Pilot Scale Production of Single Cell Oil by Apiotrichum brassicae and Pichia kudriavzevii from Acetic Acid and Propionic Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Yeast Strains
2.2. Media
2.3. Cultivation in Laboratory Scale
2.4. Cultivation in Small Pilot Scale
2.5. Cultivation in Pilot Scale
2.6. Cell Harvest
2.7. SCO Extraction
2.8. Analysis of SCO Properties
2.9. Analysis of Lipid Content and FA Composition
2.10. Analysis of VFA Concentrations
2.11. Analysis of Nitrogen, Phosphorous Content, and OD600
3. Results
3.1. Cultivation
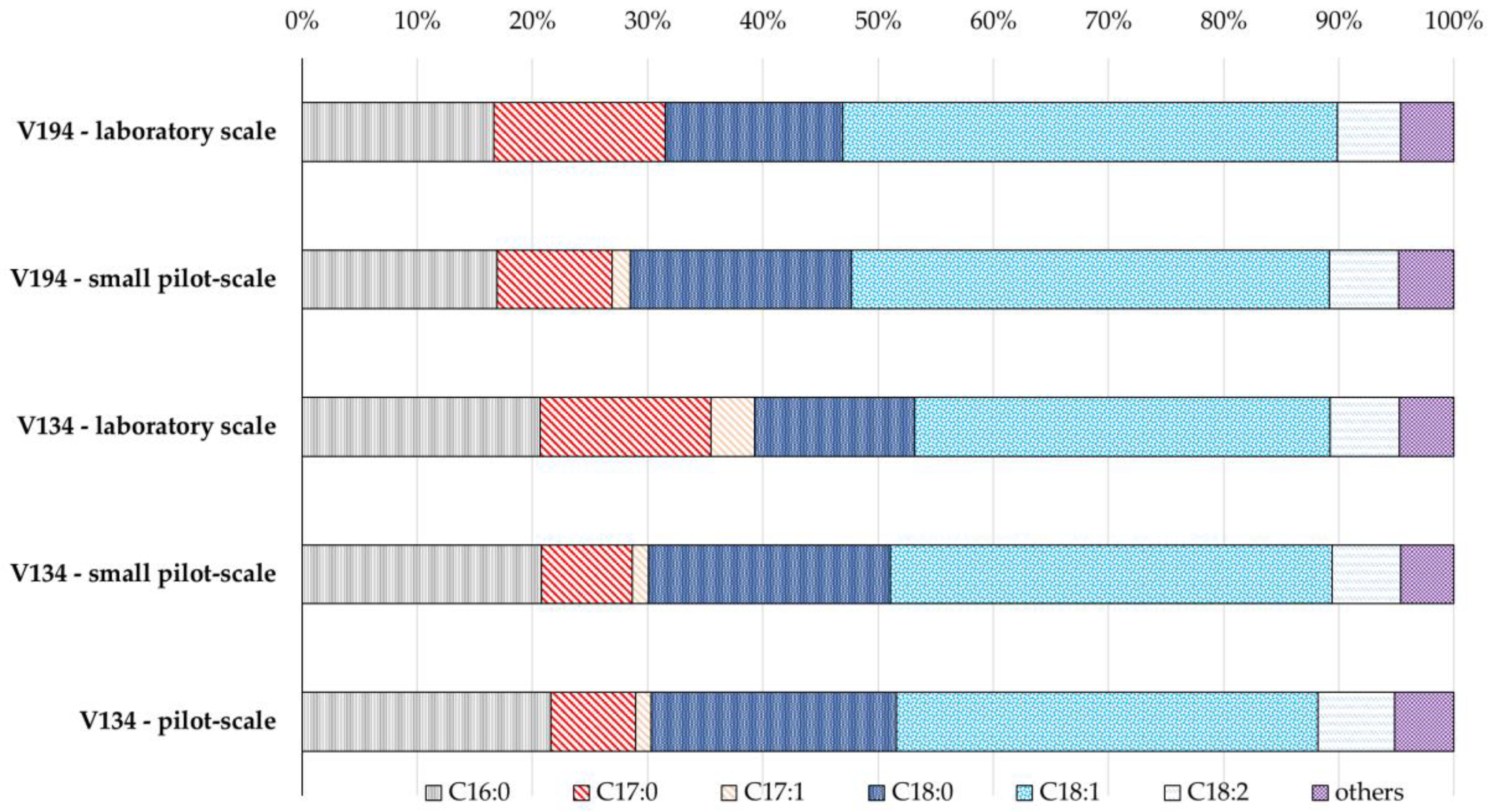
3.2. Fatty acid Spectrum
3.3. Extraction of SCO
3.4. Analysis of SCO Properties
4. Discussion
4.1. Cultivation
4.2. Extraction of SCO
4.3. Characterization of the Lipids and Economical Aspects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Papanikolaou, S.; Aggelis, G. Lipids of oleaginous yeasts. Part I: Biochemistry of single cell oil production. Eur. J. Lipid Sci. Technol. 2011, 113, 1031–1051. [Google Scholar] [CrossRef]
- Robles-Iglesias, R.; Naveira-Pazos, C.; Fernández-Blanco, C.; Veiga, M.C.; Kennes, C. Factors affecting the optimisation and scale-up of lipid accumulation in oleaginous yeasts for sustainable biofuels production. Renew. Sustain. Energy Rev. 2023, 171, 113043. [Google Scholar] [CrossRef]
- Moon, N.J.; Hammond, E.G.; Glatz, B.A. Conversion of Cheese Whey and Whey Permeate to Oil and Single-Cell Protein. J. Dairy Sci. 1978, 61, 1537–1547. [Google Scholar] [CrossRef]
- Gallego-García, M.; Moreno, A.D.; González, A.; Negro, M.J. Efficient use of discarded vegetal residues as cost-effective feedstocks for microbial oil production. Biotechnol. Biofuels Bioprod. 2023, 16, 21. [Google Scholar] [CrossRef] [PubMed]
- Muscat, A.; De Olde, E.M.; de Boer, I.; Ripoll-Bosch, R. The battle for biomass: A systematic review of food-feed-fuel competition. Glob. Food Secur. 2020, 25, 100330. [Google Scholar] [CrossRef]
- Poontawee, R.; Limtong, S. Feeding Strategies of Two-Stage Fed-Batch Cultivation Processes for Microbial Lipid Production from Sugarcane Top Hydrolysate and Crude Glycerol by the Oleaginous Red Yeast Rhodosporidiobolus fluvialis. Microorganisms 2020, 8, 151. [Google Scholar] [CrossRef] [PubMed]
- André, A.; Chatzifragkou, A.; Diamantopoulou, P.; Sarris, D.; Philippoussis, A.; Galiotou-Panayotou, M.; Komaitis, M.; Papanikolaou, S. Biotechnological conversions of bio-diesel-derived crude glycerol by Yarrowia lipolytica strains. Eng. Life Sci. 2009, 9, 468–478. [Google Scholar] [CrossRef]
- Gong, Z.; Zhou, W.; Shen, H.; Yang, Z.; Wang, G.; Zuo, Z.; Hou, Y.; Zhao, Z.K. Co-fermentation of acetate and sugars facilitating microbial lipid production on acetate-rich biomass hydrolysates. Bioresour. Technol. 2016, 207, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Ananthi, V.; Siva Prakash, G.; Chang, S.W.; Ravindran, B.; Nguyen, D.D.; Vo, D.-V.N.; La, D.D.; Bach, Q.-V.; Wong, J.; Kumar Gupta, S.; et al. Enhanced microbial biodiesel production from lignocellulosic hydrolysates using yeast isolates. Fuel 2019, 256, 115932. [Google Scholar] [CrossRef]
- Park, G.W.; Chang, H.N.; Jung, K.; Seo, C.; Kim, Y.-C.; Choi, J.H.; Woo, H.C.; Hwang, I. Production of microbial lipid by Cryptococcus curvatus on rice straw hydrolysates. Process Biochem. 2017, 56, 147–153. [Google Scholar] [CrossRef]
- Krikigianni, E.; Matsakas, L.; Rova, U.; Christakopoulos, P.; Patel, A. Investigating the Bioconversion Potential of Volatile Fatty Acids: Use of Oleaginous Yeasts Rhodosporidium toruloides and Cryptococcus curvatus towards the Sustainable Production of Biodiesel and Odd-Chain Fatty Acids. Appl. Sci. 2022, 12, 6541. [Google Scholar] [CrossRef]
- Gao, R.; Li, Z.; Zhou, X.; Bao, W.; Cheng, S.; Zheng, L. Enhanced lipid production by Yarrowia lipolytica cultured with synthetic and waste-derived high-content volatile fatty acids under alkaline conditions. Biotechnol. Biofuels 2020, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Llamas, M.; Tomás-Pejó, E.; González-Fernández, C. Volatile fatty acids from organic wastes as novel low-cost carbon source for Yarrowia lipolytica. New Biotechnol. 2020, 56, 123–129. [Google Scholar] [CrossRef]
- Fontanille, P.; Kumar, V.; Christophe, G.; Nouaille, R.; Larroche, C. Bioconversion of volatile fatty acids into lipids by the oleaginous yeast Yarrowia lipolytica. Bioresour. Technol. 2012, 114, 443–449. [Google Scholar] [CrossRef]
- Tomás-Pejó, E.; Morales-Palomo, S.; González-Fernández, C. Microbial lipids from organic wastes: Outlook and challenges. Bioresour. Technol. 2021, 323, 124612. [Google Scholar] [CrossRef] [PubMed]
- Velghe, F.; De Wilde, F.; Snellinx, S.; Farahbakhsh, S.; Belderbos, E.; Peral, C.; Wiedemann, A.; Hiessl, S.; Michels, J.; Pierrard, M.-A.; et al. Volatile fatty acid platform-a cornerstone for the circular bioeconomy. FEMS Microbiol. Lett. 2021, 368, fnab056. [Google Scholar] [CrossRef]
- Chakraborty, S. Exploring Volatile Fatty Acids (VFAs) as a Novel Substrate for Microbial Oil Production. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2015. [Google Scholar]
- Huang, X.-F.; Liu, J.-N.; Lu, L.-J.; Peng, K.-M.; Yang, G.-X.; Liu, J. Culture strategies for lipid production using acetic acid as sole carbon source by Rhodosporidium toruloides. Bioresour. Technol. 2016, 206, 141–149. [Google Scholar] [CrossRef]
- Kolouchová, I.; Schreiberová, O.; Sigler, K.; Masák, J.; Řezanka, T. Biotransformation of volatile fatty acids by oleaginous and non-oleaginous yeast species. FEMS Yeast Res. 2015, 15, fov076. [Google Scholar] [CrossRef]
- Fei, Q.; Chang, H.N.; Shang, L.; Choi, J.; Kim, N.; Kang, J. The effect of volatile fatty acids as a sole carbon source on lipid accumulation by Cryptococcus albidus for biodiesel production. Bioresour. Technol. 2011, 102, 2695–2701. [Google Scholar] [CrossRef]
- Miranda, C.; Bettencourt, S.; Pozdniakova, T.; Pereira, J.; Sampaio, P.; Franco-Duarte, R.; Pais, C. Modified high-throughput Nile red fluorescence assay for the rapid screening of oleaginous yeasts using acetic acid as carbon source. BMC Microbiol. 2020, 20, 60. [Google Scholar] [CrossRef]
- Bettencourt, S.; Miranda, C.; Pozdniakova, T.A.; Sampaio, P.; Franco-Duarte, R.; Pais, C. Single Cell Oil Production by Oleaginous Yeasts Grown in Synthetic and Waste-Derived Volatile Fatty Acids. Microorganisms 2020, 8, 1809. [Google Scholar] [CrossRef] [PubMed]
- Burgstaller, L.; Löffler, S.; De Marcellis, L.; Ghassemi, K.; Neureiter, M. The influence of different carbon sources on growth and single cell oil production in oleaginous yeasts Apiotrichum brassicae and Pichia kudriavzevii. New Biotechnol. 2022, 69, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-Z.; Wang, Q.-M.; Göker, M.; Groenewald, M.; Kachalkin, A.V.; Lumbsch, H.T.; Millanes, A.M.; Wedin, M.; Yurkov, A.M.; Boekhout, T.; et al. Towards an integrated phylogenetic classification of the Tremellomycetes. Stud. Mycol. 2015, 81, 85–147. [Google Scholar] [CrossRef] [PubMed]
- Franklin, S.; Decker, S.M.; Wee, J. Fuel and Chemical Production from Oleaginous Yeast. US Patent US 2011/0252696 A1, 2011. [Google Scholar]
- Sankh, S.; Thiru, M.; Saran, S.; Rangaswamy, V. Biodiesel production from a newly isolated Pichia kudriavzevii strain. Fuel 2013, 106, 690–696. [Google Scholar] [CrossRef]
- Bardhan, P.; Gupta, K.; Kishor, S.; Chattopadhyay, P.; Chaliha, C.; Kalita, E.; Goud, V.V.; Mandal, M. Oleaginous yeasts isolated from traditional fermented foods and beverages of Manipur and Mizoram, India, as a potent source of microbial lipids for biodiesel production. Ann. Microbiol. 2020, 70, 27. [Google Scholar] [CrossRef]
- Prabhu, K.; Jayakumar, A.; Sreelakshmi, K.P.; Raha, A.; Maitra, M.; Radha, P. Utilization of microbial oil produced from Pichia kudriavzevii NCIM 3653 using paper mill sludge as an alternative substrate for biodiesel synthesis. Biofuels 2021, 12, 1309–1316. [Google Scholar] [CrossRef]
- Garcia-Ochoa, F.; Gomez, E. Bioreactor scale-up and oxygen transfer rate in microbial processes: An overview. Biotechnol. Adv. 2009, 27, 153–176. [Google Scholar] [CrossRef]
- Schmid, M.T.; Sykacek, E.; O’Connor, K.; Omann, M.; Mundigler, N.; Neureiter, M. Pilot scale production and evaluation of mechanical and thermal properties of P(3HB) from Bacillus megaterium cultivated on desugarized sugar beet molasses. J. Appl. Polym. Sci. 2022, 139, 51503. [Google Scholar] [CrossRef]
- Khot, M.; Raut, G.; Ghosh, D.; Alarcón-Vivero, M.; Contreras, D.; Ravikumar, A. Lipid recovery from oleaginous yeasts: Perspectives and challenges for industrial applications. Fuel 2020, 259, 116292. [Google Scholar] [CrossRef]
- Dong, T.; Knoshaug, E.P.; Pienkos, P.T.; Laurens, L.M. Lipid recovery from wet oleaginous microbial biomass for biofuel production: A critical review. Appl. Energy 2016, 177, 879–895. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Method Number 920.159: Iodine Absorption Number of Oils and Fats; Wijs method; AOAC: Rockville, MD, USA, 2015. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). Official Method Number 965.33: Peroxide Value of Oils and Fats; AOAC: Rockville, MD, USA, 2000. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). Official Method Number 940.28: Fatty Acids (Free) in Crude and Refined Oils; AOAC: Rockville, MD, USA, 2003. [Google Scholar]
- Meesters, P.A.E.P.; Huijberts, G.N.M.; Eggink, G. High-cell-density cultivation of the lipid accumulating yeast Cryptococcus curvatus using glycerol as a carbon source. Appl. Microbiol. Biotechnol. 1996, 45, 575–579. [Google Scholar] [CrossRef]
- Sáez-Plaza, P.; Michałowski, T.; Navas, M.J.; Asuero, A.G.; Wybraniec, S. An Overview of the Kjeldahl Method of Nitrogen Determination. Part I. Early History, Chemistry of the Procedure, and Titrimetric Finish. Crit. Rev. Anal. Chem. 2013, 43, 178–223. [Google Scholar] [CrossRef]
- Kyriakidis, N.B.; Katsiloulis, T. Calculation of iodine value from measurements of fatty acid methyl esters of some oils: Comparison with the relevant American Oil Chemists Society method. J. Am. Oil Chem. Soc. 2000, 77, 1235–1238. [Google Scholar] [CrossRef]
- Zhang, N.; Li, Y.; Wen, S.; Sun, Y.; Chen, J.; Gao, Y.; Sagymbek, A.; Yu, X. Analytical methods for determining the peroxide value of edible oils: A mini-review. Food Chem. 2021, 358, 129834. [Google Scholar] [CrossRef] [PubMed]
- Fernando, S.; Karra, P.; Hernandez, R.; Jha, S. Effect of incompletely converted soybean oil on biodiesel quality. Energy 2007, 32, 844–851. [Google Scholar] [CrossRef]
- Christophe, G.; Deo, J.L.; Kumar, V.; Nouaille, R.; Fontanille, P.; Larroche, C. Production of oils from acetic acid by the oleaginous yeast Cryptococcus curvatus. Appl. Biochem. Biotechnol. 2012, 167, 1270–1279. [Google Scholar] [CrossRef]
- Béligon, V.; Poughon, L.; Christophe, G.; Lebert, A.; Larroche, C.; Fontanille, P. Improvement and modeling of culture parameters to enhance biomass and lipid production by the oleaginous yeast Cryptococcus curvatus grown on acetate. Bioresour. Technol. 2015, 192, 582–591. [Google Scholar] [CrossRef]
- Chi, Z.; Zheng, Y.; Ma, J.; Chen, S. Oleaginous yeast Cryptococcus curvatus culture with dark fermentation hydrogen production effluent as feedstock for microbial lipid production. Int. J. Hydrog. Energy 2011, 36, 9542–9550. [Google Scholar] [CrossRef]
- Llamas, M.; Magdalena, J.A.; González-Fernández, C.; Tomás-Pejó, E. Volatile fatty acids as novel building blocks for oil-based chemistry via oleaginous yeast fermentation. Biotechnol. Bioeng. 2020, 117, 238–250. [Google Scholar] [CrossRef]
- Davies, R.; Holdsworth, J.; Reader, S. The effect of low oxygen uptake rate on the fatty acid profile of the oleaginous yeast Apiotrichum curvatum. Appl. Microbiol. Biotechnol. 1990, 33, 569–573. [Google Scholar] [CrossRef]
- Bonturi, N.; Matsakas, L.; Nilsson, R.; Christakopoulos, P.; Miranda, E.; Berglund, K.; Rova, U. Single Cell Oil Producing Yeasts Lipomyces starkeyi and Rhodosporidium toruloides: Selection of Extraction Strategies and Biodiesel Property Prediction. Energies 2015, 8, 5040–5052. [Google Scholar] [CrossRef]
- Halim, R.; Papachristou, I.; Chen, G.Q.; Deng, H.; Frey, W.; Posten, C.; Silve, A. The effect of cell disruption on the extraction of oil and protein from concentrated microalgae slurries. Bioresour. Technol. 2022, 346, 126597. [Google Scholar] [CrossRef] [PubMed]
- Knothe, G. Structure indices in FA chemistry. How relevant is the iodine value? J. Am. Oil Chem. Soc. 2002, 79, 847–854. [Google Scholar] [CrossRef]
- Bouaid, A.; Martinez, M.; Aracil, J. Long storage stability of biodiesel from vegetable and used frying oils. Fuel 2007, 86, 2596–2602. [Google Scholar] [CrossRef]
- Sahoo, P.K.; Das, L.M.; Babu, M.; Naik, S.N. Biodiesel development from high acid value polanga seed oil and performance evaluation in a CI engine. Fuel 2007, 86, 448–454. [Google Scholar] [CrossRef]
- Atadashi, I.M.; Aroua, M.K.; Abdul Aziz, A.R.; Sulaiman, N. Production of biodiesel using high free fatty acid feedstocks. Renew. Sustain. Energy Rev. 2012, 16, 3275–3285. [Google Scholar] [CrossRef]
- Probst, K.V.; Schulte, L.R.; Durrett, T.P.; Rezac, M.E.; Vadlani, P.V. Oleaginous yeast: A value-added platform for renewable oils. Crit. Rev. Biotechnol. 2016, 36, 942–955. [Google Scholar] [CrossRef]
- Abreu, S.; Park, Y.-K.; Pires de Souza, C.; Vidal, L.; Chaminade, P.; Nicaud, J.-M. Lipid Readjustment in Yarrowia lipolytica Odd-Chain Fatty Acids Producing Strains. Biomolecules 2022, 12, 1026. [Google Scholar] [CrossRef]
 ) and single cell oil (SCO,
) and single cell oil (SCO,  ) are given as concentrations in g/L; the concentration of phosphate (
) are given as concentrations in g/L; the concentration of phosphate ( ) is given as concentration in mg P/L. (a) shows the course of the fermentation process in laboratory scale over 72 h. Cultivations were performed in duplicate. (b) shows the course of a fermentation process in small pilot scale over 96 h.
) is given as concentration in mg P/L. (a) shows the course of the fermentation process in laboratory scale over 72 h. Cultivations were performed in duplicate. (b) shows the course of a fermentation process in small pilot scale over 96 h.
 ) and single cell oil (SCO,
) and single cell oil (SCO,  ) are given as concentrations in g/L; the concentration of phosphate (
) are given as concentrations in g/L; the concentration of phosphate ( ) is given as concentration in mg P/L. (a) shows the course of the fermentation process in laboratory scale over 72 h. Cultivations were performed in duplicate. (b) shows the course of a fermentation process in small pilot scale over 96 h.
) is given as concentration in mg P/L. (a) shows the course of the fermentation process in laboratory scale over 72 h. Cultivations were performed in duplicate. (b) shows the course of a fermentation process in small pilot scale over 96 h.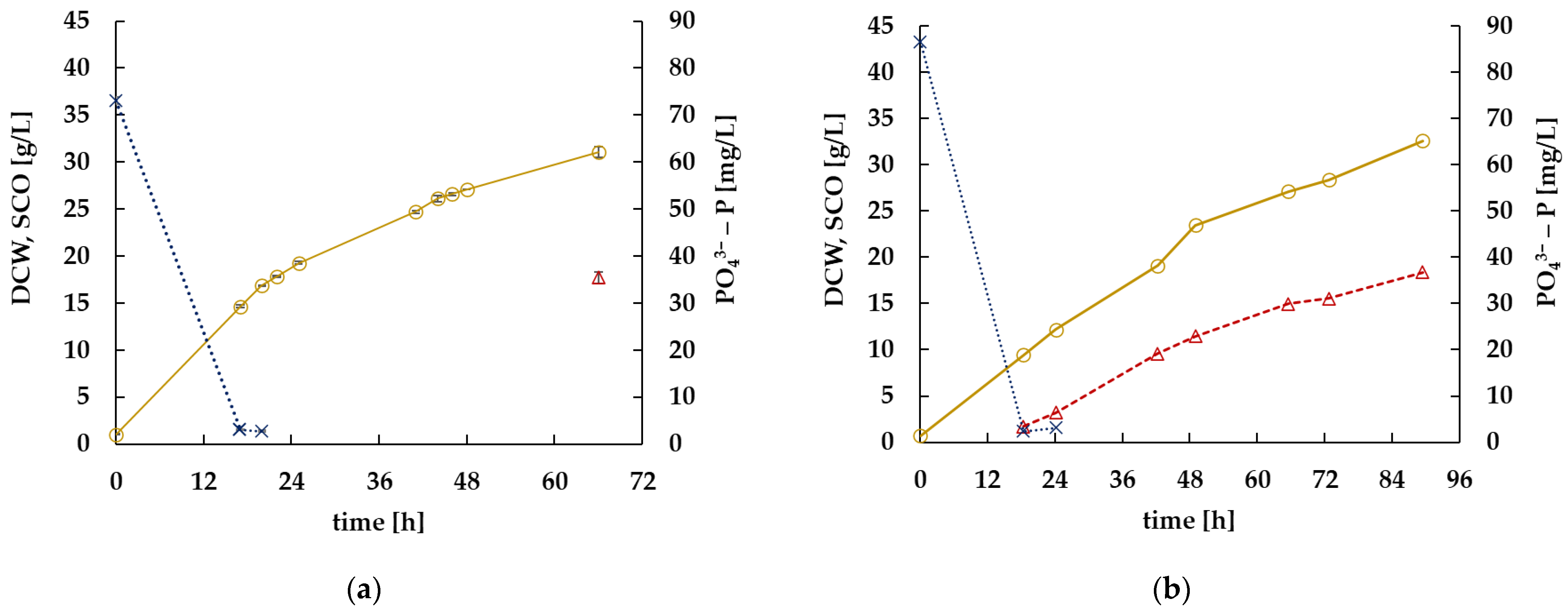
| Strain | Scale | DCW [g/L] | SCO [g/100 g DCW] | SCO [g/L] | YP/S [gSCO/gVFA] | Productivity [gSCO/Lh] |
|---|---|---|---|---|---|---|
| V194 | laboratory scale | 33.2 ± 0.1 | 57.9 ± 2.2 | 19.2 ± 0.7 | 0.162 ± 0.005 | 0.296 ± 0.010 |
| small pilot scale | 31.1 | 57.1 | 17.8 | 0.167 | 0.201 | |
| V134 | laboratory scale | 31.1 ± 0.6 | 56.9 ± 0.9 | 17.7 ± 0.6 | 0.174 ± 0.005 | 0.268 ± 0.009 |
| small pilot scale | 32.6 | 56.4 | 18.4 | 0.176 | 0.205 | |
| pilot scale | 36.9 | 57.8 | 21.3 | 0.179 | 0.152 |
| Condition of Biomass | Pretreatment Method | Extracted Lipids [g/100 g DCW] | Recovery 1 [%] |
|---|---|---|---|
| - | 17.80 ± 0.49 | 36.82 ± 1.02 | |
| lyophilized | bead milling | 22.98 ± 0.11 | 47.54 ± 0.22 |
| bead milling + induced autolysis | 38.05 ± 0.14 | 78.72 ± 0.29 | |
| wet | acid hydrolysis | 35.13 ± 0.25 | 72.68 ± 0.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burgstaller, L.; Oliver, L.; Dietrich, T.; Neureiter, M. Pilot Scale Production of Single Cell Oil by Apiotrichum brassicae and Pichia kudriavzevii from Acetic Acid and Propionic Acid. Appl. Sci. 2023, 13, 4674. https://doi.org/10.3390/app13084674
Burgstaller L, Oliver L, Dietrich T, Neureiter M. Pilot Scale Production of Single Cell Oil by Apiotrichum brassicae and Pichia kudriavzevii from Acetic Acid and Propionic Acid. Applied Sciences. 2023; 13(8):4674. https://doi.org/10.3390/app13084674
Chicago/Turabian StyleBurgstaller, Lukas, Laura Oliver, Thomas Dietrich, and Markus Neureiter. 2023. "Pilot Scale Production of Single Cell Oil by Apiotrichum brassicae and Pichia kudriavzevii from Acetic Acid and Propionic Acid" Applied Sciences 13, no. 8: 4674. https://doi.org/10.3390/app13084674
APA StyleBurgstaller, L., Oliver, L., Dietrich, T., & Neureiter, M. (2023). Pilot Scale Production of Single Cell Oil by Apiotrichum brassicae and Pichia kudriavzevii from Acetic Acid and Propionic Acid. Applied Sciences, 13(8), 4674. https://doi.org/10.3390/app13084674





