yields and economic return over 4 years of cultivation. Sci. Total Environ. 2020, 724, 138153. [Google Scholar] [CrossRef]
- Ye, L.; Camps-Arbestain, M.; Shen, Q.; Lehmann, J.; Singh, B.; Sabir, M. Biochar effects on crop yields with and without fertilizer: A meta-analysis of field studies using separate controls. Soil Use Manag. 2020, 36, 2–18. [Google Scholar] [CrossRef]
- Arif, M.; Ali, S.; Ilyas, M.; Riaz, M.; Akhtar, K.; Ali, K.; Adnan, M.; Fahad, S.; Khan, I.; Shah, S.; et al. Enhancing phosphorus availability, soil organic carbon, maize productivity and farm profitability through biochar and organic-inorganic fertilizers in an irrigated maize agroecosystem under semi-arid climate. Soil Use Manag. 2021, 37, 104–119. [Google Scholar] [CrossRef]
- Chen, H.; Yang, X.; Wang, H.; Sarkar, B.; Shaheen, S.M.; Gielen, G.; Bolan, N.; Guo, J.; Che, L.; Sun, H.; et al. Animal carcass-and wood-derived biochars improved nutrient bioavailability, enzyme activity, and plant growth in metal-phthalic acid ester co-contaminated soils: A trial for reclamation and improvement of degraded soils. J. Environ. Manag. 2020, 261, 110246. [Google Scholar] [CrossRef] [PubMed]
- Campos, P.; Miller, A.Z.; Knicker, H.; Costa-Pereira, M.F.; Merino, A.; De la Rosa, J.M. Chemical, physical and morphological properties of biochars produced from agricultural residues: Implications for their use as soil amendment. Waste Manag. 2020, 105, 256–267. [Google Scholar] [CrossRef]
- Kroeger, J.E.; Pourhashem, G.; Medlock, K.B.; Masiello, C.A. Water cost savings from soil biochar amendment: A spatial analysis. GCB—Bioenergy 2021, 13, 133–142. [Google Scholar] [CrossRef]
- Fazal, T.; Razzaq, A.; Javed, F.; Hafeez, A.; Rashid, N.; Amjad, U.S.; Rehman, M.S.U.; Faisal, A.; Rehman, F. Integration Adsorption and Photocatalysis: A cost effective Strategy for Textile wastewater Treatment using Hybrid Biochar-TiO2 Composite. J. Hazard. Mater. 2020, 390, 121623. [Google Scholar] [CrossRef]
- Mojiri, A.; Ohashi, A.; Ozaki, N.; Aoi, Y.; Kindaichi, T. Integrated anammox-biochar in synthetic wastewater treatment: Performance and optimization by artificial neural network. J. Clean. Prod. 2020, 243, 118638. [Google Scholar] [CrossRef]
- Kaetzl, K.; Lübken, M.; Nettmann, E.; Krimmler, S.; Wichern, M. Slow sand filtration of raw wastewater using biochar as an alternative filtration media. Sci. Rep. 2020, 10, 1229. [Google Scholar] [CrossRef]
- Saarela, T.; Lafdani, E.K.; Laurén, A.; Pumpanen, J.; Palviainen, M. Biochar as adsorbent in purification of clear-cut forest runoff water: Adsorption rate and adsorption capacity. Biochar 2020, 2, 227–237. [Google Scholar] [CrossRef]
- Mehmood, I.; Qiao, L.; Chen, H.; Tang, Q.; Woolf, D.; Fan, M. Biochar addition leads to more soil organic carbon sequestration under a maize-rice cropping system than continuous flooded rice. Agric. Ecosyst. Environ. 2020, 298, 106965. [Google Scholar] [CrossRef]
- Yu, J.; Wu, Z.; An, X.; Tian, F.; Yu, B. Trace metal elements mediated co-pyrolysis of biomass and bentonite for the synthesis of biochar with high stability. Sci. Total Environ. 2021, 774, 145611. [Google Scholar] [CrossRef] [PubMed]
- Ghassemi-Golezani, K.; Farhangi-Abriz, S.; Abdoli, S. How can biochar-based metal oxide nanocomposites counter salt toxicity in plants? Environ. Geochem. Health 2020, 43, 2007–2023. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Tan, X.; Gu, Y.; Liu, S.; Liu, Y.; Hu, X.; Li, J.; Zhou, Y.; Liu, S.; He, Y. Rice waste biochars produced at different pyrolysis temperatures for arsenic and cadmium abatement and detoxification in sediment. Chemosphere 2020, 250, 126268. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Strezov, V.; Weldekidan, H.; He, J.; Singh, S.; Kan, T.; Dastjerdi, B. Lignocellulose biomass pyrolysis for bio-oil production: A review of biomass pre-treatment methods for production of drop-in fuels. Renew. Sust. Energ. Rev. 2020, 123, 109763. [Google Scholar] [CrossRef]
- Dimitriadis, A.; Chrysikou, L.P.; Meletidis, G.; Terzis, G.; Auersvald, M.; Kubička, D.; Bezergianni, S. Bio-based refinery intermediate production via hydrodeoxygenation of fast pyrolysis bio-oil. Renew. Energ. 2021, 168, 593–605. [Google Scholar] [CrossRef]
- Laougé, Z.B.; Çığgın, A.S.; Merdun, H. Optimization and characterization of bio-oil from fast pyrolysis of Pearl Millet and Sida cordifolia L. by using response surface methodology. Fuel 2020, 274, 117842. [Google Scholar] [CrossRef]
- Asimakopoulos, K.; Gavala, H.N.; Skiadas, I.V. Reactor systems for syngas fermentation processes: A review. Chem. Eng. J. 2018, 348, 732–744. [Google Scholar] [CrossRef]
- Jing, Y.; Campanaro, S.; Kougias, P.; Treu, L.; Angelidaki, I.; Zhang, S.; Luo, G. Anaerobic granular sludge for simultaneous biomethanation of synthetic wastewater and CO with focus on the identification of CO-converting microorganisms. Water Res. 2017, 126, 19–28. [Google Scholar] [CrossRef]
- Sforza, E.; Barbera, E.; Girotto, F.; Cossu, R.; Bertucco, A. Anaerobic digestion of lipid-extracted microalgae: Enhancing nutrient recovery towards a closed loop recycling. Biochem. Eng. J. 2017, 121, 139–146. [Google Scholar] [CrossRef]
- Monlau, F.; Sambusiti, C.; Ficara, E.; Aboulkas, A.; Barakat, A.; Carrère, H. New opportunities for agricultural digestate valorization: Current situation and perspectives. Energy Environ. Sci. 2015, 8, 2600–2621. [Google Scholar] [CrossRef]
- Peng, X.; Nges, I.A.; Liu, J. Improving methane production from wheat straw by digestate liquor recirculation in continuous stirred tank processes. Renew. Energy 2016, 85, 12–18. [Google Scholar] [CrossRef]
- Thygesen, O.; Sommer, S.G.; Shin, S.G.; Triolo, J.M. Residual biochemical methane potential (BMP) of concentrated digestate from full-scale biogas plants. Fuel 2014, 132, 44–46. [Google Scholar] [CrossRef]
- Zheng, Z.; Cai, Y.; Zhao, Y.; Meng, X.; Zhang, Y.; Lu, C.; Hu, Y.; Cui, Z.; Wang, X. Achieve clean and efficient biomethane production by matching between digestate recirculation and straw-to-manure feeding ratios. J. Clean. Prod. 2020, 263, 121414. [Google Scholar] [CrossRef]
- Akhiar, A.; Battimelli, A.; Torrijos, M.; Carrere, H. Comprehensive characterization of the liquid fraction of digestates from full-scale anaerobic co-digestion. Waste Manag. 2016, 59, 118–128. [Google Scholar] [CrossRef]
- Li, X.; Guo, J.; Dong, R.; Ahring, B.K.; Zhang, W. Properties of plant nutrient: Comparison of two nutrient recovery techniques using liquid fraction of digestate from anaerobic digester treating pig manure. Sci. Total Environ. 2016, 544, 774–781. [Google Scholar] [CrossRef]
- Kocatürk-Schumacher, N.P.; Zwart, K.; Bruun, S.; Jensen, L.S.; Sørensen, H.; Brussard, L. Recovery of nutrients from the liquid fraction of digestate: Use of enriched zeolite and biochar as nitrogen fertilizers. J. Plant Nutr. Soil Sci. 2019, 182, 187–195. [Google Scholar] [CrossRef]
- Fernandes, F.; Silkina, A.; Fuentes-Grünewald, C.; Wooda, E.E.; Ndovela, V.L.S.; Oatley-Radcliffe, D.L.; Lovitt, R.W.; Llewellyn, C.A. Valorising nutrient-rich digestate: Dilution, settlement and membrane filtration processing for optimisation as a waste-based media for microalgal cultivation. Waste Manag. 2020, 118, 197–208. [Google Scholar] [CrossRef]
- Cammileri-Rumbau, M.S.; Soler-Cabezasb, J.L.; Christensena, K.V.; Norddahla, B.; Mendoza-Rocab, J.A.; Vincent-Velab, M.C. Application of aquaporin-based forward osmosis membranes for processing of digestate liquid fractions. Chem. Eng. J. 2019, 371, 583–592. [Google Scholar] [CrossRef]
- Cammileri-Rumbau, M.S.; Norddahla, B.; Weib, J.; Christensena, K.V.; Søtofta, L.F. Microfiltration and ultrafiltration as a post-treatment of biogas plant digestates for producing concentrated fertilizers. Desalin. Water Treat. 2015, 55, 1639–1653. [Google Scholar] [CrossRef]
- Świątczak, P.; Cydzik-Kwiatkowska, A.; Zielińska, M. Treatment of Liquid Phase of Digestate from Agricultural Biogas Plant in a System with Aerobic Granules and Ultrafiltration. Water 2019, 11, 104. [Google Scholar] [CrossRef]
- Yue, C.; Dong, H.; Chen, Y.; Shang, B.; Wang, Y.; Wang, S.; Zhu, Z. Direct Purification of Digestate Using Ultrafiltration Membranes: Influence of Pore Size on Filtration Behavior and Fouling Characteristics. Membranes 2021, 11, 179. [Google Scholar] [CrossRef] [PubMed]
- Adam, G.; Mottet, A.; Lemaigre, S.; Tsachidou, B.; Trouvé, E.; Delfosse, P. Fractionation of anaerobic digestates by dynamic nanofiltration and reverse osmosis: An industrial pilot case evaluation for nutrient recovery. J. Environ. Chem. Eng. 2018, 6, 6723–6732. [Google Scholar] [CrossRef]
- Zhan, Y.; Yin, F.; Yue, C.; Zhu, J.; Zhu, Z.; Zou, M.; Dong, H. Effect of Pretreatment on Hydraulic Performance of the Integrated Membrane Process for Concentrating Nutrient in Biogas Digestate from Swine Manure. Membranes 2020, 10, 249. [Google Scholar] [CrossRef] [PubMed]
- Blandin, G.; Ferrari, F.; Lesage, G.; Le-Clech, P.; Héran, M.; Martinez-Lladó, X. Forward Osmosis as Concentration Process: Review of Opportunities and Challenges. Membranes 2020, 10, 284. [Google Scholar] [CrossRef]
- Balkenov, A.; Anuarbek, A.; Satayeva, A.; Kim, J.; Inglezakis, V.; Arkhangelsky, E. Complex organic fouling and effect of silver nanoparticles on aquaporin forward osmosis membranes. J. Water Process Eng. 2020, 34, 101177. [Google Scholar] [CrossRef]
- Sciarria, T.P.; Vacca, G.; Tambone, F.; Trombino, L.; Adani, F. Nutrient recovery and energy production from digestate using microbial electrochemical 3 technologies (METs). J. Clean. Prod. 2018, 208, 1022–1029. [Google Scholar] [CrossRef]
- Li, X.; Zhao, X.; Zhou, X.; Yang, B. Phosphate recovery from aqueous solution via struvite crystallization based on electrochemical-decomposition of nature magnesite. J. Clean. Prod. 2021, 292, 126039. [Google Scholar] [CrossRef]
- Romero-Güiza, M.S.; Astals, S.; Mata-Alvarez, J.; Chimenos, J.M. Feasibility of coupling anaerobic digestion and struvite precipitation in the same reactor: Evaluation of different magnesium sources. Chem. Eng. J. 2015, 270, 542–548. [Google Scholar] [CrossRef]
- Astals, S.; Martínez-Martorell, M.; Huete-Hernández, S.; Aguilar-Pozo, V.B.; Dosta, J.; Chimenos, J.M. Nitrogen recovery from pig slurry by struvite precipitation using a low-cost magnesium oxide. Sci. Total Environ. 2021, 768, 144284. [Google Scholar] [CrossRef]
- Johansson, S.; Ruscalleda, M.; Saerens, B.; Colprim, J. Potassium recovery from centrate: Taking advantage of autotrophic nitrogen removal for multi-nutrient recovery. J. Chem. Technol. Biotechnol. 2019, 94, 819–828. [Google Scholar] [CrossRef]
- Shashvatt, U.; Benoit, J.; Aris, H.; Blaney, L. CO2-assisted phosphorus extraction from poultry litter and selective recovery of struvite and potassium struvite. Water Res. 2018, 143, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Giannis, A.; Zhang, J.; Chang, V.W.-C.; Wang, J.-Y. Characterization of induced struvite formation from source-separated urine using seawater and brine as magnesium sources. Chemosphere 2013, 93, 2738–2747. [Google Scholar] [CrossRef] [PubMed]
- Lahav, O.; Telzhensky, M.; Zewuhn, A.; Gendel, Y.; Gerth, J.; Calmano, W.; Birnhack, L. Struvite recovery from municipal-wastewater sludge centrifuge supernatant using seawater NF concentrate as a cheap Mg(II) source. Sep. Purif. Technol. 2013, 108, 103–110. [Google Scholar] [CrossRef]
- Heraldy, E.; Rahmawati, F.; Heriyanto; Putra, D.P. Preparation of struvite from desalination waste. J. Environ. Chem. Eng. 2017, 5, 1666–1675. [Google Scholar] [CrossRef]
- Huang, H.M.; Xiao, X.M.; Yang, L.P.; Yan, B. Removal of ammonium from rare-earth wastewater using natural brucite as a magnesium source of struvite precipitation. Water Sci. Technol. 2011, 63, 468–474. [Google Scholar] [CrossRef]
- Li, B.; Boiarkina, I.; Yu, W.; Young, B. A new thermodynamic approach for struvite product quality prediction. Environ. Sci. Pollut. Res. 2019, 26, 3954–3964. [Google Scholar] [CrossRef]
- Huang, H.; Xiao, D.; Zhang, Q.; Ding, L. Removal of ammonia from landfill leachate by struvite precipitation with the use of low-cost phosphate and magnesium sources. J. Environ. Manag. 2014, 145, 191–198. [Google Scholar] [CrossRef]
- Rahman, M.M.; Liua, Y.H.; Kwag, J.-H.; Ra, C.S. Recovery of struvite from animal wastewater and its nutrient leaching loss in soil. J. Hazard. Mater. 2011, 186, 2026–2030. [Google Scholar] [CrossRef]
- Szymańska, M.; Szara, E.; Wąs, A.; Sosulski, T.; van Pruissen, G.W.P.; Cornellisen, R.L. Struvite—An Innovative Fertilizer from Anaerobic Digestate Produced in a Bio-Refinery. Energies 2019, 12, 296. [Google Scholar] [CrossRef]
- Limoli, A.; Langone, M.; Andreottola, G. Ammonia removal from raw manure digestate by means of a turbulent mixing stripping process. J. Environ. Manag. 2016, 176, 1–10. [Google Scholar] [CrossRef]
- Shi, L.; Simplicio, W.S.; Wu, G.; Hu, Z.; Hu, H.; Zhan, X. Nutrient Recovery from Digestate of Anaerobic Digestion of Livestock Manure: A Review. Curr. Pollut. Rep. 2018, 4, 74–83. [Google Scholar] [CrossRef]
- Vaneeckhaute, C.; Lebuf, V.; Michels, E.; Belia, E.; Vanrolleghem, P.A.; Tack, F.M.G.; Meers, E. Nutrient Recovery from Digestate: Systematic Technology Review and Product Classification. Waste Biomass Valor. 2017, 8, 21–40. [Google Scholar] [CrossRef]
- Baldi, M.; Collivignarelli, M.C.; Abbà, A.; Benigna, I. The Valorization of Ammonia in Manure Digestate by Means of Alternative Stripping Reactors. Sustainability 2018, 10, 3073. [Google Scholar] [CrossRef]
- Simonič, T.; Simonič, M. Vacuum Evaporation of Liquid Fraction of Digestate. Kem. Ind. 2018, 67, 85–93. [Google Scholar] [CrossRef]
- Scarlat, N.; Dallemand, J.-F.; Fahl, F. Biogas: Developments and perspectives in Europe. Renew. Energy 2018, 129, 457–472. [Google Scholar] [CrossRef]
- Mannelli, S.; Monni, M.; ISAAC. Increasing Social Awareness and Acceptance of Biogas and Biomethane. Deliverable D.5.1: Report on Digestate and by-Products. Project No. 691875. EU Horizon 2020. 2016. Available online: https://cordis.europa.eu/project/id/691875 (accessed on 17 February 2022).
- Scarlat, N.; Dallemand, J.-F.; Monforti-Ferrario, F.; Nita, V. The role of biomass and bioenergy in a future bioeconomy: Policies and facts. Environ. Dev. 2015, 15, 3–34. [Google Scholar] [CrossRef]
- Pecorini, I.; Peruzzi, E.; Albini, E.; Doni, S.; Macci, C.; Masciandaro, G.; Iannelli, R. Evaluation of MSW Compost and Digestate Mixtures for a Circular Economy Application. Sustainability 2020, 12, 3042. [Google Scholar] [CrossRef]
- Egene, C.E.; Sigurnjak, I.; Regelink, I.C.; Schoumans, O.F.; Adani, F.; Michels, E.; Sleutel, S.; Tack, F.M.G.; Meers, E. Solid fraction of separated digestate as soil improver: Implications for soil fertility and carbon sequestration. J. Soils Sediments 2021, 21, 678–688. [Google Scholar] [CrossRef]
- EBA (European Biogas Association) Statistical Report 2018. Available online: https://www.europeanbiogas.eu/eba-statistical-report-2018/ (accessed on 17 February 2022).
- EBA (European Biogas Association) Statistical Report 2019. Available online: https://www.europeanbiogas.eu/eba-statistical-report-2019/ (accessed on 17 February 2022).
- Deng, L.; Liu, Y.; Wang, W. Utilization of Digestate. In Biogas Technology; Deng, L., Liu, Y., Wang, W., Eds.; Springer Nature: Singapore, 2020; pp. 319–363. [Google Scholar] [CrossRef]
- Wood Environment & Infrastructure Solutions UK Limited. Digestate and Compost as Fertilisers: Risk Assessment and Risk Management Options. Available online: https://ec.europa.eu/search/?queryText=Digestate+and+compost+as+fertilisers%3A+Risk+assessment+and+risk+management+options&query_source=europa_default&filterSource=europa_default&swlang=en&more_options_language=en&more_options_f_formats=&more_options_date= (accessed on 18 February 2022).
- EBA (European Biogas Association) EBA Digestate Factsheet: The Value of Organic Fertilisers for Europe’s Economy, Society and Environment. Available online: https://www.europeanbiogas.eu/category/publications/ (accessed on 18 February 2022).
- Skic, K.; Sokołowska, Z.; Boguta, P.; Skic, A. The effect of application of digestate and agro-food industry sludges on Dystric Cambisol porosity. PLoS ONE 2020, 15, e0238469. [Google Scholar] [CrossRef]
- Baştabak, B.; Koçar, G. A review of the biogas digestate in agricultural framework. J. Mater. Cycles Waste Manag. 2020, 22, 1318–1327. [Google Scholar] [CrossRef]
- Ehmann, A.; Thumm, U.; Lewandowski, I. Fertilizing Potential of Separated Biogas Digestates in Annual and Perennial Biomass Production Systems. Front. Sustain. Food Syst. 2018, 2, 12. [Google Scholar] [CrossRef]
- Wysocka-Czubaszek, A. Dynamics of Nitrogen Transformations in the Soil Fertilized with Digestate from Agricultural Biogas Plant. J. Ecol. Eng. 2019, 20, 108–117. [Google Scholar] [CrossRef]
- Möller, K. Effects of anaerobic digestion on soil carbon and nitrogen turnover, N emissions, and soil biological activity. A review. Agron. Sustain. Dev. 2015, 35, 1021–1041. [Google Scholar] [CrossRef]
- Barłóg, P.; Hlisnikovský, L.; Kunzová, E. Effect of Digestate on Soil Organic Carbon and Plant-Available Nutrient Content Compared to Cattle Slurry and Mineral Fertilization. Agronomy 2020, 10, 379. [Google Scholar] [CrossRef] [Green Version]
- Różyło, K.; Świeca, M.; Gawlik-Dziki, U.; Kwiecińska-Poppe, E.; Andruszczak, S.; Kraska, P. Phytochemical properties and heavy metal accumulation in wheat grain after three years’ fertilization with biogas digestate and mineral waste. Agric. Food Sci. 2017, 26, 148–159. [Google Scholar] [CrossRef]
- Yu, F.-B.; Luo, X.-P.; Song, C.-F.; Zhang, M.-X.; Shan, S.-D. Concentrated biogas slurry enhanced soil fertility and tomato quality. Acta Agric. Scand. Sect. B Soil Plant Sci. 2010, 60, 262–268. [Google Scholar] [CrossRef]
- You, L.; Shuqin, Y.; Hulyun, L.; Chutian, W.; Zengliang, Z.; Ling, Z.; Dongan, H. Effects of biogas slurry fertilization on fruit economic traits and soil nutrients of Camellia oleifera Abel. PLoS ONE 2019, 14, e0208289. [Google Scholar] [CrossRef]
- Ivezić, V.; Zebec, V.; Popović, B.; Engler, M.; Teklić, T.; Lončarić, Z.; Karalić, K. Potential of Industrial By-Products as Liming Materials and Digestate as Organic Fertilizer and Their Effect on Soil Properties and Yield of Alfalfa (Medicago sativa L.). Sustainability 2021, 13, 11016. [Google Scholar] [CrossRef]


| Phase of AD | Input | Microorganisms Involved | Output |
|---|---|---|---|
| Hydrolysis & Acidogenesis | Proteins | Clostridium sp. | Amino acids Sugars |
| Proteus vulgaris | |||
| Peptococcus sp. | |||
| Bacteriodes sp. | |||
| Bacillus sp. | |||
| Vibrio sp. | |||
| Carbohydrates | Clostridium sp. | Amino acids Sugars | |
| Acetovibrio calluliticus | |||
| Staphylococcus sp. | |||
| Bacteriodes sp. | |||
| Lipids | Clostridium sp. | Higher fatty acids Alcohols Amino acids Sugars | |
| Micrococcus sp. | |||
| Staphylococcus sp. | |||
| Acetogenesis | Amino acids Sugars | Lactobacillus sp. | Acetate Hydrogen |
| Escherichia sp | |||
| Staphylococcus sp. | |||
| Micrococcus sp. | |||
| Bacillus sp. | |||
| Pseudomonas sp. | |||
| Desulfovibrio sp. | |||
| Selenomonas sp. | |||
| Veillonella sp. | |||
| Sarcina sp. | |||
| Streptococcus sp. | |||
| Desulfobacter sp. | |||
| Desulfuromonas sp. | |||
| Clostridium sp. Eubacterium sp. Streptococcus sp. | Intermediates | ||
| Zymomonas mobilis | Alcohols | ||
| Higher fatty acids Alcohols | Clostridium sp. | Hydrogen Acetate Intermediates | |
| Syntrophomonas wolfei | |||
| Intermediates | Syntrophomonas wolfei | Acetate Hydrogen | |
| Syntrophobacter wolinii | |||
| Methanogenesis | Acetate (Hydrogen) | Clostridium aceticum | Hydrogen (Acetate) |
| Acetate Hydrogen | Methanothrix sp. | Methane | |
| Methanosarcina sp. | |||
| Methanospirillum sp. | |||
| Mathanobacterium sp | |||
| Methanobrevibacterium sp. | |||
| Methanoplanus sp. |
| Substrates | AD Process Conditions | The Aim of the Study | Results | Ref. |
|---|---|---|---|---|
| Corn stalks, tomato residues (stalks and leaves), dairy manure. | Batch mesophilic AD; feedstock to inoculum ratio: 4 (based on VS), TS of mixture: 20%. | Influence of digestion time on the performance of subsequent DG composting. | DG composting causes benefits on GI, pH, EC, and reduced GHG emissions compared to composting with raw feedstocks. | [51] |
| Olive wastes and citrus pulp mixed with straw, livestock wastes, and cheese whey. | Two biogas plants: (I) mesophilic regime, pH 7.8, HRT: 60 days. (II) mesophilic regime, pH 8.0, HRT: 60 days. | Influence of mixtures of substrates in order to produce a more stable DG with compatible soil use as fertilizer. | Increased OM content and optimization of nutrient balance positively affected soil fertility. Solid fractions increased soil stability and humification rates. | [52] |
| PM and FVW, dairy sludge effluent (inoculum). | Two semi-continuous stirred tank mesophilic reactors (bench-scale). TS of substrate: 8%. | Influence of PM mono-digestion and PM + FVW co-digestion on DG quality. | EC, COD, Mn, Ca and Zn values were statistically higher in PM than in PM-FVW DG. Both DGs showed high EC values. | [53] |
| MAR & TWAS. R1-untreated MAR; R2- MAR treated with KOH; R3-MAR/TWAS. | Pretreatment: (I) mechanical: knife, vibro, and planetary ball mill; (II) chemical: (a) H3PO4, (b) KOH; (III) thermal. BMP: mesophilic regime, continuous stirring. | Effect of pretreatment on DG quality. | Nutrient concentrations in R2 DG are lower than in R1, except for K (brought by the KOH). R3 DG contained high concentrations of NH4+, P, and K compared to R1. R2 was more stabilized and can be more beneficial for soil in the long term. | [54] |
| OFMSW, sludge, cattle slurry. | The mesophilic regime in both reactor types. | Monitoring of fecal indicators, pathogens, HM concentration, and fertilizing performance of DGs. | The presence of Salmonella and other pathogens, and high levels of Cu, Ni, and Zn in some DGs. A significant positive effect on plant growth observed with the DG from a lab-scale reactor. | [55] |
| PM, sunflower hulls, and seed sludge (inoculum). | Batch AD in the mesophilic regime. Substrates mixed in 6 different proportions (w/w). | Influence of anaerobically digested different substrates mixtures on DG characteristics. | The DG contained low amounts of HM and a high concentration of Zn. | [56] |
| Food and garden waste from the food industry and households. | Ground substrates were heated to 137 °C for 24 min at 2.4 bar and digested (mesophilic regime, full-scale AD). | Composition of separated liquid and solid DG fractions (concentration and seasonal variation of HM, organic pollutants, pesticides, and E. coli and B. cereus). | According to Norwegian regulation—higher Zn concentration; hazardous organic pollutants, two fungicide types, most frequently found in both fractions. Viable B. cereus detected in liquid phase; no DNA or viable cells of E. coli detected. | [57] |
| CM, PL, PS, and onion waste | Batch mesophilic digestion, manual agitation, HRT: 60 days. Manure: onion waste ratio 5:1 (w/w). | Influence of chemical and spectroscopic characteristics of AD substrates on soil biological activity; growth dynamic of lettuce and digested wastes incorporation into the soil. | Low C/N, high NH4+-N/N ratio, a greater proportion of short-chain organic acids, and greater stability of DGs if compared to fresh manures. Soils amended with DGs showed less CO2 emission than soils amended with manures. | [58] |
| 20 different DGs from different biogas plants-10 PS and 10 COM | Biogas plants operate under mesophilic or thermophilic conditions. | Influence of chemical characteristics of DGs on soil microbial activities, i.e., PAO and soil respiration | DGs contained significantly higher NH3 concentrations, but lower TC and VFA concentrations if compared to PS and COM. The DGs showed both stimulating and inhibiting effects on PAO, while all COM and PS except one showed inhibiting effects on PAO. | [59] |
| APW and DM | Lab batch mesophilic AD. APW/DM wet weight ratios: 1:0, 3:1, 1:1, 1:3, and 0:1. The initial inoculum of each bottle was 30% (w/w). | The performance of AD based on the pH, TKN, CODt, and VS removal rate. Evaluation of the stability of DG by the TG and DSC analysis. | High stability of the DG was obtained after AD of APW and DM. Single digestion of the APW and DM was incomplete compared with the mixture thereby leading to the lower stability of DG. | [60] |
| Parameter | Unit | Values | References |
|---|---|---|---|
| EC | μS cm−1 | 100–642 | [54,61] |
| pH | - | 5.6–8.6 | [51,54,61,62,63,64] |
| DM | % | 0.7–90 | [51,54,61,62,63,64] |
| OM | % DM | 15.6–98.0 | [54,61] |
| Total C | % DM | 10.4–58.7 | [51,54,61,63,64,65] |
| Total N | % DM | 0.2–20.5 | [51,54,61,62,63,64,65] |
| NH4+-N | g kg−1 DM | 2.1–17.9 | [54,61,66] |
| Ca | g kg−1 DM | 0.6–98.5 | [54,62,63,64,65] |
| K | g kg−1 DM | 0.9–110.5 | [54,61,62,63,64,65] |
| Mg | g kg−1 DM | 0.1–14.1 | [54,62,63,65] |
| P | g kg−1 DM | 0.1–54.0 | [54,62,63,64] |
| HM (mg kg−1 DM) | Values | References |
|---|---|---|
| Cd | 0.18–5.0 | [55,56,57,61,62,65] |
| Pb | 0.02–126 | [55,56,57,61,62] |
| Cu | 1.4–681.0 | [55,56,57,61,62,63] |
| Hg | 0.05–1.34 | [55,56,57,61] |
| Ni | 0.51–355.9 | [55,56,57,61] |
| Zn | 0.81–4019 | [55,56,57,61,62,63] |
| Cr | 0.06–560.3 | [55,56,57] |
| Fe | 371–29 837 | [55,62,63,65] |
| Mn | 31.5–96.5 | [65] |
| Substrate | Process Conditions of Composting | The Aim of the Study | Results | Ref. |
|---|---|---|---|---|
| DG (household wastes) | Mixing ratios WC:DG = 1:1, 2:1, 3:1, 4:1, 5:1 in volume; aeration rate: 7, 15 and 30 Lh−1 kg OM0 | Influence of different sizes of BA and different mixing ratios of DG and BA on compost quality. | Higher mixing ratios of substrates increased O2 utilization and self-heating potential, and reduced gas emissions. 15 Lh−1 kg OM0 assured O2 supply, self-heating, and limits NH3 emission. | [94] |
| DG | Concentrations of OS in DG: 0, 10, 20 and 30; C/N ratio: 25–30; aeration rate: 0.15 Lmin−1 kg−1 TS | Influence of OS as BA on composting and different mixing ratios of DG and BA on compost quality. | Improved OM degradation promoted the transformation of NH4+-N to NH3−-N and enhanced composting performance. GI revealed improvement in compost quality. | [104] |
| Solid residue DG | DG was mixed with equal parts of peat and composted for 80 days | Evaluation of composting parameters of DG from 3 depths: T of composting pile, TOC, TN, TK, TP, pH, ECs, NO3–-N, and NH4+-N. | NH4+-N, TOC, and C/N decreased with the composting process, while TP, TK, and NO3–-N increased. GI and SGI showed that raw DGs were toxic to plants, but the GI and SGI increased during the composting process. | [105] |
| DG fraction (agricultural waste and distillery stillage) | DG was composted for 51 days in 165 L composting bioreactors. Aeration was performed on days 16 and 34 by manual mixing. | Composting parameters: dry matter, OM, pH, ECs, and T; daily measurements of gaseous emissions. | DG shows a very good structure and proper C/N ratio for composting. Production of compost from DG could be a good solution for managing digested waste. | [106] |
| The solid phase of DG (maize silage, peach juice pulp, cattle slurry) | Lab-batch experiment which lasted 96 h. Moisture = 50 and 70%DM (w/w); C/N ratio: 28, 31, 33 and 36; OM = 88.07%; T measured every 15 min; aeration for 2 min by opening the Dewar jars | Optimization of pH, C/N ratio, and moisture values to maximize self-heating activity (Dewar tests) to establish the startup conditions to transfer the procedure to an industrial scale. | The optimal conditions (pH = 7.7, moisture = 50%) obtained experimentally were used to develop a mathematical model which for process optimization. The conclusions should be validated on an industrial scale to check reproducibility and compliance with standards of stabilization, sterilization and compost quality. | [103] |
| Fresh, air-dried, and oven-dried DG (municipal organic waste) | A part of solid DG was taken for air-drying (20–30 °C), a part was treated in open heaps for 6 weeks, and the third part was dried at 70 °C in a laboratory oven until the weight of DG remained constant. | Influence of drying, composting, and sieving on final DG properties, nutrient availability, HM, and C elution. | Sieving of composted DG showed that HM concentration increases with decreasing mesh sizes. The element concentration is higher in composted batches, while the water-extractability of nutrients, HM, and C is significantly lower in composted over dried DGs. A significant correlation was found between the dissolution of Zn, Ni, Ca, and Mg, pH of eluate, and DOC release (R > 0.7, p < 0.05). | [107] |
| A solid fraction obtained by mechanical separation of DG produced by AD of PS, ECs, and AIR | DG and LBA mixture ratio 4:1 (w/w), lab-scale adiabatic reactor (90 days). Aeration: 14–16% O2 during the bio-oxidative phase, (during 6 to 12 days) after which the material was placed in a plastic container (curing phase; wetting and turning weekly). | Evaluation of compost and composting quality of solid fraction of DG—chemical and biological characterization. | DG produced by AD had high biological stability with a PDRI close to 1000 mgO2 kgVS−1h−1. Subsequent composting of a mixture of DG and LBA did not give remarkably different results and led only to a slight modification of the characteristics of the initial non-composted mixtures. The composts obtained fully respected the legal limits for high-quality compost. | [108] |
| A solid fraction obtained by mechanical separation of DG produced by AD of PS | Different mixtures of DG and BA: WSD for P1 and P2, EGM for P3, VSP for P4, and PPP for P5; thermo-composters, natural ventilation. Aeration and T control were maintained by turning. Moisture = 40–70%. | The feasibility of the treatment of the solid fraction of a PS DG by co-composting with different BA, and determination of the final characteristics of the produced composts. | The composts showed suitable physical properties, and a degree of stability and maturity for their potential use as growing media. Also, the type of BA strongly influenced the development of composting and the final properties of the composts, showing the mixtures with WSD and VP the most suitable characteristics. | [109] |
| SD from a WTP, and an DG from a biogas plant treating CM | SD and DG mixed with WSD as BA, turned 3 times a day to homogenize the mixture and maintain adequate O2 levels; moisture = 55%. | OM degradation and microbial community dynamics during the thermophilic phase of composting. | Variations in T, pH, moisture, and bacterial profiles were similar in both processes. SD constituted more than 20 bacterial phyla, DG was represented by 7 phyla. | [110] |
| Ten SW and five DGs (AW, MSW, biowastes, SS, and GW) | Aerobic treatment for 31 days. The mixing ratios of BA (oak wood chips) to substrates ranged from 0.4 to 1.1 (fresh mass basis). Mixtures were turned twice (after 10 and 20 days of the process) by emptying the reactors, mixing their load, and refilling. | Determination of volatile compounds (chemical composition) and odor emissions (concentrations) upon composting of different DGs and SWs. | A total of 60 chemical compounds were identified and quantified. Terpenes, oxygenated compounds, and ammonia exhibited the largest cumulative mass emission. The composting process of SWs accounted for OEFs ranging from 65 to 3089 OUE g−1 OM0; DGs showed a lower odor emission potential with OEF ranging from 8.6 to 30.5 OUE g−1 OM0. Volatile S compounds were the main odorants (POi = 54–99%). | [111] |
| Fertilization Parameters | The Aim of the Study | Results | Ref. |
|---|---|---|---|
| Three-year application of 30 and 60 m3 ha−1 DG. | Determination of physicochemical properties of highly acidic, silty loam soils with low macronutrient content, the yield and nutritional value of switchgrass. | The 60 m3 ha−1 DG application significantly reduced soil acidity, improved its sorption properties, increased the soil OM, K, and Zn content, significantly increased switchgrass yield, the number of panicles per plant, panicle height, crude ash, and protein content from the 1st cut, and the content of protein, P, and Mg in biomass from the second cut. | [65] |
| Three-year application of (a) 5.1 t DM ha−1 DG, and (b) 155 t DM ha−1 MS + 5.1 t DM ha−1 DG. | Influence of DG and MS fertilization on wheat yield and the level of major nutrients in grain, and tracking of bioaccumulation of HM in wheat grains during 3 growing seasons (2013–2016). | In all years fertilization with MS+DG significantly increased the grain yield compared to controls, in 2015 compared to NPK, and the content of TPt, wet gluten, and phenols in grain compared to NPK. MS fertilization had a positive effect on the total ACI of grain in the 1st year. HM concentration in soil and grains was lowest in the DG treatments. | [198] |
| The experiment consisted of 4 treatments: CT, BS, CBS, and CMm. | Influence of treatments on soil properties, tomato fruit quality, and composition of microflora in both nonrhizosphere and rhizosphere soils. | In comparison to CT and CMm, treatments with BS and CBS significantly improved the content of soil available N, P, K, and EC. OM increased by different degrees, while pH values declined from 5.43 to 5.22. In the application of BS, total concentrations of N and P decreased by 4.82% and 3.45%, respectively. | [199] |
| Five different treatments: B0-no BS; B1-10 kg of BS/plant/year; B2-20 kg of BS/plant/year; B3-30 kg of BS/plant/year; B4-40 kg of BS/plant/year. | Influence of BS applications on the soil nutrients, the fruit yield, and fruit quality of C. oleifera. | Fertilization with BS significantly improved available N, P, and K concentrations in soils, and significantly improved the plant yield. The oil yield also showed a correlation to the promotion of available N, P, and K in rhizosphere soils. Fertilization with 30 kg/plant/year above (treatments B3 and B4) had the highest fresh fruit yield, fresh seed rate, and dry seed rate, and resulted in a higher oil yield per plant. | [200] |
| Three-year application of (a) carbocalk, (b) filter dust, (c) wood ash, (d) blast furnace slag; at each site, the field trial was divided into two sides, with and without solid DG. | Evaluation and comparison of the effectiveness of four liming materials in combination with and without solid DG as organic fertilizer as a measure to raise the soil pH to the optimum (pH = 6–7). Alfalfa was planted as a test crop. | All four liming materials raised the pH of the soil, whereas wood ash showed to be the best while blast furnace slag was the worst. The yield of alfalfa increased with the application of all four lime materials. The highest yields were achieved with the application of wood ash. Application of liming materials with solid DG increased soil OM and had slightly higher yields compared to liming materials without solid DG. | [201] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovačić, Đ.; Lončarić, Z.; Jović, J.; Samac, D.; Popović, B.; Tišma, M. Digestate Management and Processing Practices: A Review. Appl. Sci. 2022, 12, 9216. https://doi.org/10.3390/app12189216
Kovačić Đ, Lončarić Z, Jović J, Samac D, Popović B, Tišma M. Digestate Management and Processing Practices: A Review. Applied Sciences. 2022; 12(18):9216. https://doi.org/10.3390/app12189216
Chicago/Turabian StyleKovačić, Đurđica, Zdenko Lončarić, Jurica Jović, Danijela Samac, Brigita Popović, and Marina Tišma. 2022. "Digestate Management and Processing Practices: A Review" Applied Sciences 12, no. 18: 9216. https://doi.org/10.3390/app12189216
APA StyleKovačić, Đ., Lončarić, Z., Jović, J., Samac, D., Popović, B., & Tišma, M. (2022). Digestate Management and Processing Practices: A Review. Applied Sciences, 12(18), 9216. https://doi.org/10.3390/app12189216







