Abstract
Among inorganic contaminants, arsenic (As) is known for its toxicity and the risks to the environment and human health that could derive from its presence. Phytoremediation represents an effective strategy for the removal of arsenic from contaminated soil, provided that suitable plant species and adequate operational plans are exploited. With reference to a disused area located in Southern Italy which was the subject of a previous study, in this work, new strategies were investigated to further improve the effectiveness of a phytoremediation plan for the removal of arsenic. The usefulness of Cannabis sativa (hemp) and Zea mays (corn) was evaluated in this work by microcosm (300 g of mixed soil per test) and mesocosm (4 kg of mixed soil + 1 kg of inert gravel per test) experiments. The addition of arsenic-tolerant bacteria isolated from the rhizosphere of native herbaceous species grown in the contaminated soil was employed to promote plant growth, while different mixtures of mobilizing agents were tested to improve arsenic bioavailability. After the combined treatment, the arsenic content in the aerial parts of the plants increased by about 10 times in the case of corn (from 1.23 to 10.41 mg kg−1) and by about 8 times in the case of hemp (from 1.05 to 8.12 mg kg−1).
1. Introduction
Nowadays, human activities that cause direct or indirect release of organic and inorganic pollutants into the environment affect almost every aspect of modern society, from the process industry to transportation to agricultural practices, to name a few. These practices, too often accompanied by incorrect waste-disposal policies without adequate recovery of resources [1,2], end up having increasingly significant effects on the ecosystem and human health [3].
The need to contain these problems as much as possible has led to the development of a wide range of physicochemical and biological approaches for the recovery of contaminated water [4,5,6,7] and soils [8,9,10,11].
Arsenic (As) is among the most toxic inorganic pollutants and is unfortunately a very widespread problem as it is commonly involved in electronics (as a semiconductor), in the metallurgical industry (to improve hardness and thermal resistance in metal alloys, as well as in gold and lead mining) and in the pharmaceutical and agricultural sectors (some of its compounds are used as pesticides) [12].
Apart from its toxicity, which makes this contaminant particularly dangerous for human health in case of direct contact [13], arsenic can be assimilated through the food chain and from drinking water, with consequent bioaccumulation and occasional biomagnification [14], causing neurotoxicity and cardiovascular diseases [15,16] and, even worse, carcinogenic effects [17].
Arsenic retention in the soil is related to soil properties, such as pH, organic matter content [18], clay minerals and redox conditions. Metal speciation strongly influences its mobility and bioavailability: As(III) and As(V) are the most important and common inorganic forms and also the most toxic in soil [19].
Exposure to arsenic negatively affects almost all body systems in humans [20]; for this reason, arsenic is one of WHO’s 10 chemicals of major public health concern [21]. Along the same lines, Italian legislation limits the concentration of As in public, private and residential green land to 20 mg/kg [22].
In the case of soils contaminated by As or other potentially toxic metals, some technical limitations considerably reduce the range of possible intervention options [11]: to date, soil excavation and landfilling, as well as soil washing/flushing with stabilization/inertization, are still the most common solutions. As a potential alternative, the use of ElectroKinetic Remediation Technology (EKRT) is a promising in situ approach [23].
However, in recent years, the growing sensitivity towards environmental issues has highlighted an aspect rarely taken into consideration, namely, that most of the remediation activities have an environmental impact in themselves, as they require the use of chemical products or processes which require the consumption of raw materials and energy [24]. To avoid compromising the sustainability of approaches [24] and even the generation of harmful side effects [25], an increasing number of technologies aim to provide nature-based solutions (NBSs) [26]. Approaches of this type are generally more ecological and sustainable, as they aim not only to eliminate or reduce contamination but also to minimize the environmental impacts (emissions, energy consumption, waste production, etc.) and create synergies between different sectors and activities (protection of ecosystems and the promotion of a circular economy, climate change mitigation and resilience) [27].
Among NBS remediation measures, phytoremediation certainly occupies a leading position. It includes a variety of remediation technologies that employ plant species to remove organic and inorganic contaminants from soil and other environmental matrices (sediments, water) [28,29,30].
Their low cost, simplicity of operation and environmental benefits [31,32] are just some of the advantages offered by these phyto-technologies, and their overall sustainability and effectiveness can be further increased when used in combination with other approaches [33,34]. In this regard, the use of green roofs [35] has also intensified in recent years. Such solutions are known to be able to improve the energy and environmental performance of urban environments [36,37] by combining phytoremediation techniques in urban areas with other approaches aimed at mitigating climate change [38].
Phytoextraction is considered a non-invasive technique for removing potentially toxic metals from contaminated soil in an ecological and economical way by adsorption from the roots and accumulation by translocation in the different tissues of the plant [39,40].
Several studies have explored the possibility of using fast-growing and highly tolerant species assisted by chelating/mobilizing agents to increase the uptake of metals from soil, to be used in place of hyper-accumulative species that generally suffer from low biomass production and slow growth rates [41,42].
In fact, the chelating/mobilizing agents have the precise function of promoting the release in soil solutions of metal ions originally bound to soil particles, thus increasing their bioavailability for adsorption by plants [43], since plants can absorb only those species that are present in soluble form in soil solutions [44,45].
Another possible strategy to aid phytoextraction is the use of plant-growth-promoting bacteria, such as those that populate the rhizosphere (PGPR) [46]. These rhizobacteria can act by facilitating the bioavailability of soil nutrients and/or by modulating the production and levels of phytohormones in plants [47]. In some cases, they can also promote the mobility and bioavailability of metals in the soil, increasing their absorption by plants [48,49].
In the first part of this work, we showed how a treatment with KHC2O4 allowed an increase in the bioavailability of arsenic and its bio-adsorption by plants, especially in the presence of PGPR [50]. As a continuation of the previous study, this work aimed to investigate the single and synergistic effect of PGPR and different chelating agents on the growth of two tolerant species, Cannabis sativa (hemp) and Zea mays (corn), and their uptake of As, in order to evaluate their potential use in the site from which the As-contaminated soils were removed.
The selection of bacterial strains capable of improving the efficiency of phytoremediation is a fundamental step, made non-trivial by the specificity of the action of PGPR and their effectiveness, which depend on numerous factors related to the complex interactions between soil and plants [47]. In this study, the addition of a selected mixture of potassium hydrogen oxalate (KHC2O4) and ascorbic acid (C6H8O6) allowed an increase in the bioavailability of the target contaminant and the obtainment of detectable levels of phytoextraction without the creation of percolation problems. The optimization of the process, achieved through microcosm and mesocosm tests, made it possible to outline the application protocol for a field test.
2. Materials and Methods
2.1. Site Description, Soil Characterization and Evaluation of As Bioavailability
As described in the first part of this work [50] (which also provides details on the methodologies used), the contaminated site under investigation is located in Southern Italy and includes two different areas: the first (contaminated in various ways) once housed a chemical plant, while the second is a vegetated area that presents only arsenic contamination, about 1000 m from the former chemical plant (external area). In this second work, attention was focused only on the external area. Using a small excavator, soil samples were taken from sections 0.3 to 0.6 m, 0.6 to 1.0 m and 1.0 to 1.6 m (the presence of a layer of rocks prevented us from reaching sampling depths greater than 1.6 m). Unlike the individual study of each sample discussed in the first work, in this case, the different soil samples were gathered and thoroughly mixed to obtain a single sample which was then analyzed to quantify the total and potentially bioavailable arsenic content. This was carried out to bring the experimentation closer to the conditions of a future field test, which is usually not performed with several meter-by-meter planting approaches but by preparing the entire area for planting in the same way. However, it should be reiterated that mesocosm tests remain small-scale tests compared to field tests.
Since the bioavailability tests previously detailed and performed in [50] had shown that arsenic subjected to the remediation treatment was difficult to mobilize, adsorption of arsenic to the iron oxides/hydroxides present in the soil was hypothesized. Therefore, in addition to the most widely used chemical additive (0.05 M and 1 M KH2PO4), other solutions were tested, demonstrating, in particular, the effectiveness of 0.2 M potassium hydrogen oxalate (KHC2O4). In an attempt to further improve the extractive efficacy, the use of ascorbic acid (C6H8O6), alone or in combination with oxalate, was investigated in this work to identify the best possible combination of the two reagents capable of maximizing the bioavailability of arsenic. All tests were carried out in triplicate, considering a soil: extracting solution ratio of 1:25 and a contact time of 4 h.
2.2. Phytotoxicity Tests
Phytotoxicity tests based on the inhibition of germination and root extension of Lepidium sativum L. [51] were performed. A quantity of 10 g of As-contaminated soil was saturated with deionized water and placed in a Petri dish; a Whatman #1 filter paper with 10 seeds of Lepidium sativum L. was then placed on it. The plates (five replicates performed simultaneously) were placed in a climatic chamber for 72 h in the dark, at a temperature of 25 ± 1 °C. At the end of the test, the germinated seeds were counted, and root elongation was measured. The germination index (GI%) and root elongation inhibition (Inh%) were estimated based on the relationships expressed in Equations (1) and (2), where Gs and Gc are, respectively, the average numbers of germinated seeds in the contaminated soil samples and the negative control, while Ls and Lc are the mean root lengths (mm) for the contaminated soil samples and the negative control, respectively.
High values of GI% indicate reduced phytotoxicity of the contaminated soil, while high values of Inh% are associated with worse soil quality. These indices are useful as part of a preliminary investigation to evaluate the toxicity of soils.
2.3. Preparation of the Bacterial Consortium
Endophytic bacteria were isolated from the roots of hemp seedlings grown on soil characterized by significant arsenic contamination (about 1000 mg kg−1 [52]). The protocol described in [49] was followed to isolate the endophytes. In short, after removing the soil by repeatedly rinsing with tap water, the roots of the plants were sterilized by treatment with 70% EtOH for 5 min, with 0.1% NaClO for 2 min and again with 70% EtOH for another 5 min, and then washed with sterile water. The roots were then minced with a sterile scalpel, placed in sterile flasks containing TYEG (Trypticase Yeast Extract Glucose) and incubated overnight at 30 °C.
Serial dilutions (10−4, 10−6, 10−8) were then prepared with the suspension, and quantities of 100 µL of each dilution (in triplicate) were spread on R2A agar plates (Merck®, DA, DE, Darmstadt, Germany). Then, 100 μL of the third-rinse water was plated to confirm the efficiency of external root sterilization. Colonies of the isolates appeared after approximately five days. About 50 colonies were randomly selected and propagated to obtain pure cultures.
2.4. Characterization of Bacterial Isolates
The genomic DNA of the isolated strains was prepared with the Maxwell 16 system (Promega®, Madison, WI, USA), and the 16S rRNA fragments, amplified via polymerase chain reaction (PCR), were analyzed with a DNA sequencer (ABI model 3500 Genetic Analyzer). A SeqMan application (DNASTAR® v.11.2.1, Madison, WI, USA) from Lasergene (Madison, WI, USA) was employed to modify and assemble the obtained nucleotide sequences. The homology comparison (BLAST analysis) was performed, exploiting the National Center for Biotechnology Information server (NCBI, www.ncbi.nlm.nih.gov/blast/Blast.cgi, accessed on 1 August 2022). A collection of seven isolates belonging to the Comamonadaceae, Microbacteriaceae, Bacillaceae and Micrococcaceae families was obtained. These strains were then subjected to a series of in vitro assays to evaluate their plant-growth-promoting (PGP) potential, as reported in [49]. The list of isolates and their PGP properties are shown in Figure 1.
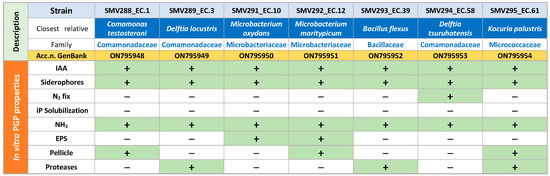
Figure 1.
List of arsenic-tolerant bacterial endophytes isolated from the roots of hemp seedlings grown on an As-contaminated soil and the results of the tests performed on them. Genbank accession numbers and in vitro PGP properties are shown.
All strains selected for the preparation of the microbial inoculum showed at least four growth-promoting properties; in particular, all of them produced 3-indol acetic acid (IAA), siderophores and ammonia.
2.5. Preparation of the Bacterial Consortium
The seven selected bacterial strains were shown to possess 4 to 5 PGP properties and all were used to prepare the microbial consortium. To optimize the growth of each strain, the seven bacteria were grown separately in LB medium for 72 h. The cell pellets were pooled and resuspended in a protective medium containing 1% sodium glutamate, 7% sucrose and 5% dextran, then frozen and freeze-dried by vacuum exposure in small aliquots for 48 h. The freeze-drying procedure was performed to allow the preservation of the inocula until they were used. Aliquots of the lyophilized bacterial consortium (about 108 CFUs per gram of soil) were then added to the pots for the phytoremediation tests.
2.6. Preparation of Microcosms
Microcosm tests represent the first stage in treatability studies by phytoremediation, as they aim at selecting the most suitable plants and treatments for the specific contaminated soil and evaluating the potential toxic effects of contaminants. The microcosm tests were set up using 300 g of mixed soil per test; different combinations of potassium hydrogen oxalate (KHC2O4) and ascorbic acid (C6H8O6) were used as mobilizing agents. The following conditions were investigated:
- Plant species: C. sativa (10 seeds) and Z. mays (6 seeds); the seeds were germinated on cotton and then subsequently transplanted;
- Four different treatments for each type of plant, i.e., a control (CT) and treatments with 0.1 M KHC2O4 + 0.01 M C6H8O6, 0.2 M KHC2O4 + 0.01 M C6H8O6 and 0.2 M KHC2O4 + 0.02 M C6H8O6;
- Administration of mobilizing agents: 5 mL of solution for 5 days + irrigation according to the needs of the plants;
- PGPR: added to all microcosms (except CTs);
- Fertilization: a few days after transplanting the plants, 12 mL of a 0.06% urea solution was added to the microcosms.
As was mentioned, the chosen plants had already been successfully tested for arsenic phytoextraction [52,53]. All tests were performed in triplicate, with a total of 24 microcosms, for a duration of 30 days. The treatment was administered about 10 days after the transplant, with the mobilizing agent added over the course of 5 days in order to dilute the effect of a possible too-rapid absorption of the As. In these tests, nitrogen fertilization with urea was also used to improve the agronomic properties of the soil. At the end of the test, the plants were collected, the roots were separated from the aerial parts and the plant samples thus obtained were carefully washed and prepared for analysis.
2.7. Preparation of Mesocosms
Mesocosm tests represent a scale up from microcosm tests; since the tests are carried out in larger pots, mesocosm tests allow study of the growth of plants at a more realistic scale, over a longer time period, and evaluation of the efficiency of phytoextraction in a more realistic way. In addition, the pots are equipped with a leachate collector to evaluate possible leaching of contaminants. Mesocosm tests were carried out to verify the possibility of percolation of As following the addition of the mobilizing agents. The same plant species (hemp and corn) investigated in the microcosm tests were used, together with a 0.2 M KHC2O4 + 0.01 M C6H8O6 mixture added as a mobilizer. The experimental conditions were the following:
- Soil: 4 kg of mixed soil + 1 kg of inert gravel per test; gravel was placed at the base of the pot to facilitate drainage;
- Plant species: C. sativa (20 seeds) and Z. mays (10 seeds); the seeds were germinated on cotton and then subsequently transplanted;
- Two different treatments (about 10 days after the transplant) for each type of plant, i.e., a control (CT) and a treatment with 0.2 M KHC2O4 + 0.01 M C6H8O6 (hereinafter referred to as Ox 0.2/AA 0.01);
- Administration of mobilizing agents: 50 mL of solution for 5 days + irrigation according to the needs of the plants;
- PGPR: added to all mesocosms (except CTs);
- Fertilization: a few days after plant transplantation, 10 + 10 mL of a 0.036% urea solution was added to the mesocosms in 2 days.
All tests were performed in triplicate, with a total of 12 mesocosms, for a duration of 60 days. The tests aimed at evaluating not only the growth of plants for longer times but also the possible effects of the percolation of arsenic resulting from the treatment with mobilizing agents. In fact, the formation of leachate was monitored following daily irrigation (administered according to the needs of the plants). At the end of the test, following the harvesting of the plants, the mesocosms were saturated with water to simulate extreme conditions that could derive from particularly intense atmospheric phenomena.
At the end of the growing period, the plants were collected, and plant samples were prepared as previously described.
2.8. Arsenic Analysis
Plant samples were mineralized and analyzed to determine the amounts of accumulated As: a weighted amount of dried plant tissue was ground and then digested in a Teflon vial with a 2.5:1 mixture of HNO3 and H2O2, using an ETHOS-900 microwave system (MILESTONE S.r.l., Bergamo, Italy) with pulsed emission. After digestion, the samples were diluted to 25 mL with milliQ water and analyzed by ICP-OES (Varian AX Liberty), using a method for the generation of hydrides [54].
2.9. Quality Assurance and Quality Control
QA/QC evaluations were performed by testing two standard solutions every 10 samples, using certified reference materials (CRM ERM—CC141 for soil and CRM ERM—CD281 for plants). The detection limit for As was 5 μg L−1. Recovery from spiked samples ranged from 94% to 101%, with a relative standard deviation (RSD) of 1.87% of the mean.
2.10. Statistical Analysis
All data relating to the phytoextraction tests are reported as the averages of three tests conducted in parallel; the analyses were also performed in triplicate, with mean values ± standard deviations (SDs) recorded. Statistical data analysis was performed using Statistica version 6.0 (StatSoft, Inc., Tulsa, OK, USA). The effects of the treatments were analyzed using one-way analysis of variance (ANOVA, San Francisco, CA, USA), comparing the differences between means and performing a post hoc analysis of variance using Tukey’s honestly significant differences test (p < 0.05) [55].
3. Results and Discussion
3.1. Soil Analysis
The main characteristics of the soil were given in [50] and the results of the arsenic extraction tests performed on the mixed soil are reported in Table 1.

Table 1.
Potentially bioavailable arsenic and iron contents (mg kg−1) in the mixed soil collected at the site under investigation. Values are reported as means (n = 3) ± SDs.
The As content of the soil was found to be 37.5 mg kg−1, but the potentially bioavailable portion estimated using 0.1 M KH2PO4 (which is the typical mobilizing agent of As) was equal to only 0.72 mg kg−1.
These results suggest the presence of particularly strong bonds which cause arsenic to be retained by soil particles and, in particular, a very strong interaction between arsenic and iron oxides/hydroxides.
The values in brackets indicate the quantities of As and Fe extracted by the action of a solution of oxalate only. The data in Table 1 allowed us to confirm the synergistic action of a mixture of the two mobilizing agents, which appears to be capable of destroying a considerable amount of iron oxides/hydroxides on which As is adsorbed with very strong bonds.
After evaluating different mobilizing agents of arsenic and considering that the exchange reactions with the phosphate did not produce satisfactory results, it was decided to try to destroy the iron oxides/hydroxides on which arsenic preferentially adsorbs (see [50] for more details).
To this end, the use of oxalate as a mobilizing agent effectively increased the bioavailability of the pollutant. Oxalate reacts with amorphous iron oxides and, as a result, arsenic is released into the soil solution, from which it can be absorbed by plants:
(FexOy)AsO4 + C2O42− → Fe2(C2O4)3 + AsO43−
However, to try to further increase the bioavailability of arsenic (the values in brackets in the central column of Table 1 show that a maximum of 6.5% mobilization of the total was obtained), it was decided to also add a reducing agent, such as ascorbic acid, as briefly explained below.
In contaminated soils, arsenic is often associated with oxides/hydroxides of various kinds and also with sulfides that may be present. Among these, iron oxides/hydroxides are the primary minerals that control the concentration of As in the different phases of the soil since, in general, they are abundant and have a high affinity for As.
The dissolution of iron oxides can occur through various reactions. It has been shown that the addition of a strong acid is ineffective because the soil surface under acidic conditions becomes positively charged, thus attracting the negative charge of As oxyanions [56]. Furthermore, it has been observed that arsenic can be effectively released from the soil through the reductive dissolution of iron oxides. In natural environments, the progressive dissolution of iron (hydr)oxides is a consequence of the action of organic ligands, such as oxalate and ascorbate, which are produced by biological activity [57]. The oxalate anion (C2O42−) is an organic ligand naturally present in the soil that can form stable complexes with various metals, including iron. Under acidic conditions, oxalate can effectively adsorb onto the positively charged surfaces of iron oxides. The formation of stable complexes with iron leads to the dissolution of the iron oxides themselves. Furthermore, oxalate is a mild reducing agent which can also promote the breakdown of iron oxides by reductive dissolution.
The ascorbate anion (C6H7O6−) is a moderate reducing agent which significantly promotes the reductive dissolution of iron oxide [58,59].
The combination of chelating and reducing agents can therefore promote the dissolution of iron oxides, causing the release of arsenic associated with them through synergistic action. The use of organic reagents, such as oxalate and ascorbate, is to be considered as a practice with high environmental compatibility, since the two substances are biodegradable and have very low toxicities; moreover, their addition does not affect the stability of soil structures.
The extraction of iron with oxalate and ascorbate is influenced by the properties of soil and depends on pH. In general, it is observed that:
- -
- The solubilization of As and Fe with oxalate is more effective at a pH of about 2;
- -
- At pH 7, the quantity of solubilized As tends to decrease;
- -
- At a very basic pH, unlike Fe, solubilized As tends to increase because a direct ion exchange mechanism can occur between the oxyanions of As and the hydroxyl groups of the iron oxy-hydroxides.
When soil is treated with a mixture of ascorbate and oxalate, the iron (Fe2+) that becomes available through the reductive action of the ascorbate can form complexes with the oxalate (for example, Fe(C2O4) and [Fe(C2O4)2]2−) as a function of the molar ratio between species. When the Ox/Fe ratio is close to 1, the formation of the Fe(C2O4) complex is dominant, while higher Ox/Fe ratios favor the formation of [Fe(C2O4)2]2−. Fe(C2O4) tends to precipitate due to low solubility (the solubility of the dihydrated salt Fe(C2O4) × 2H2O is only 0.97 g L−1 at 25 °C [60]).
The use of a mixture of oxalate and ascorbate also tends to increase arsenic extraction, indicating a synergistic action resulting from the combination of the two reagents. Therefore, the presence of a sufficient amount of binder (oxalate) is important to favor the extraction of arsenic.
In summary, oxalate extracts iron from the soil mainly through reactions in which it acts as a binder, while ascorbate acts as a reducing agent. Following the dissolution of iron oxides/hydroxides, the previously adsorbed As is released into the soil solution.
The extraction of As through the combination of chelating and reducing agents is therefore influenced both by the reducing power of the reducing agent and by the stability of the complex that the chelating agent forms with Fe. A strong reducing agent is theoretically capable of extracting As from the soil with all types of chelating agents, while a mild reducing agent requires the use of a strong chelating agent.
Based on these considerations, arsenic and iron extractability tests were also carried out on the mixed soil using different oxalate–ascorbate combinations before starting the microcosm tests.
3.2. Phytotoxicity Test
Since the data presented in Table 1 show a maximum value for the quantity of As made available corresponding to the use of the 0.2 M KHC2O4 + 0.01 M C6H8O6 solution, phytotoxicity tests were performed to evaluate the growth of plants under these conditions.
The GI% and Inh% data reported in Figure 2 allow the comparison of the phytotoxicities of the contaminated soil with and without treatment with the mixture of extracting agents and an inert non-contaminated support. The absence of significant levels of phytotoxicity is evident (high values of GI% and low values of Inh% are indicative of limited phytotoxicity). The results are correlated with low values for the bioavailability of As, which do not determine significant toxic effects in the species used.
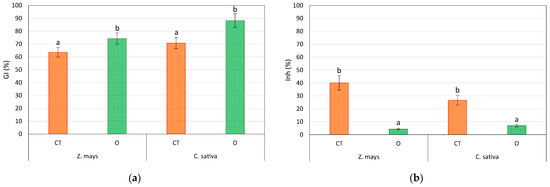
Figure 2.
(a) Germination index (GI%) and (b) root elongation inhibition (Inh%) for Z. mays and C. sativa grown on soil control (CT), As-contaminated soil and As-contaminated soil treated with a 0.2 M KHC2O4 + 0.01 M C6H8O6 solution (O). Values are the means of three replicates and the error bars show the standard deviations. Values with different letters are significantly different at the 5% probability level (Tukey’s test).
Although the treatment with oxalate and ascorbic acid increases the bioavailability of As, the amount of pollutant released as a result of the treatments remains very low, and the positive effects of PGPR are therefore predominant.
3.3. Microcosm Tests with Oxalate + Ascorbic Acid Mixtures
The biomass values obtained for the plants cultivated in the controls and in the soils treated with the different mixtures of potassium acid oxalate and ascorbic acid (and in the presence of PGPR) are shown in Figure 3. The related weight concentrations of arsenic absorbed by the roots and shoots of Z. mays and C. sativa are collected in Table 2.
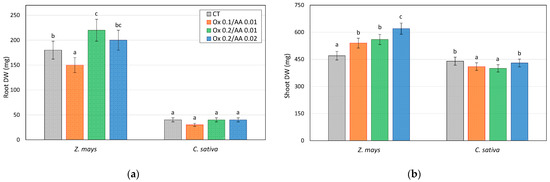
Figure 3.
Dry weights (mg pot−1) of (a) roots and (b) shoots of Z. mays and C. sativa grown on soil control (CT) and As-contaminated soils treated with the different mixtures of oxalate and ascorbic acid (microcosm tests). Values are the means of three replicates and the error bars show the standard deviations. Values with different letters are significantly different at the 5% probability level (Tukey’s test).

Table 2.
As concentrations (mg kg−1) in roots and shoots of Z. mays and C. sativa grown on As-contaminated soils treated with different mixtures of oxalate and ascorbic acid (microcosm tests). The values are the means of three replicates and the error bars show ± standard deviations. The different letters within the same column represent different significance levels at p < 0.05 (Tukey’s test).
As evidenced by the results of the Tukey’s test (letters associated with the data in Figure 3/Table 2), the biomass values obtained for the treated plants do not appear to have been significantly influenced by the treatments carried out (this applies in particular to the roots). This confirms the non-toxicity of the treatments and highlights that the quantity of arsenic absorbed by the plants does not create problems for their growth and development.
For both plant species investigated, the best combination of the two additives turns out to be 0.2 M oxalate + 0.01 M ascorbic acid, as this is the treatment that allows maximization of the concentrations of As in plants, both in the aerial parts and in the roots. It is interesting to note the increase in As content compared with the untreated soil and also compared with the microcosms investigated in the first part of this work [50], in which ascorbic acid was not used. Although it is difficult to predict whether this effect will be replicable in the field, it must be said that this is a consequence of the dissolution of a certain amount of iron oxides/hydroxides, since the extractability of arsenic with these mobilizing agents was accompanied by a greater solubilization of iron, as shown in Table 1.
The effects of the treatments on the concentration of arsenic in plants were also highlighted by the values for total removal (obtained by multiplying the concentration of arsenic by the biomass produced); the results obtained are shown in Figure 4.
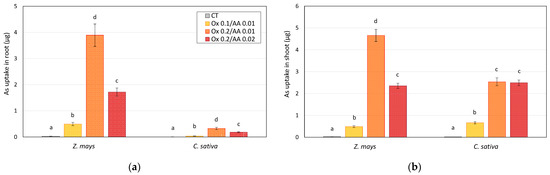
Figure 4.
Arsenic uptake by (a) roots and (b) shoots of Z. mays and C. sativa grown on As-contaminated soils treated with different mixtures of oxalate and ascorbic acid (microcosm tests). The values are the means of three replicates and the error bars show the standard deviations. Values with different letters are significantly different at the 5% probability level (Tukey’s test).
The arsenic adsorption data confirm what has already been said regarding the data in Table 2. Furthermore, at the microcosm level, the corn plants seemed to possess a slightly higher As-extraction capacity than the hemp plants.
3.4. Mesocosms Tests with 0.2 M Oxalate + 0.01 M Ascorbic Acid
The average biomass values for aerial and root parts obtained from plants grown in the control soils and in soils treated with the leaching solution (0.2 M potassium acid oxalate and 0.01 M ascorbic acid) in the presence of PGPR are shown in Figure 5.
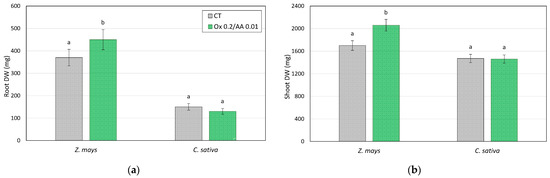
Figure 5.
Dry weights (mg pot−1) of (a) roots and (b) shoots of Z. mays and C. sativa grown on soil control (CT) and soils treated with the 0.2 M oxalate and 0.01 M ascorbic acid solution (mesocosm tests). Values are the means of three replicates and the error bars show the standard deviations. Values with different letters are significantly different at the 5% probability level (Tukey’s test).
The data obtained in the mesocosm tests confirmed what has already been seen in the microcosm tests, namely, that the treatment with the leaching solution does not significantly influence the production of biomass. The data relating to the absorption of As by plants are shown in Table 3.

Table 3.
As concentrations (mg kg−1) in roots and shoots of Z. mays and C. sativa grown on soil control (CT) and soils treated with the 0.2 M oxalate and 0.01 M ascorbic acid solution (mesocosm tests). The values are the means of three replicates and the error bars show ± standard deviations. The different letters within the same column represent different significance levels at p < 0.05 (Tukey’s test).
On the basis of the results obtained, it was confirmed that the addition of the oxalate + ascorbic acid leaching solution is able to increase the bioavailability of arsenic, thus allowing its absorption by plants. In particular, corn appears to have a greater absorption capacity than hemp. However, in a field application, an important role is played by the biomass produced, which has a decisive impact on total removal. The total arsenic removal values during the mesocosm tests shown in Figure 6 confirm the efficacy of treatment with the mobilizing agents. In quantitative terms, the amount of As removed was not particularly high (a few micrograms adsorbed per plant), but it is necessary to take into account the reduced growth of plants in the mesocosms. Therefore, it is safe to assume that the uptake will increase in field tests, where plants will be able to develop more.
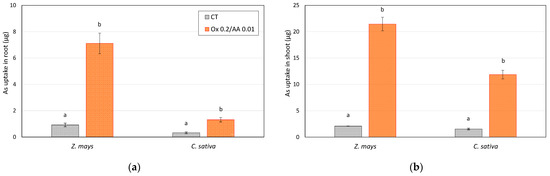
Figure 6.
Arsenic uptake by (a) roots and (b) shoots of Z. mays and C. sativa grown on soil controls (CT) and soils treated with the 0.2 M oxalate and 0.01 M ascorbic acid solution (mesocosm tests). The values are the means of three replicates and the error bars show the standard deviations. Values with different letters are significantly different at the 5% probability level (Tukey’s test).
3.5. Evaluation of the Production of Leachates
The main purpose of the mesocosm tests was to evaluate the possible effects of arsenic percolation resulting from treatment with mobilizing agents. Leachate formation during plant growth following daily irrigation was therefore monitored. The mesocosms were irrigated with tap water according to the needs of the plants, basically on a daily basis. Particular attention was paid to the formation of leachates following the addition of the leaching solution (about 10 days after transplanting the seedlings); none, however, was observed.
Once the plants were harvested at the end of the test (2 months), the mesocosms were saturated by adding 1 L of water to them, and the leachates formed were collected. This test was not intended to simulate a normal situation that can occur in the field but aimed to evaluate the maximum amount of As leached following an extreme atmospheric phenomenon. The data obtained are shown in Table 4.

Table 4.
Volume of leachates formed (mL) and concentrations of As (mg L−1). The values are the means of three replicates and the error bars show ± standard deviations. The different letters within the same column represent different significance levels at p < 0.05 (Tukey’s test).
The quantities of As found in the leached solutions were rather low and, in any case, comparable to those for the untreated control soil; therefore, the occurrence of problems in the field test under “normal” weather conditions can be excluded.
3.6. Technical Specification Proposal for the Field Test
A phytoremediation test in the field (covering about 700 square meters) is planned to be carried out in the external area with the aim of verifying on a real scale the results obtained in the microcosm and mesocosm tests, in which the treatments with the chosen mobilizing agents favored the absorption of the bioavailable fractions of the contaminant by the plants, while the use of PGPR favored their growth and, consequently, greater removal of arsenic. Corn and hemp will be used for the field test, but a third crop (to be defined) will also be required to overcome the problems related to the seasonality of crop cycles.
4. Conclusions
Microcosm (300 g of soil per test) and mesocosm (4 kg of soil per test) tests were carried out to evaluate the leaching efficacy of dilute solutions of potassium hydrogen oxalate and ascorbic acid in different ratios. All trials were conducted in the presence of a consortium of arsenic-resistant PGPR.
The tests confirmed that the combined use of the two organic compounds allows a significant increase in the bioavailability of arsenic in the soils under study and, therefore, absorption by plants. Following treatment with 0.2 M oxalate and 0.01 M ascorbic acid, the arsenic contents in the aerial parts of the plants increased almost tenfold (from 1.23 to 10.41 mg kg−1 in Z. mays and from 1.05 to 8.12 mg kg−1 in C. sativa). All trials in pots revealed that Z. mays performed better than C. sativa.
Despite the greater bioavailability of arsenic in the soils treated with the leaching solution, no percolation phenomena were observed.
Although the plants used are particularly suitable for the geographical and climatic conditions of the area, the results obtained confirm that the assisted phytoremediation approach (a nature-based solution) is effective and sustainable in dealing with arsenic contamination.
Author Contributions
Conceptualization, E.F. and G.P.; methodology, E.F., M.B. and G.P.; validation, E.F., G.P. and M.V.; formal analysis, M.B. and M.V.; investigation, E.F. and M.B.; resources, E.F., G.P. and M.V.; data curation, S.F. and M.V.; writing—original draft preparation, E.F. and M.V.; writing—review and editing, S.F. and M.V.; visualization, M.V.; supervision, M.V.; project administration, G.P.; funding acquisition, E.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Eni S.p.A, Research & Technological Innovation Department, San Donato Milanese (Italy), and fully funded by Syndial S.p.A. (now Eni Rewind S.p.A.), agreement number 3500038857.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the present article.
Acknowledgments
The authors thank Irene Rosellini, IRET-CNR, for technical assistance in the phytoremediation experiments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vocciante, M.; Meshalkin, V. An accurate inverse model for the detection of leaks in sealed landfills. Sustainability 2020, 12, 5598. [Google Scholar] [CrossRef]
- Cirrincione, L.; La Gennusa, M.; Peri, G.; Rizzo, G.; Scaccianoce, G. The landfilling of municipal solid waste and the sustainability of the related transportation activities. Sustainability 2022, 14, 5272. [Google Scholar] [CrossRef]
- Cachada, A.; Rocha-Santos, T.A.P.; Duarte, A.C. Soil and pollution: An introduction to the main issues. In Soil Pollution: From Monitoring to Remediation; Duarte, A.C., Cachada, A., Rocha-Santos, T.A.P., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 1–28. [Google Scholar]
- Farhadian, M.; Vachelard, C.; Duchez, D.; Larroche, C. In situ bioremediation of monoaromatic pollutants in groundwater: A review. Biores. Technol. 2008, 99, 5296–5308. [Google Scholar] [CrossRef] [PubMed]
- Pietrelli, L.; Ferro, S.; Reverberi, A.P.; Vocciante, M. Removal of polyethylene glycols from wastewater: A comparison of different approaches. Chemosphere 2021, 273, 129725. [Google Scholar] [CrossRef] [PubMed]
- Vocciante, M.; De Folly D’Auris, A.; Reverberi, A.P. A novel graphene-based sorbent for oil spill cleanup. Materials 2022, 5, 609. [Google Scholar] [CrossRef]
- Reverberi, A.P.; Vocciante, M.; Salerno, M.; Soda, O.; Fabiano, B. A sustainable, top-down mechanosynthesis of carbohydrate-functionalized silver nanoparticles. React. Chem. Eng. 2022, 7, 888–897. [Google Scholar] [CrossRef]
- Khan, F.I.; Husain, T.; Hejazi, R. An overview and analysis of site remediation technologies. J. Environ. Manag. 2004, 71, 95–122. [Google Scholar] [CrossRef]
- Pavel, L.V.; Gavrilescu, M. Overview of ex situ decontamination techniques for soil cleanup. Environ. Eng. Manag. J. 2008, 7, 815–834. [Google Scholar] [CrossRef]
- Li, C.; Zhou, K.; Qin, W.; Tian, C.; Qi, M.; Yan, X.; Han, W. A review on heavy metals contamination in soil: Effects, sources, and remediation techniques. Soil Sediment Contam. 2019, 28, 380–394. [Google Scholar] [CrossRef]
- Song, P.; Xu, D.; Yue, J.; Ma, Y.; Dong, S.; Feng, J. Recent advances in soil remediation technology for heavy metal contaminated sites: A critical review. Sci. Total Environ. 2022, 838, 156417. [Google Scholar] [CrossRef]
- Pietrelli, L.; Ippolito, N.M.; Ferro, S.; Dovì, V.G.; Vocciante, M. Removal of Mn and As from drinking water by red mud and pyrolusite. J. Environ. Manag. 2019, 237, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Garelick, H.; Jones, H.; Dybowska, A.; Valsami-Jones, E. Arsenic pollution sources. In Arsenic Pollution and Remediation: An International Perspective; Garelick, H., Jones, H., Eds.; Springer: New York, NY, USA, 2008; pp. 17–60. [Google Scholar]
- Duruibe, J.O.; Ogwuegbu, M.O.C.; Egwurugwu, J.N. Heavy metal pollution and human biotoxic effects. Int. J. Phys. Sci. 2007, 2, 112–118. [Google Scholar]
- Hughes, M.F.; Beck, B.D.; Chen, Y.; Lewis, A.S.; Thomas, D.J. Arsenic exposure and toxicology: A historical perspective. Toxicol. Sci. 2011, 123, 305–332. [Google Scholar] [CrossRef]
- Fernández, M.I.; López, J.F.; Vivaldi, B.; Coz, F. Long-term impact of arsenic in drinking water on bladder cancer health care and mortality rates 20 years after end of exposure. J. Urol. 2012, 187, 856–861. [Google Scholar] [CrossRef] [PubMed]
- Straif, K.; Benbrahim-Tallaa, L.; Baan, R.; Grosse, Y.; Secretan, B.; El Ghissassi, F.; Bouvard, V.; Guha, N.; Freeman, C.; Galichet, L. A review of human carcinogens—Part C: Metals, arsenic, dusts, and fibres. Lancet Oncol. 2009, 10, 453–454. [Google Scholar] [CrossRef]
- Bauer, M.; Blodau, C. Mobilization of arsenic by dissolved organic matter from iron oxides, soils and sediments. Sci. Total Environ. 2006, 354, 179–190. [Google Scholar] [CrossRef]
- Dixit, S.; Hering, J.G. Comparison of arsenic(V) and arsenic(III) sorption onto iron oxide minerals: Implications for arsenic mobility. Environ. Sci. Technol. 2003, 37, 4182–4189. [Google Scholar] [CrossRef]
- Rahaman, M.S.; Rahman, M.M.; Mise, N.; Sikder, T.; Ichihara, G.; Uddin, M.K.; Kurasaki, M.; Ichihara, S. Environmental arsenic exposure and its contribution to human diseases, toxicity mechanism and management. Environ. Pollut. 2021, 289, 117940. [Google Scholar] [CrossRef]
- CHeMyCAl. 2016. Available online: https://chemycal.com/news/a6321e58-dfbf-4e30-914d-62d270622b65/WHO_identifies_ten_chemicals_of_major_public_health_concern__International_Programme_on_Chemical_Safety (accessed on 1 September 2022).
- D.Lgs. 152/06 (Parte IV—Titolo V Allegato 5, Tabella 1). Available online: https://www.arpa.veneto.it/temi-ambientali/siti-contaminati/file-e-allegati/normativa/procedura-di-bonifica/Dlgs_152_2006_Parte%20IV_Titolo%20V_allegati.pdf/view (accessed on 3 September 2022).
- Vocciante, M.; Bagatin, R.; Ferro, S. Enhancements in electrokinetic remediation technology: Focus on water management and wastewater recovery. Chem. Eng. J. 2017, 309, 708–716. [Google Scholar] [CrossRef]
- Vocciante, M.; Dovì, V.G.; Ferro, S. Sustainability in ElectroKinetic Remediation processes: A critical analysis. Sustainability 2021, 13, 770. [Google Scholar] [CrossRef]
- Pietrelli, L.; Ferro, S.; Reverberi, A.; Pietro; Vocciante, M. Removal and recovery of heavy metals from tannery sludge subjected to plasma pyro-gasification process. J. Clean. Prod. 2020, 273, 123166. [Google Scholar] [CrossRef]
- Song, Y.; Kirkwood, N.; Maksimović, Č.; Zhen, X.; O’Connor, D.; Jin, Y.; Hou, D. Nature based solutions for contaminated land remediation and brownfield redevelopment in cities: A review. Sci. Total Environ. 2019, 663, 568–579. [Google Scholar] [CrossRef] [PubMed]
- Grifoni, M.; Franchi, E.; Fusini, D.; Vocciante, M.; Barbafieri, M.; Pedron, F.; Rosellini, I.; Petruzzelli, G. Soil remediation: Towards a resilient and adaptive approach to deal with the ever-changing environmental challenges. Environments 2022, 9, 18. [Google Scholar] [CrossRef]
- Franchi, E.; Cardaci, A.; Pietrini, I.; Fusini, D.; Conte, A.; De Folly D’Auris, A.; Grifoni, M.; Pedron, F.; Barbafieri, M.; Petruzzelli, G.; et al. Nature-based solutions for restoring an agricultural area contaminated by an oil spill. Plants 2022, 11, 2250. [Google Scholar] [CrossRef]
- Hou, D.; Bolan, N.S.; Tsang, D.C.W.; Kirkham, M.B.; O’Connor, D. Sustainable soil use and management: An interdisciplinary and systematic approach. Sci. Total Environ. 2020, 729, 138961. [Google Scholar] [CrossRef]
- O’Connor, D.; Zheng, X.; Hou, D.; Shen, Z.; Li, G.; Miao, G.; O’Connell, S.; Guo, M. Phytoremediation: Climate change resilience and sustainability assessment at a coastal brownfield redevelopment. Environ. Int. 2019, 130, 104945. [Google Scholar] [CrossRef]
- Vocciante, M.; Caretta, A.; Bua, L.; Bagatin, R.; Franchi, E.; Petruzzelli, G.; Ferro, S. Enhancements in phytoremediation technology: Environmental assessment including different options of biomass disposal and comparison with a consolidated approach. J. Environ. Manag. 2019, 237, 560–568. [Google Scholar] [CrossRef]
- Vocciante, M.; De Follis D’Auris, A.; Franchi, E.; Petruzzelli, G.; Ferro, S. CO2 footprint analysis of consolidated and innovative technologies in remediation activities. J. Clean. Prod. 2021, 297, 126723. [Google Scholar] [CrossRef]
- Pedron, F.; Grifoni, M.; Barbafieri, M.; Petruzzelli, G.; Franchi, E.; Samà, C.; Gila, L.; Zanardi, S.; Palmery, S.; Proto, A.; et al. New light on phytoremediation: The use of luminescent solar concentrators. Appl. Sci. 2021, 11, 1923. [Google Scholar] [CrossRef]
- Conte, A.; Chiaberge, S.; Pedron, F.; Barbafieri, M.; Petruzzelli, G.; Vocciante, M.; Franchi, E.; Pietrini, I. Dealing with complex contamination: A novel approach with a combined bio-phytoremediation strategy and effective analytical techniques. J. Environ. Manage. 2021, 288, 112381. [Google Scholar] [CrossRef]
- Cirrincione, L.; La Gennusa, M.; Marino, C.; Nucara, A.; Marvuglia, A.; Peri, G. Passive components for reducing environmental impacts of buildings: Analysis of an experimental green roof. In Proceedings of the 2020 IEEE 20th Mediterranean Electrotechnical Conference, Palermo, Italy, 16–18 June 2020; pp. 494–499. [Google Scholar] [CrossRef]
- Pandey, V.C.; Gajic, G.; Sharma, P.; Roy, M. Adaptive phytoremediation practices for sustaining ecosystem services. In Adaptive Phytoremediation Practices: Resilience to Climate Change; Elsevier: Amsterdam, The Netherlands, 2022; pp. 181–225. [Google Scholar] [CrossRef]
- Cirrincione, L.; Di Dio, S.; Peri, G.; Scaccianoce, G.; Schillaci, D.; Rizzo, G. A win-win scheme for improving the environmental sustainability of university commuters’ mobility and getting environmental credits. Energies 2022, 15, 396. [Google Scholar] [CrossRef]
- Cirrincione, L.; Marvuglia, A.; Scaccianoce, G. Assessing the effectiveness of green roofs in enhancing the energy and indoor comfort resilience of urban buildings to climate change: Methodology proposal and application. Build. Environ. 2021, 205, 108198. [Google Scholar] [CrossRef]
- Ashraf, S.S.; Ali, Q.; Zahir, Z.A.; Ashraf, S.S.; Asghar, H.N. Phytoremediation: Environmentally sustainable way for reclamation of heavy metal polluted soils. Ecotoxicol. Environ. Saf. 2019, 174, 714–727. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.; Daverey, A. Phytoremediation: A multidisciplinary approach to clean up heavy metal contaminated soil. Environ. Technol. Innov. 2020, 18, 100774. [Google Scholar] [CrossRef]
- Sheoran, V.; Singh Sheoran, A.; Poonia, P. Factors affecting phytoextraction: A review. Pedosphere 2016, 26, 148–166. [Google Scholar] [CrossRef]
- Barbafieri, M.; Pedron, F.; Petruzzelli, G.; Rosellini, I.; Franchi, E.; Bagatin, R.; Vocciante, M. Assisted phytoremediation of a multi-contaminated soil: Investigation on arsenic and lead combined mobilization and removal. J. Environ. Manag. 2017, 203, 316–329. [Google Scholar] [CrossRef]
- Evangelou, M.W.H.; Ebel, M.; Schaeffer, A. Chelate assisted phytoextraction of heavy metals from soil. Effect, mechanism, toxicity, and fate of chelating agents. Chemosphere 2007, 68, 989–1003. [Google Scholar] [CrossRef]
- Pedron, F.; Grifoni, M.; Barbafieri, M.; Petruzzelli, G.; Rosellini, I.; Franchi, E.; Bagatin, R.; Vocciante, M. Applicability of a Freundlich-like model for plant uptake at an industrial contaminated site with a high variable arsenic concentration. Environments 2017, 4, 67. [Google Scholar] [CrossRef]
- Petruzzelli, G.; Grifoni, M.; Barbafieri, M.; Rosellini, I.; Pedron, F. Sorption: Release processes in soil-the basis of phytoremediation efficiency. In Phytoremediation; Ansari, A.A., Gill, S.S., Gill, R., Lanza, G.R., Newman, L., Eds.; Springer: Cham, Switzerland, 2018; pp. 91–112. [Google Scholar] [CrossRef]
- Manoj, S.R.; Karthik, C.; Kadirvelu, K.; Arulselvi, P.I.; Shanmugasundaram, T.; Bruno, B.; Rajkumar, M. Understanding the molecular mechanisms for the enhanced phytoremediation of heavy metals through plant growth promoting rhizobacteria: A review. J. Environ. Manag. 2020, 254, 109779. [Google Scholar] [CrossRef]
- Vocciante, M.; Grifoni, M.; Fusini, D.; Petruzzelli, G.; Franchi, E. The role of plant growth-promoting rhizobacteria (PGPR) in mitigating plant’s environmental stresses. Appl. Sci. 2022, 12, 1231. [Google Scholar] [CrossRef]
- Abbaszadeh-Dahaji, P.; Omidvari, M.; Ghorbanpour, M. Increasing phytoremediation efficiency of heavy metal-contaminated soil using PGPR for sustainable agriculture. In Plant-Microbe Interaction: An Approach to Sustainable Agriculture; Choudhary, D.K., Varma, A., Narendra, T., Eds.; Springer: Singapore, 2017; pp. 187–204. [Google Scholar] [CrossRef]
- Pietrini, I.; Grifoni, M.; Franchi, E.; Cardaci, A.; Pedron, F.; Barbafieri, M.; Petruzzelli, G.; Vocciante, M. Enhanced lead phytoextraction by endophytes from indigenous plants. Soil Syst. 2021, 5, 55. [Google Scholar] [CrossRef]
- Franchi, E.; Barbafieri, M.; Petruzzelli, G.; Ferro, S.; Vocciante, M. Screening of plants and indigenous bacteria to improve arsenic phytoextraction. Appl. Sci. 2022, 12, 7267. [Google Scholar] [CrossRef]
- ISO 18763; 2016 Soil Quality—Determination of the Toxic Effects of Pollutants on Germination and Early Growth of Higher Plants. International Organization for Standardization: Geneva, Switzerland, 2016.
- Picchi, C.; Giorgetti, L.; Morelli, E.; Landi, M.; Rosellini, I.; Grifoni, M.; Franchi, E.; Petruzzelli, G.; Barbafieri, M. Cannabis sativa L. and Brassica juncea L. grown on arsenic-contaminated industrial soil: Potentiality and limitation for phytoremediation. Environ. Sci. Pollut. Res. Int. 2022, 29, 15983–15998. [Google Scholar] [CrossRef] [PubMed]
- Franchi, E.; Cosmina, P.; Pedron, F.; Rosellini, I.; Barbafieri, M.; Petruzzelli, G.; Vocciante, M. Improved arsenic phytoextraction by combined use of mobilizing chemicals and autochthonous soil bacteria. Sci. Total Environ. 2019, 655, 328. [Google Scholar] [CrossRef] [PubMed]
- Sparks, D.L. Methods of soil analysis, Part 3. Chemical Methods. In Soil Science Society of America Book Series; Soil Science Society of America: Madison, WI, USA, 1998. [Google Scholar]
- Haynes, W. Tukey’s Test. In Encyclopedia of Systems Biology; Dubitzky, W., Wolkenhauer, O., Cho, K.H., Yokota, H., Eds.; Springer: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Violante, A.; Pigna, M.; Del Gaudio, S. Adsorption-desorption processes of arsenate in soil environments. In Soil Abiotic & Biotic Interactions and the Impact on the Ecosystem & Human Welfare; Huang, P.M., Bollag, J.-M., Violante, A., Vityakon, P., Eds.; Science Publishers: Enfield, NH, USA, 2005; pp. 269–299. [Google Scholar]
- Dehner, C.A.; Awaya, J.D.; Maurice, P.A.; DuBois, J.L. Roles of siderophores, oxalate, and ascorbate in mobilization of iron from hematite by the aerobic bacterium Pseudomonas mendocina. Appl. Environ. Microbiol. 2010, 76, 2041–2048. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Zhang, G.; Lin, J.; Zeng, X.; Ma, X.; Wang, X.; Wang, S.; Jia, Y. The stability of Fe(III)-As(V) co-precipitate in the presence of ascorbic acid: Effect of pH and Fe/As molar ratio. Chemosphere 2019, 218, 670–679. [Google Scholar] [CrossRef]
- Larsen, O.; Postma, D.; Jakobsen, R. The reactivity of iron oxides towards reductive dissolution with ascorbic acid in a shallow sandy aquifer (Rømø, Denmark). Geochim. Cosmochim. Acta 2006, 70, 4827–4835. [Google Scholar] [CrossRef]
- Müller, H.; Bourcet, L.; Hanfland, M. Iron(II) oxalate dehydrate—Humboldtine: Synthesis, spectroscopic and structural properties of a versatile precursor for high pressure research. Minerals 2021, 11, 113. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).