Featured Application
Microbial lipids from Yarrowia lipolytica are suitable feedstocks for the biodiesel industry. Two-stage batch cultures (growth phase in glucose or glycerol, followed by a lipogenic phase in volatile fatty acids) are suitable strategies for the improvement of lipids production.
Abstract
Microbial lipids produced by Y. lipolytica have the potential to be used as feedstock for the biodiesel industry, but the high costs of pure substrates used for its production are limiting the potential of this application. Volatile fatty acids (VFAs), obtained in anaerobic fermentation of organic wastes, are inexpensive carbon sources for the cost-effective production of microbial lipids. In this work, two-stage batch cultures were tested as a strategy to improve lipids production by Y. lipolytica W29. The process consists of a first growth phase in glucose or glycerol, followed by a lipogenic phase in VFAs medium composed of a mixture of acetate, propionate, and butyrate. The addition of three pulses of 6 g·L−1 VFAs mixture, or a single pulse of 18 g·L−1 VFAs mixture, in the lipogenic phase boosted microbial lipids production (23–25%, w/w) and prevented lipids mobilization. Microbial lipids synthesized in such conditions are mainly composed of oleic acid (54%) with an unsaturated/saturated fraction above 78%. The main properties of biodiesel produced from Y. lipolytica W29 lipids are within the ranges of the EU biodiesel standard EN 14214.
1. Introduction
Microbial lipids, also known as microbial oils, bio-oils, or single-cell oils (SCO), have been under the spotlight during the last decades owing to their potential application as food supplements and nutraceuticals—polyunsaturated fatty acids (PUFA), such as those belonging to the omega-3 and omega-6 lipids—or as a renewable energy source for the biodiesel industry—saturated (SFA) and monounsaturated (MUFA) fatty acids [1].
Oleaginous yeasts are able to accumulate more than 20% (w/w) of their dry weight as intracellular lipids, mainly in the form of triacylglycerols (TAG). Comparatively to other oleaginous microorganisms (e.g., filamentous fungi or microalgae) or vegetable crops, yeasts have many attractive characteristics including high biomass and lipids productivity, utilization of several substrates, less seasonal or climate dependence, short life cycle, higher metal ion tolerance and the relative ease of culturing [2,3]. In particular, the non-conventional yeast Yarrowia lipolytica has been considered a model yeast for microbial lipids production, owing to its superior capacity for lipids accumulation and ability to use various and cheap raw materials, such as wastes and by-products of the agro-industrial sector [4]. Lipids accumulated by Y. lipolytica have, in general, a similar fatty acid composition and energy value to common vegetable oils, making them a promising feedstock for biodiesel production [1].
Biodiesel is a non-toxic, sulfur-free, renewable, and biodegradable fuel, and its combustion emits fewer greenhouse gases than petroleum diesel [5]. Biodiesel is mainly obtained from vegetable oils, but the limited supply and high cost of these feedstocks make its production not economically viable [6]. Biodiesel derived from Y. lipolytica lipids is a promising technology and does not compete with arable land for food production as in the case of crop-based oil resources used as raw material for biodiesel production. However, the high costs of carbon and nitrogen sources, the amount of lipids accumulated per unit of cellular mass, and the post-processing costs associated with oil extraction from cells are still challenges for the large-scale production of microbial lipids [7]. In this regard, the use of abundant, renewable, and low-value raw materials as substrates for microbial lipids production decreases the overall costs and enables the development of a more sustainable and affordable process within the circular bioeconomy assumptions.
Volatile fatty acids (VFAs), generated as intermediates during anaerobic fermentation of organic wastes, have been selected as a renewable and low-cost feedstock for microbial lipids production by Yarrowia species [8,9,10,11]. Regardless of the substrate and anaerobic fermentation conditions, VFAs comprise short-chain organic acids (C2–C6), specifically, acetic (30–80%), propionic (5–32%), and butyric (11–30%) acids [9,12]. Compared to sugar-based substrates, these organic acids have higher theoretical conversion yields and shorter metabolic pathways to intracellular lipids [13]. The main strategies reported so far have studied the conversion of VFAs to microbial lipids in batch cultures [9,10,11,12]. Previous works have demonstrated that the two-stage batch strategy (growth phase in glucose, followed by the addition of VFAs for lipid synthesis) enhances microbial lipids production by Y. lipolytica NCYC 2904 [9]. Thus, this study aims to evaluate the ability of Y. lipolytica W29 to synthesize intracellular lipids from a VFA mixture (acetate, propionate, and butyrate) during the lipogenic phase after yeast growth in glucose or glycerol. For the first time, a two-stage batch strategy with 3 pulses of 6 g·L−1 VFA mixture was tested. Yarrowia lipolytica W29 was able to assimilate 18 g·L−1 VFAs without the inhibition of VFA uptake rate and cellular growth, producing higher amounts of microbial lipids. The estimation of the properties of biodiesel produced from Y. lipolytica W29 lipids indicates that these lipids have a high value for the biodiesel industry. The results obtained are quite promising for the development of a more economical and sustainable bioprocess for the production of microbial lipids by a Y. lipolytica wild strain from VFAs, particularly in future works using organic wastes-derived VFAs.
2. Materials and Methods
2.1. Yeast Strain and Pre-Inoculum Preparation
Yarrowia lipolytica W29 pre-grew in YPD medium (10 g·L−1 yeast extract, 20 g·L−1 peptone, 20 g·L−1 glucose) and was preserved in cryostocks (800 μL of yeast culture, 200 μL pure glycerol) at −80 °C. One cryostock was used to inoculate the pre-inoculum (YPD medium), which was incubated at 170 rpm and 27 °C in an orbital incubator. After 19 h of growth, yeast cells were centrifuged and resuspended in the culture medium at an initial biomass concentration of 0.5 g·L−1.
2.2. Microbial Lipids Production in Two-Stage Batch Cultures
Two-stage batch cultures were firstly carried out in 500-mL baffled Erlenmeyer flasks filled with 200 mL of medium. Yarrowia lipolytica W29 cells grew for 24 h in glucose or glycerol medium (20 g·L−1 glucose or glycerol, 0.5 g·L−1 yeast extract, 1.7 g·L−1 YNB without amino acids and without ammonium sulfate, and ammonium sulfate to obtain an initial C/N mass ratio of 75)—growth phase. This phase was followed by the addition of (a) one pulse of 6 g·L−1 VFA mixture (2 g·L−1 propionate, 2 g·L−1 butyrate, and 2 g·L−1 acetate); or (b) 3 pulses of 6 g·L−1 VFA mixture (24 h, 48 h, 72 h)—lipogenic phase. Additionally, an experiment with a single pulse of 18 g·L−1 VFA mixture (6 g·L−1 propionate, 6 g·L−1 butyrate, and 6 g·L−1 acetate) after yeast growth in glucose for 24 h was also performed. All experiments were carried out at an initial pH of 6, at 27 °C and 170 rpm in an orbital incubator.
2.3. Analytical Methods
Samples were taken at appropriate intervals for the quantification of biomass, glucose, glycerol, and VFAs concentration, intracellular lipids content, and long-chain fatty acids (LCFA) composition. Biomass concentration was quantified by optical density (λ = 600 nm) and converted to cellular dry weight (g·L−1) by a previously constructed calibration curve. Glycerol, glucose, and VFAs (propionate, butyrate, and acetate) concentrations were quantified by HPLC in an Aminex HPX-87H column (300 mm × 7.8 mm, 8 μm particle size) at 60 °C and coupled with UV and RI detectors. The mobile phase was H2SO4 5 mM at a flow rate of 0.5 mL·min−1.
Intracellular lipids were measured in freeze-dried cells (10 mg) after extraction with chloroform and methanol (2 mL, 1:1 v/v), and quantified by the phosphovanillin colorimetric method [14]. Freeze-dried cells and solvents were vortex-mixed for 3 min, and 250 μL of the mixture was heated at 100 °C until the evaporation of the solvents. Then, 100 μL of H2SO4 98% were added to the solution and heated at 100 °C for 15 min. After cooling to room temperature, 2.4 mL of phosphovanillin reagent (1.2 g·L−1 vanillin dissolved in orthophosphoric acid 85%) were added, and the mixture rested for 15 min allowing the reaction between the phosphovanillin solution and the lipids, producing a pink color. The final absorbance was measured at 490 nm and converted to lipids concentration (g·L−1) by a calibration curve using olive oil as standard.
The LCFA composition of intracellular lipids was measured in freeze-dried cells after extraction with chloroform, and derivatization into their fatty acid methyl esters (FAME) with a mixture of methanol–sulfuric acid (85:15, v/v), using pentadecanoic acid (C15:0) as internal standard [14]. FAME in the organic phase was analyzed by GC using a capillary column (TRACSIL TRWAX 30 m × 0.25 mm × 0.25 mm) and an FID detector. Helium at a flow rate of 1 mL·min−1 was used as the carrier gas, and the temperatures of the injector and the detector were 220 °C and 250 °C, respectively. For total separation of FAME, a temperature gradient (50 °C for 2 min, followed by an increase of 10 °C·min−1 up to 225 °C, which was maintained by 10 min) was used. The identification of FAME was performed by the comparison of the retention times with a mixture of FAME standards. The relative amount of each FAME (%, w/w) was defined as the ratio between its concentration (g·L−1) and the total FAME detected in the sample.
2.4. Biodiesel Properties
The principal properties of biodiesel (cetane number, higher heating value, cloud point, oxidative stability, iodine value, cold filter plugging point, degree of unsaturation, kinematic viscosity, density, pour point, and saponification value) were estimated by considering the fatty acids composition of microbial lipids, using the software BiodieselAnalyzer© Ver. 2.2 (available on “http://www.brteam.ir/biodieselanalyzer”, accessed on 10 July 2022) [15].
2.5. Statistical Analysis
The results of all experiments are presented as the mean of two independent biological replicates. One-way analysis of variance (ANOVA) was used for the statistical analysis of the data, and the significant differences among means (p < 0.05) were identified by Tukey’s multi-range test (Statgraphics Centurion XVI software, Version 16.2.04).
3. Results
3.1. Microbial Lipids Production in Two-Stage Batch Cultures
The potential of Y. lipolytica W29 to produce microbial lipids using VFAs as substrate was evaluated in two-stage batch cultures (TSC). Yarrowia lipolytica W29 grew for 24 h in glucose or glycerol (growth phase) to obtain a higher cell density, followed by the addition of one pulse of 6 g·L−1 VFA mixture (lipogenic phase) to produce microbial lipids. During the first phase, yeast growth was similar in both substrates, no lag phase was observed, and the biomass concentration obtained after 24 h of cultivation was similar (Figure 1a,b). Glucose and glycerol were completely consumed after 24 h with a similar uptake rate (Table 1). In the lipogenic phase, Y. lipolytica cells continued to grow due to the addition of VFAs that were also used for biomass proliferation, but with a lower specific growth rate. The stationary growth phase was reached at 48 h in both experiments—coinciding with the VFA consumption—and the final biomass concentration and the global biomass yield were statistically equal (p ≥ 0.05). VFAs were consumed by the yeast cells with different uptake rates, depending on the substrate used in the growth phase (Table 1). However, in both conditions, a preference of the yeast for acetate was noted, the latter being completely consumed 12 h after the VFAs addition. Butyrate took 24 h to be assimilated in both experiments, whereas butyrate was consumed after 24 h and 30 h of VFAs addition to glucose (Figure 1a) and glycerol (Figure 1b) media, respectively.
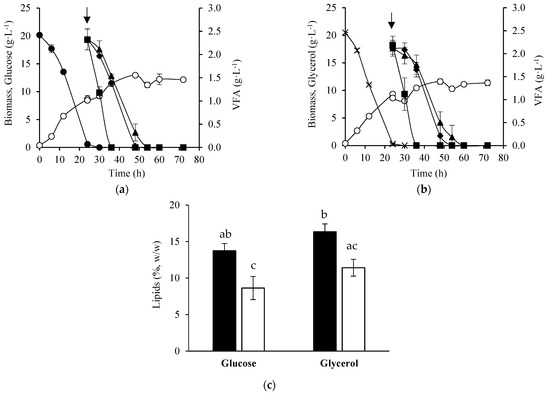
Figure 1.
Time course of cellular growth (○), VFAs (■, acetate; ▲, propionate; ♦, butyrate), glucose (●), and glycerol (x) concentration obtained in two-stage batch cultures of Y. lipolytica W29. After 24 h of growth in glucose (a) or glycerol (b), a mixture of 6 g·L−1 VFAs (2 g·L−1 acetate, 2 g·L−1 propionate, and 2 g·L−1 butyrate) was added to the culture medium. Lipids content of Y. lipolytica cells at 48 h (black bars) and 72 h (white bars) of cultivation (c). The error bars represent the standard deviation of two independent replicates.

Table 1.
Values of global biomass yield (YX/S), maximum lipids concentration and substrate (glucose or glycerol) uptake rate, and VFAs (RVFA) obtained in Y. lipolytica W29 two-stage batch cultures. A mixture of 6 g·L−1 VFAs (2 g·L−1 acetate, 2 g·L−1 propionate, and 2 g·L−1 butyrate) was added in each pulse. Data are average ± standard deviation of two independent replicates. Values followed by the same letter in each column do not present statistically significant differences (p ≥ 0.05).
The lipids content of Y. lipolytica W29 cells was not affected by the substrate used during the growth phase. Regardless of the substrate used in the growth phase, the yeast cells accumulated higher amounts of lipids at 48 h of cultivation (24 h after the VFAs addition), decreasing 1.5 times at 72 h (48 h after the VFAs addition) (Figure 1c). In all conditions, the maximum lipids production occurred at the end of the exponential growth phase, and utilization of intracellular lipids for metabolic activities was observed in the stationary phase owing to the complete depletion of the substrates (Figure 1a,b).
As VFAs were completely consumed 30 h after the addition, and a lipid mobilization was observed in the lipogenic phase, two-stage batch cultures with 3 pulses of 6 g·L−1 VFA mixture (24 h, 48 h, 72 h) were carried out as an attempt to prevent substrate depletion, lipids turnover, and to boost lipids production. No enhancement in global biomass yield was attained in the experiments with 3 pulses compared to cultures with a single pulse (Table 1), but a higher biomass concentration was reached in the glycerol medium after 3 pulses of VFAs (Figure 2b). Yarrowia lipolytica cells continued to grow after the addition of the first two pulses of VFAs, particularly in the experiments in which the growth phase was carried out with glycerol. However, yeast cells reached the stationary growth phase after the third pulse in both conditions, despite the almost total VFA consumption (Figure 2a,b). Moreover, with the addition of two further pulses, VFAs were consumed at a similar uptake rate to that observed after the first pulse. These results suggest that VFAs were channeled towards cellular maintenance, lipids biosynthesis, or other metabolites instead of cell proliferation.
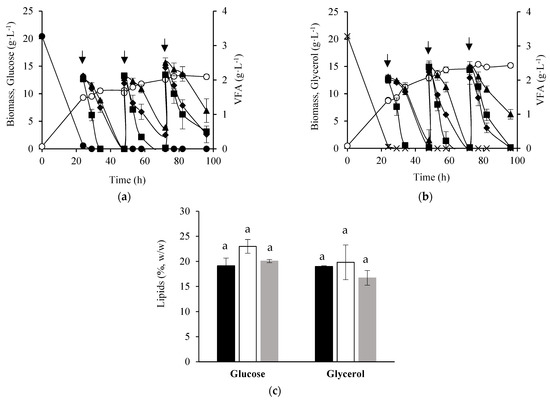
Figure 2.
Time course of cellular growth (○), VFAs (■, acetate; ▲, propionate; ♦, butyrate), glucose (●), and glycerol (x) concentration obtained in two-stage batch cultures of Y. lipolytica W29. After 24 h of growth in glucose (a) or glycerol (b), three pulses of a mixture of 6 g·L−1 VFAs (2 g·L−1 acetate, 2 g·L−1 propionate, and 2 g·L−1 butyrate) was added to the culture medium at 24 h, 48 h, and 72 h. Lipids content of Y. lipolytica cells at 48 h (black bars), 72 h (white bars), and 96 h (grey bars) of cultivation (c). The error bars represent the standard deviation of two independent replicates. Bars with the same letter do not present statistically significant differences (p ≥ 0.05).
The accumulation of microbial lipids occurred simultaneously with yeast growth, and the lipids content was similar throughout the lipogenic phase, demonstrating that the cultures with 3 pulses of VFAs were an effective strategy to prevent lipid turnover (Figure 2c). Moreover, an approximately 2-fold improvement in lipid concentration was attained in cultures with 3 pulses, compared to those obtained in cultures with a single pulse (Table 1).
As Y. lipolytica demonstrated the ability to consume a total VFA concentration of 18 g·L−1 (divided into 3 pulses) and with a higher lipids productivity, an additional experiment was carried out with a 24 h first stage of yeast growth in glucose, followed by the addition of a single pulse of 18 g·L−1 VFAs (mixtures of acetate, butyrate, and propionate with 6 g·L−1 of each one) in the second stage. From an operational point of view, and considering the industrial production of lipids, a single addition would be more advantageous. Moreover, other Y. lipolytica strains have already shown the ability to assimilate high VFA amounts without inhibitory effects [8]. Yarrowia lipolytica was able to consume 18 g·L−1 VFAs without inhibitory effects on cellular growth (Figure 3a) and VFA uptake rate. No statistical differences were observed in the VFA uptake rate between experiments with a pulse of 18 g·L−1 VFAs (0.19 g·L−1·h−1 ± 0.002 g·L−1·h−1) and with a pulse of 6 g·L−1 VFAs. As occurred in the previous experiments, the yeast cells demonstrated a preference for acetate, which was totally consumed 48 h after the pulse. By contrast, butyrate took 72 h to be completely assimilated, and a small amount of propionate (1.5 g·L−1) remained in the medium at the end of the cultivation. Other studies have also reported that Y. lipolytica strains assimilate acetic acid faster than propionate and butyrate [8,9,10]. The global biomass yield in these experiments (0.28 g·g−1 ± 0.01 g·g−1) was lower than those obtained with a single pulse of 6 g·L−1 VFAs and three pulses of 6 g·L−1 VFAs each, suggesting that VFAs were more directed to lipids synthesis than cellular growth. In fact, the lipids content (Figure 3b) was higher (p < 0.05) than that attained in the previous two-stage batch culture strategies. Moreover, no significant mobilization of lipids was observed during the cultivation, except for the last point (96 h) owing to the depletion of VFAs. The maximum lipids concentration was similar after the addition of one pulse (18 g·L−1) or three pulses of VFAs, and no differences in the maximum lipids productivity were observed between these two-stage batch strategies. Thus, a two-stage batch culture with a single pulse of 18 g·L−1 VFAs is an efficient strategy to improve lipids production without significant lipids mobilization in the lipogenic phase.
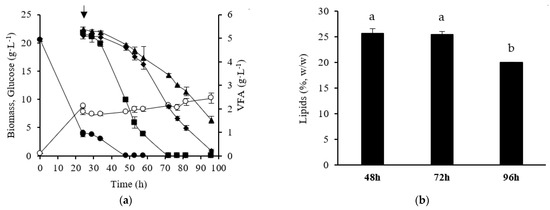
Figure 3.
Time course of cellular growth (○), VFAs (■, acetate; ▲, propionate; ♦, butyrate), and glucose (●) concentration obtained in two-stage batch cultures of Y. lipolytica W29 (a). After 24 h of growth in glucose, one single pulse of a mixture of 18 g·L−1 VFAs was added to the culture medium. Lipids content of Y. lipolytica cells obtained at 48 h, 72 h, and 96 h of cultivation (b). The error bars represent the standard deviation of two independent replicates. Bars with the same letter do not present statistically significant differences (p ≥ 0.05).
Microbial lipids produced in two-stage batch cultures of Y. lipolytica W29 (growth phase in glucose followed by the addition of 18 g·L−1 VFAs) were mainly composed of oleic acid (54% ± 1%), followed by linoleic (15.1% ± 0.2%), palmitic (11.9% ± 0.4%), stearic (7.7% ± 1.0%), and palmitoleic (5.5% ± 0.2%) acids. Due to the presence of propionate in the lipogenic phase [8], odd-chain fatty acids such as heptadecenoic (3.8% ± 0.4%) and margaric (2.2% ± 0.3%) acid were also produced by Y. lipolytica. Lipids produced in these conditions have a significantly higher unsaturated fraction (>78%, p < 0.05) than saturated one.
3.2. Biodiesel Properties Obtained from Y. lipolytica W29 Lipids
Based on the fatty acid composition of Y. lipolytica W29 lipids, synthesized in two-stage batch cultures with a pulse of 18 g·L−1 VFAs, several properties that assess the quality of biodiesel were estimated using the BiodieselAnalyzer© software. The main parameters predicted—density, kinematic viscosity, cetane number, oxidative stability, and iodine value—are within the ranges of the EU biodiesel standard EN 14214 (Table 2). Moreover, some of the parameters, namely density, kinematic viscosity, cetane number, higher heating value, oxidative stability, and iodine value are comparable with those of biodiesel produced from lipids of other Y. lipolytica strains growing in VFAs [9] and non-detoxified liquid wheat straw hydrolysate [16].

Table 2.
Biodiesel properties obtained from Y. lipolytica W29 lipids estimated by the BiodieselAnalyzer© software, and comparison with other Y. lipolytica strains and the EU biodiesel standard EN 14214.
4. Discussion
The amount of microbial lipids produced in batch cultures is usually low owing to the difficulties of optimizing the culture and operational conditions for simultaneous cellular growth (nitrogen-rich media) and lipids synthesis (nitrogen-limited media) [17]. Two-stage batch cultures, an approach that encompasses two distinct phases—the growth phase (1st phase), followed by the lipid synthesis in the lipogenic phase (2nd phase)—was proved to be advantageous for increasing lipids production by Y. lipolytica NCYC 2904 [9], Y. lipolytica MUCL 28849 [18], Cryptococcus curvatus [19], and Apiotrichum porosum [20] (Table 3). Thus, in an attempt to achieve high lipids concentration in Y. lipolytica W29 cultures, several experiments were carried out in which the cell proliferation phase in glucose or glycerol was followed by the lipogenic phase in VFAs. Regardless of the substrate (glucose or glycerol) used in the growth phase, and the two-stage batch strategy (single pulse of 6 g·L−1 VFAs or three pulses of 6 g·L−1 VFAs each), Y. lipolytica W29 was able to accumulate lipids using VFAs as a sole carbon source (Table 1). However, two distinct patterns of lipids production were observed according to the addition strategy: (1) in experiments with a single VFAs pulse, lipids content decreased from 48 h to 72 h of cultivation; and (2) in experiments with 3 pulses of VFAs, a slight increase of lipids content occurred from 48 h to 72 h, without a further significant decrease. In the first strategy, VFAs were completely consumed after 24 h of addition (48 h of cultivation) and no other carbon source remained in the culture medium until the end of the experiments (Figure 1). Thus, lipids synthesized by Y. lipolytica W29 up to 48 h were mobilized for cellular metabolic activities during the stationary growth phase. By contrast, the addition of 3 pulses made it possible to maintain the VFA concentration above zero during 96 h of cultivation (Figure 2), which contributed to preventing lipid turnover. Moreover, the maximum lipids content was reached in the stationary growth phase, indicating that the VFAs added were channeled towards lipids biosynthesis instead of biomass production. Furthermore, the maximum lipids content obtained in experiments with 3 pulses was 1.6 times higher than that attained with a single pulse. The excess of a carbon source and depletion of nitrogen are the principal factors that trigger the onset of lipogenesis in Y. lipolytica growing in hydrophilic substrates [21]. In fact, in experiments with a single pulse of 18 g·L−1 VFAs, a higher synthesis of lipids was obtained compared to the other two-stage batch strategies, particularly 24 h after the addition of VFAs. By contrast, this was the experiment where lower biomass production was reached. In this condition, there was a residual amount of glucose in the medium at the moment of VFAs addition, and a higher amount of VFAs was supplemented at once, resulting in a higher carbon/nitrogen (C/N) ratio, and favoring lipids production. According to the literature, in a de novo lipid biosynthetic pathway—as occurs from glucose and acetate—high C/N ratios favor lipids synthesis instead of yeast cell proliferation. This metabolic route is generally induced by the limitation of nitrogen, leading to a decrease in yeast growth, since this is an essential nutrient for nucleic acid and protein synthesis. Yet, the carbon sources continue to be assimilated and are channeled for lipids biosynthesis [17,21]. It has been reported that the biosynthesis of microbial lipids by Y. lipolytica from butyrate and propionate is an ex novo metabolic pathway [22], which is independent of the C/N ratio [21]. However, in this study, a clear preference for acetate—which is uptaken via the de novo pathway—was observed. Similar results were obtained by Pereira et al. [9], demonstrating that the C/N ratio is an important factor affecting lipids production by Y. lipolytica strains during the lipogenic phase.

Table 3.
Comparison of biomass and microbial lipids concentration obtained by different microbial species from VFAs in two-stage batch and two-stage fed-batch cultures.
Microbial lipids synthesized by Y. lipolytica W29 in two-stage batch cultures were mainly composed of unsaturated fatty acids (>78%), oleic acid being the predominant fatty acid. The high resemblance between the composition of Y. lipolytica lipids and vegetable oils makes these bio-oils a potential substitute for vegetable oils as feedstock for biodiesel production. Moreover, the odd-chain fatty acids (heptadecenoic and margaric acids) synthesized in these conditions will improve the fuel properties of biodiesel produced from Y. lipolytica lipids [23]. Other Y. lipolytica strains, growing in VFAs [8,9,24], glycerol [25], or in hydrophobic substrates—waste cooking oils [14] and lard [26]—accumulated preferentially unsaturated fatty acids (oleic and linoleic acids). This behavior suggests that lipogenic features of Y. lipolytica prevail over the type of substrate.
The potential of biodiesel to replace fossil-based fuel is evaluated by several parameters, namely kinematic viscosity, density, cetane number, iodine value, and oxidation stability, among others. These properties are affected by the fatty acid composition of oils used in the transesterification reactions, namely chain length, unsaturation degree, and number and position of double bonds. High saturated fatty acids, for example, avoid the auto-oxidation of biofuel and increase its shelf life, while the amount of unsaturated fatty acids has an effect on biodiesel’s cold flow plugging properties [16]. The cetane number of diesel fuel is an index of the ignition quality (ignition delay characteristics) and defines the quality of the combustion process in a diesel engine, affecting the stability, noise, and CO emissions. A low cetane number (long ignition delay, particularly in cold environments) implies that the majority of injected biodiesel burns explosively, resulting in loud diesel combustion noise, white smoke, and hydrocarbon emissions. By contrast, a high cetane number indicates a shorter ignition delay and a more uniform and complete burning of injected fuel, leading to better exhaust air quality. However, a cetane number higher than 65 results in excessive heating of the injector and in a reduction of biofuel efficiency [16,27]. The estimated cetane number for biodiesel produced from Y. lipolytica W29 lipids (56) meets this criterion and is comparable to the cetane number of biodiesel obtained from different vegetable oils [27], and bio-oils produced by Y. lipolytica NCYC 2904 [9]. The high heating value of biodiesel describes the heating energy released during the combustion, i.e., the efficiency of the biofuel. The value predicted herein (38 MJ·kg−1) is in the range of others found in the literature for biodiesel produced from pure and used vegetable oils, and bio-oils of Y. lipolytica strains grown in wheat straw hydrolysate [28,29] and VFAs [9]. The cold filter plugging point defines the lowest temperature, expressed in degrees Celsius (°C), at which a given volume of biodiesel easily flows through a standardized filtration device in a specific time when cooled under certain conditions [16]. Though there are no specific recommendations for the cold filter plugging point, the lower this value is, the better the cold flow properties of the biofuel. This is especially important in cold temperate countries since biodiesel with a high cold filter plugging point will clog the fuel line and filters more easily. The value of the cold filter plugging point estimated in this work (−0.6 °C) is lower than the value obtained using vegetable oils [27] and bio-oils of Trichosporon sp. [30], and higher than those obtained with lipids of other Y. lipolytica strains [9,16]. The degree of unsaturation and number of double bonds in fatty acids of oils has negative effects on the oxidative stability and biodiesel shelf life, since oxidatively unstable biodiesel leads to the formation of gums and sedimentation, decreasing the engine performance [16]. The oxidative stability obtained herein (10.4 h) is similar to that of biodiesel produced using lipids of different Y. lipolytica strains growing in VFAs [8] and detoxified and non-detoxified liquid wheat straw hydrolysate [31]. The kinematic viscosity of biodiesel, related to the ability of flowing, increases at low temperatures and affects the quantity and quality of injection and ignition. On the hand, lower values of kinematic viscosity result in fine particles of biodiesel with high speed and low mass [16]. The value of kinematic viscosity of biodiesel obtained from microbial lipids of Y. lipolytica W29 (this study) was similar to that for Y. lipolytica NCYC 2904 [9], R. fluviale, R. toruloides, and Lipomyces starkeyi, and higher than Y. lipolytica strains growing in detoxified and non-detoxified liquid wheat straw hydrolysate [16]. Other estimated properties, such as iodine value and density fall within a narrow range of the EU biodiesel standard EN 14214 and are similar to those of biodiesel produced from common vegetable oils and other oleaginous yeasts [16,27]. Therefore, the intracellular lipids of Y. lipolytica W29 accumulated from VFAs in two-stage batch cultures have a high potential to be used as feedstock for high-quality biodiesel production. Yet, it is possible to make the process even more economically competitive by increasing the concentration of lipids produced (grams of lipids per liter of medium) if high-cell density cultures were attained (e.g., using a fed-batch mode for biomass production).
5. Conclusions
The results demonstrate the potential of Y. lipolytica W29 to grow and produce microbial lipids from a mixture of VFAs (acetate, propionate, and butyrate) in two-stage batch cultures—growth phase in glucose or glycerol, followed by the lipogenic phase in VFAs. A single pulse of 6 g·L−1 VFAs strategy led to the mobilization of microbial lipids synthesized owing to the complete consumption of VFAs after 48 h of cultivation. However, the addition of a higher amount of VFAs (3 pulses of 6 g·L−1 VFAs each or 1 pulse of 18 g·L−1 VFAs) avoided the complete depletion of VFAs for the duration of the experiments, increased the C/N ratio, boosted the production of microbial lipids, and prevented the lipid turnover. Yarrowia lipolytica lipids, rich in unsaturated fatty acids (particularly oleic acid) and odd-chain fatty acids (heptadecenoic and margaric acids), are a promising feedstock for the bioenergy industry. The biodiesel produced from these lipids meets the criteria of the EU biodiesel standard EN 14214, demonstrating the potential of Y. lipolytica W29 as a pivot in the production of feedstock for high-quality biofuels from low-cost substrates, according to the biorefinery and bioeconomy circular guidelines. The results described herein open new perspectives on the development of an integrated bioprocess, in which crude glycerol (a by-product of the biodiesel industry) or glucose-rich by-products (e.g., lignocellulosic biomass hydrolysates) could be the substrate for cellular growth, and organic wastes-derived VFAs (obtained by anaerobic fermentation) will be used for lipids synthesis. In this sense, future research will focus on the two-stage batch production of microbial lipids by Y. lipolytica from a VFA-rich medium obtained in the anaerobic fermentation of food waste.
Author Contributions
Conceptualization, I.B. and M.L.; methodology, A.S.P.; validation, I.B. and M.L.; investigation, A.S.P.; resources, I.B.; writing—original draft preparation, A.S.P. and M.L.; writing—review and editing, I.B. and M.L.; supervision, I.B. and M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Portuguese Foundation for Science and Technology (FCT) under the scope of the strategic funding of UIDB/04469/2020 unit and Doctoral grant (SFRH/BD/129592/2017).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Patel, A.; Karageorgou, D.; Rova, E.; Katapodis, P.; Rova, U.; Christakopoulos, P.; Matsakas, L. An overview of potential oleaginous microorganisms and their role in biodiesel and omega-3 fatty acid-based industries. Microorganisms 2020, 8, 434. [Google Scholar] [CrossRef]
- Bao, W.; Li, Z.; Wang, X.; Gao, R.; Zhou, X.; Cheng, S.; Men, Y.; Zheng, L. Approaches to improve the lipid synthesis of oleaginous yeast Yarrowia lipolytica: A review. Renew. Sustain. Energ. Rev. 2021, 149, 111386. [Google Scholar] [CrossRef]
- Vasconcelos, B.; Teixeira, J.C.; Dragone, G.; Teixeira, J.A. Oleaginous yeasts for sustainable lipid production—from biodiesel to surf boards, a wide range of “green” applications. Appl. Microbiol. Biotechnol. 2019, 103, 3651–3667. [Google Scholar]
- Lopes, M.; Miranda, S.M.; Costa, A.R.; Pereira, A.S.; Belo, I. Yarrowia lipolytica as a biorefinery platform for effluents and solid wastes valorization—Challenges and opportunities. Crit. Rev. Biotechnol. 2022, 42, 163–183. [Google Scholar] [CrossRef]
- Zhang, L.; Loh, K.-C.; Kuroki, A.; Dai, Y.; Tong, Y.W. Microbial biodiesel production from industrial organic wastes by oleaginous microorganisms: Current status and prospects. J. Hazard. Mater. 2021, 402, 123543. [Google Scholar]
- Dharmaraja, J.; Nguyen, D.D.; Shobana, G.; Saratale, G.D.; Arvindnarayan, S.; Atabanif, A.E.; Chang, S.W.; Kumar, G. Engine performance, emission and bio characteristics of rice bran oil derived biodiesel blends. Fuel 2019, 239, 153–161. [Google Scholar]
- Jones, A.D.; Boundy-Mills, K.L.; Barla, G.F.; Kumar, S.; Ubanwa, B.; Balan, V. Microbial lipid alternatives to plant lipids. In Microbial Lipid Production. Methods and Protocols, 1st ed.; Balan, V., Ed.; Humana: New York, NY, USA, 2019; Volume 1995, pp. 1–32. [Google Scholar] [CrossRef]
- Pereira, A.S.; Miranda, S.M.; Lopes, M.; Belo, I. Factors affecting microbial lipids production by Yarrowia lipolytica strains from volatile fatty acids: Effect of co-substrates, operation mode and oxygen. J. Biotechnol. 2021, 331, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.S.; Lopes, M.; Miranda, S.M.; Belo, I. Bio-oil production for biodiesel industry by Yarrowia lipolytica from volatile fatty acids in two-stage batch culture. Appl. Microbiol. Biotechnol. 2022, 106, 2869–2881. [Google Scholar] [CrossRef]
- Gao, R.; Li, Z.; Zhou, X.; Cheng, S.; Zheng, L. Oleaginous yeast Yarrowia lipolytica culture with synthetic and food waste-derived volatile fatty acids for lipid production. Biotechnol. Biofuels 2017, 10, 247. [Google Scholar] [CrossRef]
- Llamas, M.; Dourou, M.; González-Fernández, C.; Aggelis, G.; Tomás-Pejó, E. Screening of oleaginous yeasts for lipid production using volatile fatty acids as substrate. Biomass Bioenergy 2020, 138, 105553. [Google Scholar] [CrossRef]
- Patel, A.; Sarkar, O.; Rova, U.; Christakopoulos, P.; Matsakas, L. Valorization of volatile fatty acids derived from low-cost organic waste for lipogenesis in oleaginous microorganisms—A review. Bioresour. Technol. 2021, 321, 124457. [Google Scholar] [CrossRef]
- Gong, Z.; Zhou, W.; Shen, H.; Yang, Z.; Wang, G.; Zuo, Z.; Hou, Y.; Zhao, Z.K. Co-fermentation of acetate and sugars facilitating microbial lipid production on acetate-rich biomass hydrolysates. Bioresour. Technol. 2016, 207, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.; Miranda, S.M.; Alves, J.M.; Pereira, A.S.; Belo, I. Waste cooking oils as feedstock for lipase and lipid-rich biomass production. Eur. J. Lipid Sci. Technol. 2019, 121, 1800188. [Google Scholar] [CrossRef]
- Talebi, A.F.; Tabatabaei, M.; Chisti, Y. BiodieselAnalyzer: A user-friendly software for predicting the properties of prospective biodiesel. Biofuel Res. J. 2014, 1, 55–57. [Google Scholar] [CrossRef]
- Patel, A.; Arora, N.; Mehtani, J.; Pruthi, V.; Pruthi, P.A. Assessment of fuel properties on the basis of fatty acid profiles of oleaginous yeast for potential biodiesel production. Renew. Sustain. Energy Rev. 2017, 77, 604–616. [Google Scholar] [CrossRef]
- Karamerou, E.E.; Webb, C. Cultivation modes for microbial oil production using oleaginous yeasts—A review. Biochem. Eng. J. 2019, 151, 107322. [Google Scholar] [CrossRef]
- Fontanille, P.; Kumar, V.; Christophe, G.; Nouaille, R.; Larroche, C. Bioconversion of volatile fatty acids into lipids by the oleaginous yeast Yarrowia lipolytica. Bioresour. Technol. 2012, 114, 443–449. [Google Scholar] [CrossRef]
- Chatterjee, S.; Mohan, S.V. Yeast fermentation towards biodiesel: Maximizing resource recovery by integrating with biohydrogen production in biorefinery framework. Biomass Bioenergy 2020, 142, 105747. [Google Scholar] [CrossRef]
- Qian, X.; Zhou, X.; Chen, L.; Zhang, X.; Xin, F.; Dong, W.; Zhang, W.; Ochsenreither, K.; Jiang, M. Bioconversion of volatile fatty acids into lipids by the oleaginous yeast Apiotrichum porosum DSM27194. Fuel 2021, 290, 119811. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Aggelis, G. Lipids of oleaginous yeasts. Part I: Biochemistry of single cell oil production. Eur. J. Lipid Sci. Technol. 2011, 113, 1031–1051. [Google Scholar] [CrossRef]
- Morales-Palomo, S.; González-Fernández, C.; Tomás-Pejó, E. Prevailing acid determines the efficiency of oleaginous fermentation from volatile fatty acids. J. Environ. Chem. Eng. 2022, 10, 107354. [Google Scholar] [CrossRef]
- Park, Y.K.; Dulermo, T.; Amaro, R.L.; Nicaud, J.M. Optimization of odd chain fatty acid production by Yarrowia lipolytica. Biotechnol. Biofuels 2018, 11, 158. [Google Scholar] [CrossRef] [Green Version]
- Gao, R.; Li, Z.; Zhou, X.; Bao, W.; Cheng, S.; Zheng, L. Enhanced lipid production by Yarrowia lipolytica cultured with synthetic and waste-derived high-content volatile fatty acids under alkaline conditions. Biotechnol. Biofuels 2020, 13, 3. [Google Scholar] [CrossRef]
- Poli, J.S.; da Silva, M.A.N.; Siqueira, E.P.; Pasa, V.M.D.; Rosa, C.A.; Valente, P. Microbial lipid produced by Yarrowia lipolytica QU21 using industrial waste: A potential feedstock for biodiesel production. Bioresour. Technol. 2014, 161, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.; Gomes, A.S.; Silva, C.M.; Belo, I. Microbial lipids and added value metabolites production by Yarrowia lipolytica from pork lard. J. Biotechnol. 2018, 265, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Yaşar, F. Comparision of fuel properties of biodiesel fuels produced from different oils to determine the most suitable feedstock type. Fuel 2020, 264, 116817. [Google Scholar] [CrossRef]
- Fassinou, W.F.; Sako, A.; Fofana, A.; Koua, K.B.; Toure, S. Fatty acids composition as a means to estimate the high heating value (HHV) of vegetable oils and biodiesel fuels. Energy 2010, 35, 4949–4954. [Google Scholar] [CrossRef]
- Nambou, K.; Zhao, C.; Wei, L.; Chen, J.; Imanaka, T.; Hua, Q. Designing of a “cheap to run” fermentation platform for an enhanced production of single cell oil from Yarrowia lipolytica DSM3286 as a potential feedstock for biodiesel. Bioresour. Technol. 2014, 173, 324–333. [Google Scholar] [CrossRef]
- Brar, K.K.; Sarma, A.K.; Aslam, M.; Polikarpov, I.; Chadha, B.S. Potential of oleaginous yeast Trichosporon sp., for conversion of sugarcane bagasse hydrolysate into biodiesel. Bioresour. Technol. 2017, 242, 161–168. [Google Scholar] [CrossRef]
- Yu, X.; Zheng, Y.; Dorgan, K.M.; Chen, S. Oil production by oleaginous yeasts using the hydrolysate from pretreatment of wheat straw with dilute sulfuric acid. Bioresour. Technol. 2011, 102, 6134–6140. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).