Abstract
Chemical composition, antioxidant, and antiproliferative properties of C. ladanifer crude extracts, including hexane (Hex), dichloromethane (DCM), ethyl acetate (E.A) and ethanol (EtOH) were investigated. The chemical composition of C. ladanifer crude extracts was determined by use of GC-MS, whereas DPPH and FRAP assays were employed to determine its antioxidant capacity. The obtained results showed that the ethanolic extract exhibited a significant antioxidant effect recording an IC50 value of 266.6 ± 0.828 μg/mL with DPPH assay, and a higher reducing power 0.494 ± 0.035 using the FRAP test. The extracts exhibited significant antiproliferative activity against three cancer cell lines. The DCM extract exhibited the highest total polyphenol content (76.066 ± 9.978 μg AGE/mg) and was revealed to be more effective against HepG2 (31.54 ± 0.242 μg/mL). The Hex extract that presented the highest flavonoid content (50.209 ± 3.805 μg CE/mg) exhibited the highest antiproliferative activity against 22Rv1 and MDA-MB-231 recording IC50 values 11.32 ± 2.126 μg/mL and 82.4 ± 1.124 μg/mL, respectively. All four extracts exhibited minimal toxicity against human skin-derived fibroblast cells indicating the specificity of their observed anticancer activity. GC-MS analysis identified interesting phytochemicals underlying the obtained antioxidant and cytotoxic activities. Taken together, results of the current study highlight the significance of C. ladanifer as a valuable source of antioxidant and anticancer bioactive compounds, thereby warranting further detailed investigation.
1. Introduction
The medicinal properties of plants have been around for thousands of years, and they are still widely employed today. Many of these medications began life as simple tinctures, teas and poultice-like substances before they were refined into more complex formulations. From medicinal plants came the first medicines ever discovered. Recently, novel, and significant medicines against numerous pharmacological targets have been discovered by the isolation and identification of pharmacologically active chemicals in medicinal plants [1].
Cistus plants, also commonly known as rock rose, have gained high biological interest due to their relevant aromatic and pharmacological potential applications. This genus is rich in biologically active compounds such as flavonoids, glycosides, and terpenoids, reputed to be responsible for various biological activities. Cistus species have been employed in the traditional medicine of the Mediterranean region as herbal infusions or as extracts for the treatment of a variety of skin conditions, as well as for their anti-diarrheal and anti-inflammatory properties [2,3,4,5,6,7]. Cistus ladanifer L. (Commonly known as gum rockrose, labdanum and brown-eyed rockrose), is a shrub species endogenous to the Mediterranean, European, western Africa and Asian regions, currently the subject of recent research in various pharmaceutical, cosmetic, and agri-food fields, and widely used in herbal medicine for its physiological properties. Since ancient times, C. ladanifer has been used to cure a variety of conditions, including diarrhea, dysentery, catarrh, and the pain associated with menstruation [5]. In addition to that, this species has a variety of fascinating qualities that have potential use in the culinary, medicinal, phytochemical, and biofuel sectors [8]. Moreover, this plant has significant pharmacological potential wherein several studies have reported antioxidant [5,8,9,10], anti-inflammatory, analgesic actions [11], hypoglycemic and hypolipidemic effects [12,13]. More recently, the cosmetic utility of extracts of two different Cistus species, Cistus incanus L. and Cistus ladanifer L., has been reported to possess sun protecting activity, thereby promoting their application as anti-hyperpigmentation and anti-melanoma products [14]. Antibacterial, antifungal, and antiproliferative potentials of C. ladanifer extracts were also described [5,14,15,16,17,18]. For further pharmacological evaluation of C. ladanifer, we investigated the chemical composition, antioxidant as well as antiproliferative activities against three different human cancerous cell lines including liver, prostate, and breast as reports describing the antiproliferative potential of this plant are limited [5,14,17]. To the best of our knowledge, the antiproliferative activity of C. ladanifer against liver (HepG2), prostate (22Rv1) and breast (MDA-MB-231) has not been previously investigated. Our data show potent antioxidant activities as well as specific and powerful anti-prostate cancer potential with an IC50 value as low as 11.32 ± 2.126 μg/mL. This underscores the pharmacological potential of the extracts under investigation that encourage further purification and structure–activity relationship studies.
2. Materials and Methods
2.1. Plant Material
Leaves of Cistus ladanifer were harvested from the Taza region in May 2017 (006°28.382′ E; 004°49.405′ N). The authentication of the plant was effectuated by Dr. Khabbach Abdelmajid. After being allowed to air-dry for several days at room temperature, leaves were ground into powder. Next, 20 g of air-dried aerial leaves of Cistus ladanifer were extracted using solvents (300 mL) with increasing polarity: n-hexane (Hex), dichloromethane (DCM), ethyl acetate (EA), and ethanolic (EtOH) for 5 h, in a Soxhlet extractor. At 45 °C and lowered pressure, the four extracts were concentrated, and then kept in a freezer at −20 °C until further usage.
2.2. Total Polyphenol Content
The content of phenolic compounds was quantified according to the method of [19]. Each extract (1 mg/mL) was combined with 0.10 mL Folin–Ciocalteu reagent and 0.30 mL sodium carbonate solution (2%). Next, the absorbance was spectrophotometrically read at a wavelength of 760 nm after 90 min of incubation and the concentration of phenols was measured in µg equivalents of gallic acid per mg of dry extract (μg GAE/mg of dry extract).
2.3. Total Flavonoids Content
The flavonoid contents were assessed by use of the method described in earlier published work [20]. A volume of 500 µL from each extract at (1 mg/mL) was combined with 76 µL sodium nitrite solution NaNO2 (5%), 150 µL of aluminum chloride AlCl3 (10.00%) and 500 µL NaOH (1.0 M) before being added to the previously prepared solution of nitrite NaNO2 (5%). Subsequently, the absorbance was measured at 510 nm and results were given in µg equivalent catechin per mg of dry extract (μg CE/mg of dry extract).
2.4. Antioxidant Activity
2.4.1. DPPH Test
The measurement of the antiradical activity of extracts was done by use of DPPH according to the method described in earlier study [21]. A range of concentrations (1000, 500, 250, 125, 62.12 μg/mL) of the extracts was prepared. Next, one milliliter of each extract was mixed with one milliliter of DPPH (0.05%). After 25 min of incubation, the absorbance was measured at a wavelength of 517 nm by use of an ultraviolet (UV) spectrophotometer (UNICO, USA). Ascorbic acid was used as a positive control (reference antioxidant). The DPPH radical’s % inhibition was determined using the following equation.
where A control is absorbance of the blank sample, while A sample is the absorbance of extract.
2.4.2. FRAP Test
One milliliter of the sample was treated with 2.50 mL K3Fe and 2.50 mL of 0.20 M phosphate buffer (pH = 6.6). Incubation was carried out for 30 min at 50 °C before the mixture was treated with one milliliter of trichloroacetic acid. Next, the mixture was centrifuged at 3000 rpm and 2.50 mL of the supernatant was combined with distilled water (2.50 mL) and 0.1 mL FeCl3 (0.1% w/v). Subsequently, the absorbance of the reaction medium was read at 700 nm against a similarly prepared blank that used as negative standard, while ascorbic acid was used as positive standard [22].
2.5. Cell Culture
Human prostate cancer 22Rv1, hepatocellular carcinoma HepG2, and human breast cancer MDA-MB-231 cell lines were tested for the antiproliferative efficacy of C. ladanifer extracts. Briefly, DMEM media supplemented with 10% Gibco BRL fetal serum, 1% L-glutamine, and 1% penicillin-streptomycin were used to culture cells at 37 ºC in a humidified environment of 95% air and 5% CO2.
2.6. Cell Viability Assay
Evaluation of cancer cell growth inhibition was performed by use of MTT method [23]. Briefly, 96-well plates with seeded cells (100 μL, 8 × 104 cells/well) were treated with different concentrations of reconstituted extract at concentrations ranging from 15.625 to 500 μg/mL before being incubated for 72 h at 37 °C. Untreated cells and Mitomycin were used as negative and positive control, respectively. After incubation for 72 h, 100 µL of the medium was replaced with 10 µL of MTT (5 mg/mL) reagent and plates were further incubated for 4 h. Next, the reading of plates was carried out at 570 nm using a Wallac Victor X3 multiplate reader. The following formula was used to determine the vitality of the cells:
2.7. Chemical Composition
The identification of phytocomponents present in the extracts was carried out using the GC-MS technique according to the protocol detailed in our previous studies [24]. The mass spectra were matched to the NIST database for identification of compounds [25].
2.8. Statistical Analysis
The data were presented with the means and standard deviations. The statistical analysis was carried out by use of ANOVA. When the p-value was less than 0.05, the values were statistically judged to be significant.
3. Results
3.1. Determination of Total Phenolic and Flavonoids Contents
The total phenolic (TPC) and flavonoids contents (TFC) of Cistus ladanifer extracts were determined as Gallic Acid Equivalent (GAE) by use of standard curve (R2 = 0.976). TFC was expressed as Catechin Equivalent (CE) using a calibration curve of catechin (R2 = 0.993). The obtained results are given in Table 1. The amount of TPC and TFC of all extracts ranged respectively from 67.366 ± 5.745 to 76.066 ± 9.978 μg AGE/mg of dry extract and from 35.634 ± 1.734 to 50.209 ± 3.805 μg CE/mg of dry extract. The dichloromethane extract of C. ladanifer was found to have the highest amount of phenolic contents of all the tested samples, while the hexanic extract showed the highest level of flavonoids.

Table 1.
Total phenolic and flavonoids contents of ethyl acetate, ethanolic, dichloromethane and hexanic extracts of Cistus ladanifer.
3.2. Antioxidant Activity
3.2.1. DPPH Free Radical Scavenging Activity
The free radical scavenging effect of ethyl Acetate, ethanolic, dichloromethane, and hexanic extracts of Cistus ladanifer was evaluated by use of DPPH test. As shown in Figure 1, the antioxidant activity of the four extracts increased as a function of increasing extract concentration. The IC50 values in Table 2 clearly showed that ethanolic extract was markedly a more potent scavenger of DPPH than the other extracts scoring an IC50 of 266.6 ± 0.828 μg/mL. However, the dichloromethane extract was considered a less effective radical scavenger recording an IC50 of 825.16 ± 11.18 μg/mL. The DPPH scavenging activity of ascorbic acid is shown in Figure 2.
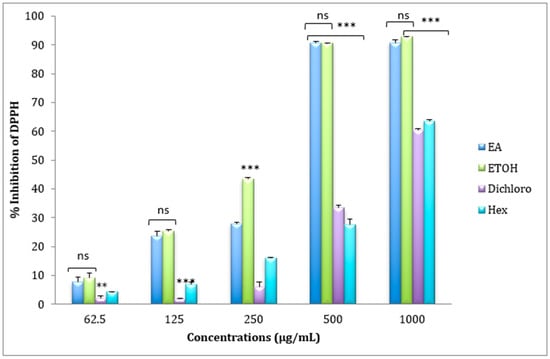
Figure 1.
Scavenging effects of Cistus ladanifer Ethyl Acetate (E.A), Ethanolic (ETOH), Dichloromethane (Dichloro), and Hexanic (Hex) extracts on DPPH radicals (mean ± SD, n = 3 experiments, ns: not significant, p values; **: p < 0.01, *** p < 0.001).

Table 2.
Antioxidant effects of C. ladanifer extracts.
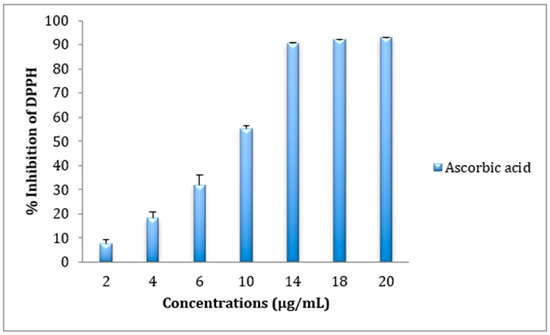
Figure 2.
DPPH radical scavenging activity of positive control Ascorbic acid.
3.2.2. Reducing Power (FRAP)
The FRAP assay was determined by the ferric reducing ability (Figure 3). As shown in Figure 3, the reducing power of extracts was dose-dependent manner. The results in Table 2, showed that the ethanolic extract demonstrated a higher reducing power that differs significantly from other extracts (p < 0.01) with the absorbance of 0.494 ± 0.035 at the highest concentration 1000 µg/mL.
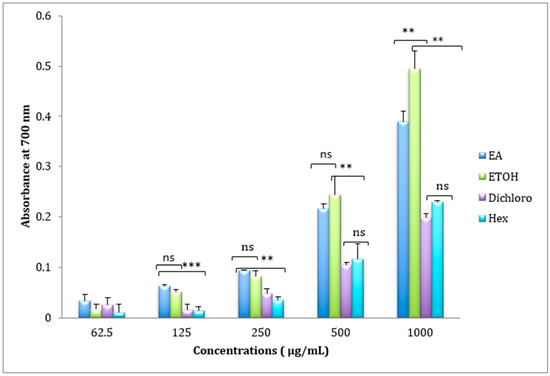
Figure 3.
Reducing power of Ethyl Acetate (E.A), Ethanolic (ETOH), Dichloromethane (Dichloro), and Hexanic extracts of Cistus ladanifer (mean ± SD, n = 3 experiments, ns: not significant, p values; **: p < 0.01, *** p < 0.001).
3.3. Evaluation of Antiproliferative Activity
Antiproliferative effect of Cistus ladanifer extracts was evaluated on 22Rv1, HepG2, and MDA-MB-231 cell lines. The viability and cytotoxicity index percentages were plotted against various extract concentrations using the MTT technique, which demonstrated time- and dose-dependent effects (Figure 4 and Figure 5). On the three tested cell lines, hexanic, dichloromethane, ethyl acetate, and ethanolic extracts exerted an antiproliferative effect after 72 h of treatment. IC50 values for the four extracts are shown in Table 3. In HepG2 cells, the antiproliferative effect of the four extracts exhibited moderate cytotoxicity, and the dichloromethane extract seems to be more active on this line scoring an IC50 of 31.54 ± 0.242 μg/mL. Ethyl acetate, hexanic, and ethanolic extracts reduced viability of this line with IC50 values of 97.74 ± 0.148; 109.6 ± 0.166 and 180.5 ± 0.64 μg/mL respectively. In both 22Rv1 and MDA-MB-231 cell lines, the hexanic extract exhibited higher antiproliferative activity compared to the other extracts scoring values of IC50 of 11.32 ± 2.126 μg/mL and 82.4 ± 1.124 μg/mL, respectively. In 22Rv1 cell line and in a dose-dependent manner, dichloromethane, ethyl acetate, and ethanolic extracts inhibit 22Rv1 cell growth with IC50 ranging from 45.96 ± 0.125 to 61.47 ± 0.551 μg/mL. In the human breast cell line MDA-MB-231, ethanolic, dichloromethane, and ethyl acetate presented an important antiproliferative activity in a dose-dependent manner growth with IC50 ranging from 144.255 ± 12.43 to 290.33 ± 68.63 μg/mL. Interestingly, 22Rv1 line was considerably affected by the four extracts, compared to the two cell lines HepG2 line and MDA-MB-231. However, there is no significant difference observed between the extracts. (p > 0.05).
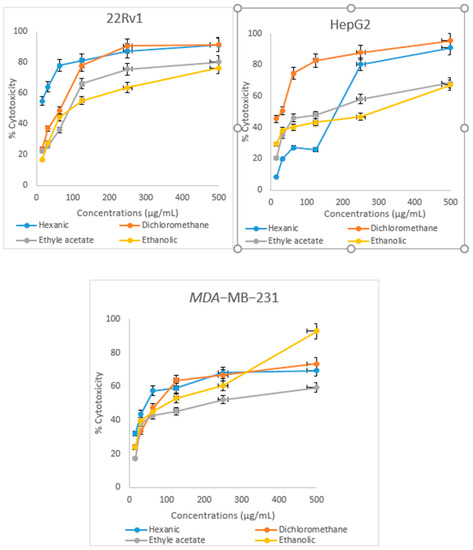
Figure 4.
Antiproliferative activity of Cistus ladanifer extracts using the MTT assay for 22Rv1, HepG2 and MDA-MB-231 cell lines.
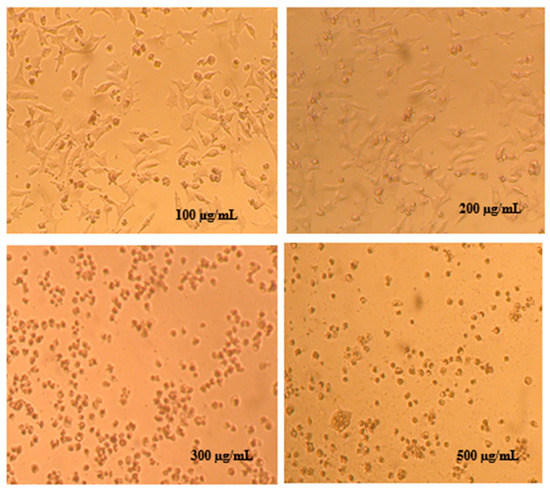
Figure 5.
Cytopathic effects in MDA-MB-231 cancer cells after 72 h post-treatment with increasing concentration of Cistus ladanifer hexanic extract.

Table 3.
IC50 values of C. ladanifer extracts in cancer cells after being treated for 72 h. Values are expressed in μg of dry extract per mL.
3.4. Chemical Composition
The chemical composition of the ethanolic extract of C. ladanifer was analyzed by GC-MS. Main active components, peak area (%), and the molecular formula (M.F) were determined (Table 4 and Figure 6). The ethanolic extract was found to be rich in important compounds known by their pharmacological activities such as octacosane, heptadecanoic acid, longifolene, ledol, and borneol identified in major amounts.

Table 4.
Chemical compounds identified in the ethanolic extract of Cistus ladanifer bar.
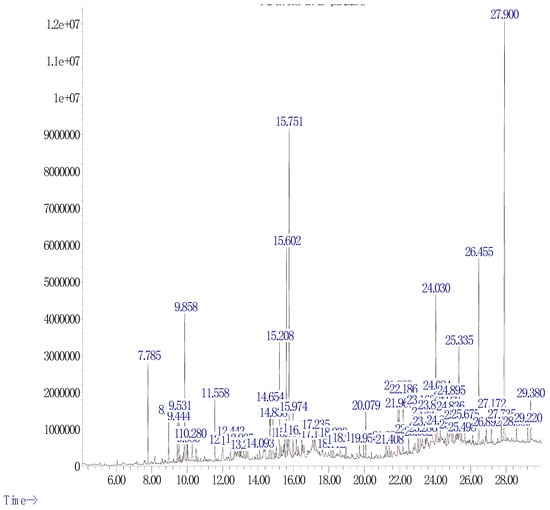
Figure 6.
Chromatogram of the characterized ethanolic extract of C. ladanifer.
4. Discussion
The biological features of phenolic compounds, such as antibacterial, antioxidant, and anticancer activity, have attracted a lot of attention [26]. The studied Cistus extracts here showed significant phenolic and flavonoid contents. The higher phenolic content of C. ladanifer was found in dichloromethane extract (76.066 ± 9.978 μg AGE/mg).
The redox properties of various compounds, which cause them to act as reducing agents, hydrogen donors, singlet oxygen quenchers, and potential metal chelators, are largely responsible for their antioxidant activity. It has been shown that this activity is responsible for preventing the excessive generation of reactive oxygen species (ROS). In addition, oxidative stress has been shown to have a significant role in the development of a number of human illnesses, including cancer, inflammation, and neurodegenerative disorders [27].
In the present work, C. ladanifer was subjected to the evaluation of its antioxidant activity and results revealed that ethanolic extract was more scavenging than other extracts scoring an IC50 of 266.6 ± 0.828 μg/mL. Our result was in agreement with previously reported findings [17], which demonstrated that ethanolic extract presents a high antioxidant activity when compared to acetone extract recording an IC50 of 7.85 µg/mL. Moreover, Amensour et al. [9] showed that methanolic extract exhibited a high antioxidant activity than ethanolic extract scoring 87.72% and 50.10% of DPPH inhibition respectively. For comparison purposes, our results showed a higher percentage of DPPH of inhibition (92.88%) with the ethanolic extract. Moreover, our findings are in agreement with Zidane study investigating the antioxidant potential of C. ladanifer [10]. In this work, it was reported that the methanolic leaf, stem, flower, and fruit extracts of C. lanadifer possessed an antioxidant activity with a percentage of inhibition 97.8%, 97.3%, 96.1%, and 95.9% respectively. A study by Gaweł-Bęben et al. [14], showed that C. ladanifer extracts from Poland exhibited a significant antioxidant activity with IC50 ranging from 4.08 to 10.20 μg/mL. The variability in the antioxidant activity of C. ladanifer extracts reported in this work can be due to the difference in extraction solvents, the difference in collection areas, and plant parts.
In the current work, FRAP assay showed that ethanolic extract possessed a higher reducing power of 0.494 ± 0.035 at the highest concentration of 1000 µg/mL when compared to other extracts. It is thus fitting that our results were in accordance with those found by Amensour et al. [9]. In addition, the aqueous extract from C. ladanifer showed a reducing power of 117.72 mmol Fe2+/100 g dry weight and 3.02 ± 0.07 mmol Fe2+/g [5,28].
Other antioxidant assays including TBARS and ORAC showed that aqueous extract presents an important antioxidant activity [5] also assays as, ABTS and Inhibition of lipid peroxidation of buffered egg, showed an important antioxidant activity of methanolic extract [9].
Antioxidant tests vary because of the different methods through which an extract interacts. Another study indicated that C. ladanifer leaves contain antioxidants such as tocopherol, ascorbic acid, and reducing sugars [29]. Based on our results, C. ladanifer extracts can serve as promising natural agent to scavenge free radicals involved in lipid oxidation in food and biological systems.
In our study, we also investigated the antiproliferative effect of C. ladanifer leaves extracts. The cell lines of hepatocellular carcinoma HepG2, the prostate cancer line 22Rv1, and the human breast MDA-MB-231 cell lines served as a model for our study. The MTT viability test demonstrated the antiproliferative potency of different extracts in a dose-dependent manner. On the HepG2 line, the dichloromethane extract proved to be more active when compared to the other extracts recording an IC50 of the order of 31.54 ± 0.242 μg/mL, as for the hexanic extract, it is more active on the lines 22Rv1 and MDA-MB-231 scoring an IC50 of the order of 11.32 ± 2.126 μg/mL and 82.4 ± 1.1243 μg/mL, respectively. Our results deserve more attention since our study is the first reporting anticancer activity of leaf extracts of C. ladanifer on cancer cell lines including HepG2, 22Rv1, and MDA-MB-231. The aqueous leaf extract of Spanish C. ladanifer was studied for its cytotoxic activity on cell lines of pancreas, breast and colon and showed IC50 values ranging from 0.49 to 16.10 mg/mL [5]. C. ladanifer extracts demonstrated antiproliferative action against two human skin cancer cells; malignant melanoma (A375) and squamous cell carcinoma (SCC-15), which, thus, confirmed our results [14].
In the case of our results, it is difficult to attribute the antiproliferative activity observed to a specific compound or group of compounds in the organic extracts of C. ladanifer leaves. Several secondary metabolites may indeed be present simultaneously in the extracts [30]. However, we can advance the hypothesis that the activity of the dichloromethane extract on the HepG2 line is probably attributed to its high polyphenol content when compared to other extracts (76.066 ± 9.978 μg AGE/mg). It has been reported that phenolic compounds enhance the apoptotic action and cause cell cycle arrest in HepG2 cells by inducing inactivation of transcription factors, which in turn activate the death pathways in liver cancer cells [31].
Moreover, the high antiproliferative potential of the hexanic extract of C. ladanifer leaves on the prostate and breast cancer cell lines can be explained by its high level of flavonoid compounds when compared to the other extracts (50.209 ± 3.805 μg CE/mg). Hormone-dependent cancer cell lines show a growth-inhibiting and cell death impact when exposed to flavonoids, which are found in many fruits and vegetables. Flavonoids, such as isoflavones, have been classified as phytoestrogens, which possess the ability to bind to the estrogen receptor and alter its activity, resulting in anti-estrogenic actions [6].
Several works have reported that antioxidant activity may correlate with anticancer potential. Reactive oxygen species (ROS) are involved in cancer, meanwhile, antioxidant agents are used to counteracting them. Plant extracts have been found to contain significant ROS scavenging and induce antiproliferative activities toward cancer cells through ROS-mediated activities [32]. In our case, there is no correlation between the two activities
The phytochemical composition of the ethanolic extract was also studied and the results showed that this extract is rich in compounds that are important from pharmacological and botanical viewpoints. These compounds are mainly constituted of octacosane, heptadecanoic acid, longifolene, ledol, and borneol, which can probably be responsible for the activities of this plant. All these compounds have been demonstrated in the literature for their biological effects including antioxidant and cytotoxic properties. The Dichloromethane and the hexanic extracts with an important antiproliferative activity were also studied for their chemical composition (data not shown) showing various classes of compounds known for their pharmacological activities including borneol, ledol, camphene and alpha tocopherol. The example of borneol, one of the important compounds found in the three extracts, is a monoterpenoid, which enhances antiproliferative activity by induction of apoptosis, reduces cancer cell growth through the triggering apoptotic cell death, and activates ROS-mediated DNA damage [33,34,35].
It was reported in many studies that C.ladanifer is a source of interesting compounds belonging to different chemical classes including kaempferol glycosides and phenolic acids (gallic and ellagic acids) with flavanol derivatives [14], tannins (the punicalagingallates and punicalines) [2,5], terpenes mainly monoterpenes and sesquiterpenes in essential oils [10], and labdane-type diterpenes in the secreted resin (Labdanum), Oxo-8-labden-15-oic acid, 7-oxo-8-labden-15-oic acid, oxocativic acid and sclareol are the main diterpenes found in labdanum extract of C. ladanifer [36]. Of note, Skorić and co-workers have reported the in vitro anticancer activity of labdane compounds derived from Cistus creticus sub. cretenicus (L.) [37]. Our extracts exhibited potent anti-prostate cancer activity. To our knowledge, this is the only report on prostate cancer cells using extracts from this specific species. The observed potent cytotoxicity against prostate cancer cells (22Rv1) could be associated with the high phenolic compounds content. In this context, polyphenol-rich extracts of two related Cistus species to the one under investigation, namely C. incanus L. and C. monspeliensis L., have been reported to be beneficial for the treatment of benign prostatic hypertrophy (BPH) condition [6]. The aerial parts of C. ladanifer are therefore considered a valuable repertoire of water-soluble polyphenolic compounds with potent antioxidant activity. Moreover, their potent and selective cytotoxic activities against other cancer cell lines requires further detailed investigation.
5. Conclusions
Cistus ladanifer, a plant known for its pharmacological activities that have been yet little explored. In this study, it is intended to investigate the chemical profile, antioxidant, and antiproliferative activities of the different extracts from C. ladanifer leaves. Our extracts showed an important antioxidant activity using DPPH and FRAP bioassays. Moreover, C. ladanifer revealed promising antiproliferative activity against HepG2, 22Rv1 and MDA-MB-231 cancer cell lines. These activities are probably associated with the characterized compounds in the plant, which are known by their biological activities as reported in previous works. Further investigations are therefore warranted aiming at identifying the responsible compounds for the reported activities.
Author Contributions
K.B., M.B.: writing the original draft, conceptualization. N.A., M.A., A.M.S.: formal analysis. H.-A.N., F.M.: formal analysis, writing—review and editing. M.E.M., M.A.M.A.-S.: writing—review and editing and formal analysis, data curation. L.B.: supervision, methodology. All authors have read and agreed to the published version of the manuscript.
Funding
This work is funded by the Researchers Supporting Project number (RSP-2022R437) King Saud University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available from the corresponding authors upon request.
Acknowledgments
The authors extend their appreciation to the Researchers Supporting Project number (RSP-2022R437) King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare that there is no conflict of interest.
References
- Tripathi, P.; Singh, A. Natural Resources From Plants in the Treatment of Cancer: An Update. Asian J. Pharm. Clin. Res. 2017, 10, 13. [Google Scholar] [CrossRef]
- Papaefthimiou, D.; Papanikolaou, A.; Falara, V.; Givanoudi, S.; Kostas, S.; Kanellis, A.K. Genus Cistus: A Model for Exploring Labdane-Type Diterpenes’ Biosynthesis and a Natural Source of High Value Products with Biological, Aromatic, and Pharmacological Properties. Front. Chem. 2014, 2, 35. [Google Scholar] [CrossRef] [PubMed]
- Barrajón-Catalán, E.; Tomás-Menor, L.; Morales-Soto, A.; Bruñá, N.M.; López, D.S.; Segura-Carretero, A.; Micol, V. Rockroses (Cistus Sp.) Oils. In Essential Oils in Food Preservation, Flavor and Safety; Elsevier: Amsterdam, The Netherlands, 2016; pp. 649–658. ISBN 978-0-12-416641-7. [Google Scholar]
- Barrajón-Catalán, E.; Fernández-Arroyo, S.; Roldán, C.; Guillén, E.; Saura, D.; Segura-Carretero, A.; Micol, V. A Systematic Study of the Polyphenolic Composition of Aqueous Extracts Deriving from Several Cistus Genus Species: Evolutionary Relationship: Polyphenolic Characterization of Cistus Aqueous Extracts. Phytochem. Anal. 2011, 22, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Barrajón-Catalán, E.; Fernández-Arroyo, S.; Saura, D.; Guillén, E.; Fernández-Gutiérrez, A.; Segura-Carretero, A.; Micol, V. Cistaceae Aqueous Extracts Containing Ellagitannins Show Antioxidant and Antimicrobial Capacity, and Cytotoxic Activity against Human Cancer Cells. Food Chem. Toxicol. 2010, 48, 2273–2282. [Google Scholar] [CrossRef] [PubMed]
- Vitali, F.; Pennisi, G.; Attaguile, G.; Savoca, F.; Tita, B. Antiproliferative and Cytotoxic Activity of Extracts from Cistus incanus L. and Cistus monspeliensis L. on Human Prostate Cell Lines. Nat. Prod. Res. 2011, 25, 188–202. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, H.; Aouadhi, C.; Kaddour, R.; Gruber, M.; Zargouni, H.; Zaouali, W.; Hamida, N.B.; Nasri, M.B.; Ouerghi, Z.; Hosni, K. Comparison of Antioxidant and Antimicrobial Activities of Two Cultivated Cistus Species from Tunisia. Biosci. J. 2016, 32, 226–237. [Google Scholar] [CrossRef]
- Raimundo, J.R.; Frazão, D.F.; Domingues, J.L.; Quintela-Sabarís, C.; Dentinho, T.P.; Anjos, O.; Alves, M.; Delgado, F. Neglected Mediterranean Plant Species Are Valuable Resources: The Example of Cistus ladanifer. Planta 2018, 248, 1351–1364. [Google Scholar] [CrossRef]
- Amensour, M.; Sendra, E.; Pérez-Alvarez, J.A.; Skali-Senhaji, N.; Abrini, J.; Fernández-López, J. Antioxidant Activity and Chemical Content of Methanol and Ethanol Extracts from Leaves of Rockrose (Cistus ladaniferus). Plant Foods Hum. Nutr. 2010, 65, 170–178. [Google Scholar] [CrossRef]
- Zidane, H.; Elmiz, M.; Aouinti, F.; Tahani, A.; Wathelet, J.; Sindic, M.; Elbachiri, A. Chemical Composition and Antioxidant Activity of Essential Oil, Various Organic Extracts of Cistus Ladanifer and Cistus Libanotis Growing in Eastern Morocco. Afr. J. Biotechnol. 2013, 12, 5314–5320. [Google Scholar] [CrossRef]
- El Hamsas El Youbi, A.; El Mansouri, L.; Boukhira, S.; Daoudi, A.; Bousta, D. In Vivo Anti-Inflammatory and Analgesic Effects of Aqueous Extract of Cistus ladanifer L. From Morocco. Am. J. Ther. 2016, 23, e1554–e1559. [Google Scholar] [CrossRef]
- El Kabbaoui, M.; Chda, A.; Azdad, O.; Mejrhit, N.; Aarab, L.; Bencheikh, R.; Tazi, A. Evaluation of Hypoglycemic and Hypolipidemic Activities of Aqueous Extract of Cistus ladaniferus in Streptozotocin-Induced Diabetic Rats. Asian Pac. J. Trop. Biomed. 2016, 6, 1044–1049. [Google Scholar] [CrossRef]
- Rauter, A.P.; Martins, A.; Borges, C.; Mota-Filipe, H.; Pinto, R.; Sepodes, B.; Justino, J. Antihyperglycaemic and Protective Effects of Flavonoids on Streptozotocin-Induced Diabetic Rats. Phytother. Res. 2010, 24, S133–S138. [Google Scholar] [CrossRef] [PubMed]
- Gaweł-Bęben, K.; Kukula-Koch, W.; Hoian, U.; Czop, M.; Strzępek-Gomółka, M.; Antosiewicz, B. Characterization of Cistus × Incanus L. and Cistus ladanifer L. Extracts as Potential Multifunctional Antioxidant Ingredients for Skin Protecting Cosmetics. Antioxidants 2020, 9, 202. [Google Scholar] [CrossRef]
- Barros, L.; Dueñas, M.; Alves, C.T.; Silva, S.; Henriques, M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Antifungal Activity and Detailed Chemical Characterization of Cistus Ladanifer Phenolic Extracts. Ind. Crops Prod. 2013, 41, 41–45. [Google Scholar] [CrossRef]
- Stępień, A.; Aebisher, D.; Department of Human Immunology, Faculty of Medicine, University of Rzeszów, Poland; Bartusik-Aebisher, D. Department of Experimental and Clinical Pharmacology, Faculty of Medicine, University of Rzeszów, Poland Biological Properties of Cistus Species. Eur. J. Clin. Exp. Med. 2018, 16, 127–132. [Google Scholar] [CrossRef]
- Andrade, D.; Gil, C.; Breitenfeld, L.; Domingues, F.; Duarte, A.P. Bioactive Extracts from Cistus ladanifer and Arbutus unedo L. Ind. Crops Prod. 2009, 30, 165–167. [Google Scholar] [CrossRef]
- Benali, T.; Bouyahya, A.; Habbadi, K.; Zengin, G.; Khabbach, A.; Achbani, E.H.; Hammani, K. Chemical Composition and Antibacterial Activity of the Essential Oil and Extracts of Cistus ladaniferus Subsp. ladanifer and Mentha suaveolens against Phytopathogenic Bacteria and Their Ecofriendly Management of Phytopathogenic Bacteria. Biocatal. Agric. Biotechnol. 2020, 28, 101696. [Google Scholar] [CrossRef]
- Aoussar, N.; Rhallabi, N.; Ait Mhand, R.; Manzali, R.; Bouksaim, M.; Douira, A.; Mellouki, F. Seasonal Variation of Antioxidant Activity and Phenolic Content of Pseudevernia Furfuracea, Evernia Prunastri and Ramalina Farinacea from Morocco. J. Saudi Soc. Agric. Sci. 2020, 19, 1–6. [Google Scholar] [CrossRef]
- Žilić, S.; Hadži-Tašković Šukalović, V.; Dodig, D.; Maksimović, V.; Maksimović, M.; Basić, Z. Antioxidant Activity of Small Grain Cereals Caused by Phenolics and Lipid Soluble Antioxidants. J. Cereal Sci. 2011, 54, 417–424. [Google Scholar] [CrossRef]
- Kosanic, M.; Rankovic, B.; Stanojkovic, T.; Vasiljevic, P.; Manojlovic, N. Biological Activities and Chemical Composition of Lichens from Serbia. Excli J. 2014, 13, 1226–1238. [Google Scholar]
- Oyaizu, M. Studies on Products of Browning Reaction. Antioxidative Activities of Products of Browning Reaction Prepared from Glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef] [Green Version]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Moussaoui, A.E.; Bourhia, M.; Jawhari, F.Z.; Es-safi, I.; Ali, S.S.; Bari, A.; Mahmood, H.M.; Bousta, D.; Bari, A. Withania Frutescens. L Extract: Phytochemical Characterization and Acute and Repeated Dose 28-Day Oral Toxicity Studies in Mice. BioMed Res. Int. 2020, 2020, 1976298. [Google Scholar] [CrossRef] [PubMed]
- van Den Dool, H.; Kratz, P.D. A Generalization of the Retention Index System Including Linear Temperature Programmed Gas-Liquid Partition Chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- El moussaoui, A.; Kadiri, M.; Bourhia, M.; Agour, A.; Salamatullah, A.M.; Alzahrani, A.; Alyahya, H.K.; Albadr, N.A.; Chedadi, M.; Sfaira, M.; et al. Promising Antioxidant and Anticorrosion Activities of Mild Steel in 1.0 M Hydrochloric Acid Solution by Withania Frutescens L. Essential Oil. Front. Chem. 2021, 9, 739273. [Google Scholar] [CrossRef]
- Bouyahya, A.; Abrini, J.; Et-Touys, A.; Bakri, Y.; Dakka, N. Indigenous Knowledge of the Use of Medicinal Plants in the North-West of Morocco and Their Biological Activities. Eur. J. Integr. Med. 2017, 13, 9–25. [Google Scholar] [CrossRef]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J.-M. Comparative Study of Antioxidant Properties and Total Phenolic Content of 30 Plant Extracts of Industrial Interest Using DPPH, ABTS, FRAP, SOD, and ORAC Assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, R.; Barros, L.; Carvalho, A.M.; Sousa, M.J.; Morais, J.S.; Ferreira, I.C.F.R. Aromatic Plants as a Source of Important Phytochemicals: Vitamins, Sugars and Fatty Acids in Cistus ladanifer, Cupressus lusitanica and Eucalyptus gunnii Leaves. Ind. Crops Prod. 2009, 30, 427–430. [Google Scholar] [CrossRef]
- Roleira, F.M.F.; Varela, C.L.; Costa, S.C.; Tavares-da-Silva, E.J. Phenolic Derivatives From Medicinal Herbs and Plant Extracts: Anticancer Effects and Synthetic Approaches to Modulate Biological Activity. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; Volume 57, pp. 115–156. ISBN 978-0-444-64057-4. [Google Scholar]
- Stagos, D.; Amoutzias, G.D.; Matakos, A.; Spyrou, A.; Tsatsakis, A.M.; Kouretas, D. Chemoprevention of Liver Cancer by Plant Polyphenols. Food Chem. Toxicol. 2012, 50, 2155–2170. [Google Scholar] [CrossRef]
- Sammar, M.; Abu-Farich, B.; Rayan, I.; Falah, M.; Rayan, A. Correlation between Cytotoxicity in Cancer Cells and Free Radical-Scavenging Activity: In Vitro Evaluation of 57 Medicinal and Edible Plant Extracts. Oncol. Lett. 2019, 18, 6563–6571. [Google Scholar] [CrossRef]
- Cao, W.; Li, Y.; Hou, Y.; Yang, M.; Fu, X.; Zhao, B.; Jiang, H.; Fu, X. Enhanced Anticancer Efficiency of Doxorubicin against Human Glioma by Natural Borneol through Triggering ROS-Mediated Signal. Biomed. Pharmacother. 2019, 118, 109261. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yin, Y.; Sun, J.; Feng, S.; Ma, J.; Fu, X.; Hou, Y.; Yang, M.; Sun, B.; Fan, C. Natural Borneol Is a Novel Chemosensitizer That Enhances Temozolomide-Induced Anticancer Efficiency against Human Glioma by Triggering Mitochondrial Dysfunction and Reactive Oxide Species-Mediated Oxidative Damage. OncoTargets Ther. 2018, 11, 5429–5439. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Lai, H.; Chen, J.; Li, L.; Wong, Y.-S.; Chen, T.; Li, X. Natural Borneol, a Monoterpenoid Compound, Potentiates Selenocystine-Induced Apoptosis in Human Hepatocellular Carcinoma Cells by Enhancement of Cellular Uptake and Activation of ROS-Mediated DNA Damage. PLoS ONE 2013, 8, e63502. [Google Scholar] [CrossRef] [PubMed]
- Frazão, D.F.; Raimundo, J.R.; Domingues, J.L.; Quintela-Sabarís, C.; Gonçalves, J.C.; Delgado, F. Cistus ladanifer (Cistaceae): A Natural Resource in Mediterranean-Type Ecosystems. Planta 2018, 247, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Skorić, M.; Todorović, S.; Gligorijević, N.; Janković, R.; Živković, S.; Ristić, M.; Radulović, S. Cytotoxic Activity of Ethanol Extracts of in Vitro Grown Cistus creticus Subsp. creticus L. on Human Cancer Cell Lines. Ind. Crops Prod. 2012, 38, 153–159. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).