Abstract
Advanced nutritional interventions are one of the key components of elite sports performance in general. Combat sports require a high percentage of muscle mass with minimum body weight to generate the maximum power possible. An adequate level of nutrition knowledge, particularly with respect to identifying energy needs while avoiding confusion over dietary supplements and false perceptions of steroid requirement, which may compromise the health condition, is of crucial importance. In this context, the aim of our work is to highlight nutritional requirements/nutritional assessment, the importance of daily dietary intake in combat players, which increasingly includes a broad range of sports nutrition supplements, and the roles of vitamins, minerals and proteins, combined with antioxidants and strength training, in muscular performance. The main nutrients required in the daily diet of combat players, the mechanisms of action, the main outcomes and possible side effects are summarized. Special attention is paid to natural supplements and their importance and advantages over synthetic ones, along with future trends of development.
Keywords:
nutrition; sports performance; dietary supplements; vitamins; minerals; proteins; nutraceuticals 1. Introduction
A dietary supplement is a commercially available product that is consumed as an addition to the usual diet and includes vitamins, minerals, herbs (botanicals), amino acids and a variety of other products. They are recommended as adjuvants in diets in order to achieve the desired ratio of macro- and micronutrients according to each individual’s need. The nutrient requirements of each individual incorporate a sum of factors, such as age, gender, body mass and composition, physiological state, growth and recovery needs and the energy expenditure of each physical activity [1]. Dietary supplementation benefits are extremely popular, especially among physically active persons, with over 80% of athletes using supplements [2]. The prime objective in nutrition supplementation among athletes is performance enhancement, but other effects must also be taken into consideration, such as maintaining optimum physical and psychical health status, gaining lean mass and improving body composition. However, not all substances that have a performance-enhancing capacity are healthy, so the vigilant eye of WADA monitors supplements and permits their use in sports only if they do not represent any health hazards. For example, anabolic–androgenic steroid consumption is known to be involved in the systemic inflammatory response and multiple-organ dysfunction in healthy individuals [3]. In a world governed by aesthetic appearance and social networks, young athletes need to be carefully advised regarding the appropriate methods for improving body composition and lowering the fat/lean mass ratio. It is mandatory that athletes across all levels of competition have an adequate level of nutrition knowledge, particularly with respect to identifying their energy needs, while avoiding confusion over dietary supplements and false perceptions of steroid requirement, which may compromise their health condition [3,4]. Educational programs on this subject are not generally available in every country, especially in developing ones, and hence, misinformation may lead to serious health problems, which, in turn, are reflected in the poor performance of combat players [5]. In this context, the aim of our work is to highlight the crucial importance of knowledge related to dietary habits, which increasingly include a broad range of sports nutrition supplements, and the roles of vitamins, minerals and proteins, combined with antioxidants and strength training, in muscular performance. Special attention is paid to natural supplements, their importance and their advantages over synthetic ones.
2. Attitudes towards Using Sports Supplements
Most commonly, the assessment of a sports diet is realized based on food records, which is considered the gold standard for this task, even though its accuracy can be affected by the subjectiveness of the participants [1]. Often, the study participants misreport their food intake by lowering the reported amounts. This misreporting seems to be proportional to the Total Energy Expenditure (TEE) [1]. Although there is a vast amount of research on sports supplements and their optimal ratios in the athlete diet, most of the time, in practice, the scientific data are not so precisely applied [6]. Table 1 illustrates the most frequently recommended nutrient intake deviations in different nutrition assessments related to different sports, according to available data in the literature.

Table 1.
Recommendations for supplement intake in different types of sports activities.
Some studies compared the meeting of nutritional requirements before and after supplementation, evidencing a lot of improvement in satisfying the calculated nutritional demands after supplementation [7,11]. This provides good support for the high popularity of sports supplement use. Consequently, the sports supplement market has developed different types of supplements in order to close the gaps in everyday nutrition. They provide targeted nutrients in an easy-to-prepare and easy-to-use form for the optimum contribution of essential nutrients. Ranging from normal foods to medical supplements, together, they form a market value of USD 40.0 billion in 2021 [12]. Being of such high financial interest, aggressive marketing campaigns often mislead those with little nutrition knowledge who end up buying products that are of no use or can lead to the disproportionality of some nutrients. Consequently, sports nutrition supplementation should be supervised by a professional who is well informed in this domain, such as a nutritionist or a doctor. Table 2 illustrates the most common categories of supplements.

Table 2.
The most common categories of supplements.
2.1. Nutritional Requirements/Nutritional Assessment and Importance of the Daily Dietary Intake of Combat Players
Olympic combat sports include judo, taekwondo, wrestling, boxing and karate, which are the most popular combat sports. They represent ~25% of the Olympic medals, and consequently, there is a special interest in any solution that might aid in their performance. Combat sports nutrition requires professional interventions in order to achieve objectives such as weight loss, muscle gain and quick recovery while maintaining health and minimizing the risk of injury.
According to AHA (American Heart Association), most combat sports involve high static–low/moderate dynamic activity, which translates, from the cardiovascular point of view, into an increased pressure load due to the long periods of maximal voluntary contraction [21]. From the exercise physiology point of view, explosive strength and power are required for punching and kicking, and isometric and concentric strength is required for grappling [22]. Depending on the duration of the matches, aerobic metabolism plays a more important role in sustaining the effort in sports with a longer match duration [23]. The aerobic metabolic system is demonstrated to play an important role during judo matches, with the involvement of lipid and protein metabolism alongside glycolysis [24]. Thus, due to the fact that all metabolic systems are involved in energy production during combat matches, nutritional recommendations should be based on a holistic view of all nutrients, packed in ideal caloric meals, in order to maintain the weight category. Regarding macronutrient intake, most of the scientific community agrees on the ideal ratios for athletes.
2.1.1. Nutrient Timing
Nutrition science has provided recommendations for the ideal timing of each nutrient in order to provide the maximum effect in the lowest caloric concentration possible. In combat sports, these recommendations are essential to be taken into consideration and applied at key moments of each player’s program. The International Society of Sports Nutrition (ISSN) published guidelines for nutrition timing that can be adapted to combat player needs. High-carbohydrate meals are to be consumed 4 h before competition and in the accumulation phase in order to provide adequate glycogen availability to support the high demand. Carbohydrates also support fast recovery, especially when consumed in combination with protein, a formula that offers the best glycogen resynthesis rate with the lowest carbohydrate load, an important goal for combat players. In combat sports, fast recovery is essential, especially after weight loss programs and between bouts when limited time is available to prepare for the next game. After protein ingestion, amino acid availability in the blood is elevated for about 4 h, so protein intake has to be distributed during the recovery phase, or low-absorption-rate proteins such as casein are recommended before sleep. Regarding caloric distribution during the day, several studies have shown favorable effects on weight loss when the greatest quantity of calories are consumed in the first part of the day and divided into multiple small meals [25].
2.1.2. Nutrition in the Training Period
The training period for combat players is composed of the accumulation and intensification phases according to the training periodization concept, and nutrition interventions have to be in accordance with them. Carbohydrates are considered to be the primary source of energy for aerobic exercise. Considering their limited storage capacity in the form of glycogen, carbohydrates play a vital role in sports performance [26]. High-glycemic-index carbohydrates, due to their rapid availability, are recommended before and during exercise to support the effort energy demand. Fast recovery can be achieved by ingesting high-glycemic-index carbohydrates immediately after the effort [27]. According to the International Society of Sports Nutrition, the recommended quantity of carbohydrates for athletes ranges from 8–12 g/kg/day [26]. In combat sports, CHO are, along with hydration, the main strategy for recovery after rapid weight loss. The proposed protocol for rapid glycogen restoration is 1.2 g/kg/h of high-glycemic-index CHO or 0.8 g/kg/h CHO combined with 0.2–0.4 g/kg/h protein in combination with 3–8 mg/kg of caffeine [26].
Protein intake in the athlete diet should be higher compared with the rest of the population (0.8–0.9 g/kg/day), ranging from 1.2–2.1 g/kg/day with a variation of up to 3.1 g/kg/day in calorie-restricted diets. Optimal protein ingestion shows multiple benefits, such as muscle gain and anabolic effects, anticatabolic effects, injury prevention and fast recovery, and plays an important role in weight loss strategies [25,28,29].
There are no major differences between athletes and the general population in terms of lipid consumption recommendations. Between 20 and 35% of daily calories should come from healthy fats. Fat-restricted diets are not recommended, taking into consideration the role of fat in many processes besides energy production, such as cell membrane synthesis, absorption of fat-soluble vitamins, hormone production and proper brain function. Considering this aspect, the combat player’s diet is often supplemented with omega-3 polyunsaturated fatty acids, with satisfactory results [30]. Lipids were proposed in many studies as the prime energy source for performance due to their high caloric densities, but the results are inferior compared with CHO in intense near-VO2max activities [31,32]. Lipids remain an important source of energy in low-intensity activities and are an essential component in every nutrition plan.
2.1.3. Rapid Weight Loss (RWL)
Nowadays, most combat sports are regulated by weight classes to ensure fair chances in combat for each fighter. Official weigh-in takes place 3–24 h before competition, depending on each sport’s rules. While this policy makes each sport event equilibrated, it imposes an extremely strict lifestyle on athletes in order to maintain their weight category. Often, between competitions, during the accumulation training phase, athletes exceed the category limit in order to gain maximum endurance, strength and power. An aggressive RWL strategy is applied before weigh-in, followed by intensive recovery between weigh-in and competition. This program plays a crucial role in sports results achievement, and any mistake made can lead to health risks, eating disorders, performance loss or disqualification. The most used strategies for RWL include an increase in training volume; the restriction of fluid, minerals (sodium) and food; and perspiration promotion techniques, such as a sauna or wearing plastic clothes [33,34,35]. Some of them may generate severe dehydration and electrolyte imbalances that can lead to adverse effects such as headache, dizziness, nausea, hot flashes, nose bleeds and, to a smaller degree, fever, disorientation and increased heart rate, fatigue, myalgia, depression and anger [36]. The average weight lost before a competition is approximately 5 kg in most combat sports, varying from 0 to 10 kg [34,35,37]. Those who rely more on aggressive weight loss strategies have been demonstrated to have scarce knowledge of nutrition principles. Excessive weight loss is rather detrimental to sports performance, with a demonstrated decline in physical and neuromuscular performance, mood and psychological states and biological profiles [38,39]. Furthermore, the long-term consequences of RWL include eating disorders such as anorexia, binge eating or bulimia, resulting in a higher percentage of obesity in former combat players. Isolated cases of death caused by severe dehydration have been reported. Wearable electrochemical sensors, such as those described by Lu et al., that monitor sweat content might trigger an alarm signal when dehydration goes too far [40]. Some recommendations can be made in order to prevent this side effect. They include planned, gradual weight loss not higher than 1 kg/week and not more than 5% of body mass and fat loss not lower than 5% for males and 12% for females. Strength training and BCAA supplementation should be considered in order to prevent muscle mass loss.
2.1.4. Precompetition Diet
The diet between the weigh-in and the actual fight can have a decisive effect on the outcome. It is known that even slight dehydration or glycogen scarcity can significantly affect performance. In this period, it is essential to restore muscle glycogen and to correct dehydration and dyselectrolytemia. In order to achieve that, beverages consumed should amount to 150% of fluid loss at least, combined with up to 12 g/kg/24h of carbohydrates. The meal before competition should take place 3–4 h before and contain 1–2 g of carbohydrates [41]. Regular sports drinks are not designed to correct such massive dehydration, as they contain only about 40 mmol of sodium. Oral rehydration solutions used to treat diarrhea are the best option for this task, as they contain up to 90 mmol of sodium. Gastrointestinal distress can occur if large amounts of fiber-rich food are ingested after a period of fasting, so limiting fiber-rich foods is a wise choice [33].
Table 3 summarizes the main nutrients required as daily intake adapted for combat players, the mechanisms of action, main outcomes and possible side effects, according to available references.

Table 3.
The main nutrients required in the daily diet of combat players, the mechanisms of action, main outcomes, targeted effects for combat players and possible side effects.
3. Importance of Vitamin and Mineral Supplements
Vitamins and minerals are part of the diet of athletes and combat players for their alleged role in improving physical performance during training and competitions.
3.1. Vitamins
Based on their solubility, vitamins are divided into two categories: water-soluble and fat-soluble. Thirteen different compounds are considered vitamins [115], as presented in Figure 1.
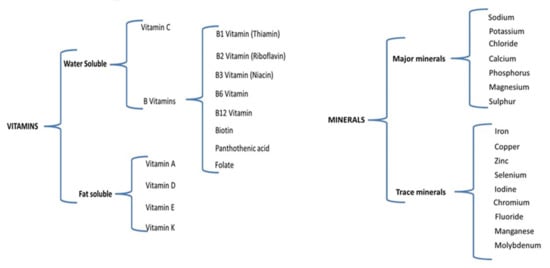
Figure 1.
Vitamin and mineral classification.
The bioavailability of vitamins is different with respect to their type: water-soluble vitamins are absorbed directly from the small intestine into the bloodstream, while fat-soluble vitamins are absorbed along with a fat-based diet in the form of chylomicrons through the lymphatic system to the bloodstream and then to the liver. On the other hand, water-soluble vitamins have little storage within the body, being lost in the urine, while fat-soluble vitamins can be easily stored in the liver and fatty tissues of the body [116].
In the case of strenuous exercises, gut absorption of vitamins and minerals is decreased, while their loss in sweat and urine is increased [83].
However, according to Grozenski and Kiel [83], the supplementation of fat-soluble vitamins in the diet of combat players has not shown any improvement in exercise performance. Moreover, toxicity may occur when consumed in excess due to their prolonged periods of storage. The recommended daily allowances (RDAs) of vitamins and minerals are determined by Commission Directive 2008/100/EC [117] amending Council of Europe Directive 90/496 and the US Food and Nutrition Council, which are considered adequate for the nutritional needs of human health.
Players put in a lot of effort during training, so they have higher nutrient requirements. Exercise requires many metabolic pathways in which micronutrients are needed, and training can lead to muscle biochemical adaptations that increase the need for micronutrients.
Książek et al. [11] investigated the levels of vitamin and mineral intake in Polish football players during the training period for one week. According to their study [11], the intake of food supplements and foodstuffs intended for the investigated players led to the correction of vitamin B2, folic acid, vitamin C and vitamin D but did not cover the daily requirement of calcium.
Vitamin A is a fat-soluble retinoid involved in night vision as an essential component of rhodopsin. Retinoic acid acts as a transcription factor ligand in cell proliferation processes. The antioxidant effect of vitamin A was noted after six weeks of supplementation in taekwondo athletes [118].
Vitamin D plays an important role in skeleton bone mineralization and calcium regulation through endocrine and autocrine mechanisms. The deficiency of vitamin D in the case of combat players led to increased risks of stress fractures, muscle injuries and respiratory infections [119]. A recent study [120] highlighted a positive impact of supplementing with 50,000 IU of vitamin D3 per week in male recreational combat athletes for six weeks, combined with six weeks of sprint interval training, as demonstrated by improved aerobic and anaerobic performance markers.
Baranauskas et al. [121] investigated the relationship between dietary supplement intake and the body composition of elite Lithuanian athletes training for Olympic Sports. The data showed that athletes consumed much more fat-soluble vitamins A and E and minerals K, Mg, P Mn, Cu and Zn compared to the recommended dietary intake (RDI), according to the Minister of Health of the Republic of Lithuania. Conversely, the diets of athletes were deficient in vitamin D, a feature often found in countries at North European latitudes. On the other hand, athletes who train and compete indoors are also prone to an increased risk of vitamin D deficiency. A diet based on fish, dairy and eggs is not sufficient to maintain an optimal level of vitamin D in athletes; instead, exposure to UVB allows sufficient maintenance of vitamin D status [122,123]. Medical supplementation with vitamin D and minerals such as iron and calcium is recommended only in the case of deficiencies and only under medical supervision [121]. Vitamin D co-supplementation with calcium prevents bone loss [124]. Positive effects on performance have been noted after vitamin D supplementation in indoor combat sports such as judo or other combat sports, especially during winter periods [125,126].
Vitamin E is a lipophilic antioxidant that prevents the formation of free radicals during intense exercise. Vitamin E also prevents the destruction of red blood cells, improving oxygen delivery to the muscles during intense exercise [124]. A study by Tiidus and Houston [85] showed that at high altitudes, vitamin E may improve exercise performance among athletes. In another recent study [86], the performance of football players was investigated, and the effect of supplementation with vitamins C and E on oxidative stress was shown in terms of delayed-onset muscle soreness. Their results support the idea that antioxidant vitamins do not exert any ergogenic effects on the football performance of young players, although they did reduce oxidative stress.
Vitamin K is involved in blood clotting and has been reported to improve the balance between bone formation and resorption in elite female athletes.
B vitamins play important roles in the body, but there are no studies to show that they are involved in sports performance. A combination of B1 and B12 vitamins or a combination of B1 and B6 vitamins and cyanocobalamin have improved fine motor skills by increasing serotonin levels, but only in some sports, such as pistol shooting and archery [127].
Vitamin C, a powerful antioxidant, is involved in numerous different metabolic processes in the body. Cannataro et al. 2022 suggested that the use of vitamin C, especially for combat sports athletes, is indicated to manage free radical production [128].
Vitamin C indirectly contributes to improving the performance of athletes through its effects on improving the immune system or through its role in iron absorption [124]. Reduced muscle damage and inflammatory responses were observed after high doses of vitamin C and E supplementation in taekwondo athletes [129].
3.2. Minerals
Minerals, similar to vitamins, are essential micronutrients required in the diet of combat players and are classified into major and trace minerals (Figure 1) depending on the amount necessary for the body.
Calcium, the most important mineral of the skeleton, is also involved in muscle contraction, heart rhythm regulation, coagulation and nerve conduction. Being involved in such important processes, calcium homeostasis is precisely regulated by calcitonin, parathyroid hormone and vitamin D. In a situation where the calcium balance is negative, the plasmatic concentrations are maintained within their required limits by mobilizing bone calcium. This fact has to be taken into consideration in athlete nutrition because proper bone mineralization is essential in fracture prevention. Calcium in combination with vitamin D was proven to increase bone mineralization and reduce fractures in a prospective study among female athletes. Although, in most cases, vitamin D is the limiting factor of bone mineralization and calcium uptake, proper calcium intake has to be taken into consideration, especially in calorie-restricted or dissociated diets, which are popular among athletes.
Magnesium is essential in sports nutrition, as it is utilized as a cofactor in over 300 enzymatic reactions, including those of ATP synthesis and muscle contraction [130]. Glucose metabolism is limited by magnesium availability, with magnesium being involved in glucose utilization and homeostasis, phosphorylation, pyruvate dehydrogenase and creatine kinase reactions [105]. Magnesium is demonstrated to promote muscle synthesis through activation of the mTOR pathway [106]. Magnesium might have a lifesaving role in sports due to its demonstrated arrhythmia prevention effects [131], given that most sudden cardiac deaths in athletes occur through arrhythmias [132]. Multiple studies have proved the efficiency of magnesium in sports-related performance, such as increases in muscle strength and walking and running speed and a decrease in lactate levels [105].
Higher training adaptation regarding erythrocyte and hemoglobin levels was observed in taekwondo athletes after magnesium supplementation [133]. In judo athletes, magnesium was demonstrated to reduce strength loss after a caloric restriction program [134].
Sodium is an important consideration because, during intense training, especially on hot days, large amounts of water and sodium are lost through perspiration [109,135], so nutrition strategies for athletes should include sodium in foods and beverages in order to minimize diuresis, thus promoting regeneration in post-training athletes [114]. In the case of intense exercises that cause considerable sodium loss through sweating, sodium intake can exceed 10 g/day.
Iron deficiency in athletes can affect muscle function and limit work capacity, resulting in impaired athletic performance [123]. The causes that lead to a low iron status are low intake of heme food, inadequate energy intake, rapid growth of the player, training at high altitudes or intense training [123]. Iron supplementation is not recommended to occur immediately after intense training, as high levels of hepcidin may interfere with iron absorption [123,136]. It is preferable for athletes to take in iron from food, especially by eating heme iron foods or combining non-heme iron foods with vitamin C foods for better absorption.
4. Importance of Protein and Amino Acid Supplements
Protein’s value for athletes and combat players has long been established. Protein has always been regarded as a critical nutritional element for athletic achievement, from Olympian coaches in ancient Greece to today’s multi-millionaire sportsmen [137]. The nature of that relevance has been a source of contention for an equal amount of time. Protein and amino acid consumption is deemed vital to effectiveness by many athletes, particularly strength and team sports competitors. Supplements containing amino acids and proteins have grown into a multibillion-dollar industry.
Proteins are demonstrated to have a synergic effect with resistance training on muscle synthesis. The recommended dose varies from 0.8 g/kg to 2g/kg depending on age, gender and body composition and can be increased up to 3 g/kg when body composition enhancement is targeted. In combat sports, these facts have important applicability in each training cycle, from the accumulation phase, where strength and muscle synthesis are targeted, to the intensification phase, where speed and tactics are exercised. In the precompetition and RWL periods, adequate protein intake is essential, as it is proven to limit muscle loss, thus sustaining the best possible body composition [44].
Nutrition science went even further and identified key amino acids that promote muscle synthesis, offering targeted supplements so that athletes can benefit without any unwanted additional calories. The amino acid content for muscle protein balance tends to be influenced by resistance training. Only necessary amino acids are required for net muscle protein synthesis [138]. As a result, non-essential amino acids are not required for the activation of muscle protein synthesis that results in net muscle protein. Even single amino acids have been shown to increase protein synthesis and potentially net muscle protein synthesis. Anthony et al. 1999 [139] found that ingesting leucine after exercising stimulates protein translation initiation mechanisms, resulting in enhanced muscle protein synthesis. Bolus dosages of single essential, but not non-essential, amino acids increased muscle protein synthesis in sedentary individuals. Individual amino acids may therefore serve as a stimulus for muscle protein synthesis and promote a positive net muscle protein balance. These findings imply that essential amino acids may increase muscle protein synthesis in two ways: (1) by providing a substrate for it and (2) by serving as a regulatory factor [67].
Acute stimulation of muscle protein synthesis and net muscle protein synthesis apparently requires small amounts of essential amino acids. Ingesting as little as 6 g of essential amino acids, both with and without carbohydrates, leads to substantial increases in muscle protein synthesis, resulting in net muscle protein synthesis. Furthermore, some evidence suggests that the response of muscle protein synthesis to essential amino acid consumption after exercise is dosage-dependent. The reaction of net muscle protein balance to two doses of 6 g of essential amino acids each was nearly double that of two doses of 6 g of miscellaneous amino acids each [140]. Each dose of combined amino acids had around 3 g of essential amino acids, which was nearly half of the quantity in the two 6 g doses. As a result, there may be a saturation point for critical amino acids over which no further stimulation is conceivable. The fact that net muscle protein synthesis was identical regardless of whether 20 g or 40 g of essential amino acids was consumed after resistance exercise offers credence to this theory [141].
The supply of intracellular amino acids may influence muscle protein synthesis and net muscle protein balance in reaction to hyperaminoacidemia. Hyperaminoacidemia is caused by the ingestion of a nutrient that enhances amino acid supply to the muscle, transit into the muscle cell and intracellular amino acid accessibility. Greater amino acid supply to the muscle and the possibility of improved muscle protein synthesis after exercise would result from increasing the blood flow and a high rate of protein synthesis caused by the exercise session. These factors explain the cumulative effect of exercise and amino acids on net muscle protein balance when considered together. There is evidence attributing the acceleration of muscle protein synthesis to changes in arterial amino acid concentrations instead of, or in addition to, increased intracellular accessibility of amino acids [142]. Borsheim et al. [143] found that when 6 g of essential amino acids was consumed after resistance training, arterial essential amino acids increased several-fold. When arterial amino acid concentrations started to decrease, net muscle protein accumulation was proportionally higher than arterial concentrations and dropped drastically. Even though arterial amino acid concentrations were still quadruple resting levels, the net muscle protein balance reverted to resting levels. Borsheim et al. [143] found that muscle intracellular amino acid concentrations were raised but did not differ from resting levels by the end of the research.
These findings are comparable to those of a recent study that showed rapid changes in the value balance but no long-term alterations in intracellular concentrations in muscle [144]. Furthermore, another work from our group found that lower blood amino acid concentrations led to lower muscle protein synthesis, while higher arterial amino acid concentrations contributed to increased synthesis [145]. In addition, protein supplementation is proven to increase lean mass and improve body composition during calorie-restricted diets [44].
5. The Roles of Creatine, Caffeine, Phosphate, Beta-Alanine and Carnitine
5.1. Creatine
Creatine supplementation is probably the most efficient way to improve strength and sports performance, promote exercise adaptation and recovery and prevent injury and neurodegenerative disease incidence, with its efficacy supported by dozens of studies. About 95% of creatine is found in skeletal muscle, playing a key role in anaerobic ergogenesis as part of the phosphagen system. In an omnivorous diet, about half of creatine is provided by the diet, and the rest is synthetized from glycine, methionine and arginine. Numerous scientific studies have proven that supplementation with this organic compound effectively increases the concentration of creatine found in muscle cells, which explains the improvement in sports performance in high-intensity exercise and leads to better training adaptation. In addition, creatine may have a beneficial effect on post-workout regeneration, thermoregulation, prevention of sports injuries, neuroprotection of the spinal cord, rehabilitation, improvement of the health of people suffering from neurodegenerative diseases, diabetes, osteoarthritis or old age and the associated loss of lean body mass. The rate of synthesis may vary indirectly proportionally to exogen intake [48]. There are several recommended protocols regarding creatine supplementation. The most common protocol includes a loading phase of 20 g of creatine divided into four meals during the day for 1 week, followed by a maintenance dose of 3–5g/day. Undoubtedly, the advantage of such a procedure is the rapid increase in creatine concentration in the muscles of about 20%, but the same effects are also obtained by supplementation with a lower dose (even 3 g/d) but for a much longer period, e.g., four weeks. The optimal solution is to take creatine in small portions with meals rich in carbohydrates, especially during the so-called loading phase, because creatine from a single dose of more than 5 grams will not be effectively absorbed by the muscle cells [61,62,63]. Producers of dietary supplements offer a number of different forms of creatine on the market, which are often even in one product (the so-called creatine stack).
Most fears regarding side effects such as kidney damage, hair loss, dehydration, fat mass increase, concerns about use in the young or elderly population, musculoskeletal injuries and muscle cramps have been infirmed in multiple studies, and creatine use is considered safe and efficient by the scientific community [48,49]. In combat sports, the main targeted advantage of creatine use is the increase in muscle mass, resulting in increased explosive power via the phosphagen system. Several studies have validated the theory, finding the applicability of creatine in most combat sports. A study performed by Sterkowicz et al. demonstrated the positive effect of creatine in judo training, with an increase in VO2max and a decrease in time to generate peak power [146]. Anaerobic performance and peak power enhancement were also observed in taekwondo athletes supplemented with creatine and bicarbonate [147]. In elite wrestlers, a significant increase in average and peak power was noted after creatine supplementation [148]. Along with anaerobic power, a slight increase in fat mass was obtained after creatine supplementation in taekwondo practitioners [149]. An increase in fat mass caused by creatine was extensively researched in other studies, which failed to validate this theory [49].
Creatinine can be a useful and safe product for all combat sports participants who want to improve important parameters of their performance, such as maximum anaerobic power.
5.2. Caffeine
Caffeine, a stimulant that is found in almost every adult’s diet, has long been known to improve athletic performance. Adenosine receptor antagonism, higher endorphin release, better neuromuscular functionality, enhanced attentiveness and a decreased impression of effort during exercise are among the processes behind these improvements [150]. Caffeine supplementation has a long research history in a variety of performance procedures, such as endurance-based circumstances, strength exercises and/or repeat-sprint activities. Current research on the efficiency of reduced caffeine doses, fluctuations in the timing of consumption before and/or during exercise, and the dearth of a requirement for the expelled time for productivity impact enhancement are all worth noting [150]. Caffeine supplementation has been shown to boost physical performance during period exercise trials, including treadmill running to exhaustion [151] and resistance training repetitions to defeat [152]. Additionally, ergogenic effects are frequently observed in competitive contexts, including actual or laboratory-simulated time trials (TTs). Ganio et al. [153] reported that caffeine consumption supplied a mean substantial advantage of ∼3.2 percent when given before and/or during endurance-based TT workouts of varying duration (5–150 min) across a multitude of exercise modalities in a systematic review of 33 trials. Caffeine dosages of ∼3–6 mg/kg of body mass (BM) in the form of anhydrous caffeine were frequently employed in studies claiming its benefits and were taken 60 minutes prior to exercise. Intriguingly, both habitual caffeine users and nonusers should certainly expect performance outcomes [154], with a recent study finding that high repetitive daily caffeine consumption (351 ± 139 mg/day) is closely linked to equivalent absolute and relative outcome advantages with low or adequate routine caffeine consumption [155]. Caffeine consumption at low levels during endurance training has also been demonstrated to improve productivity. In fact, following 80 minutes of preload cycling, 100–200 mg (~1.5–2.9 mg/kg BM) of caffeine combined with a carbohydrate–electrolyte solution resulted in a 4–7% enhancement during a following TT performed in 26–28 minutes [156]. Moreover, 200–300 mg of caffeine given as a sugar substance at the 10-kilometer mark of a 30 km cycling TT was demonstrated to boost average power output (+3.8%) for the final 10 km of the task, as well as provide a 4% improvement in peak sprint power production at the conclusion [157]. A double-blind study performed on ten elite judokas demonstrated improvement in training parameters such as number of throws and a lower fatigue index, combined with a better biochemical profile, after caffeine supplementation [158]. Another study performed on 18 judokas showed that caffeine at a dose of 4 mg/kg resulted in better performance and a lower rating of perceived exertion [159]. A meta-analysis of nine studies including 109 combat athletes found increased performance and glycolytic activity after caffeine supplementation [160].
In the WADA monitoring program, caffeine remains one of the most utilized stimulants in sports. The results of several studies also demonstrate its efficacy in sustaining better performance in combat sports, especially in aerobic conditions.
5.3. Phosphate
A number of theories suggest the promising advantages of phosphate supplementation on sports outcomes, including an increase in the frequency of ATP and PCr resynthesis; improved buffering ability to support high rates of anaerobic glycolysis; enhancement of myocardial contractility, contributing to higher cardiac efficiency; and an elevated erythrocyte 2,3 diphosphoglycerate (2,3 DPG) concentration, resulting in decreased oxygen affinity for hemoglobin and greater disassembly [18].
Recent research on phosphate supplementation (sodium, calcium or potassium phosphate) has concentrated on the physiological and resulting consequences of experimental procedures such as the 30 s Wingate test, 6–20 m (3–4 s) repetitive sprint efforts and 3–60 min time trials. Therefore, there are ambiguous data that phosphate supplementation improves performance. Phosphate has been proven in several studies to improve VO2max, the anaerobic barrier and cycling TT performance [18]. However, the level of gain from successive sprints has been demonstrated to be variable and ambiguous [161]. Furthermore, a vast collection of findings [162,163] indicate that phosphate supplementation had no effect on exercise training or outcome variables. Alterations in the supplement procedure used (variables in dose, type, exercise procedure, etc.), as well as people’s reactions to the supplement itself, are sufficient to reveal the lack of clear consistency indicated by this cumulative effort.
5.4. Beta-Alanine
Beta-alanine is a rate-limiting precursor to carnosine, an endogenous intracellular (muscle) buffer, and one of the first lines of defense against protons building up in the expanding muscles during exercise [164]. For a minimum of 2–4 weeks, routine supplementation with 3.2–6.4 g (∼65 mg/kg BM) of beta-alanine might raise muscle tissue carnosine content (65% over resting levels), enhancing tolerance for maximal activity sessions lasting 30 s to 10 min [165]. Beta-alanine supplementation has been demonstrated to provide small but noticeable advantages (∼2–3%) in both continual and sporadic exercise testing; however, sport-specific studies that emphasize the practical consequences for sporadic sports are notably absent [165]. When supplementation regimens are prolonged to 10–12 weeks (80% above resting levels), muscle carnosine concentration could be further increased; however, the relationship between muscle alterations and the amount of predicted performance remains unknown [165]. Supplementing with beta-alanine may not be as helpful in well-trained athletes as it is in less-trained athletes, possibly due to carnosine’s reduced function in intramuscular pH control in people who already have a high retention time [166]. Nevertheless, in the context of an actual competition situation, the slight performance improvements reported in well-trained athletes to date (∼0.2–1.3%) may still be significant.
To decrease the probability of health consequences, beta-alanine dosing approaches usually involve split doses absorbed throughout the day (i.e., 0.8–1.6 g every 3–4 h) and/or controlled-release preparations [165]. However, following beta-alanine supplementation, large interindividual variability in muscle carnosine synthesis has been revealed, which is thought to have an inverse relationship with (a) the individual’s preingestion carnosine levels, (b) the person’s training status and (c) the proportion of fast-twitch muscle fibers [167]. Nevertheless, an in-depth study and assessment of the existing research by Stellingwerff et al. [168] suggest that a total of 230 g of beta-alanine should be ingested, including a current consumption range of 1.6–6.4 g/day, in order to obtain around a 50% rise in muscle carnosine. In any case, where practicable, a tailored strategy for beta-alanine supplementation should be investigated [168].
A study on 23 highly trained judokas identified improvements in judo-related performance after 4-week supplementation with 6.4 g/day of beta-alanine [169]. Ten weeks of beta-alanine supplementation resulted in significant performance improvement with no significant side effects in nine male amateur boxing athletes [170]. Bicarbonate may have a synergic additive effect when used in combination with beta-alanine, as demonstrated by a study performed on 37 judo and jiu-jitsu players [171]. We can conclude that beta-alanine has a place in most combat athletes’ nutrition with ergogenic potential when used alone or in combination with other products.
5.5. Carnitine
Carnitine is a molecule found mostly (95%) in skeletal muscle, where it serves a number of key functions in substrate use. Carnitine aids the flux of carbohydrates through the citric acid cycle by aiding the migration of long-chain fatty acids into the mitochondria for beta-oxidation, as well as offering a sink for excessive synthesis of acetyl-CoA [172]. Supplementing with L-carnitine has yielded mixed results in studies. When 1 g of L-carnitine was ingested every 6 h for 2 weeks, Marconi et al. [173] observed a ∼6% rise in VO2max during graded treadmill running but no alteration in steady-state VO2 or fuel usage during submaximal (∼65% VO2max) activity. Furthermore, Greig and colleagues [174] observed that supplementing with L-carnitine at a level of 2 g/day in split doses for 2–4 weeks had no influence on VO2max or substrate metabolism. Of note, the dearth of a productivity effect reported in these studies may likely come from the fact that muscle carnitine levels do not seem to rise when utilizing these conventional supplementation regimens (i.e., up to 4 g/day for 14 days) [175]. Novakova et al. [176] found that 12 weeks of L-carnitine supplementation (2 g/day in split doses) was related to a 20% rise in plasma carnitine levels in regular meat eaters and a ∼30% rise in vegetarians. Conversely, this had no effect on the animal flesh group’s muscle carnitine concentrations and caused only a ∼13% rise in the vegetarians (who had entered the study with ∼10% lower muscle carnitine levels). During both submaximal and maximal activity assessments, there were no consequences on muscular strength, glucose metabolism or VO2 [176].
Although the results on performance are contradictory, L-carnitine may have an effect on weight loss, a very delicate task in weight category sports. The results of a meta-analysis published in 2016 illustrate positive effects of carnitine in non-athlete persons regarding weight loss. Another meta-analysis published in 2020 including an obese non-athletic population concluded a small reducing effect of carnitine on fat mass and body weight [177]. Even though further sports-focused studies are necessary to research the carnitine effect in combat sports, it remains an extensively used supplement by those who intend to lose weight.
6. The Roles of Antioxidants and Strength Training in Muscular Performance
Polyphenols are secondary metabolites of plants with high antioxidant activity, which plays an important role in the prevention and treatment of different diseases, such as cancers, cardiovascular and neurodegenerative diseases and diabetes [178]. In addition to health benefits, a polyphenol-rich diet provides performance-enhancing effects for various types of exercise [179], although opinions related to the effect of antioxidants on athletes’ performance are divided. Hence, in this section, we bring to light the latest articles arguing the role of antioxidants in athletes’ performance. From a chemical point of view, polyphenols are classified into four classes, including phenolic acids, flavonoids, stilbenes and lignans, the last two being available in low quantities and in limited food sources, so their contribution to total polyphenol intake is minor. In contrast, phenolic acids and flavonoids are predominant in the human diet and are found in a wide variety of sources, such as fruits, vegetables, coffee beans, cocoa or their derived products, including red wine, tea, juice and chocolate [180].
In plants, polyphenols are conjugated with sugar moieties (glycosides), although esterified or polymerized forms may be present. After ingested, polyphenols must be hydrolyzed into the aglycone and sugar group before absorption [92,181]. A small proportion of polyphenols is absorbed in the small intestine, with the largest amount reaching the colon, where they undergo fermentation processes by the intestinal microbiota, resulting in metabolites with different physiological effects [181]. Once in the large intestine, polyphenols act as prebiotics for certain other microorganisms [181,182].
Polyphenol effectiveness in the health condition and potential improvement in sports performance depends on their bioavailability (Figure 2).
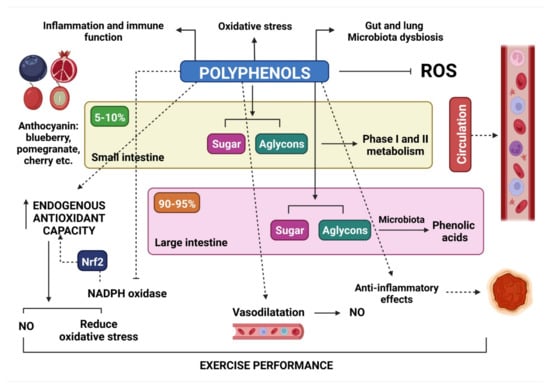
Figure 2.
The impact of polyphenols on one’s health and the possibility of improving exercise performance.
During exercise, the metabolism is accelerated, oxygen consumption is high and the production of reactive oxygen species (ROS) increases. In addition, after muscle contraction, phospholipase A2 is activated, which initiates a cascade of enzymes, resulting in the formation of ROS [183]. On the one hand, exercise-induced ROS have negative effects, causing altered cellular structure and function and, consequently, muscle damage, immune dysfunction and fatigue. On the other hand, ROS formation after exercise can also have beneficial effects, such as stimulating glycogen resynthesis and reducing susceptibility to infection while initiating and promoting adaptative responses to training, thus enhancing athletic performance [183].
Numerous endogenous defense mechanisms against ROS have been elucidated, but at the same time, dietary antioxidants also contribute to the removal of ROS. The available literature presents only a few studies related to the effect of antioxidants on athletes’ performance.
The mechanisms by which anthocyanins can improve exercise performance and their effects on blood flow, the diameter of blood vessels during exercise and endothelial nitric oxide synthase have been described in several studies [184]. Table 4 summarizes the ways in which anthocyanins and phenols influence athletes’ exercise performance. Anthocyanin-induced vasodilation may also occur as a consequence of increased nitric oxide (NO) production. Various mechanisms are proposed [92], including: (i) cyanidin-3-o-glucoside upregulates nitric oxide synthase (NOS) expression, resulting in NO production; (ii) based on chemical features, anthocyanins may reduce nitrite NO2 to NO in the stomach; and (iii) they inhibit nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and induce signaling of nuclear respiratory factor 2 (Nrf2). Enhanced endogenous antioxidant capacity correlates with NO bioavailability.

Table 4.
The effects of polyphenol supplementation from different sources on exercise performance.
It is generally accepted that flavonoids possess strong antioxidant effects, and the mechanisms are not necessarily related to the direct scavenging of ROS but rather to the indirect modulation of multiple cell signaling pathways [190,193]. The reported results may also be related to a positive adaptation of the endogenous antioxidant system in response to strength training. The authors acknowledge the variation in polyphenol types, so not all antioxidants possess similar physiological effects. However, opinions are still divided, with some authors arguing that antioxidant supplements would not be beneficial in terms of athletes’ performance. However, the strongest argument in favor of antioxidant supplementation (especially natural ones) remains the fact that antioxidant supplementation can delay fatigue and improve endurance performance [194].
There is no doubt that the physical activity of elite combat sports athletes generates oxidative stress that exceeds the endogenous antioxidant capacity to neutralize it, although a study performed on 10 judokas and another one on 12 karate athletes demonstrated that they had stronger intrinsic antioxidant activity [195,196]. Antioxidant and prooxidant activities were also demonstrated to be altered by rapid weight loss in judo athletes [197]. Supplementation with grape juice for 14 days was proven to increase antioxidant capacity, reduce lipid and DNA damage and increase upper limb strength in a double-blind study including 20 judokas [198]. Kendo athletes benefitted from reduced muscle injury after Q10 coenzyme supplementation in a double-blind 18-participant study [199]. Improved immune function and antioxidant capacity were observed after Lycium barbarum extract supplementation in taekwondo athletes [200].
Regarding strength training and muscle hypertrophy, the addition of extrinsic antioxidants may interfere with the cell signaling system and limit anabolic signals, resulting in lower muscle synthesis following a training stimulus. This theory is confirmed by the findings of a placebo-controlled study on 42 female athletes, with statistically significant lower peak torque and total work in the control group supplemented with vitamin C and E [201].
There is no doubt about the positive antioxidant effect on rapid recovery, limiting the damage generated by intense physical activity. This effect is of high utility in combat sports, where recovery times are limited. The results of a double-blind randomized study on 18 elite taekwondo athletes revealed a significantly better biological profile in the control group after supplementation with vitamins C and E, with lower values of muscle damage markers such as creatine kinase, myoglobin and hemolysis [129]. Multiple studies support the positive vitamin E effect at high altitudes, limiting the deformation of erythrocytes [202].
Consequently, although they may have detrimental effects on muscle hypertrophy, short-term and targeted supplementation of antioxidants are important aids for endurance and fast recovery, which is an important aspect for combat players between weigh-in and competition.
7. Natural Supplements: Importance and Advantages over Synthetic Ones
Some nutritional supplements have a direct impact on athletic performance by increasing various rate-limiting processes, while others have an indirect impact on physical performance by influencing factors such as inflammatory modulation, oxidative stress and signaling pathways for adaptation or their ability to support repetitive performance.
Nutritional supplements are defined as concentrated sources of nutrients, such as amino acids and/or proteins, carbohydrates, vitamins, creatine, minerals, isotonic liquids and herbal preparations, among others. Their use is widespread among elite and amateur athletes, as well as the general population [203]. The type, amount and intake timing of supplements are important for promoting optimal health and performance of players during training and competitive sports [123].
Natural supplements are obtained from different food sources in different ways, such as extraction, concentration and so on, whilst synthetic supplements are obtained by chemical synthesis in the laboratory. Natural supplements/nutrients include complex mixtures of phytochemicals that exert additive and synergistic effects resulting in health benefits [204]. Numerous metabolic pathways in which micronutrients are needed are affected by exercise during training and result in muscle biochemical adaptations that increase the need for micronutrients [205]. In the case of athletes who frequently restrict their energy intake, such as combat players who eliminate one or more food groups from their diet, micronutrient supplementation is necessary. This can lead to deficiencies in minerals such as calcium, iron or vitamins and antioxidants. Single micronutrient supplements are generally used to correct a clinically defined medical condition [205]. Among athletes, one of the most popular supplements is protein in order to increase body mass and strength. Following endurance training, high protein intake is required due to transient immune system dysfunction, increased inflammation and oxidative stress [203]. When protein in food is not available or is insufficient, then dietary supplements with high-quality ingredients can serve as a practical alternative to help athletes meet their protein needs [123].
Studies suggest that the bioavailability of natural supplements, especially of vitamins, is much higher than that of synthetic ones, probably due to their physicochemical features or the presence of additional components that can promote their absorption and potentiate their beneficial effects [206]. Synthetic vitamins mimic the natural ones found in foods and present some disadvantages from the points of view of bioavailability, efficiency, purity and possible side effects.
In this context, a new class of naturally derived metabolic regulators was promoted, generically named adaptogens, which have been shown to increase the ability of the organism to adapt to environmental factors while avoiding damage and maintaining mental abilities. A comprehensive review on adaptogen supplementation and their capacity to enhance athletic performance was performed by Melinos A. [207], who highlighted the differences between adaptogens and other metabolic regulators, such as stimulants. Various adaptogen plants were discussed in this study; for example, Rhodiola rosea, Eleutherococcus senticosus, Schizandra chinensis and Panax ginseng showed encouraging results in improving physical performance, which can be applied to combat players. A similar point of view was expressed by Sellami et al. [208] in a study including Tribulus Terrestris, Ginkgo biloba, Cordyceps Sinensis, Panax ginseng, Coffea arabica, Green tea extract, Ginger, etc. The conclusion was that Ginseng and caffeine had the most significant effect on the central nervous system by increasing alertness and reaction time, which is crucial for combat players, while other herbs seem to stimulate steroid hormone production. On the other hand, a moderate effect was noticed in terms of oxidative stress, fatigue resistance and endurance capacity.
A promising natural supplement used to optimize performance is based on beetroot juice due to its nitrate content with ergogenic effects on endurance exercise [209]. Gallardo and Coggan suggested measuring the nitrate content of any beetroot supplements used in scientific research rather than depending on the manufacturer’s claim [210].
This is an important consideration, not only for future studies in this area but also for coaches and athletes who may be attempting to optimize performance and reaction time for competition with the use of beetroot supplements, which is crucial for combat players.
Reactive oxygen species (ROS) are molecules that are produced and consumed continuously in all living organisms. During exercise, the training muscle produces much higher amounts of ROS compared to the resting muscle. Coenzyme Q10, a controversial molecule with a key role in cellular bioenergetics, being a cofactor in the mitochondrial respiratory chain to provide energy to cells, has several roles, such as lipophilic antioxidation, regeneration of vitamins C and E and reduction in inflammatory markers [211]. Although studies are conflicting regarding CoQ10 effects on sports performance, it contributes to the prevention of muscle injuries due to its antioxidant effect, anti-inflammatory action, DNA protection and modifying effect on gene expression. Other studies have shown the positive involvement of CoQ10 in sports performance through its impact on the nervous system and muscle disease, increasing resistance to oxidative stress. In addition, CoQ10 supplementation may work synergistically with other molecules (creatine, fatty acids and curcumin) in order to prevent or restore tissue after the stress produced by exercise [211,212,213].
Lycopene, a carotenoid responsible for the red color of tomatoes, is used as a supplement by athletics to reduce exercise-induced oxidative stress, cell damage and postexercise fatigue. Gholami et al. 2021 [214] investigated the effects of both tomato powder and lycopene on the antioxidant capacity and biomarkers of lipid peroxidation in response to exhaustive exercise in well-trained male athletes. Their results showed that supplementation with tomato powder for one week positively increased the total antioxidant capacity and was stronger compared to lycopene supplementation. These results suggested that lycopene exerts a synergistic effect with bioactive compounds present in tomatoes, such as polyphenols, carotenoids, ascorbic acid, α-tocopherol and folate.
8. Nanotechnological Tools for Nutraceuticals and Functional Foods
Nanotechnology is the study of the potential to image, analyze, understand and control materials at scales of 1–100 nanometers, where unexpected interfacial phenomena implement new functions. This extraordinary aptitude has resulted in a plethora of new technologies that influence practically every part of science and technology, industry, the economy, the environment and human existence [215,216]. In a visionary presentation, Feynman (1959) proposed that we should be capable of manipulating matter down to the atomic level with excellent consistency and accuracy so that innovative substances with tailored functions could be developed. The developing area in research facilities did not flourish until the mid-1980s, when essential analytical tools, including scanning tunneling microscopy and atomic force microscopy, were available [217].
8.1. Different Nano-Formulations for Encapsulation and Nutraceutical Delivery: General Properties, Advantages over Conventional Ones and Improvement of Oral Bioavailability
Previously, the majority of studies focused on putting specific beneficial compounds into colloidal delivery systems, such as β-carotene into a nanoemulsion [215], vitamin D into an emulsion, resveratrol into solid lipid NPs [217] or curcumin into protein NPs [218]. It is typically essential to integrate several bioactive compounds into a single delivery system [219]. Furthermore, combining numerous bioactive compounds in a single system may have advantages since they have synergistic effects. When taken together, their bioavailability or bioactivity is more significant than when consumed separately. A multitude of strategies can be used to produce a multi-bioactive delivery system.
Single-particle encapsulation: When the bioactives to be encapsulated have similar polarity, such as being all hydrophobic or all hydrophilic, they can be delivered in a single phase using a colloidal delivery system. Micelles, liposomes, lipid droplets and protein NPs, for example, can disperse non-polar bioactives within the same hydrophobic domain. Non-polar bioactives are generally distributed throughout the hydrophobic phase before the particles are produced. Single-particle encapsulation is typically more accessible and less expensive to implement than the method described below. Still, it is significantly less versatile when controlling the precise levels of different nutrients or nutraceuticals in a system. Depending on the particular nutritional profile sought, the entire emulsion production process would have to be repeated for each unique food item. At the moment, the most extensively used method is encapsulating nutraceutical combinations in single colloidal particles. Curcumin and resveratrol, for example, have been co-encapsulated in nanoparticles made of lipids [220], proteins [221] and polysaccharides [222]. Before making colloidal particles, the two polyphenols were mixed with the dispersed phase material. Another study found that curcumin and vitamin D could be successfully encapsulated into liposomes [223]. Curcumin and egg white peptides were co-encapsulated in chitosan and casein biopolymer particles to enable simultaneous administration [224]. Both hydrophobic nutraceuticals with complementary biological activity, coenzyme Q10 and piperine were encapsulated in biopolymer nanoparticles with a hydrophobic zein core and a hydrophilic carrageenan shell [225]. It has been demonstrated that co-encapsulation improves the chemical stability of the two bioactive components while also managing their gastrointestinal fate, such as natural antioxidants, ferulic acid and gallic acid co-encapsulated within cyclodextrin complexes [226]. According to another study, the natural antioxidants anthocyanins and curcumin can be co-encapsulated within casein micelles via electrostatic and hydrophobic interactions [227]. Co-encapsulation increased the chemical stability and in vitro bioavailability of nutraceuticals. A carotenoid antioxidant (astaxanthin) co-encapsulated with other natural antioxidants (tocotrienols, capsaicin and resveratrol) was shown to improve antioxidant activity synergistically, which was linked to molecular interactions between the nutraceuticals [228]. Lactobacillus acidophilus (probiotic) and inulin or polydextrose (prebiotics) have also been co-encapsulated into solid lipid microparticles, increasing their stability and survivability in simulated GIT conditions [229]. Other colloidal particles, such as alginate microgels [230], pea protein/alginate microgels [231] and alginate–chitosan microgels [232], have been utilized to encapsulate probiotics and prebiotics. Prebiotics within colloidal particles may boost probiotic viability by providing a source of nourishment.
Multiple-particle encapsulation: In some cases, encapsulating different bioactive compounds inside other colloidal particles can be advantageous. There are several reasons why one may desire to do so. First, it is necessary to create a variety of bioactive-loaded colloidal dispersions that can be blended in various ratios if the aim is to carefully manage the balance of different bioactive substances in a meal or beverage. Second, if two or more bioactives interact physically or chemically, it may be better to keep them separated to avoid adverse interactions. Third, because different hydrophobic bioactive may have differing solubilities in non-polar settings, colloidal particles consisting of various hydrophobic materials may be beneficial (such as other oils or hydrophobic proteins) [233]. Fourth, some hydrophobic bioactives’ chemical stability is impacted by their local molecular environment; for example, some may degrade faster in a lipid matrix than in a protein matrix. As a result, encapsulating various bioactives in separate colloidal particles with molecular environments tailored to each bioactive could be beneficial. Fifth, various bioactives may need to be released at different times or in other parts of the human body. For example, one bioactive may need to be released in the small intestine, whereas another requires release in the colon. Alternatively, one bioactive might require a burst release technique, while another might require a continuous release profile. The bioactive would be encased in colloidal particles with various release properties, such as nanodroplets for burst release and microgels for sustained release. Distinct bioactives could be contained within the same colloidal particle or across different types of colloidal particles [233]. A bioactive may be encapsulated in one emulsion, while a different kind of bioactive may be encapsulated in another. Another possibility is to encapsulate one type of bioactive in an emulsion and the other in a solid lipid nanoparticle. After that, the two colloidal delivery methods might be merged to generate a final formulation with various bioactive compounds. This technology could be particularly beneficial in personalized nutrition applications. To obtain the desired nutritional profile, a range of single bioactive-loaded delivery systems could be combined in varying doses and ratios. When combining different colloidal dispersions, it is vital to ensure that the particles are compatible; otherwise, the integrated system could be physically or chemically unstable. For example, one type of colloidal particle may promote the aggregation of a different kind of colloidal particle. If their surface charges are opposite, such as one positive and one negative, this can happen [234]. Furthermore, bioactive molecules can be prevented from migrating from one type of colloidal particle to another [235]. The entropy of mixing drives the exchange of bioactive chemicals between colloidal particles. Having all bioactive compounds randomly disseminated across all colloidal particles is better than having them concentrated in one type of particle from a thermodynamic standpoint. The bioactive’s water solubility influences the exchange kinetics. Even hydrophobic bioactives have some aqueous solubility, implying that they can pass through water, separating colloidal particles. In some situations, changing the properties of colloidal particles may be able to slow down this process. Components that bind to bioactives and limit their freedom, on the other hand, could be added to colloidal particles [236]. These processes must be understood to be adequately controlled and avoid undesired functional changes during storage. Curcumin was encapsulated in protein (zein) NPs to enhance its water mobilization and chemical stability and then combined with digestive lipid NPs to boost its bioavailability [237]. Curcumin was released from protein NPs and then solubilized in mixed micelles produced by the digestion of triacylglycerol molecules in lipid NPs under simulated gastrointestinal conditions.
Compartmentalized encapsulation: Some colloidal delivery methods, such as core–shell particles or multiple emulsions, have particles with various physicochemical properties in their interior regions. These particles usually include many bioactive chemicals with varying molecular properties. This could be required to avoid the two bioactives’ interference and manage the rates and sequence. Core–shell polymer particles have been used to encapsulate pharmaceuticals with changing polarity in the pharmaceutical industry. For example, the aqueous center of polymer core–shell particles contains doxorubicin hydrochloride (a hydrophilic chemical), while the solid shell contains paclitaxel (a hydrophobic substance). A similar method could be used to encapsulate food-grade nutraceuticals. A hydrophobic bioactive agent (α-tocopherol) can be contained within the oil droplet core. In contrast, according to studies with oil-in-water emulsions, a more amphiphilic bioactive material (resveratrol) can be discovered at the oil droplet surfaces [235]. Natural antioxidant combinations have also been co-encapsulated in multi-compartment colloidal particles. Tween 60 niosomes, for example, have been co-encapsulated with gallic acid/curcumin and ascorbic acid/quercetin [238]. The hydrophilic gallic acid and ascorbic acid were assumed to be in the hydrophilic core of the particles. In contrast, the hydrophobic curcumin and quercetin were believed to be in the hydrophobic shell. The researchers discovered that combining antioxidants in a single delivery method has synergistic antioxidant benefits.
Multiple-phase encapsulation: When bioactives have diverse polarities (for example, some are hydrophobic while others are hydrophilic), they can be found in separate phases in a colloidal particle solution. Non-polar bioactives, for instance, may disperse in the oil phase of an oil-in-water emulsion, whereas polar bioactives may dissolve in the water phase. This approach was used to dissolve fat-soluble vitamins (A or E) in the oil phase and water-soluble vitamins (C) in the water phase in nanoemulsions [239,240].
8.2. Liposomes for Nutraceutical Delivery
Several molecules gradually deteriorate and lose their nutritional content, which is a significant challenge in producing (fortified) foodstuffs and dietary supplements. Various microencapsulation methods can be implemented to enhance their stability. While the liposomal technique was initially developed for pharmaceutical applications, its benefits have recently been utilized by the food industry. Liposomes and nano-liposomes can encapsulate a broad range of nutritionally crucial elements, including essential oils, amino acids, antioxidants, enzymes, vitamins and minerals. Vitamin C, for instance, is supplied to a variety of commodities to prolong shelf life, as detailed by Schrooyen et al. [241]. Spray cooling or spray chilling and fluidized-bed coating can be used to add vitamin C to solid foods (biscuits and bread).
In contrast, liposomal encapsulation adds vitamin C to liquid products. While vitamin C is used to extend the shelf life of some foods, it is also used for its functional and pharmacological actions. Vitamin C is an antioxidant and a crucial cofactor for several enzymes responsible for physiological functions. Despite its significance in various processes, vitamin C has limited bioavailability and cannot be generated by the body on its own. Therefore, it must be consumed through the diet [242].
Even though most Western cultures now have access to vitamin C sources, including fruits and vegetables, the increasing use of microwaved foods has contributed to a rise in the prevalence of scurvy (severe vitamin C insufficiency). Vitamin C deficiency has also been linked to chronic disorders, notably obesity [243], with vitamin C supplementation improving lipid buildup and endothelial function [244]. Researchers have begun to examine liposomal formulations as a potential alternative to traditional oral vitamin C administration to enhance vitamin C bioavailability and enhance its therapeutic effects. Hickey and colleagues [245] performed the first investigation to compare the pharmacokinetics of different single doses (5, 20 and 36 g) of liposomal vitamin C to “standard” oral vitamin C (5 g). According to the study, the most significant peak plasma level (~400 uM/L) was obtained with 36 g, peaking ~5–6 h after intake. Liposomal vitamin C’s effectiveness and consequences on ischemia–reperfusion injury were studied in more studies [246]. A placebo pill or 4 g of vitamin C was administered via oral unencapsulated, liposomal or parenteral administration. Liposomal vitamin C generated higher blood levels of vitamin C than unencapsulated vitamin C, but not as high as those induced by intravenous vitamin C delivery.
Furthermore, all medications exhibited equal protection against hypoperfusion damage [247]. Because iron deficiency is a prominent cause of anemia in nondialysis chronic kidney disease (ND-CKD) patients, Pisani et al. [248] investigated whether liposomal iron improved anemia in ND-CKD patients. Ninety-nine patients with CKD and iron deficiency anemia for three months were randomly assigned 30 mg/day of oral liposomal iron (LI; n = 66) or intravenous iron gluconate (IV: total dosage = 1000 mg; n = 33) using a randomized experimental technique. After three months, both the LI (5.6%) and IV (9.3%) groups had considerably higher Hb levels than baseline, with no differences between interventions. On the other hand, the IV group exhibited a more noticeable upward trend, with markedly greater Hb in months 1 and 2 than the LI group. In contrast, while no serious negative consequences were observed, the proportion of LI individuals who suffered at least one possibly treatment-related adverse reaction (3.1%) was much lower than the IV group. Because iron deficiency and anemia are significant problems for the health and performance of great male and female athletes [249], and because liposomal iron has been demonstrated to facilitate exercise-related anemia in an in vitro model [250], more studies into liposomal iron in athletes are recommended.
8.3. Polysaccharide Nanocarriers for Nutraceuticals
Polysaccharides are lengthy chains of monosaccharide molecules that form polymeric carbohydrates. The most common polysaccharide storage in plants is starch, amylose and branched amylopectin. Polysaccharides include chitosan, alginates, pectin, dextran and inulin [251]. The polymers amylopectin (70–75 percent) and amylose (20–25 percent) make up the starch molecule. Depending on the starch supply, this ratio changes [252]. Starch in nanoparticulate form can be used as a flexible barrier packaging material in addition to its application in food. The characteristics of starches can be altered through enzymes, hydrolysis, oxidation and replacement processes. Starches and derivatives are used in several microencapsulation and nutrition delivery applications [253,254,255,256,257].
On the other hand, chitosan is a biopolymer that encapsulates active chemicals in a biodegradable and biocompatible polymer matrix. The use of chitosan to make nanoparticles necessitates the use of basic procedures. Because of its characteristics, this biopolymer can contain bioactive components such as vitamins, polyphenols, curcumin and essential oils [258,259,260].
8.4. Protein-Based Nanocarriers
Fathi et al. [261] discussed the state of the art in protein-based nanoencapsulation technologies and protein alteration methods to prolong their activity in nanocarrier systems to increase the encapsulation, retention, shielding and release of bioactive substances. Ramos et al. [262] published a literature review on the latest discoveries on nanoscale manifestations of denatured whey proteins and their agglomeration, which could help design protein nanostructures with new or significantly improved attributes for the implementation of nutraceuticals in food materials and their release. Parthasarathi and Anandharamakrishnan [263] reported a spray freeze-drying-based microencapsulation approach as a viable strategy for improving the oral bioavailability of insufficiently water-soluble bioactive substances such as vitamin E, employing whey protein concentrate as an encapsulating reagent. Under gastric digestion circumstances, substantial incorporation and sedimentation of zein NPs comprising lutein (ZLNPs) with a hydrodynamic radius of approx. 75 nm were noticed, and ZLNPs that were not entirely metabolized by gastric enzymes conformed to lipid droplets; nevertheless, when salt (i.e., high ion concentration) was removed, the aggregation was lowered, and digestion was energized.
On the other hand, lutein’s digestive stability was improved once it was encapsulated in NPs [264]. The size of egg albumin (Alb)-FA nanocomplexes made by mixing egg Alb NPs with FA was not altered when the pH was changed from 3 to 4, but it increased significantly when the pH was adjusted to 5, 6 or 7. Notwithstanding this, the bioavailability of FA in the form of digestion process nanocomplexes for Lactobacillus rhamnosus was improved.
Negatively charged (41 mV) sophorolipid-coated curcumin NPs with a particle size of 61 nm and high EE and loading capability for the amorphous form had 2.7–3.6-fold greater efficacy than curcumin in amorphous form-free curcumin crystals, which was primarily due to their higher nutrient digestibility [265]. In a system designed to simulate GI media, protein–lipid composite NPs with a three-layered structure and an inner aqueous compartment for loading the hydrophilic nutritional supplement vitamin B12 demonstrated controlled release actions. In in vivo tests, the NPs loaded with vitamin B12 led to excessive vitamin B12 levels in rats and lowered methylmalonic serum concentrations more effectively than in the control group. These NPs could be employed to improve vitamin B12 absorption when taken orally [266].
Lin et al. [267] showed improved physicochemical stability and in vitro bioavailability of vitamin D3 in maize protein hydrolysate-based vitamin D3 nanocomplexes with spherical structure and diameters of 102–121 nm. The nanocomplexation offered prominent safeguards and lowered vitamin D losses during pasteurization and also under many various sets of processing conditions in vitamin D–potato protein co-assemblies, implying that potato protein could be used as a protective barrier for carrying hydrophobic nutritional supplements adequate for the enhancement of clear beverages and other food or drink products with health-promoting properties [268].
8.5. Nutraceutical Nano-Delivery and Personalized Nutrition
The individualization of meals based on biometric data has become a significant trend in modern nutrition [269,270,271]. This study has been spurred on by technological developments in various domains, including analytical instrumentation, biotechnology, computers and data processing [269,270]. The many metabolites contained in human physiological fluids such as blood, urine and feces can now be studied in depth by researchers. Modifications in the metabolic parameters can be tracked over time and in response to certain meals or diets, as well as individuals with various lifestyles or health conditions. Thanks to advances in instrumentation, researchers can now quantify the several types of molecules found in meals and their structural structures. Advances in genetics, equipment and computer science have made it easier to swiftly and inexpensively read a person’s genetic code [272,273]. Similar methods can be used to evaluate the composition of a person’s gut microbiome to determine the microbial community that dwells in their intestines, which is vital for optimal health [274]. Biosensors, digital cameras and portable measurement devices are some of the newest technologies being developed to provide detailed information about people’s eating habits, physical activity and physiological features. Scientists can find links between food, genetics, phenotype and health status by storing vast amounts of data from many different sources in computer databases and evaluating them with modern computer algorithms [275]. Only a few companies presently provide personalized dietary advice based on a thorough examination of a person’s metabolism and microbiota. As a result, personalized dietary gadgets tailored to a particular individual’s requirements could be developed. Personalized diets could advise a person to eat more of certain food groups. On the other hand, several food and nutrition companies work on functional foods and beverages enhanced with specific nutrients or nutraceuticals. Many of these things will be dependent on colloidal delivery technologies, which can incorporate a range of bioactive compounds into foods and beverages. These individualized diets can be adapted to an individual’s needs in various ways, including combining multiple nutrients in a single delivery system or integrating multiple delivery systems containing different nutrients, as we will see later. However, there is presently no information on the appropriate type and concentration of various bioactive components to distribute to specific individuals, which will have to be discovered when colloidal delivery systems are constructed [276].
9. Safety Aspects and Limitations
There were no relevant safety data available, and no adverse effects were documented in any of the studies considered. As a result, this review study cannot comment on it. All substances discussed in this review are considered safe for humans and approved by WADA in recommended doses. Even though some products, such as whey protein, are acknowledged as safe supplements for athletes, WADA’s concern stems from the discovery of prohibited substances. As a result, athletes must be cautious when taking supplements to avoid breaking WADA rules and regulations.
There are a few drawbacks to this review that should be considered. First and foremost, the study did not address issues that existed in the primary analyses. Furthermore, the biases in the initial research were not removed in the review. Again, there would be inaccuracy due to the inability to generalize various criteria, such as age, gender or regional factors, from one study to the next. Furthermore, based on this review of sports performance and recovery among athletes when they were measured, the discussion and conclusion are drawn from this meta-analysis. As a result, this study cannot demonstrate causation between the parameters and athletes’ long-term performance and recovery.
10. Conclusions and Future Trends
To make evidence-based judgments about whether and how to use a supplement, athletes and coaches should examine the critical areas that sports nutrition research should address. Scientific data on dietary requirements and nutritional goals can be used to choose medical supplements and sports foods. Unfortunately, the variety of products on the market (even when limited to those with some evidence base) and the complexities of real-life settings in which performance supplements can make a difference restrict the ability to undertake high-quality evaluations of performance supplements. Models for performing and interpreting performance supplement studies do exist, though. Caffeine is an excellent example of how sports nutrition knowledge and practice evolve, with the possibility of always learning something new or better. A few tropical fruits that can be used for sports nutrition are banana, cherry, grape, pomegranate and watermelon. Those fruits may have the potential to be developed as a sports supplement in the form of nanoparticles, which have several training and competitive benefits for athletes. Overall, skipping protein during the peri-workout period has no adaptive effect. Stimulation of MPS in the acute time following training may not result in increases in strength, hypertrophy, body composition or performance unless other strategies are used carefully over the prolonged recovery phase.
On the other hand, the individualization of meals based on biometric data has become a significant trend in modern nutrition. The many metabolites contained in human physiological fluids such as blood, urine and feces can now be studied in depth by researchers. Nowadays, modifications in metabolic parameters can be tracked over time and in response to certain meals or diets, as well as in individuals with various lifestyles or health conditions. Thanks to advances in instrumentation, researchers can now quantify different types of molecules found in meals and their structural features. Advances in genetics, equipment and computer science have made it easier to swiftly and inexpensively read a person’s genetic code and to evaluate the composition of a person’s gut microbiome to determine the microbial community that dwells in their intestines, which is vital for optimal health. Biosensors, digital cameras and portable measurement devices are some of the newest technologies being developed to provide detailed information about people’s eating habits, physical activity and physiological features. Scientists can find links between food, genetics, phenotype and health status by storing vast amounts of data from many different sources in computer databases and evaluating them with modern computer algorithms. Only a few companies presently provide personalized dietary advice based on a thorough examination of a person’s metabolism and microbiota. As a result, personalized diets could be successfully applied to combat players, as gadgets tailored to a particular individual’s requirements could be developed, advising an athlete to consume more or less of certain food groups. On the other hand, several food and nutrition companies work on functional foods and beverages enhanced with specific nutrients or nutraceuticals based on colloidal delivery technologies, which can incorporate a wide range of bioactive compounds into foods and beverages. These individualized diets can be adapted to an individual’s needs in various ways, including combining multiple nutrients in a single delivery system or integrating multiple delivery systems containing different nutrients. However, there is presently no information on the appropriate type and concentration of various bioactive components to distribute to specific individuals, and hence, in the near future, different formulations for encapsulation and nutraceutical delivery are expected to emerge, taking into account possible advantages over conventional ones and improvement of oral bioavailability. As a result, future research should consider this much broader perspective, including advanced development in designing nanostructures with new or significantly improved attributes for the implementation of novel nutraceuticals in sports nutrition.
Author Contributions
Conceptualization, A.T., S.I.V. and S.C.; methodology, A.T., F.I., M.R.I., S.I.V. and S.C.; investigation, A.T., F.I. and M.R.I.; writing—original draft preparation, A.T., F.I., S.I.V. and M.R.I.; writing—review and editing, A.T., S.I.V. and S.C.; supervision, S.C. and S.I.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
This research did not report any data.
Acknowledgments
The publication fee for this manuscript was supported by the University of Oradea, Romania.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Capling, L.; Beck, K.; Gifford, J.; Slater, G.; Flood, V.; O’Connor, H. Validity of Dietary Assessment in Athletes: A Systematic Review. Nutrients 2017, 9, 1313. [Google Scholar] [CrossRef] [PubMed]
- Knapik, J.J.; Steelman, R.A.; Hoedebecke, S.S.; Austin, K.G.; Farina, E.K.; Lieberman, H.R. Prevalence of Dietary Supplement Use by Athletes: Systematic Review and Meta-Analysis. Sports Med. 2016, 46, 103–123. [Google Scholar] [CrossRef] [PubMed]
- Tirla, A.; Vesa, C.M.; Cavalu, S. Severe Cardiac and Metabolic Pathology Induced by Steroid Abuse in a Young Individual. Diagnostics 2021, 11, 1313. [Google Scholar] [CrossRef] [PubMed]
- Jagim, A.R.; Fields, J.B.; Magee, M.; Kerksick, C.; Luedke, J.; Erickson, J.; Jones, M.T. The Influence of Sport Nutrition Knowledge on Body Composition and Perceptions of Dietary Requirements in Collegiate Athletes. Nutrients 2021, 13, 2239. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, S.; El Koofy, N.; Moawad, E.M.I. Patterns of Nutrition and Dietary Supplements Use in Young Egyptian Athletes: A Community-Based Cross-Sectional Survey. PLoS ONE 2016, 11, e0161252. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.L.; De Souza, M.J.; Kindler, J.M.; Williams, N.I. Nutritional Practices Associated with Low Energy Availability in Division I Female Soccer Players. J. Sports Sci. 2014, 32, 1499–1509. [Google Scholar] [CrossRef] [PubMed]
- Wardenaar, F.; Brinkmans, N.; Ceelen, I.; Van Rooij, B.; Mensink, M.; Witkamp, R.; De Vries, J. Macronutrient Intakes in 553 Dutch Elite and Sub-Elite Endurance, Team, and Strength Athletes: Does Intake Differ between Sport Disciplines? Nutrients 2017, 9, 119. [Google Scholar] [CrossRef]
- Anderson, L.; Orme, P.; Naughton, R.J.; Close, G.L.; Milsom, J.; Rydings, D.; O’Boyle, A.; Di Michele, R.; Louis, J.; Hambly, C.; et al. Energy Intake and Expenditure of Professional Soccer Players of the English Premier League: Evidence of Carbohydrate Periodization. Int. J. Sport Nutr. Exerc. Metab. 2017, 27, 228–238. [Google Scholar] [CrossRef]
- Ebine, N.; Feng, J.-Y.; Homma, M.; Saitoh, S.; Jones, P.J.H. Total Energy Expenditure of Elite Synchronized Swimmers Measured by the Doubly Labeled Water Method. Eur. J. Appl. Physiol. 2000, 83, 1–6. [Google Scholar] [CrossRef]
- Jenner, S.L.; Trakman, G.; Coutts, A.; Kempton, T.; Ryan, S.; Forsyth, A.; Belski, R. Dietary Intake of Professional Australian Football Athletes Surrounding Body Composition Assessment. J. Int. Soc. Sports Nutr. 2018, 15, 43. [Google Scholar] [CrossRef]
- Książek, A.; Zagrodna, A.; Słowińska-Lisowska, M. Assessment of the Dietary Intake of High-Rank Professional Male Football Players during a Preseason Training Week. Int. J. Environ. Res. Public. Health 2020, 17, 8567. [Google Scholar] [CrossRef]
- Sports Nutrition Market Size, Share Report, 2022–2030. Available online: https://www.grandviewresearch.com/industry-analysis/sports-nutrition-market (accessed on 1 August 2022).
- Garthe, I.; Maughan, R.J. Athletes and Supplements: Prevalence and Perspectives. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 126–138. [Google Scholar] [CrossRef]
- Peeling, P.; Castell, L.M.; Derave, W.; de Hon, O.; Burke, L.M. Sports Foods and Dietary Supplements for Optimal Function and Performance Enhancement in Track-and-Field Athletes. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 198–209. [Google Scholar] [CrossRef]
- Ventura Comes, A.; Sánchez-Oliver, A.; Martínez-Sanz, J.; Domínguez, R. Analysis of Nutritional Supplements Consumption by Squash Players. Nutrients 2018, 10, 1341. [Google Scholar] [CrossRef]
- Jäger, R.; Mohr, A.E.; Carpenter, K.C.; Kerksick, C.M.; Purpura, M.; Moussa, A.; Townsend, J.R.; Lamprecht, M.; West, N.P.; Black, K.; et al. International Society of Sports Nutrition Position Stand: Probiotics. J. Int. Soc. Sports Nutr. 2019, 16, 62. [Google Scholar] [CrossRef]
- Nicolov, M.; Cocora, M.; Buda, V.; Danciu, C.; Duse, A.O.; Watz, C.; Borcan, F. Hydrosoluble and Liposoluble Vitamins: New Perspectives through ADMET Analysis. Medicina 2021, 57, 1204. [Google Scholar] [CrossRef]
- Peeling, P.; Binnie, M.J.; Goods, P.S.R.; Sim, M.; Burke, L.M. Evidence-Based Supplements for the Enhancement of Athletic Performance. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 178–187. [Google Scholar] [CrossRef]
- Fernandes, H. Dietary and Ergogenic Supplementation to Improve Elite Soccer Players’ Performance. Ann. Nutr. Metab. 2021, 77, 197–203. [Google Scholar] [CrossRef]
- Özen, A.E.; Bibiloni, M.D.M.; Pons, A.; Tur, J.A. Consumo De Alimentos Funcionales En Europa; Una Revisión Sistemática. Nutr. Hosp. 2014, 470–478. [Google Scholar] [CrossRef]
- Levine, B.D.; Baggish, A.L.; Kovacs, R.J.; Link, M.S.; Maron, M.S.; Mitchell, J.H. Eligibility and Disqualification Recommendations for Competitive Athletes With Cardiovascular Abnormalities: Task Force 1: Classification of Sports: Dynamic, Static, and Impact: A Scientific Statement From the American Heart Association and American College of Cardiology. Circulation 2015, 132. [Google Scholar] [CrossRef]
- Barley, O.R.; Chapman, D.W.; Guppy, S.N.; Abbiss, C.R. Considerations When Assessing Endurance in Combat Sport Athletes. Front. Physiol. 2019, 10, 205. [Google Scholar] [CrossRef] [PubMed]
- Slimani, M.; Davis, P.; Franchini, E.; Moalla, W. Rating of Perceived Exertion for Quantification of Training and Combat Loads During Combat Sport-Specific Activities: A Short Review. J. Strength Cond. Res. 2017, 31, 2889–2902. [Google Scholar] [CrossRef] [PubMed]
- Degoutte, F. Energy Demands during a Judo Match and Recovery. Br. J. Sports Med. 2003, 37, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Kreider, R.B.; Wilborn, C.D.; Taylor, L.; Campbell, B.; Almada, A.L.; Collins, R.; Cooke, M.; Earnest, C.P.; Greenwood, M.; Kalman, D.S.; et al. ISSN Exercise & Sport Nutrition Review: Research & Recommendations. J. Int. Soc. Sports Nutr. 2010, 7, 7. [Google Scholar] [CrossRef]
- Kerksick, C.M.; Arent, S.; Schoenfeld, B.J.; Stout, J.R.; Campbell, B.; Wilborn, C.D.; Taylor, L.; Kalman, D.; Smith-Ryan, A.E.; Kreider, R.B.; et al. International Society of Sports Nutrition Position Stand: Nutrient Timing. J. Int. Soc. Sports Nutr. 2017, 14, 33. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.; Rollo, I. Carbohydrate Nutrition and Team Sport Performance. Sports Med. 2015, 45, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Huecker, M.; Sarav, M.; Pearlman, M.; Laster, J. Protein Supplementation in Sport: Source, Timing, and Intended Benefits. Curr. Nutr. Rep. 2019, 8, 382–396. [Google Scholar] [CrossRef]
- Passariello, C.L.; Marchionni, S.; Carcuro, M.; Casali, G.; Pasqua, A.D.; Hrelia, S.; Malaguti, M.; Lorenzini, A. The Mediterranean Athlete’s Nutrition: Are Protein Supplements Necessary? Nutrients 2020, 12, 3681. [Google Scholar] [CrossRef]
- Philpott, J.D.; Witard, O.C.; Galloway, S.D.R. Applications of Omega-3 Polyunsaturated Fatty Acid Supplementation for Sport Performance. Res. Sports Med. 2019, 27, 219–237. [Google Scholar] [CrossRef]
- Burke, L.M.; Whitfield, J.; Heikura, I.A.; Ross, M.L.R.; Tee, N.; Forbes, S.F.; Hall, R.; McKay, A.K.A.; Wallett, A.M.; Sharma, A.P. Adaptation to a Low Carbohydrate High Fat Diet Is Rapid but Impairs Endurance Exercise Metabolism and Performance despite Enhanced Glycogen Availability. J. Physiol. 2021, 599, 771–790. [Google Scholar] [CrossRef]
- Burke, L.M. Ketogenic Low-CHO, High-fat Diet: The Future of Elite Endurance Sport? J. Physiol. 2021, 599, 819–843. [Google Scholar] [CrossRef]
- Reale, R.; Slater, G.; Burke, L.M. Acute-Weight-Loss Strategies for Combat Sports and Applications to Olympic Success. Int. J. Sports Physiol. Perform. 2017, 12, 142–151. [Google Scholar] [CrossRef]
- Dugonjić, B.; Krstulović, S.; Kuvačić, G. Rapid Weight Loss Practices in Elite Kickboxers. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 583–588. [Google Scholar] [CrossRef]
- Figlioli, F.; Bianco, A.; Thomas, E.; Stajer, V.; Korovljev, D.; Trivic, T.; Maksimovic, N.; Drid, P. Rapid Weight Loss Habits before a Competition in Sambo Athletes. Nutrients 2021, 13, 1063. [Google Scholar] [CrossRef]
- Lakicevic, N.; Matthews, J.J.; Artioli, G.G.; Paoli, A.; Roklicer, R.; Trivic, T.; Bianco, A.; Drid, P. Patterns of Weight Cycling in Youth Olympic Combat Sports: A Systematic Review. J. Eat. Disord. 2022, 10, 75. [Google Scholar] [CrossRef]
- Roklicer, R.; Lakicevic, N.; Stajer, V.; Trivic, T.; Bianco, A.; Mani, D.; Milosevic, Z.; Maksimovic, N.; Paoli, A.; Drid, P. The Effects of Rapid Weight Loss on Skeletal Muscle in Judo Athletes. J. Transl. Med. 2020, 18, 142. [Google Scholar] [CrossRef]
- Zubac, D.; Karnincic, H.; Sekulic, D. Rapid Weight Loss Is Not Associated With Competitive Success in Elite Youth Olympic-Style Boxers in Europe. Int. J. Sports Physiol. Perform. 2018, 13, 860–866. [Google Scholar] [CrossRef]
- Zubac, D.; Šimunič, B.; Buoite Stella, A.; Morrison, S.A. Neuromuscular Performance after Rapid Weight Loss in Olympic-Style Boxers. Eur. J. Sport Sci. 2020, 20, 1051–1060. [Google Scholar] [CrossRef]
- Lu, H.; He, B.; Gao, B. Emerging Electrochemical Sensors for Life Healthcare. Eng. Regen. 2021, 2, 175–181. [Google Scholar] [CrossRef]
- Hoffman, J.R.; Maresh, C.M. Nutrition and Hydration Issues for Combat Sport Athletes. Strength Cond. J. 2011, 33, 10–17. [Google Scholar] [CrossRef]
- Ilha, J.; do Espírito-Santo, C.C.; de Freitas, G.R. MTOR Signaling Pathway and Protein Synthesis: From Training to Aging and Muscle Autophagy. In Muscle Atrophy; Xiao, J., Ed.; Advances in Experimental Medicine and Biology; Springer: Singapore, 2018; Volume 1088, pp. 139–151. ISBN 9789811314346. [Google Scholar]
- Phillips, S.M. Dietary Protein Requirements and Adaptive Advantages in Athletes. Br. J. Nutr. 2012, 108, S158–S167. [Google Scholar] [CrossRef]
- Jäger, R.; Kerksick, C.M.; Campbell, B.I.; Cribb, P.J.; Wells, S.D.; Skwiat, T.M.; Purpura, M.; Ziegenfuss, T.N.; Ferrando, A.A.; Arent, S.M.; et al. International Society of Sports Nutrition Position Stand: Protein and Exercise. J. Int. Soc. Sports Nutr. 2017, 14, 20. [Google Scholar] [CrossRef]
- Saunders, M.; Luden, N.; DeWitt, C.; Gross, M. Amanda Dillon Rios Protein Supplementation During or Following a Marathon Run Influences Post-Exercise Recovery. Nutrients 2018, 10, 333. [Google Scholar] [CrossRef]
- Vasconcelos, Q.D.J.S.; Bachur, T.P.R.; Aragão, G.F. Whey Protein Supplementation and Its Potentially Adverse Effects on Health: A Systematic Review. Appl. Physiol. Nutr. Metab. 2021, 46, 27–33. [Google Scholar] [CrossRef]
- Butts, J.; Jacobs, B.; Silvis, M. Creatine Use in Sports. Sports Health Multidiscip. Approach 2018, 10, 31–34. [Google Scholar] [CrossRef]
- Kreider, R.B.; Kalman, D.S.; Antonio, J.; Ziegenfuss, T.N.; Wildman, R.; Collins, R.; Candow, D.G.; Kleiner, S.M.; Almada, A.L.; Lopez, H.L. International Society of Sports Nutrition Position Stand: Safety and Efficacy of Creatine Supplementation in Exercise, Sport, and Medicine. J. Int. Soc. Sports Nutr. 2017, 14, 18. [Google Scholar] [CrossRef]
- Antonio, J.; Candow, D.G.; Forbes, S.C.; Gualano, B.; Jagim, A.R.; Kreider, R.B.; Rawson, E.S.; Smith-Ryan, A.E.; VanDusseldorp, T.A.; Willoughby, D.S.; et al. Common Questions and Misconceptions about Creatine Supplementation: What Does the Scientific Evidence Really Show? J. Int. Soc. Sports Nutr. 2021, 18, 13. [Google Scholar] [CrossRef]
- Campbell, B.; Kreider, R.B.; Ziegenfuss, T.; La Bounty, P.; Roberts, M.; Burke, D.; Landis, J.; Lopez, H.; Antonio, J. International Society of Sports Nutrition Position Stand: Protein and Exercise. J. Int. Soc. Sports Nutr. 2007, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- Holeček, M. Side Effects of Amino Acid Supplements. Physiol. Res. 2022, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Fedewa, M.V.; Spencer, S.O.; Williams, T.D.; Becker, Z.E.; Fuqua, C.A. Effect of Branched-Chain Amino Acid Supplementation on Muscle Soreness Following Exercise: A Meta-Analysis. Int. J. Vitam. Nutr. Res. 2019, 89, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Shao, J.; Wu, C.-Y.; Shu, L.; Dong, W.; Liu, Y.; Chen, M.; Wynn, R.M.; Wang, J.; Wang, J.; et al. Targeting BCAA Catabolism to Treat Obesity-Associated Insulin Resistance. Diabetes 2019, 68, 1730–1746. [Google Scholar] [CrossRef]
- Shou, J.; Chen, P.-J.; Xiao, W.-H. The Effects of BCAAs on Insulin Resistance in Athletes. J. Nutr. Sci. Vitaminol. 2019, 65, 383–389. [Google Scholar] [CrossRef]
- Viribay, A.; Burgos, J.; Fernández-Landa, J.; Seco-Calvo, J.; Mielgo-Ayuso, J. Effects of Arginine Supplementation on Athletic Performance Based on Energy Metabolism: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 1300. [Google Scholar] [CrossRef]
- Pahlavani, N.; Entezari, M.H.; Nasiri, M.; Miri, A.; Rezaie, M.; Bagheri-Bidakhavidi, M.; Sadeghi, O. The Effect of L-Arginine Supplementation on Body Composition and Performance in Male Athletes: A Double-Blinded Randomized Clinical Trial. Eur. J. Clin. Nutr. 2017, 71, 544–548. [Google Scholar] [CrossRef]
- Maughan, R.J.; Burke, L.M.; Dvorak, J.; Larson-Meyer, D.E.; Peeling, P.; Phillips, S.M.; Rawson, E.S.; Walsh, N.P.; Garthe, I.; Geyer, H.; et al. IOC Consensus Statement: Dietary Supplements and the High-Performance Athlete. Br. J. Sports Med. 2018, 52, 439–455. [Google Scholar] [CrossRef]
- Ramezani Ahmadi, A.; Rayyani, E.; Bahreini, M.; Mansoori, A. The Effect of Glutamine Supplementation on Athletic Performance, Body Composition, and Immune Function: A Systematic Review and a Meta-Analysis of Clinical Trials. Clin. Nutr. 2019, 38, 1076–1091. [Google Scholar] [CrossRef]
- Córdova-Martínez, A.; Caballero-García, A.; Bello, H.J.; Pérez-Valdecantos, D.; Roche, E. Effect of Glutamine Supplementation on Muscular Damage Biomarkers in Professional Basketball Players. Nutrients 2021, 13, 2073. [Google Scholar] [CrossRef]
- Kaczka, P.; Michalczyk, M.M.; Jastrząb, R.; Gawelczyk, M.; Kubicka, K. Mechanism of Action and the Effect of Beta-Hydroxy-Beta-Methylbutyrate (HMB) Supplementation on Different Types of Physical Performance—A Systematic Review. J. Hum. Kinet. 2019, 68, 211–222. [Google Scholar] [CrossRef]
- Bear, D.E.; Langan, A.; Dimidi, E.; Wandrag, L.; Harridge, S.D.R.; Hart, N.; Connolly, B.; Whelan, K. β-Hydroxy-β-Methylbutyrate and Its Impact on Skeletal Muscle Mass and Physical Function in Clinical Practice: A Systematic Review and Meta-Analysis. Am. J. Clin. Nutr. 2019, 109, 1119–1132. [Google Scholar] [CrossRef]
- Halson, S.L. Sleep in Elite Athletes and Nutritional Interventions to Enhance Sleep. Sports Med. 2014, 44, 13–23. [Google Scholar] [CrossRef]
- Sawicka, A.K.; Renzi, G.; Olek, R.A. The Bright and the Dark Sides of L-Carnitine Supplementation: A Systematic Review. J. Int. Soc. Sports Nutr. 2020, 17, 49. [Google Scholar] [CrossRef] [PubMed]
- Fielding, R.; Riede, L.; Lugo, J.; Bellamine, A. L-Carnitine Supplementation in Recovery after Exercise. Nutrients 2018, 10, 349. [Google Scholar] [CrossRef] [PubMed]
- Alghannam, A.F.; Ghaith, M.M.; Alhussain, M.H. Regulation of Energy Substrate Metabolism in Endurance Exercise. Int. J. Environ. Res. Public. Health 2021, 18, 4963. [Google Scholar] [CrossRef] [PubMed]
- Cavalu, S.; Popa, A.; Bratu, I.; Borodi, G.; Maghiar, A. New Evidences of Key Factors Involved in "Silent Stones" Etiopathogenesis and Trace Elements: Microscopic, Spectroscopic, and Biochemical Approach. Biol. Trace Elem. Res. 2015, 168, 311–320. [Google Scholar] [CrossRef]
- Fuchs, C.J.; Gonzalez, J.T.; Loon, L.J.C. Fructose Co-ingestion to Increase Carbohydrate Availability in Athletes. J. Physiol. 2019, 597, 3549–3560. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, J.T.; Wallis, G.A. Carb-Conscious: The Role of Carbohydrate Intake in Recovery from Exercise. Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 364–371. [Google Scholar] [CrossRef]
- Newell, M.; Wallis, G.; Hunter, A.; Tipton, K.; Galloway, S. Metabolic Responses to Carbohydrate Ingestion during Exercise: Associations between Carbohydrate Dose and Endurance Performance. Nutrients 2018, 10, 37. [Google Scholar] [CrossRef]
- Pickering, C.; Grgic, J. Caffeine and Exercise: What Next? Sports Med. 2019, 49, 1007–1030. [Google Scholar] [CrossRef]
- Wikoff, D.; Welsh, B.T.; Henderson, R.; Brorby, G.P.; Britt, J.; Myers, E.; Goldberger, J.; Lieberman, H.R.; O’Brien, C.; Peck, J.; et al. Systematic Review of the Potential Adverse Effects of Caffeine Consumption in Healthy Adults, Pregnant Women, Adolescents, and Children. Food Chem. Toxicol. 2017, 109, 585–648. [Google Scholar] [CrossRef]
- Cappelletti, S.; Daria, P.; Sani, G.; Aromatario, M. Caffeine: Cognitive and Physical Performance Enhancer or Psychoactive Drug? Curr. Neuropharmacol. 2015, 13, 71–88. [Google Scholar] [CrossRef]
- Chen, Q.; Li, Z.; Pinho, R.A.; Gupta, R.C.; Ugbolue, U.C.; Thirupathi, A.; Gu, Y. The Dose Response of Taurine on Aerobic and Strength Exercises: A Systematic Review. Front. Physiol. 2021, 12, 700352. [Google Scholar] [CrossRef]
- Kurtz, J.A.; VanDusseldorp, T.A.; Doyle, J.A.; Otis, J.S. Taurine in Sports and Exercise. J. Int. Soc. Sports Nutr. 2021, 18, 39. [Google Scholar] [CrossRef]
- Waldron, M.; Patterson, S.D.; Tallent, J.; Jeffries, O. The Effects of an Oral Taurine Dose and Supplementation Period on Endurance Exercise Performance in Humans: A Meta-Analysis. Sports Med. 2018, 48, 1247–1253. [Google Scholar] [CrossRef]
- Grgic, J.; Rodriguez, R.F.; Garofolini, A.; Saunders, B.; Bishop, D.J.; Schoenfeld, B.J.; Pedisic, Z. Effects of Sodium Bicarbonate Supplementation on Muscular Strength and Endurance: A Systematic Review and Meta-Analysis. Sports Med. 2020, 50, 1361–1375. [Google Scholar] [CrossRef]
- Saunders, B.; Sale, C.; Harris, R.C.; Sunderland, C. Sodium Bicarbonate and High-Intensity-Cycling Capacity: Variability in Responses. Int. J. Sports Physiol. Perform. 2014, 9, 627–632. [Google Scholar] [CrossRef]
- Hilton, N.P.; Leach, N.K.; Sparks, S.A.; Gough, L.A.; Craig, M.M.; Deb, S.K.; McNaughton, L.R. A Novel Ingestion Strategy for Sodium Bicarbonate Supplementation in a Delayed-Release Form: A Randomised Crossover Study in Trained Males. Sports Med. Open 2019, 5, 4. [Google Scholar] [CrossRef]
- Jones, A.M.; Vanhatalo, A.; Seals, D.R.; Rossman, M.J.; Piknova, B.; Jonvik, K.L. Dietary Nitrate and Nitric Oxide Metabolism: Mouth, Circulation, Skeletal Muscle, and Exercise Performance. Med. Sci. Sports Exerc. 2021, 53, 280–294. [Google Scholar] [CrossRef]
- Zamani, H.; de Joode, M.E.J.R.; Hossein, I.J.; Henckens, N.F.T.; Guggeis, M.A.; Berends, J.E.; de Kok, T.M.C.M.; van Breda, S.G.J. The Benefits and Risks of Beetroot Juice Consumption: A Systematic Review. Crit. Rev. Food Sci. Nutr. 2021, 61, 788–804. [Google Scholar] [CrossRef]
- Galior, K.; Grebe, S.; Singh, R. Development of Vitamin D Toxicity from Overcorrection of Vitamin D Deficiency: A Review of Case Reports. Nutrients 2018, 10, 953. [Google Scholar] [CrossRef]
- Koundourakis, N.E.; Avgoustinaki, P.D.; Malliaraki, N.; Margioris, A.N. Muscular Effects of Vitamin D in Young Athletes and Non-Athletes and in the Elderly. Hormones 2017, 15, 471–488. [Google Scholar] [CrossRef]
- Grozenski, A.; Kiel, J. Basic Nutrition for Sports Participation, Part 2: Vitamins and Minerals. Curr. Sports Med. Rep. 2020, 19, 508–510. [Google Scholar] [CrossRef]
- Braakhuis, A.J. Effect of Vitamin C Supplements on Physical Performance. Curr. Sports Med. Rep. 2012, 11, 180–184. [Google Scholar] [CrossRef]
- Tiidus, P.M.; Houston, M.E. Vitamin E Status and Response to Exercise Training. Sports Med. 1995, 20, 12–23. [Google Scholar] [CrossRef]
- De Oliveira, D.C.X.; Rosa, F.T.; Simões-Ambrósio, L.; Jordao, A.A.; Deminice, R. Antioxidant Vitamin Supplementation Prevents Oxidative Stress but Does Not Enhance Performance in Young Football Athletes. Nutrition 2019, 63–64, 29–35. [Google Scholar] [CrossRef]
- Peuhkuri, K.; Sihvola, N.; Korpela, R. Diet Promotes Sleep Duration and Quality. Nutr. Res. 2012, 32, 309–319. [Google Scholar] [CrossRef]
- Woolf, K.; Manore, M.M. B-Vitamins and Exercise: Does Exercise Alter Requirements? Int. J. Sport Nutr. Exerc. Metab. 2006, 16, 453–484. [Google Scholar] [CrossRef]
- Rafeq, Z.; Roh, J.D.; Guarino, P.; Kaufman, J.; Joseph, J. Adverse Myocardial Effects of B-Vitamin Therapy in Subjects with Chronic Kidney Disease and Hyperhomocysteinaemia. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Bacharach, R.; Lowden, M.; Ahmed, A. Pyridoxine Toxicity Small Fiber Neuropathy With Dysautonomia: A Case Report. J. Clin. Neuromuscul. Dis. 2017, 19, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Leite, G.S.F.; Resende Master Student, A.S.; West, N.P.; Lancha, A.H. Probiotics and Sports: A New Magic Bullet? Nutrition 2019, 60, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Kashi, D.S.; Shabir, A.; Da Boit, M.; Bailey, S.J.; Higgins, M.F. The Efficacy of Administering Fruit-Derived Polyphenols to Improve Health Biomarkers, Exercise Performance and Related Physiological Responses. Nutrients 2019, 11, 2389. [Google Scholar] [CrossRef]
- Bowtell, J.; Kelly, V. Fruit-Derived Polyphenol Supplementation for Athlete Recovery and Performance. Sports Med. 2019, 49, 3–23. [Google Scholar] [CrossRef]
- D’Angelo, S. Polyphenols: Potential Beneficial Effects of These Phytochemicals in Athletes. Curr. Sports Med. Rep. 2020, 19, 260–265. [Google Scholar] [CrossRef]
- Mason, S.A.; Trewin, A.J.; Parker, L.; Wadley, G.D. Antioxidant Supplements and Endurance Exercise: Current Evidence and Mechanistic Insights. Redox Biol. 2020, 35, 101471. [Google Scholar] [CrossRef]
- Spain, R.I.; Andeen, N.K.; Gibson, P.C.; Samuels, M.H.; Morris, C.D.; Solomon, A.J.; Solomon, R.; Waslo, C.; Avasare, R.S. Lipoic Acid Supplementation Associated with Neural Epidermal Growth Factor-like 1 (NELL1)–Associated Membranous Nephropathy. Kidney Int. 2021, 100, 1208–1213. [Google Scholar] [CrossRef]
- Derosa, G.; D’Angelo, A.; Preti, P.; Maffioli, P. Safety and Efficacy of Alpha Lipoic Acid During 4 Years of Observation: A Retrospective, Clinical Trial in Healthy Subjects in Primary Prevention. Drug Des. Devel. Ther. 2020, 14, 5367–5374. [Google Scholar] [CrossRef]
- Faria, V.S.; Pejon, T.M.M.; Gobatto, C.A.; de Araujo, G.G.; Cornachione, A.S.; Beck, W.R. Acute Melatonin Administration Improves Exercise Tolerance and the Metabolic Recovery after Exhaustive Effort. Sci. Rep. 2021, 11, 19228. [Google Scholar] [CrossRef]
- Paryab, N.; Taheri, M.; H’Mida, C.; Irandoust, K.; Mirmoezzi, M.; Trabelsi, K.; Ammar, A.; Chtourou, H. Melatonin Supplementation Improves Psychomotor and Physical Performance in Collegiate Student-Athletes Following a Sleep Deprivation Night. Chronobiol. Int. 2021, 38, 753–761. [Google Scholar] [CrossRef]
- Atkinson, G.; Drust, B.; Reilly, T.; Waterhouse, J. The Relevance of Melatonin to Sports Medicine and Science. Sports Med. 2003, 33, 809–831. [Google Scholar] [CrossRef]
- Andersen, L.P.H.; Gögenur, I.; Rosenberg, J.; Reiter, R.J. The Safety of Melatonin in Humans. Clin. Drug Investig. 2016, 36, 169–175. [Google Scholar] [CrossRef]
- Heffernan, S.; Horner, K.; De Vito, G.; Conway, G. The Role of Mineral and Trace Element Supplementation in Exercise and Athletic Performance: A Systematic Review. Nutrients 2019, 11, 696. [Google Scholar] [CrossRef]
- Wessels, I.; Maywald, M.; Rink, L. Zinc as a Gatekeeper of Immune Function. Nutrients 2017, 9, 1286. [Google Scholar] [CrossRef]
- Koehler, K.; Parr, M.K.; Geyer, H.; Mester, J.; Schänzer, W. Serum Testosterone and Urinary Excretion of Steroid Hormone Metabolites after Administration of a High-Dose Zinc Supplement. Eur. J. Clin. Nutr. 2009, 63, 65–70. [Google Scholar] [CrossRef]
- Zhang, Y.; Xun, P.; Wang, R.; Mao, L.; He, K. Can Magnesium Enhance Exercise Performance? Nutrients 2017, 9, 946. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Q.; Zhang, Z.; Fu, R.; Zhou, T.; Long, C.; He, T.; Yang, D.; Li, Z.; Peng, S. Magnesium Supplementation Enhances MTOR Signalling to Facilitate Myogenic Differentiation and Improve Aged Muscle Performance. Bone 2021, 146, 115886. [Google Scholar] [CrossRef]
- Van Laecke, S. Hypomagnesemia and Hypermagnesemia. Acta Clin. Belg. 2019, 74, 41–47. [Google Scholar] [CrossRef]
- Sale, C.; Elliott-Sale, K.J. Nutrition and Athlete Bone Health. Sports Med. 2019, 49, 139–151. [Google Scholar] [CrossRef]
- Gogojewicz, A.; Śliwicka, E.; Durkalec-Michalski, K. Assessment of Dietary Intake and Nutritional Status in CrossFit-Trained Individuals: A Descriptive Study. Int. J. Environ. Res. Public. Health 2020, 17, 4772. [Google Scholar] [CrossRef]
- Raizel, R.; da Godois, A.M.; Coqueiro, A.Y.; Voltarelli, F.A.; Fett, C.A.; Tirapegui, J.; de Ravagnani, F.C.P.; de Coelho-Ravagnani, C.F. Pre-Season Dietary Intake of Professional Soccer Players. Nutr. Health 2017, 23, 215–222. [Google Scholar] [CrossRef]
- Benardot, D. Advanced Sports Nutrition, 2nd ed.; Human Kinetics: Champaign, IL, USA, 2012. [Google Scholar]
- López-Seoane, J.; Martinez-Ferran, M.; Romero-Morales, C.; Pareja-Galeano, H. N-3 PUFA as an Ergogenic Supplement Modulating Muscle Hypertrophy and Strength: A Systematic Review. Crit. Rev. Food Sci. Nutr. 2021, 1–21. [Google Scholar] [CrossRef]
- Exercise and Fluid Replacement. Med. Sci. Sports Exerc. 2007, 39, 377–390. [CrossRef]
- Shirreffs, S.M.; Sawka, M.N. Fluid and Electrolyte Needs for Training, Competition, and Recovery. J. Sports Sci. 2011, 29, S39–S46. [Google Scholar] [CrossRef] [PubMed]
- Van der Beek, E.J. Vitamin Supplementation and Physical Exercise Performance. J. Sports Sci. 1991, 9, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Nutrition: Science and Everyday Application, V. 1.0—Simple Book Publishing. Available online: https://openoregon.pressbooks.pub/nutritionscience/ (accessed on 1 August 2022).
- Dozele Zilnice Recomandate de Vitamine Si Minerale | Gama Serv - Furnizor Ingrediente Industria Alimentara Si a Vinului. Available online: https://www.gama-serv.ro/dsm-nutritional-products/doza-zilnica-recomandata (accessed on 19 May 2022).
- Patlar, S.; Boyali, E.; Baltaci, A.K.; Mogulkoc, R. The Effect of Vitamin A Supplementation on Various Elements in Elite Taekwondo Players. Biol. Trace Elem. Res. 2011, 139, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Goolsby, M.A.; Boniquit, N. Bone Health in Athletes. Sports Health 2017, 9, 108–117. [Google Scholar] [CrossRef]
- Marley, A.; Grant, M.C.; Babraj, J. Vitamin D3 Supplementation Combined with Sprint Interval Training Improves Aerobic and Anaerobic Exercise Performance over Sprint Interval Training Alone in Recreational Combat Sport Athletes. Sci. Sports 2022, 37, 217.e1–217.e10. [Google Scholar] [CrossRef]
- Baranauskas, M.; Jablonskienė, V.; Abaravičius, J.A.; Stukas, R. Actual Nutrition and Dietary Supplementation in Lithuanian Elite Athletes. Medicina 2020, 56, 247. [Google Scholar] [CrossRef]
- Larson-Meyer, D.E.; Willis, K.S. Vitamin D and Athletes. Curr. Sports Med. Rep. 2010, 9, 220–226. [Google Scholar] [CrossRef]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. American College of Sports Medicine Joint Position Statement. Nutrition and Athletic Performance. Med. Sci. Sports Exerc. 2016, 48, 543–568. [Google Scholar] [CrossRef]
- Kerksick, C.M.; Wilborn, C.D.; Roberts, M.D.; Smith-Ryan, A.; Kleiner, S.M.; Jäger, R.; Collins, R.; Cooke, M.; Davis, J.N.; Galvan, E.; et al. ISSN Exercise & Sports Nutrition Review Update: Research & Recommendations. J. Int. Soc. Sports Nutr. 2018, 15, 38. [Google Scholar] [CrossRef]
- Marley, A.; Grant, M.C.; Babraj, J. Weekly Vitamin D 3 Supplementation Improves Aerobic Performance in Combat Sport Athletes. Eur. J. Sport Sci. 2021, 21, 379–387. [Google Scholar] [CrossRef]
- Jung, H.C.; Seo, M.W.; Lee, S.; Jung, S.W.; Song, J.K. Correcting Vitamin D Insufficiency Improves Some But Not All Aspects of Physical Performance During Winter Training in Taekwondo Athletes. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 635–643. [Google Scholar] [CrossRef]
- Bonke, D.; Nickel, B. Improvement of Fine Motoric Movement Control by Elevated Dosages of Vitamin B1, B6, and B12 in Target Shooting. Int. J. Vitam. Nutr. Res. Suppl. Int. Z. Vitam.- Ernahrungsforschung Suppl. 1989, 30, 198–204. [Google Scholar]
- Cannataro, R.; Straface, N.; Cione, E. Nutritional Supplements in Combat Sports: What We Know and What We Do. Hum. Nutr. Metab. 2022, 29, 200155. [Google Scholar] [CrossRef]
- Chou, C.-C.; Sung, Y.-C.; Davison, G.; Chen, C.-Y.; Liao, Y.-H. Short-Term High-Dose Vitamin C and E Supplementation Attenuates Muscle Damage and Inflammatory Responses to Repeated Taekwondo Competitions: A Randomized Placebo-Controlled Trial. Int. J. Med. Sci. 2018, 15, 1217–1226. [Google Scholar] [CrossRef]
- Gröber, U.; Schmidt, J.; Kisters, K. Magnesium in Prevention and Therapy. Nutrients 2015, 7, 8199–8226. [Google Scholar] [CrossRef]
- Fairley, J.L.; Zhang, L.; Glassford, N.J.; Bellomo, R. Magnesium Status and Magnesium Therapy in Cardiac Surgery: A Systematic Review and Meta-Analysis Focusing on Arrhythmia Prevention. J. Crit. Care 2017, 42, 69–77. [Google Scholar] [CrossRef]
- Kochi, A.N.; Vettor, G.; Dessanai, M.A.; Pizzamiglio, F.; Tondo, C. Sudden Cardiac Death in Athletes: From the Basics to the Practical Work-Up. Medicina 2021, 57, 168. [Google Scholar] [CrossRef]
- Cinar, V.; Nizamlioglu, M.; Mogulkoc, R.; Baltaci, A.K. Effects of Magnesium Supplementation on Blood Parameters of Athletes at Rest and after Exercise. Biol. Trace Elem. Res. 2007, 115, 205–212. [Google Scholar] [CrossRef]
- Matias, C.N.; Santos, D.A.; Monteiro, C.P.; Silva, A.M.; de Raposo, M.F.; Martins, F.; Sardinha, L.B.; Bicho, M.; Laires, M.J. Magnesium and Strength in Elite Judo Athletes According to Intracellular Water Changes. Magnes. Res. 2010, 23, 138–141. [Google Scholar]
- Zagrodna (Kopeć), A.; Książek, A.; Lisowska, M. An Assessment of Diet among High—Rank Professional Judo Athletes. J. Combat Sports Martial Arts 2014, 5, 37–41. [Google Scholar] [CrossRef]
- Peeling, P.; Sim, M.; Badenhorst, C.E.; Dawson, B.; Govus, A.D.; Abbiss, C.R.; Swinkels, D.W.; Trinder, D. Iron Status and the Acute Post-Exercise Hepcidin Response in Athletes. PLoS ONE 2014, 9, e93002. [Google Scholar] [CrossRef]
- Kårlund, A.; Gómez-Gallego, C.; Turpeinen, A.M.; Palo-oja, O.-M.; El-Nezami, H.; Kolehmainen, M. Protein Supplements and Their Relation with Nutrition, Microbiota Composition and Health: Is More Protein Always Better for Sportspeople? Nutrients 2019, 11, 829. [Google Scholar] [CrossRef]
- Phillips, S.M.; Tang, J.E.; Moore, D.R. The Role of Milk- and Soy-Based Protein in Support of Muscle Protein Synthesis and Muscle Protein Accretion in Young and Elderly Persons. J. Am. Coll. Nutr. 2009, 28, 343–354. [Google Scholar] [CrossRef]
- Anthony, J.C.; Anthony, T.G.; Layman, D.K. Leucine Supplementation Enhances Skeletal Muscle Recovery in Rats Following Exercise. J. Nutr. 1999, 129, 1102–1106. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.L.; Tipton, K.D.; Chinkes, D.L.; Wolf, S.E.; Wolfe, R.R. Independent and Combined Effects of Amino Acids and Glucose after Resistance Exercise. Med. Sci. Sports Exerc. 2003, 35, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Tipton, K.D.; Wolfe, R.R. Protein and Amino Acids for Athletes. J. Sports Sci. 2004, 22, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Church, D.D.; Hirsch, K.R.; Park, S.; Kim, I.-Y.; Gwin, J.A.; Pasiakos, S.M.; Wolfe, R.R.; Ferrando, A.A. Essential Amino Acids and Protein Synthesis: Insights into Maximizing the Muscle and Whole-Body Response to Feeding. Nutrients 2020, 12, 3717. [Google Scholar] [CrossRef] [PubMed]
- Børsheim, E.; Tipton, K.D.; Wolf, S.E.; Wolfe, R.R. Essential Amino Acids and Muscle Protein Recovery from Resistance Exercise. Am. J. Physiol.-Endocrinol. Metab. 2002, 283, E648–E657. [Google Scholar] [CrossRef]
- Tipton, K.D.; Rasmussen, B.B.; Miller, S.L.; Wolf, S.E.; Owens-Stovall, S.K.; Petrini, B.E.; Wolfe, R.R. Timing of Amino Acid-Carbohydrate Ingestion Alters Anabolic Response of Muscle to Resistance Exercise. Am. J. Physiol.-Endocrinol. Metab. 2001, 281, E197–E206. [Google Scholar] [CrossRef]
- Cinta-Pinzaru, S.; Cavalu, S.; Leopold, N.; Petry, R.; Kiefer, W. Raman and surface-enhanced Raman spectroscopy of tempyo spin labelled ovalbumin. J. Mol. Struct. 2001, 565, 225–229. [Google Scholar] [CrossRef]
- Sterkowicz, S.; Tyka, A.K.; Chwastowski, M.; Sterkowicz-Przybycień, K.; Tyka, A.; Klys, A. The Effects of Training and Creatine Malate Supplementation during Preparation Period on Physical Capacity and Special Fitness in Judo Contestants. J. Int. Soc. Sports Nutr. 2012, 9, 41. [Google Scholar] [CrossRef]
- Sarshin, A.; Fallahi, V.; Forbes, S.C.; Rahimi, A.; Koozehchian, M.S.; Candow, D.G.; Kaviani, M.; Khalifeh, S.N.; Abdollahi, V.; Naderi, A. Short-Term Co-Ingestion of Creatine and Sodium Bicarbonate Improves Anaerobic Performance in Trained Taekwondo Athletes. J. Int. Soc. Sports Nutr. 2021, 18, 10. [Google Scholar] [CrossRef]
- Kocak, S.; Karli, U. Effects of High Dose Oral Creatine Supplementation on Anaerobic Capacity of Elite Wrestlers. J. Sports Med. Phys. Fitness 2003, 43, 488. [Google Scholar]
- De Oca, R.M.M.; Farfán-González, F.; Camarillo-Romero, S. Efectos De La Suplementación Con Creatina En Practicantes De. Nutr. Hosp. 2013, 28, 391–399. [Google Scholar] [CrossRef]
- Burke, L.M. Caffeine and Sports Performance. Appl. Physiol. Nutr. Metab. 2008, 33, 1319–1334. [Google Scholar] [CrossRef]
- Cesareo, K.R.; Mason, J.R.; Saracino, P.G.; Morrissey, M.C.; Ormsbee, M.J. The Effects of a Caffeine-like Supplement, TeaCrine®, on Muscular Strength, Endurance and Power Performance in Resistance-Trained Men. J. Int. Soc. Sports Nutr. 2019, 16, 47. [Google Scholar] [CrossRef]
- Ma, X.; Chen, H.; Cao, L.; Zhao, S.; Zhao, C.; Yin, S.; Hu, H. Mechanisms of Physical Fatigue and Its Applications in Nutritional Interventions. J. Agric. Food Chem. 2021, 69, 6755–6768. [Google Scholar] [CrossRef]
- Ganio, M.S.; Klau, J.F.; Casa, D.J.; Armstrong, L.E.; Maresh, C.M. Effect of Caffeine on Sport-Specific Endurance Performance: A Systematic Review. J. Strength Cond. Res. 2009, 23, 315–324. [Google Scholar] [CrossRef]
- Goldstein, E.R.; Ziegenfuss, T.; Kalman, D.; Kreider, R.; Campbell, B.; Wilborn, C.; Taylor, L.; Willoughby, D.; Stout, J.; Graves, B.S.; et al. International Society of Sports Nutrition Position Stand: Caffeine and Performance. J. Int. Soc. Sports Nutr. 2010, 7, 5. [Google Scholar] [CrossRef]
- Gonçalves, L.D.S.; Painelli, V.D.S.; Yamaguchi, G.; Oliveira, L.F.D.; Saunders, B.; da Silva, R.P.; Maciel, E.; Artioli, G.G.; Roschel, H.; Gualano, B. Dispelling the Myth That Habitual Caffeine Consumption Influences the Performance Response to Acute Caffeine Supplementation. J. Appl. Physiol. 2017, 123, 213–220. [Google Scholar] [CrossRef]
- Loureiro, L.M.R.; Reis, C.E.G.; da Costa, T.H.M. Effects of Coffee Components on Muscle Glycogen Recovery: A Systematic Review. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Paton, C.; Costa, V.; Guglielmo, L. Effects of Caffeine Chewing Gum on Race Performance and Physiology in Male and Female Cyclists. J. Sports Sci. 2015, 33, 1076–1083. [Google Scholar] [CrossRef] [PubMed]
- Carmo, K.E.O.; Pérez, D.I.V.; Valido, C.N.; dos Santos, J.L.; Miarka, B.; Mendes-Netto, R.S.; Leite, M.M.R.; Antoniêtto, N.R.; Aedo-Muñoz, E.A.; Brito, C.J. Caffeine Improves Biochemical and Specific Performance after Judo Training: A Double-Blind Crossover Study in a Real Judo Training Situation. Nutr. Metab. 2021, 18, 15. [Google Scholar] [CrossRef] [PubMed]
- Astley, C.; Souza, D.; Polito, M. Acute Caffeine Ingestion on Performance in Young Judo Athletes. Pediatr. Exerc. Sci. 2017, 29, 336–340. [Google Scholar] [CrossRef]
- López-González, L.M.; Sánchez-Oliver, A.J.; Mata, F.; Jodra, P.; Antonio, J.; Domínguez, R. Acute Caffeine Supplementation in Combat Sports: A Systematic Review. J. Int. Soc. Sports Nutr. 2018, 15, 60. [Google Scholar] [CrossRef]
- Kopec, B.J.; Dawson, B.T.; Buck, C.; Wallman, K.E. Effects of Sodium Phosphate and Caffeine Ingestion on Repeated-Sprint Ability in Male Athletes. J. Sci. Med. Sport 2016, 19, 272–276. [Google Scholar] [CrossRef]
- Brewer, C.P.; Dawson, B.; Wallman, K.E.; Guelfi, K.J. Effect of Sodium Phosphate Supplementation on Cycling Time Trial Performance and VO2 1 and 8 Days Post Loading. J. Sports Sci. Med. 2014, 13, 529. [Google Scholar]
- West, J.S.; Ayton, T.; Wallman, K.E.; Guelfi, K.J. The Effect of 6 Days of Sodium Phosphate Supplementation on Appetite, Energy Intake, and Aerobic Capacity in Trained Men and Women. Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 422–429. [Google Scholar] [CrossRef]
- Lancha, A.H., Jr.; de Painelli, V.S.; Saunders, B.; Artioli, G.G. Nutritional Strategies to Modulate Intracellular and Extracellular Buffering Capacity During High-Intensity Exercise. Sports Med. 2015, 45, 71–81. [Google Scholar] [CrossRef]
- Saunders, B.; Elliott-Sale, K.; Artioli, G.G.; Swinton, P.A.; Dolan, E.; Roschel, H.; Sale, C.; Gualano, B. β-Alanine Supplementation to Improve Exercise Capacity and Performance: A Systematic Review and Meta-Analysis. Br. J. Sports Med. 2017, 51, 658–669. [Google Scholar] [CrossRef]
- Bellinger, P.M. β-Alanine Supplementation for Athletic Performance: An Update. J. Strength Cond. Res. 2014, 28, 1751–1770. [Google Scholar] [CrossRef]
- Nassis, G.P.; Sporer, B.; Stathis, C.G. β-Alanine Efficacy for Sports Performance Improvement: From Science to Practice. Br. J. Sports Med. 2017, 51, 626–627. [Google Scholar] [CrossRef]
- Stellingwerff, T.; Decombaz, J.; Harris, R.C.; Boesch, C. Optimizing Human in vivo Dosing and Delivery of β-Alanine Supplements for Muscle Carnosine Synthesis. Amino Acids 2012, 43, 57–65. [Google Scholar] [CrossRef]
- De Kratz, C.A.; de Painelli, V.S.; de Nemezio, K.M.A.; da Silva, R.P.; Franchini, E.; Zagatto, A.M.; Gualano, B.; Artioli, G.G. Beta-Alanine Supplementation Enhances Judo-Related Performance in Highly-Trained Athletes. J. Sci. Med. Sport 2017, 20, 403–408. [Google Scholar] [CrossRef]
- Kim, K.-J.; Song, H.-S.; Yoon, D.H.; Fukuda, D.H.; Kim, S.H.; Park, D.-H. The Effects of 10 Weeks of β-Alanine Supplementation on Peak Power, Power Drop, and Lactate Response in Korean National Team Boxers. J. Exerc. Rehabil. 2018, 14, 985–992. [Google Scholar] [CrossRef]
- Tobias, G.; Benatti, F.B.; de Painelli, V.S.; Roschel, H.; Gualano, B.; Sale, C.; Harris, R.C.; Lancha, A.H.; Artioli, G.G. Additive Effects of Beta-Alanine and Sodium Bicarbonate on Upper-Body Intermittent Performance. Amino Acids 2013, 45, 309–317. [Google Scholar] [CrossRef]
- Stephens, F.B.; Constantin-Teodosiu, D.; Greenhaff, P.L. New Insights Concerning the Role of Carnitine in the Regulation of Fuel Metabolism in Skeletal Muscle: Carnitine Metabolism in Skeletal Muscle. J. Physiol. 2007, 581, 431–444. [Google Scholar] [CrossRef]
- Marconi, C.; Sassi, G.; Carpinelli, A.; Cerretelli, P. Effects Ofl-Carnitine Loading on the Aerobic and Anaerobic Performance of Endurance Athletes. Eur. J. Appl. Physiol. 1985, 54, 131–135. [Google Scholar] [CrossRef]
- Greig, C.; Finch, K.M.; Jones, D.A.; Cooper, M.; Sargeant, A.J.; Forte, C.A. The Effect of Oral Supplementation with L-Carnitine on Maximum and Submaximum Exercise Capacity. Eur. J. Appl. Physiol. 1987, 56, 457–460. [Google Scholar] [CrossRef]
- Barnett, C.; Costill, D.L.; Vukovich, M.D.; Cole, K.J.; Goodpaster, B.H.; Trappe, S.W.; Fink, W.J. Effect of L-Carnitine Supplementation on Muscle and Blood Camitine Content and Lactate Accumulation during High-Intensity Sprint Cycling. Int. J. Sport Nutr. 1994, 4, 280–288. [Google Scholar] [CrossRef]
- Novakova, K.; Kummer, O.; Bouitbir, J.; Stoffel, S.D.; Hoerler-Koerner, U.; Bodmer, M.; Roberts, P.; Urwyler, A.; Ehrsam, R.; Krähenbühl, S. Effect of L-Carnitine Supplementation on the Body Carnitine Pool, Skeletal Muscle Energy Metabolism and Physical Performance in Male Vegetarians. Eur. J. Nutr. 2016, 55, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Talenezhad, N.; Mohammadi, M.; Ramezani-Jolfaie, N.; Mozaffari-Khosravi, H.; Salehi-Abargouei, A. Effects of L-Carnitine Supplementation on Weight Loss and Body Composition: A Systematic Review and Meta-Analysis of 37 Randomized Controlled Clinical Trials with Dose-Response Analysis. Clin. Nutr. ESPEN 2020, 37, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The Effects of Polyphenols and Other Bioactives on Human Health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef] [PubMed]
- Shaw, K.A.; Zello, G.A.; Rodgers, C.D.; Warkentin, T.D.; Baerwald, A.R.; Chilibeck, P.D. Benefits of a Plant-Based Diet and Considerations for the Athlete. Eur. J. Appl. Physiol. 2022, 122, 1163–1178. [Google Scholar] [CrossRef] [PubMed]
- Marranzano, M.; Rosa, R.L.; Malaguarnera, M.; Palmeri, R.; Tessitori, M.; Barbera, A.C. Polyphenols: Plant Sources and Food Industry Applications. Curr. Pharm. Des. 2018, 24, 4125–4130. [Google Scholar] [CrossRef] [PubMed]
- Sorrenti, V.; Fortinguerra, S.; Caudullo, G.; Buriani, A. Deciphering the Role of Polyphenols in Sports Performance: From Nutritional Genomics to the Gut Microbiota toward Phytonutritional Epigenomics. Nutrients 2020, 12, 1265. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, A.; Minihane, A.-M. The Role of Metabolism (and the Microbiome) in Defining the Clinical Efficacy of Dietary Flavonoids. Am. J. Clin. Nutr. 2017, 105, 10–22. [Google Scholar] [CrossRef]
- Braakhuis, A.J.; Hopkins, W.G. Impact of Dietary Antioxidants on Sport Performance: A Review. Sports Med. 2015, 45, 939–955. [Google Scholar] [CrossRef]
- Cook, M.D.; Myers, S.D.; Gault, M.L.; Edwards, V.C.; Willems, M.E.T. Dose Effects of New Zealand Blackcurrant on Substrate Oxidation and Physiological Responses during Prolonged Cycling. Eur. J. Appl. Physiol. 2017, 117, 1207–1216. [Google Scholar] [CrossRef]
- Willems, M.E.T.; Myers, S.D.; Gault, M.L.; Cook, M.D. Beneficial Physiological Effects With Blackcurrant Intake in Endurance Athletes. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 367–374. [Google Scholar] [CrossRef]
- Clifford, T.; Mitchell, N.; Scott, A. The influence of different sources of polyphenols on sub-maximal cycling and time trial performance. 2013. J. Athl. Enhanc. 2013, 2, S10. [Google Scholar] [CrossRef]
- Howatson, G.; McHugh, M.P.; Hill, J.A.; Brouner, J.; Jewell, A.P.; van Someren, K.A.; Shave, R.E.; Howatson, S.A. Influence of Tart Cherry Juice on Indices of Recovery Following Marathon Running. Scand. J. Med. Sci. Sports 2010, 20, 843–852. [Google Scholar] [CrossRef]
- Levers, K.; Dalton, R.; Galvan, E.; O’Connor, A.; Goodenough, C.; Simbo, S.; Mertens-Talcott, S.; Rasmussen, C.; Greenwood, M.; Riechman, S.; et al. Effects of Powdered Montmorency Tart Cherry Supplementation on Acute Endurance Exercise Performance in Aerobically Trained Individuals. J. Int. Soc. Sports Nutr. 2016, 13, 22. [Google Scholar] [CrossRef]
- Keane, K.M.; Bailey, S.J.; Vanhatalo, A.; Jones, A.M.; Howatson, G. Effects of Montmorency Tart Cherry (L. Prunus Cerasus) Consumption on Nitric Oxide Biomarkers and Exercise Performance. Scand. J. Med. Sci. Sports 2018, 28, 1746–1756. [Google Scholar] [CrossRef]
- Decroix, L.; Tonoli, C.; Soares, D.D.; Descat, A.; Drittij-Reijnders, M.-J.; Weseler, A.R.; Bast, A.; Stahl, W.; Heyman, E.; Meeusen, R. Acute Cocoa Flavanols Intake Has Minimal Effects on Exercise-Induced Oxidative Stress and Nitric Oxide Production in Healthy Cyclists: A Randomized Controlled Trial. J. Int. Soc. Sports Nutr. 2017, 14, 28. [Google Scholar] [CrossRef]
- Allgrove, J.; Farrell, E.; Gleeson, M.; Williamson, G.; Cooper, K. Regular Dark Chocolate Consumption’s Reduction of Oxidative Stress and Increase of Free-Fatty-Acid Mobilization in Response to Prolonged Cycling. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 113–123. [Google Scholar] [CrossRef]
- Lafay, S.; Jan, C.; Nardon, K.; Lemaire, B.; Ibarra, A.; Roller, M.; Houvenaeghel, M.; Juhel, C.; Cara, L. Grape Extract Improves Antioxidant Status and Physical Performance in Elite Male Athletes. J. Sports Sci. Med. 2009, 8, 468. [Google Scholar]
- Kang, S.W.; Hahn, S.; Kim, J.-K.; Yang, S.-M.; Park, B.-J.; Chul Lee, S. Oligomerized Lychee Fruit Extract (OLFE) and a Mixture of Vitamin C and Vitamin E for Endurance Capacity in a Double Blind Randomized Controlled Trial. J. Clin. Biochem. Nutr. 2012, 50, 106–113. [Google Scholar] [CrossRef][Green Version]
- Powers, S.; Nelson, W.B.; Larson-Meyer, E. Antioxidant and Vitamin D Supplements for Athletes: Sense or Nonsense? J. Sports Sci. 2011, 29, S47–S55. [Google Scholar] [CrossRef] [PubMed]
- Antonioni, A.; Fantini, C.; Dimauro, I.; Caporossi, D. Redox Homeostasis in Sport: Do Athletes Really Need Antioxidant Support? Res. Sports Med. 2019, 27, 147–165. [Google Scholar] [CrossRef]
- Jemili, H.; Mejri, M.; Bouhlel, E.; Amri, M. Biochemical Status, Oxidative and Antioxidant Responses after 3-Month Specific Training in Elite Karate Athletes. Physiol. Int. 2017, 104, 344–354. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Knapik, K.; Sieroń, K.; Wojtyna, E.; Onik, G.; Romuk, E.; Birkner, E.; Stanek, A.; Kawczyk-Krupka, A.; Plinta, R.; Sieroń, A. Precompetitional Weight Reduction Modifies Prooxidative-Antioxidative Status in Judokas. Oxid. Med. Cell. Longev. 2019, 2019, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Goulart, M.J.V.C.; Pisamiglio, D.S.; Möller, G.B.; Dani, C.; Alves, F.D.; Bock, P.M.; Schneider, C.D. Effects of Grape Juice Consumption on Muscle Fatigue and Oxidative Stress in Judo Athletes: A Randomized Clinical Trial. An. Acad. Bras. Ciênc. 2020, 92, e20191551. [Google Scholar] [CrossRef]
- Kon, M.; Tanabe, K.; Akimoto, T.; Kimura, F.; Tanimura, Y.; Shimizu, K.; Okamoto, T.; Kono, I. Reducing Exercise-Induced Muscular Injury in Kendo Athletes with Supplementation of Coenzyme Q 10. Br. J. Nutr. 2008, 100, 903–909. [Google Scholar] [CrossRef]
- Ma, J.-J.; Zhang, W.-J.; Yang, Y.; Yuan, X.-G. Effects of Lycium Barbarum Polysaccharide on the Immune Function and Antioxidant of Taekwondo Athletes. Chin. J. Appl. Physiol. 2019, 35, 513–516. [Google Scholar]
- Dutra, M.T.; Alex, S.; Mota, M.R.; Sales, N.B.; Brown, L.E.; Bottaro, M. Effect of Strength Training Combined with Antioxidant Supplementation on Muscular Performance. Appl. Physiol. Nutr. Metab. 2018, 43, 775–781. [Google Scholar] [CrossRef]
- Higgins, M.R.; Izadi, A.; Kaviani, M. Antioxidants and Exercise Performance: With a Focus on Vitamin E and C Supplementation. Int. J. Environ. Res. Public. Health 2020, 17, 8452. [Google Scholar] [CrossRef]
- Kioukia-Fougia, N.; Georgiadis, N.; Tsarouhas, K.; Vasilaki, F.; Fragiadaki, P.; Meimeti, E.; Tsitsimpikou, C. Synthetic and Natural Nutritional Supplements: Health “Allies” or Risks to Public Health? Recent Pat. Inflamm. Allergy Drug Discov. 2017, 10, 72–85. [Google Scholar] [CrossRef]
- Liu, R.H. Health Benefits of Fruit and Vegetables Are from Additive and Synergistic Combinations of Phytochemicals. Am. J. Clin. Nutr. 2003, 78, 517S–520S. [Google Scholar] [CrossRef]
- Lukaski, H.C. Vitamin and Mineral Status: Effects on Physical Performance. Nutr. Burbank Los Angel. Cty. Calif 2004, 20, 632–644. [Google Scholar] [CrossRef]
- Lindschinger, M.; Tatzber, F.; Schimetta, W.; Schmid, I.; Lindschinger, B.; Cvirn, G.; Fuchs, N.; Markolin, G.; Lamont, E.; Wonisch, W. Bioavailability of natural versus synthetic B vitamins and their effects on metabolic processes. MMW Fortschr. Med. 2020, 162, 17–27. [Google Scholar] [CrossRef]
- Molinos Domene, Á. Effects of Adaptogen Supplementation on Sport Performance. A Recent Review of Published Studies. J. Hum. Sport Exerc. 2013, 8, 1054–1066. [Google Scholar] [CrossRef]
- Sellami, M.; Slimeni, O.; Pokrywka, A.; Kuvačić, G.; D Hayes, L.; Milic, M.; Padulo, J. Herbal Medicine for Sports: A Review. J. Int. Soc. Sports Nutr. 2018, 15, 14. [Google Scholar] [CrossRef]
- Wong, T.H.; Sim, A.; Burns, S.F. The Effect of Beetroot Ingestion on High-Intensity Interval Training: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 3674. [Google Scholar] [CrossRef]
- Gallardo, E.J.; Coggan, A.R. What Is in Your Beet Juice? Nitrate and Nitrite Content of Beet Juice Products Marketed to Athletes. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Drobnic, F.; Lizarraga, M.A.; Caballero-García, A.; Cordova, A. Coenzyme Q10 Supplementation and Its Impact on Exercise and Sport Performance in Humans: A Recovery or a Performance-Enhancing Molecule? Nutrients 2022, 14, 1811. [Google Scholar] [CrossRef] [PubMed]
- Guescini, M.; Tiano, L.; Genova, M.L.; Polidori, E.; Silvestri, S.; Orlando, P.; Fimognari, C.; Calcabrini, C.; Stocchi, V.; Sestili, P. The Combination of Physical Exercise with Muscle-Directed Antioxidants to Counteract Sarcopenia: A Biomedical Rationale for Pleiotropic Treatment with Creatine and Coenzyme Q10. Oxid. Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Parohan, M.; Sarraf, P.; Javanbakht, M.H.; Foroushani, A.R.; Ranji-Burachaloo, S.; Djalali, M. The Synergistic Effects of Nano-Curcumin and Coenzyme Q10 Supplementation in Migraine Prophylaxis: A Randomized, Placebo-Controlled, Double-Blind Trial. Nutr. Neurosci. 2021, 24, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Gholami, F.; Antonio, J.; Evans, C.; Cheraghi, K.; Rahmani, L.; Amirnezhad, F. Tomato Powder Is More Effective than Lycopene to Alleviate Exercise-Induced Lipid Peroxidation in Well-Trained Male Athletes: Randomized, Double-Blinded Cross-over Study. J. Int. Soc. Sports Nutr. 2021, 18, 17. [Google Scholar] [CrossRef]
- Qian, C.; Decker, E.A.; Xiao, H.; McClements, D.J. Physical and Chemical Stability of β-Carotene-Enriched Nanoemulsions: Influence of PH, Ionic Strength, Temperature, and Emulsifier Type. Food Chem. 2012, 132, 1221–1229. [Google Scholar] [CrossRef]
- Yang, Y.; McClements, D.J. Encapsulation of Vitamin E in Edible Emulsions Fabricated Using a Natural Surfactant. Food Hydrocoll. 2013, 30, 712–720. [Google Scholar] [CrossRef]
- Gokce, E.H.; Korkmaz, E.; Dellera, E.; Sandri, G.; Cristina Bonferoni, M.; Ozer, O. Resveratrol-Loaded Solid Lipid Nanoparticles versus Nanostructured Lipid Carriers: Evaluation of Antioxidant Potential for Dermal Applications. Int. J. Nanomedicine 2012, 7, 1841–1850. [Google Scholar] [CrossRef]
- Pan, K.; Zhong, Q.; Baek, S.J. Enhanced Dispersibility and Bioactivity of Curcumin by Encapsulation in Casein Nanocapsules. J. Agric. Food Chem. 2013, 61, 6036–6043. [Google Scholar] [CrossRef]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. A Practical Guide for Designing Effective Nutraceutical Combinations in the Form of Foods, Beverages, and Dietary Supplements against Chronic Degenerative Diseases. Trends Food Sci. Technol. 2019, 88, 179–193. [Google Scholar] [CrossRef]
- Nasr, M. Development of an Optimized Hyaluronic Acid-Based Lipidic Nanoemulsion Co-Encapsulating Two Polyphenols for Nose to Brain Delivery. Drug Deliv. 2016, 23, 1444–1452. [Google Scholar] [CrossRef]
- Liu, F.; Ma, D.; Luo, X.; Zhang, Z.; He, L.; Gao, Y.; McClements, D.J. Fabrication and Characterization of Protein-Phenolic Conjugate Nanoparticles for Co-Delivery of Curcumin and Resveratrol. Food Hydrocoll. 2018, 79, 450–461. [Google Scholar] [CrossRef]
- Guo, C.; Yin, J.; Chen, D. Co-Encapsulation of Curcumin and Resveratrol into Novel Nutraceutical Hyalurosomes Nano-Food Delivery System Based on Oligo-Hyaluronic Acid-Curcumin Polymer. Carbohydr. Polym. 2018, 181, 1033–1037. [Google Scholar] [CrossRef]
- ChavesMA, O.P.L.; CG, J. Structural Characterization Ofmultilamellar Liposomes Coencapsulating Curcumin and Vitamin D-3. Colloids Surf. 2018, 549, 112–121. [Google Scholar] [CrossRef]
- Du, Z.; Liu, J.; Zhai, J.; Huang, H.; Wei, S.; Zhang, T.; Ding, L.; Liu, B. Fabrication of N-Acetyl-L-Cysteine and L-Cysteine Functionalized Chitosan-Casein Nanohydrogels for Entrapment of Hydrophilic and Hydrophobic Bioactive Compounds. Food Hydrocoll. 2019, 96, 377–384. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, Y.; Qing, J.; Han, Y.; McClements, D.J.; Gao, Y. Core-Shell Nanoparticles for Co-Encapsulation of Coenzyme Q10 and Piperine: Surface Engineering of Hydrogel Shell around Protein Core. Food Hydrocoll. 2020, 103. [Google Scholar] [CrossRef]
- Olga, G.; Styliani, C.; Ioannis, R.G. Coencapsulation of Ferulic and Gallic Acid in Hp-b-Cyclodextrin. Food Chem. 2015, 185, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Huang, J.; Bao, C.; Jiao, L.; Zhao, H.; Du, Y.; Fazheng, R.; Li, Y. Enzymatically Partially Hydrolyzed α-Lactalbumin Peptides for Self-Assembled Micelle Formation and Their Application for Coencapsulation of Multiple Antioxidants. J. Agric. Food Chem. 2018, 66, 12921–12930. [Google Scholar] [CrossRef] [PubMed]
- Fukuta, T.; Hirai, S.; Yoshida, T.; Maoka, T.; Kogure, K. Enhancement of Antioxidative Activity of Astaxanthin by Combination with an Antioxidant Capable of Forming Intermolecular Interactions. Free Radic. Res. 2020, 54. [Google Scholar] [CrossRef] [PubMed]
- Okuro, P.K.; Thomazini, M.; Balieiro, J.C.C.; Liberal, R.D.C.O.; Fávaro-Trindade, C.S. Co-Encapsulation of Lactobacillus Acidophilus with Inulin or Polydextrose in Solid Lipid Microparticles Provides Protection and Improves Stability. Food Res. Int. 2013, 53, 96–103. [Google Scholar] [CrossRef]
- Atia, A.; Gomaa, A.; Fliss, I.; Beyssac, E.; Garrait, G.; Subirade, M. A Prebiotic Matrix for Encapsulation of Probiotics: Physicochemical and Microbiological Study. J. Microencapsul. 2016, 33, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Klemmer, K.J.; Korber, D.R.; Low, N.H.; Nickerson, M.T. Pea Protein-Based Capsules for Probiotic and Prebiotic Delivery. Int. J. Food Sci. Technol. 2011, 46, 2248–2256. [Google Scholar] [CrossRef]
- Cook, M.T.; Tzortzis, G.; Charalampopoulos, D.; Khutoryanskiy, V.V. Microencapsulation of a Synbiotic into PLGA/Alginate Multiparticulate Gels. Int. J. Pharm. 2014, 466, 400–408. [Google Scholar] [CrossRef]
- McClements, D.J. Advances in Nanoparticle and Microparticle Delivery Systems for Increasing the Dispersibility, Stability, and Bioactivity of Phytochemicals. Biotechnol. Adv. 2020, 38. [Google Scholar] [CrossRef]
- Mao, Y.; McClements, D.J. Modulation of Bulk Physicochemical Properties of Emulsions by Hetero-Aggregation of Oppositely Charged Protein-Coated Lipid Droplets. Food Hydrocoll. 2011, 25, 1201–1209. [Google Scholar] [CrossRef]
- Gao, S.; Decker, E.A.; McClements, D.J. Molecular Exchange Processes in Mixed Oil-in-Water Nanoemulsions: Impact on Droplet Size and Composition. J. Food Eng. 2019, 250, 1–8. [Google Scholar] [CrossRef]
- McClements, D.J. Food Emulsions: Principles, Practice, and Techniques; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Zou, L.; Zheng, B.; Zhang, R.; Zhang, Z.; Liu, W.; Liu, C.; Xiao, H.; McClements, D.J. Enhancing the Bioaccessibility of Hydrophobic Bioactive Agents Using Mixed Colloidal Dispersions: Curcumin-Loaded Zein Nanoparticles plus Digestible Lipid Nanoparticles. Food Res. Int. 2016, 81, 74–82. [Google Scholar] [CrossRef]
- Tavano, L.; Muzzalupo, R.; Picci, N.; De Cindio, B. Co-Encapsulation of Antioxidants into Niosomal Carriers: Gastrointestinal Release Studies for Nutraceutical Applications. Colloids Surf. B Biointerfaces 2014, 114, 82–88. [Google Scholar] [CrossRef]
- Chopra, H.; Bibi, S.; Islam, F.; Ahmad, S.U.; Olawale, O.A.; Alhumaydhi, F.A.; Marzouki, R.; Baig, A.A.; Emran, T. Bin Emerging Trends in the Delivery of Resveratrol by Nanostructures: Applications of Nanotechnology in Life Sciences. J. Nanomater. 2022, 2022. [Google Scholar] [CrossRef]
- Islam, F.; Shohag, S.; Uddin, M.J.; Islam, M.R.; Nafady, M.H.; Akter, A.; Mitra, S.; Roy, A.; Emran, T.B.; Cavalu, S. Exploring the Journey of Zinc Oxide Nanoparticles (ZnO-NPs) toward Biomedical Applications. Materials 2022, 15, 2160. [Google Scholar] [CrossRef]
- Schrooyen, P.M.M.; van der Meer, R.; de Kruif, C.G. Microencapsulation: Its Application in Nutrition. Proc. Nutr. Soc. 2001, 60, 475–479. [Google Scholar] [CrossRef]
- Fenech, M.; Amaya, I.; Valpuesta, V.; Botella, M.A. Vitamin C Content in Fruits: Biosynthesis and Regulation. Front. Plant Sci. 2019, 9. [Google Scholar] [CrossRef]
- Frei, B.; Birlouez-Aragon, I.; Lykkesfeldt, J. Authors’ Perspective: What Is the Optimum Intake of Vitamin C in Humans? Crit. Rev. Food Sci. Nutr. 2012, 52, 815–829. [Google Scholar] [CrossRef]
- Zaric, B.; Obradovic, M.; Trpkovic, A.; Banach, M.; Mikhailidis, D.P.; Isenovic, E.R. Endothelial Dysfunction in Dyslipidaemia: Molecular Mechanisms and Clinical Implications. Curr. Med. Chem. 2020, 27, 1021–1040. [Google Scholar] [CrossRef]
- Łukawski, M.; Dałek, P.; Borowik, T.; Foryś, A.; Langner, M.; Witkiewicz, W.; Przybyło, M. New Oral Liposomal Vitamin C Formulation: Properties and Bioavailability. J. Liposome Res. 2020, 30, 227–234. [Google Scholar] [CrossRef]
- Khudhair, D.H.; Al-Gareeb, A.I.; Al-kuraishy, H.M.; El-Kadem, A.H.; Elekhnawy, E.; Negm, W.A.; Saber, S.; Cavalu, S.; Tirla, A.; Alotaibi, S.S.; et al. Combination of Vitamin C and Curcumin Safeguards Against Methotrexate-Induced Acute Liver Injury in Mice by Synergistic Antioxidant Effects. Front. Med. 2022, 9, 866343. [Google Scholar] [CrossRef]
- Hickey, S.; Roberts, H.J.; Miller, N.J. Pharmacokinetics of Oral Vitamin C. J. Nutr. Environ. Med. 2008, 17, 169–177. [Google Scholar] [CrossRef]
- Pisani, A.; Riccio, E.; Sabbatini, M.; Andreucci, M.; Del Rio, A.; Visciano, B. Effect of Oral Liposomal Iron versus Intravenous Iron for Treatment of Iron Deficiency Anaemia in CKD Patients: A Randomized Trial. Nephrol. Dial. Transplant. 2015, 30, 645–652. [Google Scholar] [CrossRef]
- Eiduson, R.; Heeney, M.M.; Kao, P.C.; London, W.B.; Fleming, M.D.; Shrier, L.A. Prevalence and Predictors of Iron Deficiency in Adolescent and Young Adult Outpatients: Implications for Screening. Clin. Pediatr. 2022, 61, 66–75. [Google Scholar] [CrossRef]
- Sumi, K.; Munakata, K.; Konno, S.; Ashida, K.; Nakazato, K. Inorganic Iron Supplementation Rescues Hematological Insufficiency Even Under Intense Exercise Training in a Mouse Model of Iron Deficiency with Anemia. Biol. Trace Elem. Res. 2021, 199, 2945–2960. [Google Scholar] [CrossRef]
- De Vos, P.; Faas, M.M.; Spasojevic, M.; Sikkema, J. Encapsulation for Preservation of Functionality and Targeted Delivery of Bioactive Food Components. Int. Dairy J. 2010, 20, 292–302. [Google Scholar] [CrossRef]
- Oh, E.J.; Choi, S.J.; Lee, S.J.; Kim, C.H.; Moon, T.W. Modification of Granular Corn Starch with 4-α-Glucanotransferase from Thermotoga Maritima: Effects on Structural and Physical Properties. J. Food Sci. 2008, 73. [Google Scholar] [CrossRef]
- Le Corre, D.; Bras, J.; Dufresne, A. Starch Nanoparticles: A Review. Biomacromolecules 2010, 11, 1139–1153. [Google Scholar] [CrossRef]
- Kanakdande, D.; Bhosale, R.; Singhal, R.S. Stability of Cumin Oleoresin Microencapsulated in Different Combination of Gum Arabic, Maltodextrin and Modified Starch. Carbohydr. Polym. 2007, 67, 536–541. [Google Scholar] [CrossRef]
- Krishnan, S.; Kshirsagar, A.C.; Singhal, R.S. The Use of Gum Arabic and Modified Starch in the Microencapsulation of a Food Flavoring Agent. Carbohydr. Polym. 2005, 62, 309–315. [Google Scholar] [CrossRef]
- Hornung, P.S.; Granza, A.G.; de Oliveira, C.S.; Lazzarotto, M.; Schnitzler, E. Study of the Effects of Ultraviolet Light and Sodium Hypochlorite Solutions on Properties of Cassava Starch Granules. Food Biophys. 2015, 10, 368–374. [Google Scholar] [CrossRef]
- Hornung, P.S.; de Oliveira, C.S.; Lazzarotto, M.; da Lazzarotto, S.R.S.; Schnitzler, E. Investigation of the Photo-Oxidation of Cassava Starch Granules. J. Therm. Anal. Calorim. 2016, 123, 2129–2137. [Google Scholar] [CrossRef]
- Luo, Y. Nutrient Delivery Systems: The Future Strategy for Chronic Diseases. Austin J Nutri Food Sci 2014, 2, 2. [Google Scholar]
- Cho, H.T.; Salvia-Trujillo, L.; Kim, J.; Park, Y.; Xiao, H.; McClements, D.J. Droplet Size and Composition of Nutraceutical Nanoemulsions Influences Bioavailability of Long Chain Fatty Acids and Coenzyme Q10. Food Chem. 2014, 156, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Zhou, B.; He, L.; An, Y.; Lin, L.; Li, Y.; Liu, S.; Chen, Y.; Li, B. Fabrication of Zein/Quaternized Chitosan Nanoparticles for the Encapsulation and Protection of Curcumin. RSC Adv. 2015, 5, 13891–13900. [Google Scholar] [CrossRef]
- Fathi, M.; Donsi, F.; McClements, D.J. Protein-Based Delivery Systems for the Nanoencapsulation of Food Ingredients. Compr. Rev. Food Sci. Food Saf. 2018, 17, 920–936. [Google Scholar]
- Ramos, O.L.; Pereira, R.N.; Martins, A.; Rodrigues, R.; Fuciños, C.; Teixeira, J.A.; Pastrana, L.; Malcata, F.X.; Vicente, A.A. Design of Whey Protein Nanostructures for Incorporation and Release of Nutraceutical Compounds in Food. Crit. Rev. Food Sci. Nutr. 2017, 57, 1377–1393. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathi, S.; Anandharamakrishnan, C. Enhancement of Oral Bioavailability of Vitamin E by Spray-Freeze Drying of Whey Protein Microcapsules. Food Bioprod. Process. 2016, 100, 469–476. [Google Scholar] [CrossRef]
- Cheng, C.J.; Ferruzzi, M.; Jones, O.G. Fate of Lutein-Containing Zein Nanoparticles Following Simulated Gastric and Intestinal Digestion. Food Hydrocoll. 2019, 87, 229–236. [Google Scholar] [CrossRef]
- Peng, S.; Li, Z.; Zou, L.; Liu, W.; Liu, C.; McClements, D.J. Enhancement of Curcumin Bioavailability by Encapsulation in Sophorolipid-Coated Nanoparticles: An in Vitro and in Vivo Study. J. Agric. Food Chem. 2018, 66, 1488–1497. [Google Scholar] [CrossRef]
- Liu, G.; Huang, W.; Babii, O.; Gong, X.; Tian, Z.; Yang, J.; Wang, Y.; Jacobs, R.L.; Donna, V.; Lavasanifar, A.; et al. Novel Protein-Lipid Composite Nanoparticles with an Inner Aqueous Compartment as Delivery Systems of Hydrophilic Nutraceutical Compounds. Nanoscale 2018, 10, 10629–10640. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, Y.H.; Yang, X.Q.; Guo, J.; Wang, J.M. Corn Protein Hydrolysate as a Novel Nano-Vehicle: Enhanced Physicochemical Stability and in Vitro Bioaccessibility of Vitamin D3. LWT-Food Sci. Technol. 2016, 72, 510–517. [Google Scholar] [CrossRef]
- David, S.; Livney, Y.D. Potato Protein Based Nanovehicles for Health Promoting Hydrophobic Bioactives in Clear Beverages. Food Hydrocoll. 2016, 57, 229–235. [Google Scholar] [CrossRef]
- Gan, J.; Siegel, J.B.; German, J.B. Molecular Annotation of Food – Towards Personalized Diet and Precision Health. Trends Food Sci. Technol. 2019, 91, 675–680. [Google Scholar] [CrossRef]
- De Toro-Martín, J.; Arsenault, B.J.; Després, J.P.; Vohl, M.C. Precision Nutrition: A Review of Personalized Nutritional Approaches for the Prevention and Management of Metabolic Syndrome. Nutrients 2017, 9, 913. [Google Scholar] [CrossRef]
- Braconi, D.; Bernardini, G.; Millucci, L.; Santucci, A. Foodomics for Human Health: Current Status and Perspectives. Expert Rev. Proteomics 2018, 15, 153–164. [Google Scholar] [CrossRef]
- Suárez, M.; Caimari, A.; del Bas, J.M.; Arola, L. Metabolomics: An Emerging Tool to Evaluate the Impact of Nutritional and Physiological Challenges. TrAC Trends Anal. Chem. 2017, 96, 79–88. [Google Scholar] [CrossRef]
- Valdés, A.; Cifuentes, A.; León, C. Foodomics Evaluation of Bioactive Compounds in Foods. TrAC Trends Anal. Chem. 2017, 96, 2–13. [Google Scholar] [CrossRef]
- Young, V.B. The Role of the Microbiome in Human Health and Disease: An Introduction for Clinicians. BMJ Online 2017, 356. [Google Scholar] [CrossRef]
- Tao, D.; Yang, P.; Feng, H. Utilization of Text Mining as a Big Data Analysis Tool for Food Science and Nutrition. Compr. Rev. Food Sci. Food Saf. 2020, 19, 875–894. [Google Scholar] [CrossRef]
- McClements, D.J. Nano-Enabled Personalized Nutrition: Developing Multicomponent-Bioactive Colloidal Delivery Systems. Adv. Colloid Interface Sci. 2020, 282, 102211. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).