Metal–Organic Frameworks as Powerful Heterogeneous Catalysts in Advanced Oxidation Processes for Wastewater Treatment
Abstract
:1. Introduction
2. MOFs
2.1. Most Useful Types of MOF for Wastewater
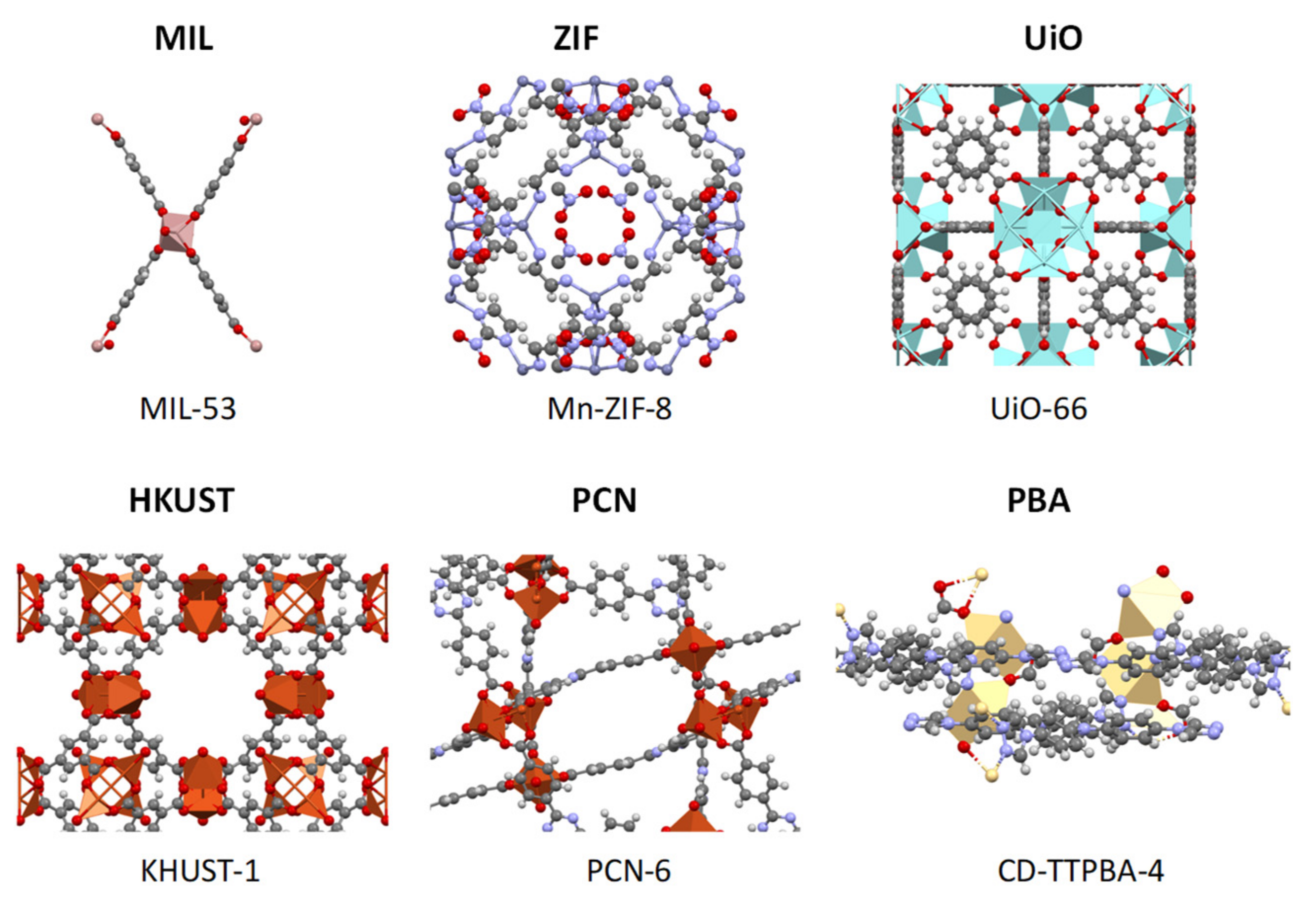
2.1.1. MIL
| MIL | Pollutant | Processes | Reference |
|---|---|---|---|
| AgCl/MIL-100(Fe) | Sulfamethazine (SMT) | Photocatalytic | [28] |
| BC@FexC | Norfloxacin | Catalytic activation of peroxydisulfate (PDS) | [29] |
| NCQDs/MIL-101(Fe) | Tetracycline (TC) | Photo-Fenton and Photocatalytic | [30] |
| MIL-101(Cr)@AC | Sulfacetamide | Adsorption | [31] |
| MIL-125(Ti) | TC | Photocatalytic | [32] |
| MIL-125(Ti)/BiOI | Rhodamine B and TC | Photocatalytic | [33] |
| MIL-53(Al)@SiO2 | Bisphenol A | Photocatalytic | [34] |
| MIL-53(Al)-NDC | Tinidazole | Adsorption | [35] |
| Mn-MIL-53(Fe) | TC | Catalytic activation of PMS | [36] |
| (PDI)/MIL-101(Cr) | Iohexol | Photocatalytic activation of PDS | [36] |
2.1.2. ZIF
| ZIF | Pollutant | Processes | Reference |
|---|---|---|---|
| CZC600-30 | TC and methylene blue | Photocatalytic | [46] |
| MoS2@ZNC-2 | phenol | Electro-catalytic | [47] |
| N@ZIF-67 | ciprofloxacin | Catalytic activation of PMS | [48] |
| ZIF-67@WA | TC | Adsorption | [49] |
| ZIF-9@GEL and ZIF-12@GEL | p-nitrophenol | Catalytic activation of PMS | [50] |
| ZIF-8 | TC and oxytetracycline | Adsorption | [51] |
| ZnCDs/ZnO@ZIF-8 | TC | Photocatalytic | [52] |
| Zn2SnO4/SnO2@ZIF-8 | TC | Photocatalytic | [53] |
| ZnO/ZIF-9 | TC | Photocatalytic | [54] |
| ZnO@ZIF-67 | TC | Adsorption | [55] |
2.1.3. UiO
2.1.4. HKUST
3. Fe-MOF Synthesis
3.1. Solvothermal Synthesis
3.2. Microwave-Assisted Synthesis
| MOF | Organic Ligand | Metal Source | Solvent | Procedure | Reference |
|---|---|---|---|---|---|
| MIL-100-1 | Trimesic acid (H3BTC) | FeCl3 | Water | H3BTC and FeCl3 (molar ratio 1:1) were mixed and transfer in a Teflon autoclave and placed in an oven (200 °C, 8 h). After cooling at room temperature, it was purified and dried at 80 °C under a vacuum overnight. | [90] |
| MIL-100-2 | H3BTC | Fe(NO3)3 | Water | H3BTC and Fe(NO3)3 (molar ratio 1:1) were mixed in water following the same procedure as MIL-100-1. | [90] |
| MIL-100-3 | H3BTC | Fe2(SO4)3 | Water | H3BTC and Fe2(SO4)3 (molar ratio 1:1) were mixed in water following the same procedure as MIL-100-1. | [90] |
| Fe/Co-MOF-x | Terephthalic acid (BDC) | FeCl3·6H2O and CoCl2·6H2O | DMF | Metal ions (Fe3+ and Co2+) and BDC were dissolved in DMF with a molar ratio of 1:1. Once NaOH solution was added, it was put in an oven at 100 °C for 10 h. When the reaction was completed, the resulting solid was recovered, cleaned and dried in a vacuum oven at 60 °C. | [91] |
| MIL-53(Fe)-NH2 | 2-aminoterephthalic acid (NH2-BDC) | FeCl3·6H2O | HCl | A reaction mixture of NH2-BDC, HCl and FeCl3·6H2O (molar ratio 1:1:1) was loaded in a 10 mL glass vial, sonicated for 1 min in an ultrasound bath and placed in a microwave. Then, it was heated to the reaction temperature as fast as possible with different power impulses. Then, it was cooled down to 65 °C by an airflow. The resulting product was washed. | [99] |
| Fe-MOF | BDC | FeCl3·6H2O | DMF | FeCl3·6H2O and BDC (molar ratio 1:1) were dissolved in 50 mL DMF. Then, 10 mL NaOH solution was added. Then it was placed in an oven at 100 °C for 10 h. After the reactor cooled, the resulting solid was recovered, cleaned and dried in a vacuum oven at 60 °C. | [91] |
| Fe-MOF | H2BDC | FeSO4·7H2O | DMF | A total of 20 mL DMF with H2BDC (2.6734 g) was stirred for 30 min, while FeSO4·7H2O (3.2133 g) was dissolved in 20 mL of deionized water. Then both dissolutions were mixed and stirred for 30 min, and then transferred to the reactor (150 °C for 12 h). Finally, the product was filtered, washed and dried (70 °C for 5 h). | [100] |
| Zr/Fe-MOFs/GO | BDC | FeSO4·7H2O and zirconium acetate | DMF | A hydrothermal process was used to synthesize the compound. Two solutions, BDC and DMF (ratio: 3.3215 g: 10 mL) and FeSO4·7H2O and zirconium acetate. (ratio: 2.8133 g:2.6 mL) were mixed and transferred to a reactor (120 °C for 10 h). Then, the reaction mixture was filtered, washed and dried (80 °C for 12 h). | [101] |
| Mg/Fe-MOF | BDC | Fe(NO3)3·9H2O and MgCl2·6H2O | DMF and acetonitrile | Fe(NO3)3·9H2O, MgCl2·6H2O and BDC (molar ratio 10:1:12.4) and 40 mL DMF were mixed. Next, 40 mL of acetonitrile was added and the mixture was placed into a Teflon flask in an oven (150 °C for 12 h). After cooling, the precipitate was collected, washed and dried at 150 °C under a vacuum. | [102] |
| Cu/Fe-MOF | BDC | Fe(NO3)3·9H2O and Cu(NO3)2·3H2O | DMF and acetonitrile | Fe(NO3)3·9H2O, Cu(NO3)2·3H2O and BDC (molar ratio 10:1:12.4) and 40 mL DMF were mixed. Next, 40 mL of acetonitrile was added and the mixture was placed into a Teflon flask in an oven (150 °C for 12 h). After cooling, the precipitate was collected, washed and dried at 150 °C under a vacuum. | [102] |
| MIL-100(Fe)/rGO | H3BTC | Fe(NO3)3·9H2O | Water | Fe(NO3)3·9H2O (14.43 g) and H3BTC (5.04 g) were dissolved in water (36 mL). The rGO (50 mg) was dispersed in distilled water (100 mL) under ultrasonication for 1 h, and both dissolutions were mixed for 1 h. Then it was transferred to a Teflon-lined stainless-steel autoclave (150 °C for 15 h). After that, the product was collected, washed and dried at 90 °C overnight. | [103] |
| Cu-doped MIL-101(Fe) | H2BDC | FeCl3·6H2O and Cu(OAc)2 | DMF | A total of 1.35 g of FeCl3·6H2O, 415 mg of H2BDC and 181.6 mg of Cu(OAc)2 were added to 30 mL of DMF. Then it was transferred to a Teflon-lined autoclave and left at 110 °C for 20 h with a heating rate of 5 °C/min. After the reaction was over, the solid product was collected, washed and dried at 80 °C overnight. | [104] |
3.3. Electrochemical Synthesis
| MOF | Organic Ligand | Metal Source | Solvent | Procedure | Reference |
|---|---|---|---|---|---|
| Fe3O4@MIL–100(Fe) | H3BTC | Fe3O4 nanoparticles | Water | H3BTC (1.0 g), Fe3O4 microspheres (0.6 g) and water (12.5 mL) were reacted under microwave at 150 °C for 30 min (300 W). The solid was collected, washed and dried in a vacuum at 60 °C. | [105] |
| NH2-MIL-101(Fe) | NH2-BDC | FeCl3·6H2O | DMF | Dissolved FeCl3·6H2O and acetic acid in DMF under sonication, followed by the addition of NH2-BDC. Then, it was placed in a microwave reactor (110 °C and 45 min). After that, the reactor was cooled down to room temperature, the precipitates were collected, cleaned and finally dried under vacuum at 60 °C overnight. | [114] |
| MIL-88A(Fe) | Fumaric acid | FeCl3·6H2O | The fumaric acid solution was added into a FeCl3·6H2O solution (ratio molar 1:1). Then it was transferred into a microwave oven and heated for 3 min. Finally, the formed precipitate was collected, washed and dried at 70 °C. | [97] | |
| Fe-MOFs | H3BTC | FeSO4 7H2O | FeSO4 7H2O and H3BTC (with a ratio molar of 1.5:1), stainless-steel balls and deionized water were combined in a tetrafluoroethylene milling pot. Stir milling was performed at 200 rpm and the microwave oven was started concurrently for 40 min. The solid was filtered, washed, added to a beaker containing ethanol and then stirred with a magnetic stirrer for 3 h. This mixture was then filtered and dried. | [115] | |
| NH2-MIL-88B(Fe) | NH2-BDC | FeCl3·6H2O | DMF | FeCl3·6H2O, NH2-BDC and DMF (molar ratio of 1:1:282) were mixed for 30 min and the solution was degassed by shaking in an ultrasonic bath for 5 min. Then, it was transferred into a Teflon autoclave and placed in a microwave oven (150 °C, 20 min and 600 W). The final product was purified and dried under a vacuum at 60 °C overnight. | [98] |
3.4. Sonochemical Synthesis
3.5. Mechanochemical Synthesis
3.6. Dry-Gel Synthesis
3.7. Other Synthesis Methods of Fe-MOF
4. Application of Fe-MOF in Advanced Oxidation Processes
4.1. Catalytic AOP
4.2. Photo-Based AOPs
4.3. Electrochemical AOP
4.3.1. Electro-Fenton
4.3.2. Photoelectro-Fenton
4.4. Ozone-Based AOP
| MOF | Pollutants | AOP | Removal (%Degradation/Time (min)) | Reuses (n° Cycles/% Degradation) | Reference |
|---|---|---|---|---|---|
| 3 % Fe-MOF/CM | TC | Photocatalytic/H2O2 | 100%/60 min | 4/90% | [179] |
| g-C3N4/NH2-MIL-101(Fe) | Acetaminophen | Photocatalytic/H2O2 | 94%/60 min | 10/>85% | [216] |
| g-C3N4/PDI@NH2-MIL-53(Fe) | TC | Photocatalytic/H2O2 | 90%/60 min | 5/- | [185] |
| g-C3N4/PDI@NH2-MIL-53(Fe) | Carbamazepine (CBZ) | Photocatalytic/H2O2 | 75%/150 min | 5/- | [185] |
| g-C3N4/PDI@NH2-MIL-53(Fe) | BPA | Photocatalytic/H2O2 | 100%/10 min | 5/- | [185] |
| g-C3N4/PDI@NH2-MIL-53(Fe) | 4-NP | Photocatalytic/H2O2 | 100%/30 min | 5/- | [185] |
| MIL-88A(Fe)/MC | Oxytetracycline (OTC) | Photoactivated sulfate radical | 98.2%/240 min | 30/83.7% | [187] |
| MIL-88A(Fe)/MC | TC | Photoactivated sulfate radical | 98.3%/240 min | 30/78.8% | [187] |
| MIL-88A(Fe)/MC | Chlortetracycline (CTC) | Photoactivated sulfate radical | 100%/240 min | 30/88.1% | [187] |
| 6%MIL-88A@BCN | Phenol | Photocatalytic/H2O2 | 92.7%/30 min | 5/93–88% | [217] |
| 6%MIL-88A@RCN | Phenol | Photocatalytic/H2O2 | 91.1%/30 min | 5/92–87% | [217] |
| Bi5O7I@MIL-100(Fe) | Doxycycline | Photoactivated sulfate radical | 100%/130 min | 5/100–90% | [218] |
| BiOBr/MIL-53(Fe) | CBZ | Photocatalytic/H2O2 | 85%/100 min | 0 | [219] |
| Fe3O4@MIL-53(Fe) | Ibuprofen | Photocatalytic/H2O2 | 99%/60 min | 5/95% | [220] |
| Fe-UiO-66 | Sulfameter | Photoactivated sulfate radical | 90%/300 min | 5/99–95% | [189] |
| MIL-101(Fe)/TiO2 | TC | Photocatalytic/H2O2 | 92.8%/10 min | 5/93–90% | [221] |
| PDINH/MIL-88A | Chloroquine phosphate | Photoactivated sulfate radical | 94.6%/30 min | 5/93.8% | [188] |
| AFG@30MIL-101(Fe) | Diazinon | Photo-Fenton | 100%/105 min | 4/100–97% | [222] |
| AFG@30MIL-101(Fe) | Atrazine | Photo-Fenton | 81%/105 min | 4/81–75% | [222] |
| Cu2O/MIL(Fe/Cu) | Thiacloprid | Photo-Fenton | 82.3%/80 min | 10/>95% | [223] |
| Co-Fe PBAs | Levofloxacin Hydrochloride | Fenton-like with PMS | 97.6%/30 min | 5/83.7% | [164] |
| CUMSs/MIL-101(Fe,Cu) | CIP | Fenton-like | 93.5%/30 min | 4/88.4% | [224] |
| Fe(PyBDC) | SMX (Sulfamethoxazole) | Fenton-like with PS | 98.7%/180 min. | 2/0%, it can not be reused | [163] |
| Basolite F-300 | Antipyrine | Fenton-like with PMS | 100%/300 min | 4/93% | [225] |
| Basolite F-300 | Escherichia coli | Fenton-like with PMS | 100%/5 min | 4/100% | [225] |
| Fe@MesoC | SMX | Fenton | 100%/120 min | 3/85.2% | [160] |
| Fe0.75Cu0.25(BDC) | SMX | Fenton-like | 100%/120 min | 3/99–98% | [161] |
| Fe3O4@MIL-100(Fe) | Levofloxacin | Photo-Fenton | 93.4%/180 min | 5/>80% | [226] |
| FeII-MIL-53(Fe) | 4-NP | Fenton-like | 95.2%/120 min | 5/89% | [227] |
| Fe-BDC-NH2 | Bisphenol A (BPA) | Fenton-like | 95%/10 min | 5/>90% | [162] |
| Fe-ISAs@CN | Sulfadiazine (SDZ) | Fenton-like | 96%/60 min | 5/>70% | [228] |
| Fe-Pd@C nanomaterial | Phenol | Fenton | 95%/60 min | 5/75% | [136] |
| Fe-TCPP-3 | CIP | Photo-Fenton | 73%/30 min | 0 | [229] |
| M.MIL-100(Fe)@ZnO NS | Phenol, BPA and atrazine | Photo-Fenton | 92%/120 min (mean value of all pollutants) | 5/>85% (mean value of all pollutants) | [171] |
| MIL-100(Fe)-M (H3BTC/4 NaOH) | Sodium sulfadiazine | Photo-Fenton | 95%/240 min | 5/95–90% | [230] |
| MIL-101(Fe)-NH2@Al2O3(MA) | Norfloxacin | Photo-Fenton | 97.3%/100 min | 10/97% | [231] |
| MIL-53(Fe)@PES | CBZ | Photo-Fenton | 99%/60 min | 5/80% | [232] |
| r-MIL-88A-Fe | Phenol | Fenton | 100%/15 min | 3/97% | [158] |
| Ti3C2Tx/MIL-53(Fe) hybrid | TC | Photo-Fenton | 90%/80 min | 5/85% | [233] |
| VC@Fe3O4 nanoparticles | SDZ | Fenton-like | 56.6%/90 min | 3/26.2% | [234] |
| CMIL-100(Fe)@PCM25 | Napropamide | Electro-Fenton | 97%/60 min | 3/95–85% | [200] |
| Cu0.33Fe0.67NBDC-300/GF | SMX | Electro-Fenton | 100%/75 min | 5/95% | [201] |
| Fe bpydc | Bezafibrate | Photo-electro-Fenton | 96%/90 min | 3/67% | [195] |
| Fe/Fe3C@PC | SMT | Electro-Fenton | 99.2%/60 min | 2/57% | [196] |
| FeCu@PC | SMT | Photo-electro-Fenton | 99.9%/60 min | 5/97% | [206] |
| MOF-525-Fe/Zr@CF | SMX | Photo-electro-Fenton | 97.3%/180 min | 4/96.6% | [205] |
| FeS2/C | Fluoxetine | Electro-Fenton | 91%/60 min | 5/61% | [202] |
| Mn/Fe@PC | Triclosan | Electro-Fenton | 100%/120 min | 6/99% | [235] |
| Fe2+/NDCA | Dimethyl phthalate | Electro-Fenton | 100%/50 min | 5/95–90% | [236] |
| Fe2+/NDCA | 3-Chlorophenol | Electro-Fenton | 100%/30 min | 0 | [236] |
| Fe2+/NDCA | BPA | Electro-Fenton | 100%/10 min | 0 | [236] |
| Fe2+/NDCA | SMX | Electro-Fenton | 100%/15 min | 0 | [236] |
| NH2-MIL(Fe)-88B (nano-ZVI@C-N) | Gemfibrozil | Electro-Fenton | 95%/60 min | 5/80–75% | [203] |
5. Conclusions
- -
- Eco-friendly preparation of MOFs: search for alternative methods for MOF synthesis with high yield and using environmentally friendly and harmless solvents.
- -
- Bimetallic MOFs: application of various transition metals in the synthesis of the MOF enhancing their properties for the AOPs;
- -
- New composite materials hybridized with MOFs: exploration of new materials to be introduced on MOFs adding new functions and achieving high-performance of the catalyst.
- -
- Shelf life and reusability: these are prerequisites for long-term industrial use. The improvement of the stability of MOFs for their use in aqueous environments is required because most of them are unstable in water and decompose. Moreover, their recovery and reuse in several cycles or continuous treatment is a crucial factor in the scale-up of the processes.
- -
- Emerging contaminants treatment and real wastewater use: mostly dye degradation is studied using MOF, but emerging contaminants are barely studied, and it is required to give more attention to these compounds in the future. Another consideration is the evaluation of real wastewater in the treatments due to most of the actual ones are performed using simulated ones.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sales, H.B.; Menezes, R.R.; Neves, G.A.; de Souza, J.J.N.; Ferreira, J.M.; Chantelle, L.; de Oliveira, A.L.M.; Lira, H.d.L. Development of Sustainable Heterogeneous Catalysts for the Photocatalytic Treatment of Effluents. Sustainability 2020, 12, 7393. [Google Scholar] [CrossRef]
- Natarajan, S.; Bajaj, H.C.; Tayade, R.J. Recent Advances Based on the Synergetic Effect of Adsorption for Removal of Dyes from Waste Water Using Photocatalytic Process. J. Environ. Sci. 2018, 65, 201–222. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Lai, C.; Liu, Y.; Zeng, G.; Huang, D.; Zhang, C.; Qin, L.; Hu, L.; Zhou, C.; Xiong, W. Metal-Organic Frameworks for Highly Efficient Heterogeneous Fenton-like Catalysis. Coord. Chem. Rev. 2018, 368, 80–92. [Google Scholar] [CrossRef]
- Dhaka, S.; Kumar, R.; Deep, A.; Kurade, M.B.; Ji, S.W.; Jeon, B.H. Metal–Organic Frameworks (MOFs) for the Removal of Emerging Contaminants from Aquatic Environments. Coord. Chem. Rev. 2019, 380, 330–352. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Reactive Species in Advanced Oxidation Processes: Formation, Identification and Reaction Mechanism. Chem. Eng. J. 2020, 401, 126158. [Google Scholar] [CrossRef]
- De Rosa, S.; Giordano, G.; Granato, T.; Katovic, A.; Siciliano, A.; Tripicchio, F. Chemical Pretreatment of Olive Oil Mill Wastewater Using a Metal-Organic Framework Catalyst. J. Agric. Food Chem. 2005, 53, 8306–8309. [Google Scholar] [CrossRef]
- Zorainy, M.Y.; Sheashea, M.; Kaliaguine, S.; Gobara, M.; Bo, D.C. Facile Solvothermal Synthesis of a MIL-47 (V) Metal–Organic Framework for a High-Performance Epoxy/MOF Coating with Improved Anticorrosion Properties. RSC Adv. 2022, 12, 9008–9022. [Google Scholar] [CrossRef]
- Pasandideh, Y.; Razmi, H. Introduction of a Zn-Based Metal–Organic Framework @ Biomass Porous Activated Carbon as a High-Sensitive Coating for a Stainless Steel SPME Fiber: Application to the Simultaneous Analysis of Nonsteroidal Anti-Inflammatory Drugs. BMC Chem. 2022, 16, 25. [Google Scholar] [CrossRef]
- El Taher, B.J.; Sabouni, R.; Ghommem, M. Luminescent Metal Organic Framework for Selective Detection of Mercury in Aqueous Media: Microwave-Based Synthesis and Evaluation. Colloids Surf. A Physicochem. Eng. Asp. 2020, 607, 125477. [Google Scholar] [CrossRef]
- Zhong, Q.; Xu, C.; Liu, Y.; Ji, Q.; Xu, Z.; Sun, D.; Zhou, S.; Yang, B.; Dai, Y.; Qi, C.; et al. Defect-Engineered FeSe2−x@C with Porous Architecture for Enhanced Peroxymonosulfate-Based Advanced Oxidation Processes. Appl. Catal. B Environ. 2022, 309, 121259. [Google Scholar] [CrossRef]
- Kampouraki, Z.C.; Giannakoudakis, D.A.; Nair, V.; Hosseini-Bandegharaei, A.; Colmenares, J.C.; Deliyanni, E.A. Metal Organic Frameworks as Desulfurization Adsorbents of DBT and 4,6-DMDBT from Fuels. Molecules 2019, 24, 4525. [Google Scholar] [CrossRef]
- Pi, Y.; Li, X.; Xia, Q.; Wu, J.; Li, Y.; Xiao, J.; Li, Z. Adsorptive and Photocatalytic Removal of Persistent Organic Pollutants (POPs) in Water by Metal-Organic Frameworks (MOFs). Chem. Eng. J. 2018, 337, 351–371. [Google Scholar] [CrossRef]
- Hou, J.; Wan, J.; Yan, Z.; Wang, Y.; Ma, Y.; Xie, Y.; Chen, H.; Xue, Y. A Novel Polydopamine-Modified Metal Organic Frameworks Catalyst with Enhanced Catalytic Performance for Efficient Degradation of Sulfamethoxazole in Wastewater. Chemosphere 2022, 297, 134100. [Google Scholar] [CrossRef]
- Li, J.; Zhu, W.; Gao, Y.; Lin, P.; Liu, J.; Zhang, J.; Huang, T. The Catalyst Derived from the Sulfurized Co-Doped Metal–Organic Framework (MOF) for Peroxymonosulfate (PMS) Activation and Its Application to Pollutant Removal. Sep. Purif. Technol. 2022, 285, 120362. [Google Scholar] [CrossRef]
- Singh, S.; Numan, A.; Cinti, S. Point-of-Care for Evaluating Antimicrobial Resistance through the Adoption of Functional Materials. Anal. Chem. 2022, 94, 26–40. [Google Scholar] [CrossRef]
- Zhao, F.; Fang, S.; Gao, Y.; Bi, J. Removal of Aqueous Pharmaceuticals by Magnetically Functionalized Zr-MOFs: Adsorption Kinetics, Isotherms, and Regeneration. J. Colloid Interface Sci. 2022, 615, 876–886. [Google Scholar] [CrossRef]
- Yadav, A.; Bagotia, N.; Sharma, A.K.; Kumar, S. Advances in Decontamination of Wastewater Using Biomass-Based composites: A Critical Review. Sci. Total Environ. 2021, 784, 147108. [Google Scholar] [CrossRef]
- Yuan, S.; Feng, L.; Wang, K.; Pang, J.; Bosch, M.; Lollar, C.; Sun, Y.; Qin, J.; Yang, X.; Zhang, P.; et al. Stable Metal–Organic Frameworks: Design, Synthesis, and Applications. Adv. Mater. 2018, 30, 1704303. [Google Scholar] [CrossRef]
- The Cambridge Crystallographic Data Center (CCDC) CSD MOF Collection. Available online: https://www.ccdc.cam.ac.uk/Community/csd-community/csd-mof-collection/ (accessed on 8 November 2021).
- Li, Y.; Jing, X.; Li, Q.; Shen, Y.; Fang, Q. Well-Defined Bimetal Oxides Derived from Prussian Blue Analogues with Regulable Active Sites for Phosphate Removal. J. Colloid Interface Sci. 2022, 622, 390–401. [Google Scholar] [CrossRef]
- Hariri, R.; Dehghanpour, S. Adsorptive Removal and Visible-Light Photocatalytic Degradation of Large Cationic and Anionic Dyes Induced by Air-Bubbles in the Presence of a Magnetic Porphyrinic Metal-Organic Framework (Fe3O4@SiO2@PCN-222(Fe)). J. Phys. Chem. Solids 2021, 155, 110126. [Google Scholar] [CrossRef]
- Kirchon, A.; Zhang, P.; Li, J.; Joseph, E.A.; Chen, W.; Zhou, H.C. Effect of Isomorphic Metal Substitution on the Fenton and Photo-Fenton Degradation of Methylene Blue Using Fe-Based Metal-Organic Frameworks. ACS Appl. Mater. Interfaces 2020, 12, 9292–9299. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hou, S.; Liu, D.; Zhong, C. Effective Adsorption of Cefradine from Wastewater with a Stable Zirconium Metal–Organic Framework. Ind. Eng. Chem. Res. 2018, 57, 15132–15137. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, H.; Dai, W.; Wei, Y.; Wang, Y.; Zhang, Y.; Zhi, L.; Huang, H.; Gao, Z. A Metal-Organic Framework with Large 1-D Channels and Rich OH Sites for High-Efficiency Chloramphenicol Removal from Water. J. Colloid Interface Sci. 2018, 526, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Shan, Q.; Fang, Y.; Zhao, N.; Feng, X. Shape-Controlled Mn–Fe PBA Derived Micromotors for Organic Pollutant Removal. New J. Chem. 2022, 46, 8611–8618. [Google Scholar] [CrossRef]
- Khan, N.A.; Najam, T.; Shah, S.S.A.; Hussain, E.; Ali, H.; Hussain, S.; Shaheen, A.; Ahmad, K.; Ashfaq, M. Development of Mn-PBA on GO Sheets for Adsorptive Removal of Ciprofloxacin from Water: Kinetics, Isothermal, Thermodynamic and Mechanistic Studies. Mater. Chem. Phys. 2020, 245, 122737. [Google Scholar] [CrossRef]
- Yang, S.; Li, X.; Zeng, G.; Cheng, M.; Huang, D.; Liu, Y.; Zhou, C.; Xiong, W.; Yang, Y.; Wang, W.; et al. Materials Institute Lavoisier (MIL) Based Materials for Photocatalytic Applications. Coord. Chem. Rev. 2021, 438, 213874. [Google Scholar] [CrossRef]
- Ning, R.; Pang, H.; Yan, Z.; Lu, Z.; Wang, Q.; Wu, Z.; Dai, W.; Liu, L.; Li, Z.; Fan, G.; et al. An Innovative S-Scheme AgCl/MIL-100 (Fe) Heterojunction for Visible-Light-Driven Degradation of Sulfamethazine and Mechanism Insight. J. Hazard. Mater. 2022, 435, 129061. [Google Scholar] [CrossRef]
- Tong, J.; Chen, L.; Cao, J.; Yang, Z.; Xiong, W.; Jia, M.; Xiang, Y.; Peng, H. Biochar Supported Magnetic MIL-53-Fe Derivatives as an Efficient Catalyst for Peroxydisulfate Activation towards Antibiotics Degradation. Sep. Purif. Technol. 2022, 294, 121064. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, C.; Li, D.; Liu, Y. In Situ-Generated H2O2 with NCQDs/MIL-101(Fe) by Activating O2: A Dual Effect of Photocatalysis and Photo-Fenton for Efficient Removal of Tetracyline at Natural PH. Appl. Surf. Sci. 2022, 592, 153312. [Google Scholar] [CrossRef]
- Zhou, Q.; Liu, G. Urea-Functionalized MIL-101(Cr)@AC as a New Adsorbent to Remove Sulfacetamide in Wastewater Treatment. Ind. Eng. Chem. Res. 2020, 59, 12056–12064. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, X.; Bi, F.; Zhang, X.; Yang, Y.; Wang, Y. A Facile Synthesis for Uniform Tablet-like TiO2/C Derived from Materials of Institut Lavoisier-125(Ti) (MIL-125(Ti)) and Their Enhanced Visible Light-Driven Photodegradation of Tetracycline. J. Colloid Interface Sci. 2020, 571, 275–284. [Google Scholar] [CrossRef]
- Hu, Q.; Dong, J.; Chen, Y.; Yi, J.; Xia, J.; Yin, S.; Li, H. In-Situ Construction of Bifunctional MIL-125(Ti)/BiOI Reactive Adsorbent/Photocatalyst with Enhanced Removal Efficiency of Organic Contaminants. Appl. Surf. Sci. 2022, 583, 152423. [Google Scholar] [CrossRef]
- Chatterjee, A.; Jana, A.K.; Basu, J.K. A Novel Synthesis of MIL-53(Al)@SiO2: An Integrated Photocatalyst Adsorbent to Remove Bisphenol a from Wastewater. New J. Chem. 2020, 44, 18892–18905. [Google Scholar] [CrossRef]
- Li, G.; Zhao, H.; Guo, P.; Liu, D. Effective Removal of Tinidazole by MIL-53(Al)-NDC Metal-Organic Framework from Aqueous Solution. J. Solid State Chem. 2022, 310, 123066. [Google Scholar] [CrossRef]
- Yu, J.; Cao, J.; Yang, Z.; Xiong, W.; Xu, Z.; Song, P.; Jia, M.; Sun, S.; Zhang, Y.; Zhu, J. One-Step Synthesis of Mn-Doped MIL-53(Fe) for Synergistically Enhanced Generation of Sulfate Radicals towards Tetracycline Degradation. J. Colloid Interface Sci. 2020, 580, 470–479. [Google Scholar] [CrossRef]
- Bergaoui, M.; Khalfaoui, M.; Awadallah-F, A.; Al-Muhtaseb, S. A Review of the Features and Applications of ZIF-8 and Its Derivatives for Separating CO2 and Isomers of C3- and C4- Hydrocarbons. J. Nat. Gas Sci. Eng. 2021, 96, 104289. [Google Scholar] [CrossRef]
- Wang, H.; Pei, X.; Kalmutzki, M.J.; Yang, J.; Yaghi, O.M. Large Cages of Zeolitic Imidazolate Frameworks. Acc. Chem. Res. 2022, 55, 707–721. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Lv, Z.; Xie, Y.; Shu, J.; Alsaedi, A.; Hayat, T.; Chen, C. Exploration of the Adsorption Performance and Mechanism of Zeolitic Imidazolate Framework-8@graphene Oxide for Pb(II) and 1-Naphthylamine from Aqueous Solution. J. Colloid Interface Sci. 2019, 542, 410–420. [Google Scholar] [CrossRef]
- Xue, W.; Zhou, Q.; Li, F.; Ondon, B.S. Zeolitic Imidazolate Framework-8 (ZIF-8) as Robust Catalyst for Oxygen Reduction Reaction in Microbial Fuel Cells. J. Power Sources 2019, 423, 9–17. [Google Scholar] [CrossRef]
- Shafiq, S.; Al-Maythalony, B.A.; Usman, M.; Ba-Shammakh, M.S.; Al-Shammari, A.A. ZIF-95 as a Filler for Enhanced Gas Separation Performance of Polysulfone Membrane. RSC Adv. 2021, 11, 34319–34328. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Oveisi, M.; Bakhtiari, M.; Hayati, B.; Shekarchi, A.A.; Bagheri, A.; Rahimi, S. Environmentally Friendly Ultrasound-Assisted Synthesis of Magnetic Zeolitic Imidazolate Framework-Graphene Oxide Nanocomposites and Pollutant Removal from Water. J. Mol. Liq. 2019, 282, 115–130. [Google Scholar] [CrossRef]
- Wang, Y.; Dai, X.; Zhan, Y.; Ding, X.; Wang, M.; Wang, X. In Situ Growth of ZIF-8 Nanoparticles on Chitosan to Form the Hybrid Nanocomposites for High-Efficiency Removal of Congo Red. Int. J. Biol. Macromol. 2019, 137, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Chen, C.; Wang, X.; Hu, K.; Xu, Z. ZIF-8 Prepared in Ionic Liquid Microemulsions for Efficient Capture of Phosphate from Water. J. Chem. Sci. 2022, 134, 62. [Google Scholar] [CrossRef]
- Miao, M.; Mu, L.; Cao, S.; Yang, Y.; Feng, X. Dual-Functional CDs@ZIF-8/Chitosan Luminescent Film Sensors for Simultaneous Detection and Adsorption of Tetracycline. Carbohydr. Polym. 2022, 291, 119587. [Google Scholar] [CrossRef]
- Liu, S.; Ning, Y.; Qi, X.; Zhao, J.; Fu, Y.; Zhang, B.; Gao, J.; Miao, J.; Song, J.; Huo, Q. CdS-Modified ZIF-8-Derived Porous Carbon for Organic Pollutant Degradations under Visible-Light Irradiation. Res. Chem. Intermed. 2021, 47, 4193–4211. [Google Scholar] [CrossRef]
- Fan, L.; Gong, Y.; Wan, J.; Wei, Y.; Shi, H.; Liu, C. Flower-like Molybdenum Disulfide Decorated ZIF-8-Derived Nitrogen-Doped Dodecahedral Carbon for Electro-Catalytic Degradation of Phenol. Chemosphere 2022, 298, 134315. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Thai, V.A.; Chen, C.W.; Huang, C.P.; Doong, R.; Chen, L.; Dong, C. Di N-Doping Modified Zeolitic Imidazole Framework-67 (ZIF-67) for Enhanced Peroxymonosulfate Activation to Remove Ciprofloxacin from Aqueous Solution. Sep. Purif. Technol. 2022, 288, 120719. [Google Scholar] [CrossRef]
- Chen, G.; He, S.; Shi, G.; Ma, Y.; Ruan, C.; Jin, X.; Chen, Q.; Liu, X.; Dai, H.; Chen, X.; et al. In-Situ Immobilization of ZIF-67 on Wood Aerogel for Effective Removal of Tetracycline from Water. Chem. Eng. J. 2021, 423, 130184. [Google Scholar] [CrossRef]
- Ren, W.; Gao, J.; Lei, C.; Xie, Y.; Cai, Y.; Ni, Q.; Yao, J. Recyclable Metal-Organic Framework/Cellulose Aerogels for Activating Peroxymonosulfate to Degrade Organic Pollutants. Chem. Eng. J. 2018, 349, 766–774. [Google Scholar] [CrossRef]
- Li, N.; Zhou, L.; Jin, X.; Owens, G.; Chen, Z. Simultaneous Removal of Tetracycline and Oxytetracycline Antibiotics from Wastewater Using a ZIF-8 Metal Organic-Framework. J. Hazard. Mater. 2019, 366, 563–572. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, X.; Mei, Y.; Wang, D.; Ji, C. ZnCDs/ZnO@ZIF-8 Zeolite Composites for the Photocatalytic Degradation of Tetracycline. Catalysts 2021, 11, 934. [Google Scholar] [CrossRef]
- Wu, J.; Jin, Y.; Wu, D.; Yan, X.; Ma, N.; Dai, W. Well-Construction of Zn2SnO4/SnO2@ZIF-8 Core-Shell Hetero-Structure with Efficient Photocatalytic Activity towards Tetracycline under Restricted Space. Chin. J. Chem. Eng. 2022; in press. [Google Scholar] [CrossRef]
- Liu, N.; Shang, Q.; Gao, K.; Cheng, Q.; Pan, Z. Construction of ZnO/ZIF-9 Heterojunction Photocatalyst: Enhanced Photocatalytic Performance and Mechanistic Insight. New J. Chem. 2020, 44, 6384–6393. [Google Scholar] [CrossRef]
- Ahmadi, S.A.R.; Kalaee, M.R.; Moradi, O.; Nosratinia, F.; Abdouss, M. Synthesis of Novel Zeolitic Imidazolate Framework (ZIF-67)—Zinc Oxide (ZnO) Nanocomposite (ZnO@ZIF-67) and Potential Adsorption of Pharmaceutical (Tetracycline (TCC)) from Water. J. Mol. Struct. 2022, 1251, 132013. [Google Scholar] [CrossRef]
- Ru, J.; Wang, X.; Wang, F.; Cui, X.; Du, X.; Lu, X. UiO Series of Metal-Organic Frameworks Composites as Advanced Sorbents for the Removal of Heavy Metal Ions: Synthesis, Applications and Adsorption Mechanism. Ecotoxicol. Environ. Saf. 2021, 208, 111577. [Google Scholar] [CrossRef]
- Yuan, N.; Gong, X.; Sun, W.; Yu, C. Chemosphere Advanced Applications of Zr-Based MOFs in the Removal of Water Pollutants. Chemosphere 2021, 267, 128863. [Google Scholar] [CrossRef]
- Zhuang, S.; Cheng, R.; Wang, J. Adsorption of Diclofenac from Aqueous Solution Using UiO-66-Type Metal- Organic Frameworks. Chem. Eng. J. 2019, 359, 354–362. [Google Scholar] [CrossRef]
- He, X.; Deng, F.; Shen, T.; Yang, L.; Chen, D.; Luo, J.; Luo, X.; Min, X.; Wang, F. Journal of Colloid and Interface Science Exceptional Adsorption of Arsenic by Zirconium Metal-Organic Frameworks: Engineering Exploration and Mechanism Insight. J. Colloid Interface Sci. 2019, 539, 223–234. [Google Scholar] [CrossRef]
- Hu, D.; Song, X.; Wu, S.; Yang, X.; Zhang, H.; Chang, X.; Jia, M. Solvothermal Synthesis of Co-Substituted Phosphomolybdate Acid Encapsulated in the UiO-66 Framework for Catalytic Application in Olefin Epoxidation. Chin. J. Catal. 2021, 42, 356–366. [Google Scholar] [CrossRef]
- Abdelmoaty, A.S.; El, S.T.; Nady, W.; Adly, F. High Performance of UiO-66 Metal—Organic Framework Modified with Melamine for Uptaking of Lead and Cadmium from Aqueous Solutions. J. Inorg. Organomet. Polym. Mater. 2022, 32, 2557–2567. [Google Scholar] [CrossRef]
- Sena, Ş.; Selin, Ş. Acid-Modulated Zirconium Based Metal Organic Frameworks for Removal of Organic Micropollutants. J. Environ. Chem. Eng. 2020, 8, 103901. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, Y.; Wang, J.; Liu, F.; Hu, N.; Pei, H.; Yang, W.; Li, Z.; Suo, Y.; Wang, J. High Effective Adsorption/Removal of Illegal Food Dyes from Contaminated Aqueous Solution by Zr-MOFs (UiO-67). Food Chem. 2018, 254, 241–248. [Google Scholar] [CrossRef]
- Dong, X.; Lin, Y.; Ma, Y.; Zhao, L. Ce-Doped UiO-67 Nanocrystals with Improved Adsorption Property for Removal of Organic Dyes. RSC Adv. 2019, 9, 27674–27683. [Google Scholar] [CrossRef]
- Safaralizadeh, E.; Mahjoub, A.R.; Fazlali, F.; Bagheri, H. Facile Construction of C3N4-TE@TiO2/UiO-66 with Double Z-Scheme Structure as High Performance Photocatalyst for Degradation of Tetracycline. Ceram. Int. 2021, 47, 2374–2387. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, N.; Gan, C.; Liu, Y.; Chen, L.; Zhang, C. Materials Science in Semiconductor Processing Synthesis of In2S3/UiO-66 Hybrid with Enhanced Photocatalytic Activity towards Methyl Orange and Tetracycline Hydrochloride Degradation under Visible-Light Irradiation. Mater. Sci. Semicond. Process. 2019, 91, 212–221. [Google Scholar] [CrossRef]
- Sun, J.; Feng, S.; Feng, S. Hydrothermally Synthesis of MWCNT/N-TiO2/UiO-66-NH2 Ternary Composite with Enhanced Photocatalytic Performance for Ketoprofen. Inorg. Chem. Commun. 2020, 111, 107669. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, C.; Wang, C.; Hu, Q.; Ding, L. 0D/3D NiCo2O4/Defected UiO-66 Catalysts for Enhanced Degradation of Tetracycline in Peroxymonosulfate/Simulated Sunlight Systems: Degradation Mechanisms and Pathways. Chemosphere 2022, 299, 134322. [Google Scholar] [CrossRef] [PubMed]
- Uba, Z.; Ramli, A.; Jumbri, K.; Soraya, N.; Ahmad, H.; Hana, N.; Abu, H.; Saad, B. Optimization Studies and Artificial Neural Network Modeling for Pyrene Adsorption onto UiO-66 (Zr) and NH2 -UiO-66 (Zr) Metal Organic Frameworks. Polyhedron 2020, 192, 114857. [Google Scholar] [CrossRef]
- Gotthardt, M.A.; Schoch, R.; Wolf, S.; Bauer, M.; Kleist, W. Synthesis and Characterization of Bimetallic Metal–Organic Framework Cu–Ru-BTC with HKUST-1 Structure. Dalton Trans. 2015, 44, 2052–2056. [Google Scholar] [CrossRef]
- Guo, L.; Du, J.; Li, C.; He, G.; Xiao, Y. Facile Synthesis of Hierarchical Micro-Mesoporous HKUST-1 by a Mixed-Linker Defect Strategy for Enhanced Adsorptive Removal of Benzothiophene from Fuel. Fuel 2021, 300, 120955. [Google Scholar] [CrossRef]
- Nobar, S.N. Cu-BTC Synthesis, Characterization and Preparation for Adsorption Studies. Mater. Chem. Phys. 2018, 213, 343–351. [Google Scholar] [CrossRef]
- Qiu, S.; Du, J.; Xiao, Y.; Zhao, Q.; He, G. Hierarchical Porous HKUST-1 Fabricated by Microwave-Assisted Synthesis with CTAB for Enhanced Adsorptive Removal of Benzothiophene from Fuel. Sep. Purif. Technol. 2021, 271, 118868. [Google Scholar] [CrossRef]
- Forsyth, C.; Taras, T.; Johnson, A.; Zagari, J.; Collado, C.; Hoffmann, M.M.; Reed, C.R. Microwave Assisted Surfactant-Thermal Synthesis of Metal-Organic Framework Materials. Appl. Sci. 2020, 10, 4563. [Google Scholar] [CrossRef]
- Zhang, J.; Su, C.; Xie, X.; Liu, P.; Huq, M.E. Enhanced Visible Light Photocatalytic Degradation of Dyes in Aqueous Solution Activated by HKUST-1: Performance and Mechanism. RSC Adv. 2020, 10, 37028–37034. [Google Scholar] [CrossRef]
- Pan, J.; Bai, X.; Li, Y.; Yang, B.; Yang, P.; Yu, F.; Ma, J. HKUST-1 Derived Carbon Adsorbents for Tetracycline Removal with Excellent Adsorption Performance. Environ. Res. 2022, 205, 112425. [Google Scholar] [CrossRef]
- Wu, Y.; Li, X.; Zhao, H.; Yao, F.; Cao, J.; Chen, Z. Core-Shell Structured Cu2O@ HKUST-1 Heterojunction Photocatalyst with Robust Stability for Highly Efficient Tetracycline Hydrochloride Degradation under Visible Light. Chem. Eng. J. 2021, 426, 131255. [Google Scholar] [CrossRef]
- Wu, G.; Ma, J.; Li, S.; Guan, J.; Jiang, B.; Wang, L.; Li, J.; Wang, X.; Chen, L. Magnetic Copper-Based Metal Organic Framework as an Effective and Recyclable Adsorbent for Removal of Two Fluoroquinolone Antibiotics from Aqueous Solutions. J. Colloid Interface Sci. 2018, 528, 360–371. [Google Scholar] [CrossRef]
- Yang, S.; Tang, R.; Dai, Y.; Wang, T.; Zeng, Z.; Zhang, L. Fabrication of Cellulose Acetate Membrane with Advanced Ultrafiltration Performances and Antibacterial Properties by Blending With. Sep. Purif. Technol. 2021, 279, 119524. [Google Scholar] [CrossRef]
- Bai, K.; Fan, S.; Chen, Y.; Wang, Y.; Chen, J.; Mai, Z.; Liu, J.; Deng, L. Membrane Adsorber with Hierarchically Porous HKUST-1 Immobilized in Membrane Pores by Flowing Synthesis. J. Membr. Sci. 2022, 650, 120424. [Google Scholar] [CrossRef]
- Seetharaj, R.; Vandana, P.V.; Arya, P.; Mathew, S. Dependence of Solvents, PH, Molar Ratio and Temperature in Tuning Metal Organic Framework Architecture. Arab. J. Chem. 2019, 12, 295–315. [Google Scholar] [CrossRef]
- Sharanyakanth, P.S.; Radhakrishnan, M. Synthesis of Metal-Organic Frameworks (MOFs) and Its Application in Food Packaging: A Critical Review. Trends Food Sci. Technol. 2020, 104, 102–116. [Google Scholar] [CrossRef]
- Joseph, J.; Iftekhar, S.; Srivastava, V.; Fallah, Z.; Zare, E.N.; Sillanpää, M. Iron-Based Metal-Organic Framework: Synthesis, Structure and Current Technologies for Water Reclamation with Deep Insight into Framework Integrity. Chemosphere 2021, 284, 131171. [Google Scholar] [CrossRef]
- Rocío-Bautista, P.; Taima-Mancera, I.; Pasán, J.; Pino, V. Metal-Organic Frameworks in Green Analytical Chemistry. Separations 2019, 6, 33. [Google Scholar] [CrossRef]
- Kumar, S.; Jain, S.; Nehra, M.; Dilbaghi, N.; Marrazza, G.; Kim, K. Green Synthesis of Metal—Organic Frameworks: A State-of-the-Art Review of Potential Environmental and Medical Applications. Coord. Chem. Rev. 2020, 420, 213407. [Google Scholar] [CrossRef]
- Nunes, D.; Pimentel, A.; Santos, L.; Barquinha, P.; Pereira, L.; Fortunato, E.; Martins, R. Synthesis, Design, and Morphology of Metal Oxide Nanostructures. In Metal Oxides Nanostructures; Nunes, D., Pimentel, A., Santos, L., Pereira, L., Fortunato, E., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 21–57. [Google Scholar]
- Kashyap, A.; Singh, N.K.; Soni, M.; Soni, A. Deposition of Thin Films by Chemical Solution-Assisted Techniques. In Chemical Solution Synthesis for Materials Design and Thin Film Device Applications; Das, S., Dhara, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 79–117. [Google Scholar]
- Atikah, N.; Azuwa, M.; Nur, M.; Salehmin, I. Photocatalytic Active Metal—Organic Framework and Its Derivatives for Solar-Driven Environmental Remediation and Renewable Energy. Coord. Chem. Rev. 2022, 468, 214639. [Google Scholar] [CrossRef]
- Ghawade, S.P.; Pande, K.N.; Dhoble, S.J.; Deshmukh, A.D. Tuning the Properties of ZnS Semiconductor by the Addition of Graphene. In Woodhead Publishing Series in Electronic and Optical Materials; Pawade, V.B., Dhoble, S.J., Swart, H.C., Eds.; Woodhead Publishing: Suston, UK, 2022; pp. 351–381. [Google Scholar]
- Mohammad, N.; Abdi, J.; Oveisi, M.; Alinia, M. Metal-Organic Framework (MIL-100 (Fe)): Synthesis, Detailed Photocatalytic Dye Degradation Ability in Colored Textile Wastewater and Recycling. Mater. Res. Bull. 2018, 100, 357–366. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, C.; Zhang, Z.; Chen, Y.; Deng, W.; Chen, W. Bimetallic Fe/Co-MOFs for Tetracycline Elimination. J. Mater. Sci. 2021, 56, 15684–15697. [Google Scholar] [CrossRef]
- Dahiya, M.S.; Tomer, V.K.; Duhan, S. Metal–Ferrite Nanocomposites for Targeted Drug Delivery. In Woodhead Publishing Series in Biomaterials; Inamuddin, A., Mohammad, A., Eds.; Woodhead Publishing: Suston, UK, 2018; pp. 737–760. [Google Scholar]
- Zhang, P.; Wang, Q.; Fang, Y.; Chen, W.; Kirchon, A.A.; Baci, M.; Feng, M.; Sharma, V.K.; Zhou, H. Metal-Organic Frameworks for Capture and Degradation of Organic Pollutants. In Metal-Organic Frameworks (MOFs) for Environmental Applications; Ghosh, S.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 203–229. [Google Scholar]
- Zhao, Z.; Li, H.; Zhao, K.; Wang, L.; Gao, X. Microwave-Assisted Synthesis of MOFs: Rational Design via Numerical Simulation. Chem. Eng. J. 2022, 428, 131006. [Google Scholar] [CrossRef]
- Kumar, A.; Kuang, Y.; Liang, Z.; Sun, X. Materials Today Nano Microwave Chemistry, Recent Advancements, and Eco-Friendly Microwave-Assisted Synthesis of Nanoarchitectures and Their Applications: A Review. Mater. Today Nano 2020, 11, 100076. [Google Scholar] [CrossRef]
- Jiang, D.; Fang, D.; Zhou, Y.; Wang, Z.; Yang, Z.; Zhu, J. Strategies for Improving the Catalytic Activity of Metal-Organic Frameworks and Derivatives in SR-AOPs: Facing Emerging Environmental Pollutants. Environ. Pollut. 2022, 306, 119386. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Amira, M.F.; Seleim, S.M.; Mohamed, A.K. Amino-Decorated Magnetic Metal-Organic Framework as a Potential Novel Platform for Selective Removal of Chromium (Vl), Cadmium (II) and Lead (II). J. Hazard. Mater. 2020, 381, 120979. [Google Scholar] [CrossRef]
- He, J.; Zhang, Y.; Zhang, X.; Huang, Y. Highly Efficient Fenton and Enzyme-Mimetic Activities of NH2-MIL-88B(Fe) Metal Organic Framework for Methylene Blue Degradation. Sci. Rep. 2018, 8, 5159. [Google Scholar] [CrossRef] [PubMed]
- Arenas-Vivo, A.; Avila, D.; Horcajada, P. Phase-Selective Microwave Assisted Synthesis of Iron(III) Aminoterephthalate MOFs. Materials 2020, 13, 1469. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Nie, M.; Yang, L.; Wei, F.; Ding, B.; Chen, H.; Liu, Z.; Liang, Z. Synthesis of MOFs for RhB Adsorption from Wastewater. Inorganics 2022, 10, 27. [Google Scholar] [CrossRef]
- Chang, F.; Memon, N.; Memon, S.; Chang, A.S. Removal of Emerging Contaminants from Water by Using Fe-MOF Composite as a Sorbent. J. Iran. Chem. Soc. 2021, 18, 3249–3255. [Google Scholar] [CrossRef]
- Ngan Tran, T.K.; Ho, H.L.; Nguyen, H.V.; Tran, B.T.; Nguyen, T.T.; Thi Bui, P.Q.; Bach, L.G. Photocatalytic Degradation of Rhodamine B in Aqueous Phase by Bimetallic Metal-Organic Framework M/Fe-MOF (M=Co, Cu, and Mg). Open Chem. 2022, 20, 52–60. [Google Scholar] [CrossRef]
- Ploychompoo, S.; Chen, J.; Luo, H.; Liang, Q. Fast and Efficient Aqueous Arsenic Removal by Composites: Adsorption Performance and Mechanism. J. Environ. Sci. 2020, 91, 22–34. [Google Scholar] [CrossRef]
- Xiong, P.; Zhang, H.; Li, G.; Liao, C.; Jiang, G. Adsorption Removal of Ibuprofen and Naproxen from Aqueous Solution with Cu-Doped Mil-101 (Fe). Sci. Total Environ. 2021, 797, 149179. [Google Scholar] [CrossRef]
- Li, X.; Chen, X.; Lv, Z.; Wang, B. Ultrahigh Ciprofloxacin Accumulation and Visible-Light Photocatalytic Degradation: Contribution of Metal Organic Frameworks Carrier in Magnetic Surface Molecularly Imprinted Polymers. J. Colloid Interface Sci. 2022, 616, 872–885. [Google Scholar] [CrossRef]
- Tabuyo-martínez, M.; Cascos, V.; María, M.; Dur, J.; Avila-brande, D.; Prado-gonjal, J. Microwave-Assisted Synthesis of Thermoelectric Oxides and Chalcogenides. Ceram. Int. 2022, 48, 12331–12341. [Google Scholar] [CrossRef]
- Nguyen, H.T.T.; Tran, K.N.T.; Van Tan, L.; Tran, V.A.; Doan, V.D.; Lee, T.; Nguyen, T.D. Microwave-Assisted Solvothermal Synthesis of Bimetallic Metal-Organic Framework for Efficient Photodegradation of Organic Dyes. Mater. Chem. Phys. 2021, 272, 125040. [Google Scholar] [CrossRef]
- Abdelkareem, M.A.; Abbas, Q.; Mouselly, M.; Alawadhi, H.; Olabi, A.G. High-Performance Effective Metal–Organic Frameworks for Electrochemical Applications. J. Sci. Adv. Mater. Devices 2022, 7, 100465. [Google Scholar] [CrossRef]
- Gascon, J. Control of Interpenetration of Copper-Based MOFs on Supported Surfaces by Electrochemical Synthesis. CrystEngComm 2016, 18, 4018–4022. [Google Scholar] [CrossRef]
- Anumah, A.; Louis, H.; Hamzat, A.T.; Amusan, O.O. Metal-Organic Frameworks ( MOFs ): Recent Advances in Synthetic Methodologies and Some Applications. Chem. Methodol. 2018, 3, 283–305. [Google Scholar] [CrossRef]
- Varsha, M.V.; Nageswaran, G. Review—Direct Electrochemical Synthesis of Metal Organic Frameworks Review—Direct Electrochemical Synthesis of Metal Organic Frameworks. J. Electrochem. Soc. 2020, 167, 155527. [Google Scholar] [CrossRef]
- Ghoorchian, A.; Afkhami, A.; Madrakian, T.; Ahmadi, M. Electrochemical Synthesis of MOFs. In Metal-Organic Frameworks for Biomedical Applications; Mozafari, M., Ed.; Woodhead Publishing: Suston, UK, 2020; pp. 177–195. [Google Scholar]
- Al-kutubi, H.; Gascon, J.; Sudhçlter, E.J.R.; Rassaei, L. Electrosynthesis of Metal–Organic Frameworks: Challenges and Opportunities. ChemElectroChem 2015, 2, 462–474. [Google Scholar] [CrossRef]
- Dong, Y.; Hu, T.; Pudukudy, M.; Su, H.; Jiang, L. Influence of Microwave-Assisted Synthesis on the Structural and Textural Properties of Mesoporous MIL-101 (Fe) and NH2-MIL-101 (Fe) for Enhanced Tetracycline Adsorption. Mater. Chem. Phys. 2020, 251, 123060. [Google Scholar] [CrossRef]
- Wei, F.; Chen, D.; Liang, Z.; Zhao, S. Comparison Study on the Adsorption Capacity of Rhodamine B, Congo Red, and Orange II on Fe-MOFs. Nanomaterials 2018, 8, 248. [Google Scholar] [CrossRef]
- Jia, Z.; Hao, S.; Wen, J.; Li, S.; Peng, W.; Huang, R.; Xu, X. Electrochemical Fabrication of Metal–Organic Frameworks Membranes and Films: A Review. Microporous Mesoporous Mater. 2020, 305, 110322. [Google Scholar] [CrossRef]
- Campagnol, N.; Van Assche, T.; Boudewijns, T.; Denayer, J.; Binnemans, K.; De Vos, D.; Fransaer, J. High Pressure, High Temperature Electrochemical Synthesis of Metal–Organic Frameworks: Films of MIL-100 (Fe) and HKUST-1 in Different Morphologies. J. Mater. Chem. A 2013, 1, 5827–5830. [Google Scholar] [CrossRef]
- Wu, W.; Decker, G.E.; Weaver, A.E.; Arnoff, A.I.; Bloch, E.D.; Rosenthal, J. Facile and Rapid Room-Temperature Electrosynthesis and Controlled Surface Growth of Fe-MIL-101 and Fe-MIL-101-NH2. ACS Cent. Sci. 2021, 7, 1427–1433. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, P.; Chen, J.; Dang, X.; Hu, Y.; Ai, Y.; Zheng, D.; Chen, H. One-Step Controlled Electrodeposition of Iron-Based Binary Metal Organic Nanocomposite. Appl. Surf. Sci. 2020, 504, 144504. [Google Scholar] [CrossRef]
- Vaitsis, C.; Sourkouni, G.; Argirusis, C. Metal Organic Frameworks (MOFs) and Ultrasound: A Review. Ultrason. Sonochem. 2019, 52, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, N.; Yaser, M.; Morsali, A.; Junk, P.C.; Wang, J. Sonochemical Synthesis and Structural Characterization of a New Zn (II) Nanoplate Metal–Organic Framework with Removal Efficiency of Sudan Red and Congo Red. Ultrason. Sonochem. 2018, 45, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Mehrabadi, Z.; Farsadrooh, M.; Bafkary, R.; Derikvandi, H.; Hayati, P.; Mohammadi, K. Metal–Organic Framework. In Adsorption: Fundamental Processes and Applications; Ghaedi, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 279–387. [Google Scholar]
- Abdpour, S.; Kowsari, E.; Reza, M.; Moghaddam, A. Synthesis of MIL-100 (Fe)@ MIL-53 (Fe) as a Novel Hybrid Photocatalyst and Evaluation Photocatalytic and Photoelectrochemical Performance under Visible Light Irradiation. J. Solid State Chem. 2018, 262, 172–180. [Google Scholar] [CrossRef]
- Zadeh, M.H.; Keramati, N.; Ghazi, M.M. The Effect of Solvents on Photocatalytic Activity of Fe-BTC Metal Organic Framework Obtained via Sonochemical Method. Inorg. Nano-Metal Chem. 2019, 49, 448–454. [Google Scholar] [CrossRef]
- Taherzade, S.D.; Abbasichaleshtori, M.; Soleimannejad, J. Efficient and Ecofriendly Cellulose-Supported MIL-100(Fe) for Wastewater Treatment. RSC Adv. 2022, 12, 9023–9035. [Google Scholar] [CrossRef] [PubMed]
- Al-attri, R.; Halladj, R.; Askari, S. Green Route of Flexible Al-MOF Synthesis with Superior Properties at Low Energy Consumption Assisted by Ultrasound Waves. Solid State Sci. 2022, 123, 106782. [Google Scholar] [CrossRef]
- Vijayalakshmi, K.; Haq, L.N.U. Microwave-Sonochemical Synergistically Assisted Synthesis of Hybrid Ni-Fe3O4/ZnO Nanocomposite for Enhanced Antibacterial Performance. Mater. Today Commun. 2021, 26, 101835. [Google Scholar] [CrossRef]
- Swetha, S.; Janani, B.; Khan, S.S. A Critical Review on the Development of Metal-Organic Frameworks for Boosting Photocatalysis in the Fields of Energy and Environment. J. Clean. Prod. 2022, 333, 130164. [Google Scholar] [CrossRef]
- Sud, D.; Kaur, G. A Comprehensive Review on Synthetic Approaches for Metal-Organic Frameworks: From Traditional Solvothermal to Greener Protocols. Polyhedron 2021, 193, 114897. [Google Scholar] [CrossRef]
- Beamish-cook, J.; Shankland, K.; Murray, C.A.; Vaqueiro, P. Insights into the Mechanochemical Synthesis of MOF-74. Cryst. Growth Des. 2021, 21, 3047–3055. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, X.; Li, T.; Zhang, Y.; Xu, H.; Sun, Y.; Gu, X.; Gu, C.; Luo, J.; Gao, B. MIL Series of Metal Organic Frameworks (MOFs) as Novel Adsorbents for Heavy Metals in Water: A Review. J. Hazard. Mater. 2022, 429, 128271. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, J.; Zhang, P.; Dai, S. Mechanochemical Synthesis of Metal–Organic Frameworks. Polyhedron 2019, 162, 59–64. [Google Scholar] [CrossRef]
- Sylwia, G.; Szcze, B. Mechanochemistry: Toward Green Synthesis of Metal–Organic Frameworks. Mater. Today 2021, 46, 109–124. [Google Scholar] [CrossRef]
- Pilloni, M.; Padella, F.; Ennas, G.; Lai, S.; Bellusci, M.; Rombi, E.; Sini, F.; Pentimalli, M.; Delitala, C.; Scano, A.; et al. Liquid-Assisted Mechanochemical Synthesis of an Iron Carboxylate Metal Organic Framework and Its Evaluation in Diesel Fuel Desulfurization. Microporous Mesoporous Mater. 2015, 213, 14–21. [Google Scholar] [CrossRef]
- Jeong, H.; Lee, J. 3D-Superstructured Networks Comprising Fe-MIL-88A Metal- Organic Frameworks Under Mechanochemical Conditions. Eur. J. Inorg. Chem. 2019, 42, 4597–4600. [Google Scholar] [CrossRef]
- He, D.; Niu, H.; He, S.; Mao, L.; Cai, Y.; Liang, Y. Strengthened Fenton Degradation of Phenol Catalyzed by Core/Shell Fe e Pd @ C Nanocomposites Derived from Mechanochemically Synthesized Fe-Metal Organic Frameworks. Water Res. 2019, 162, 151–160. [Google Scholar] [CrossRef]
- Liu, X.; Liang, T.; Zhang, R.; Ding, Q.; Wu, S.; Li, C.; Lin, Y.; Ye, Y.; Zhong, Z.; Zhou, M. Iron-Based Metal—Organic Frameworks in Drug Delivery and Biomedicine. ACS Appl. Mater. Interfaces 2021, 13, 9643–9655. [Google Scholar] [CrossRef]
- Ahmed, I.; Jeon, J.; Khan, N.A.; Jhung, S.H. Synthesis of a Metal–Organic Framework, Iron-Benezenetricarboxylate, from Dry Gels in the Absence of Acid and Salt. Cryst. Growth Des. 2012, 12, 5878–5881. [Google Scholar] [CrossRef]
- Luo, Y.; Tan, B.; Liang, X.; Wang, S.; Gao, X.; Zhang, Z.; Fang, Y. Dry Gel Conversion Synthesis of Hierarchical Porous MIL-100 (Fe) and Its Water Vapor Adsorption/Desorption Performance. Ind. Eng. Chem. Res. 2019, 100, 7801–7807. [Google Scholar] [CrossRef]
- Tannert, N.; Gökpinar, S.; Hastürk, E.; Nießing, S.; Janiak, C. Microwave-Assisted Dry-Gel Conversion-a New Sustainable Route for the Rapid Synthesis of Metal–Organic Frameworks with Solvent Re-Use. Dalton Trans. 2018, 47, 9850–9860. [Google Scholar] [CrossRef]
- Troyano, J.; Çamur, C.; Garzón-Tovar, L.; Carné-Sánchez, A.; Imaz, I.; Maspoch, D. Spray-Drying Synthesis of MOFs, COFs, and Related Composites. Acc. Chem. Res. 2020, 53, 1206–1217. [Google Scholar] [CrossRef]
- Garzón-Tovar, L.; Cano-Sarabia, M.; Carné-Sánchez, A.; Carbonell, C.; Imaz, I.; Maspoch, D. A Spray-Drying Continuous-Flow Method for Simultaneous Synthesis and Shaping of Microspherical High Nuclearity MOF Beads. React. Chem. Eng. 2016, 1, 533–539. [Google Scholar] [CrossRef]
- Le, V.N.; Kwon, H.T.; Vo, T.K.; Kim, J.-H.; Kim, W.-S.; Kim, J. Microwave-Assisted Continuous Flow Synthesis of Mesoporous Metal-Organic Framework MIL-100 (Fe) and Its Application to Cu(I)-Loaded Adsorbent for CO/CO2 Separation. Mater. Chem. Phys. 2020, 253, 123278. [Google Scholar] [CrossRef]
- Tao, X.; Yuan, X.; Huang, L. Effects of Fe(II)/Fe(III) of Fe-MOFs on Catalytic Performance in Plasma/Fenton-like System. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 610, 125745. [Google Scholar] [CrossRef]
- Tao, X.; Sun, C.; Han, Y.; Huang, L.; Xu, D. The Plasma Assisted Preparation of Fe-MOFs with High Adsorption Capacity. CrystEngComm 2019, 21, 2541–2550. [Google Scholar] [CrossRef]
- Tao, X.; Yuan, X.; Huang, L.; Shang, S.; Xu, D. Fe-Based Metal-Organic Frameworks as Heterogeneous Catalysts for Highly Efficient Degradation of Wastewater in Plasma/Fenton-like Systems. RSC Adv. 2020, 10, 36363–36370. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Cong, W.; Huang, L.; Xu, D. CeO2 Photocatalysts Derived from Ce-MOFs Synthesized with DBD Plasma Method for Methyl Orange Degradation. J. Alloys Compd. 2019, 805, 1060–1070. [Google Scholar] [CrossRef]
- Tao, X.; Sun, C.; Huang, L.; Han, Y.; Xu, D. Fe-MOFs Prepared with the DBD Plasma Method for Efficient Fenton Catalysis. RSC Adv. 2019, 9, 6379–6386. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Tang, T.; Tao, X.; Huang, L.; Shang, S. Regulating N Content to Anchor Fe in Fe-MOFs: Obtaining Multiple Active Sites as Ef Fi Cient Photocatalysts. J. Taiwan Inst. Chem. Eng. 2022, 132, 104133. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of Advanced Oxidation Processes for Water and Wastewater Treatment–A Critical Review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef]
- Moreira, F.C.; Boaventura, R.A.R.; Brillas, E.; Vilar, V.J.P. Electrochemical Advanced Oxidation Processes: A Review on Their Application to Synthetic and Real Wastewaters. Appl. Catal. B Environ. 2017, 202, 217–261. [Google Scholar] [CrossRef]
- Rodríguez-Narváez, O.M.; Pérez, L.S.; Yee, N.G.; Peralta-Hernández, J.M.; Bandala, E.R. Comparison between Fenton and Fenton-like Reactions for l-Proline Degradation. Int. J. Environ. Sci. Technol. 2019, 16, 1515–1526. [Google Scholar] [CrossRef]
- Li, X.; Jie, B.; Lin, H.; Deng, Z.; Qian, J.; Yang, Y.; Zhang, X. Application of Sulfate Radicals-Based Advanced Oxidation Technology in Degradation of Trace Organic Contaminants (TrOCs): Recent Advances and Prospects. J. Environ. Manag. 2022, 308, 114664. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, S.; Yu, Z.; Zhang, L.; Wang, D.; Pang, B.; Sun, W. Application of Fe-MOFs in Advanced Oxidation Processes. Res. Chem. Intermed. 2019, 45, 3777–3793. [Google Scholar] [CrossRef]
- Psaltou, S.; Kaprara, E.; Mitrakas, M.; Zouboulis, A. Comparative Study on Heterogeneous and Homogeneous Catalytic Ozonation Efficiency in Micropollutants’ Removal. AQUA Water Infrastruct. Ecosyst. Soc. 2021, 70, 1121–1134. [Google Scholar] [CrossRef]
- Li, B.; Wang, Y.F.; Zhang, L.; Xu, H.Y. Enhancement Strategies for Efficient Activation of Persulfate by Heterogeneous Cobalt-Containing Catalysts: A Review. Chemosphere 2022, 291, 132954. [Google Scholar] [CrossRef]
- Lv, H.; Zhao, H.; Cao, T.; Qian, L.; Wang, Y.; Zhao, G. Efficient Degradation of High Concentration Azo-Dye Wastewater by Heterogeneous Fenton Process with Iron-Based Metal-Organic Framework. J. Mol. Catal. A Chem. 2015, 400, 81–89. [Google Scholar] [CrossRef]
- Liao, X.; Wang, F.; Wang, F.; Cai, Y.; Yao, Y.; Teng, B.; Hao, Q. Synthesis of (100) Surface Oriented MIL-88A-Fe with Rod-like Structure and Its Enhanced Fenton-like Performance for Phenol Removal. Appl. Catal. B Environ. 2019, 259, 118064. [Google Scholar] [CrossRef]
- Gao, C.; Chen, S.; Quan, X.; Yu, H.; Zhang, Y. Enhanced Fenton-like Catalysis by Iron-Based Metal Organic Frameworks for Degradation of Organic Pollutants. J. Catal. 2017, 356, 125–132. [Google Scholar] [CrossRef]
- Tang, J.; Wang, J. Fenton-like Degradation of Sulfamethoxazole Using Fe-Based Magnetic Nanoparticles Embedded into Mesoporous Carbon Hybrid as an Efficient Catalyst. Chem. Eng. J. 2018, 351, 1085–1094. [Google Scholar] [CrossRef]
- Tang, J.; Wang, J. Iron-Copper Bimetallic Metal-Organic Frameworks for Efficient Fenton-like Degradation of Sulfamethoxazole under Mild Conditions. Chemosphere 2020, 241, 125002. [Google Scholar] [CrossRef]
- Huang, P.; Yao, L.; Chang, Q.; Sha, Y.; Jiang, G.; Zhang, S.; Li, Z. Room-Temperature Preparation of Highly Efficient NH2-MIL-101(Fe) Catalyst: The Important Role of –NH2 in Accelerating Fe(III)/Fe(II) Cycling. Chemosphere 2022, 291, 133026. [Google Scholar] [CrossRef]
- Pu, M.; Niu, J.; Brusseau, M.L.; Sun, Y.; Zhou, C.; Deng, S. Ferrous Metal-Organic Frameworks with Strong Electron-Donating Properties for Persulfate Activation to Effectively Degrade Aqueous Sulfamethoxazole. Chem. Eng. J. 2020, 394, 125044. [Google Scholar] [CrossRef]
- Pi, Y.; Ma, L.; Zhao, P.; Cao, Y.; Gao, H.; Wang, C.; Li, Q.; Dong, S.; Sun, J. Facile Green Synthetic Graphene-Based Co-Fe Prussian Blue Analogues as an Activator of Peroxymonosulfate for the Degradation of Levofloxacin Hydrochloride. J. Colloid Interface Sci. 2018, 526, 18–27. [Google Scholar] [CrossRef]
- Khan, S.; Sayed, M.; Sohail, M.; Shah, L.A.; Raja, M.A. Advanced Oxidation and Reduction Processes. In Advances in Water Purification Techniques; Ahuja, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 135–164. [Google Scholar]
- Bedia, J.; Muelas-Ramos, V.; Peñas-Garzón, M.; Gómez-Avilés, A.; Rodríguez, J.J.; Belver, C. A Review on the Synthesis and Characterization of Metal Organic Frameworks for Photocatalytic Water Purification. Catalysts 2019, 9, 52. [Google Scholar] [CrossRef]
- Llabrés i Xamena, F.X.; Corma, A.; Garcia, H. Applications for Metal−Organic Frameworks (MOFs) as Quantum Dot Semiconductors. J. Phys. Chem. C 2007, 111, 80–85. [Google Scholar] [CrossRef]
- Boronat, M.; Climent, M.J.; Concepción, P.; Díaz, U.; García, H.; Iborra, S.; Leyva-Pérez, A.; Liu, L.; Martínez, A.; Martínez, C.; et al. A Career in Catalysis: Avelino Corma. ACS Catal. 2022, 12, 7054–7123. [Google Scholar] [CrossRef]
- Navarathna, C.M.; Dewage, N.B.; Karunanayake, A.G.; Farmer, E.L.; Perez, F.; Hassan, E.B.; Mlsna, T.E.; Pittman, C.U. Rhodamine B Adsorptive Removal and Photocatalytic Degradation on MIL-53-Fe MOF/Magnetic Magnetite/Biochar Composites. J. Inorg. Organomet. Polym. Mater. 2020, 30, 214–229. [Google Scholar] [CrossRef]
- Li, Y.; Xia, Y.; Liu, K.; Ye, K.; Wang, Q.; Zhang, S.; Huang, Y. Constructing Fe-MOF-Derived Z-Scheme Photocatalysts with Enhanced Charge Transport: Nanointerface and Carbon Sheath Synergistic Effect. ACS Appl. Mater. Interfaces 2020, 12, 25494–25502. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Chen, S.; Ye, F.; Quan, X.; Afzal, S.; Yu, H.; Zhao, X. Efficient Photo-Fenton Activity in Mesoporous MIL-100(Fe) Decorated with ZnO Nanosphere for Pollutants Degradation. Appl. Catal. B Environ. 2019, 245, 428–438. [Google Scholar] [CrossRef]
- Oladipo, A.A. MIL-53 (Fe)-Based Photo-Sensitive Composite for Degradation of Organochlorinated Herbicide and Enhanced Reduction of Cr(VI). Process Saf. Environ. Prot. 2018, 116, 413–423. [Google Scholar] [CrossRef]
- Panneri, S.; Thomas, M.; Ganguly, P.; Nair, B.N.; Mohamed, A.P.; Warrier, K.G.K.; Hareesh, U.S. C3N4 Anchored ZIF 8 Composites: Photo-Regenerable, High Capacity Sorbents as Adsorptive Photocatalysts for the Effective Removal of Tetracycline from Water. Catal. Sci. Technol. 2017, 7, 2118–2128. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Dong, Y.; Li, H.; Xia, Y.; Wang, H. A Facile Approach for the Synthesis of Z-Scheme Photocatalyst ZIF-8/g-C3N4 with Highly Enhanced Photocatalytic Activity under Simulated Sunlight. New J. Chem. 2018, 42, 12180–12187. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, X.; Song, H.; Chen, C.; Han, F.; Wen, C. Protonated Graphitic Carbon Nitride Coated Metal-Organic Frameworks with Enhanced Visible-Light Photocatalytic Activity for Contaminants Degradation. Appl. Surf. Sci. 2018, 441, 85–98. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Y.; Huang, W.; Yang, Y.; Wang, Y.; He, C.; Liu, N.; Wu, M.; Tang, L. G-C3N4/UiO-66 Nanohybrids with Enhanced Photocatalytic Activities for the Oxidation of Dye under Visible Light Irradiation. Mater. Res. Bull. 2018, 99, 349–358. [Google Scholar] [CrossRef]
- Giannakoudakis, D.A.; Travlou, N.A.; Secor, J.; Bandosz, T.J. Oxidized G-C3N4 Nanospheres as Catalytically Photoactive Linkers in MOF/g-C3N4 Composite of Hierarchical Pore Structure. Small 2017, 13, 1601758. [Google Scholar] [CrossRef]
- Shi, L.; Wang, T.; Zhang, H.; Chang, K.; Ye, J. Electrostatic Self-Assembly of Nanosized Carbon Nitride Nanosheet onto a Zirconium Metal–Organic Framework for Enhanced Photocatalytic CO2 Reduction. Adv. Funct. Mater. 2015, 25, 5360–5367. [Google Scholar] [CrossRef]
- Pan, Y.; Hu, X.; Bao, M.; Li, F.; Li, Y.; Lu, J. Fabrication of MIL-Fe (53)/Modified g-C3N4 Photocatalyst Synergy H2O2 for Degradation of Tetracycline. Sep. Purif. Technol. 2021, 279, 119661. [Google Scholar] [CrossRef]
- Jun, Y.-S.; Lee, E.Z.; Wang, X.; Hong, W.H.; Stucky, G.D.; Thomas, A. From Melamine-Cyanuric Acid Supramolecular Aggregates to Carbon Nitride Hollow Spheres. Adv. Funct. Mater. 2013, 23, 3661–3667. [Google Scholar] [CrossRef]
- Guo, R.; Nengzi, L.-C.; Chen, Y.; Song, Q.; Gou, J.; Cheng, X. Construction of High-Efficient Visible Photoelectrocatalytic System for Carbamazepine Degradation: Kinetics, Degradation Pathway and Mechanism. Chin. Chem. Lett. 2020, 31, 2661–2667. [Google Scholar] [CrossRef]
- Dowd, K.O.; Pillai, S.C. Photo-Fenton Disinfection at near Neutral PH: Process, Parameter Optimization and Recent Advances. J. Environ. Chem. Eng. 2020, 8, 104063. [Google Scholar] [CrossRef]
- Ameta, R.; Chohadia, A.K.; Jain, A.; Punjabi, P.B. Fenton and Photo-Fenton Processes. In Advanced Oxidation Processes for Waste Water Treatment; Ameta, S.C., Ameta, R., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 49–87. [Google Scholar]
- Liu, B.; Wu, Y.; Han, X.; Lv, J.; Zhang, J.; Shi, H. Facile Synthesis of G-C3N4/Amine-Functionalized MIL-101(Fe) Composites with Efficient Photocatalytic Activities under Visible Light Irradiation. J. Mater. Sci. Mater. Electron. 2018, 29, 17591–17601. [Google Scholar] [CrossRef]
- Li, Y.; Fang, Y.; Cao, Z.; Li, N.; Chen, D.; Xu, Q.; Lu, J. Construction of G-C3N4/PDI@MOF Heterojunctions for the Highly Efficient Visible Light-Driven Degradation of Pharmaceutical and Phenolic Micropollutants. Appl. Catal. B Environ. 2019, 250, 150–162. [Google Scholar] [CrossRef]
- Li, X.; Pi, Y.; Wu, L.; Xia, Q.; Wu, J.; Li, Z.; Xiao, J. Facilitation of the Visible Light-Induced Fenton-like Excitation of H2O2 via Heterojunction of g-C3N4/NH2-Iron Terephthalate Metal-Organic Framework for MB Degradation. Appl. Catal. B Environ. 2017, 202, 653–663. [Google Scholar] [CrossRef]
- Wang, J.S.; Yi, X.H.; Xu, X.; Ji, H.; Alanazi, A.M.; Wang, C.C.; Zhao, C.; Kaneti, Y.V.; Wang, P.; Liu, W.; et al. Eliminating Tetracycline Antibiotics Matrix via Photoactivated Sulfate Radical-Based Advanced Oxidation Process over the Immobilized MIL-88A: Batch and Continuous Experiments. Chem. Eng. J. 2022, 431, 133213. [Google Scholar] [CrossRef]
- Yi, X.; Ji, H.; Wang, C.; Li, Y.; Li, Y.; Zhao, C.; Wang, A.; Fu, H.; Wang, P.; Zhao, X.; et al. Photocatalysis-Activated SR-AOP over PDINH/MIL-88A ( Fe ) Composites for Boosted Chloroquine Phosphate Degradation: Performance, Mechanism, Pathway and DFT Calculations. Appl. Catal. B Environ. 2021, 293, 120229. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, Y.; Li, G. Promotion of Sulfameter Degradation by Coupling Persulfate and Photocatalytic Advanced Oxidation Processes with Fe-Doped MOFs. Sep. Purif. Technol. 2022, 282, 119632. [Google Scholar] [CrossRef]
- Ruan, Y.; Kong, L.; Zhong, Y.; Diao, Z.; Shih, K.; Wang, S.; Chen, D. Review on the Synthesis and Activity of Iron-Based Catalyst in Catalytic Oxidation of Refractory Organic Pollutants in Wastewater. J. Clean. Prod. 2021, 321, 128924. [Google Scholar] [CrossRef]
- He, Z.; Liu, Y.; Wang, J.; Lv, Y.; Xu, Y.; Jia, S. Enhanced Degradation of Old Landfill Leachate in Heterogeneous Electro–Fenton Catalyzed Using Fe3O4 Nano–Particles Encapsulated by Metal Organic Frameworks. J. Clean. Prod. 2021, 321, 128947. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, M.; Hou, C.; Chen, W.; Li, S.; Ren, R.; Li, Z. Efficient Degradation of Perfluorooctanoic Acid by Solar Photo-Electro-Fenton like System Fabricated by MOFs/Carbon Nanofibers Composite Membrane. Chem. Eng. J. 2021, 414, 128940. [Google Scholar] [CrossRef]
- Ren, G.; Zhou, M.; Liu, M.; Ma, L.; Yang, H. A Novel Vertical-Flow Electro-Fenton Reactor for Organic Wastewater Treatment. Chem. Eng. J. 2016, 298, 55–67. [Google Scholar] [CrossRef]
- Lu, J.-Y.; Yuan, Y.-R.; Hu, X.; Liu, W.-J.; Li, C.-X.; Liu, H.-Q.; Li, W.-W. MOF-Derived Fe2O3/Nitrogen/Carbon Composite as a Stable Heterogeneous Electro-Fenton Catalyst. Ind. Eng. Chem. Res. 2020, 59, 1800–1808. [Google Scholar] [CrossRef]
- Ye, Z.; Schukraft, G.E.M.; L’Hermitte, A.; Xiong, Y.; Brillas, E.; Petit, C.; Sirés, I. Mechanism and Stability of an Fe-Based 2D MOF during the Photoelectro-Fenton Treatment of Organic Micropollutants under UVA and Visible Light Irradiation. Water Res. 2020, 184, 115986. [Google Scholar] [CrossRef]
- Du, X.; Fu, W.; Su, P.; Cai, J.; Zhou, M. Internal-Micro-Electrolysis-Enhanced Heterogeneous Electro-Fenton Process Catalyzed by Fe/Fe3C@PC Core–Shell Hybrid for Sulfamethazine Degradation. Chem. Eng. J. 2020, 398, 125681. [Google Scholar] [CrossRef]
- Le, T.X.H.; Cowan, M.G.; Drobek, M.; Bechelany, M.; Julbe, A.; Cretin, M. Fe-Nanoporous Carbon Derived from MIL-53(Fe): A Heterogeneous Catalyst for Mineralization of Organic Pollutants. Nanomaterials 2019, 9, 641. [Google Scholar] [CrossRef]
- Tang, J.; Wang, J. MOF-Derived Three-Dimensional Flower-like FeCu@C Composite as an Efficient Fenton-like Catalyst for Sulfamethazine Degradation. Chem. Eng. J. 2019, 375, 122007. [Google Scholar] [CrossRef]
- Du, X.; Fu, W.; Su, P.; Zhang, Q.; Zhou, M. S-doped MIL-53 as efficient heterogeneous electro-Fenton catalyst for degradation of sulfamethazine at circumneutral pH. J. Hazard. Mater. 2022, 424, 127674. [Google Scholar] [CrossRef]
- Liu, K.; Yu, M.; Wang, H.; Wang, J.; Liu, W. Multiphase Porous Electrochemical Catalysts Derived from Iron- Based Metal − Organic Framework Compounds. Environ. Sci. Technol. 2019, 53, 6474–6482. [Google Scholar] [CrossRef]
- Fu, A.; Liu, Z.; Sun, Z. Cu/Fe Oxide Integrated on Graphite Felt for Degradation of Sulfamethoxazole in the Heterogeneous Electro-Fenton Process under near-Neutral Conditions. Chemosphere 2022, 297, 134257. [Google Scholar] [CrossRef]
- Ye, Z.; Padilla, J.A.; Xuriguera, E.; Beltran, J.L.; Alcaide, F.; Brillas, E.; Sirés, I. A Highly Stable Metal-Organic Framework-Engineered FeS2/C Nanocatalyst for Heterogeneous Electro-Fenton Treatment: Validation in Wastewater at Mild PH. Environ. Sci. Technol. 2020, 54, 4664–4674. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Padilla, J.A.; Xuriguera, E.; Brillas, E.; Sirés, I. Magnetic MIL(Fe)-Type MOF-Derived N-Doped Nano-ZVI@C Rods as Heterogeneous Catalyst for the Electro-Fenton Degradation of Gemfibrozil in a Complex Aqueous Matrix. Appl. Catal. B Environ. 2020, 266, 118604. [Google Scholar] [CrossRef]
- Du, X.; Fu, W.; Su, P.; Zhang, Q.; Zhou, M. FeMo@porous Carbon Derived from MIL-53(Fe)@MoO3 as Excellent Heterogeneous Electro-Fenton Catalyst: Co-Catalysis of Mo. J. Environ. Sci. 2023, 127, 652–666. [Google Scholar] [CrossRef]
- Qi, C.; Han, S.; Lin, J.; Cheng, J.; Du, K.; Hu, Y.; Chen, Y. Reconstruction of Electronic Structure of MOF-525 via Metalloporphyrin for Enhanced Photoelectro-Fenton Process. Catalysts 2022, 12, 671. [Google Scholar] [CrossRef]
- Du, X.; Su, P.; Fu, W.; Zhang, Q.; Zhou, M. Heterogeneous Photoelectro-Fenton Catalyzed by FeCu@PC for Efficient Degradation of Sulfamethazine. Electrochim. Acta 2022, 412, 140122. [Google Scholar] [CrossRef]
- Hang, J.; Yi, X.-H.; Wang, C.-C.; Fu, H.; Wang, P.; Zhao, Y. Heterogeneous Photo-Fenton Degradation toward Sulfonamide Matrix over Magnetic Fe3S4 Derived from MIL-100(Fe). J. Hazard. Mater. 2022, 424, 127415. [Google Scholar] [CrossRef]
- Chavoshani, A.; Hashemi, M.; Amin, M.M.; Ameta, S.C. Pharmaceuticals as Emerging Micropollutants in Aquatic Environments. In Micropollutants and Challenges; Ameta, S.C., Chavoshani, A., Hashemi, M., Amin, M.M., Eds.; Academic Press: London, UK, 2020; pp. 35–90. [Google Scholar]
- Martín-Pozo, L.; Gómez-Regalado, M.d.C.; García-Córcoles, M.T.; Zafra-Gómez, A. Removal of Quinolone Antibiotics from Wastewaters and Sewage Sludge. In Emerging Contaminants in the Environment; Sarma, H., Dominguez, D.C., Lee, W.-Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 381–406. [Google Scholar]
- Rekhate, C.V.; Srivastava, J.K. Recent Advances in Ozone-Based Advanced Oxidation Processes for Treatment of Wastewater—A Review. Chem. Eng. J. Adv. 2020, 3, 100031. [Google Scholar] [CrossRef]
- Linden, K.G.; Mohseni, M. Advanced Oxidation Processes: Applications in Drinking Water Treatment. In Comprehensive Water Quality and Purification; Ahuja, S., Ed.; Elsevier: Waltham, MA, USA, 2014; pp. 148–172. [Google Scholar]
- Ikehata, K.; Li, Y. Ozone-Based Processes. In Emerging Green Chemical Technology; Ameta, S.C., Ameta, R., Eds.; Academic Press: London, UK, 2018; pp. 115–134. [Google Scholar] [CrossRef]
- Mohebali, H.; Moussavi, G.; Karimi, M.; Giannakis, S. Catalytic Ozonation of Acetaminophen with a Magnetic, Cerium-Based Metal-Organic Framework as a Novel, Easily-Separable Nanocomposite. Chem. Eng. J. 2022, 434, 134614. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J. MOF-Derived Co3O4-C@FeOOH as an Efficient Catalyst for Catalytic Ozonation of Norfloxacin. J. Hazard. Mater. 2021, 403, 123697. [Google Scholar] [CrossRef]
- Yu, D.; Li, L.; Wu, M.; Crittenden, J.C. Enhanced Photocatalytic Ozonation of Organic Pollutants Using an Iron- Based Metal-Organic Framework. Appl. Catal. B Environ. 2019, 251, 66–75. [Google Scholar] [CrossRef]
- Pattappan, D.; Kavya, K.V.; Vargheese, S.; Kumar, R.T.R.; Haldorai, Y. Graphitic Carbon Nitride/NH2-MIL-101(Fe) Composite for Environmental Remediation: Visible-Light-Assisted Photocatalytic Degradation of Acetaminophen and Reduction of Hexavalent Chromium. Chemosphere 2022, 286, 131875. [Google Scholar] [CrossRef]
- Huang, W.; Liu, W.; Yang, Z.; Chen, Y.; Li, G.; Liao, X. MIL-88A Anchoring on Different Morphological g-C3N4 for Enhanced Fenton Performance. Microporous Mesoporous Mater. 2022, 329, 111531. [Google Scholar] [CrossRef]
- Yu, R.; Ma, R.; Wang, L.; Bai, L.; Yang, S.; Qian, J. Activation of Peroxydisulfate (PDS) by Bi5O7I@MIL-100 (Fe) for Catalytic Degradation of Aqueous Doxycycline (DOX) under UV Light Irradiation: Characteristic, Performance and Mechanism. J. Water Process Eng. 2022, 48, 102903. [Google Scholar] [CrossRef]
- Tang, L.; Lv, Z.; Xue, Y.; Xu, L.; Qiu, W.; Zheng, C. MIL-53 (Fe) Incorporated in the Lamellar BiOBr: Promoting the Visible-Light Catalytic Capability on the Degradation of Rhodamine B and Carbamazepine. Chem. Eng. J. 2019, 374, 975–982. [Google Scholar] [CrossRef]
- Liu, N.; Wang, J.; Wu, J.; Li, Z.; Huang, W.; Zheng, Y.; Lei, J.; Zhang, X.; Tang, L. Magnetic Fe3O4@MIL-53(Fe) Nanocomposites Derived from MIL-53(Fe) for the Photocatalytic Degradation of Ibuprofen under Visible Light Irradiation. Mater. Res. Bull. 2020, 132, 111000. [Google Scholar] [CrossRef]
- He, L.; Dong, Y.; Zheng, Y.; Jia, Q.; Shan, S.; Zhang, Y. A Novel Magnetic MIL-101(Fe)/TiO2 Composite for Photo Degradation of Tetracycline under Solar Light. J. Hazard. Mater. 2019, 361, 85–94. [Google Scholar] [CrossRef]
- Fakhri, H.; Farzadkia, M.; Boukherroub, R.; Srivastava, V.; Sillanpää, M. Design and Preparation of Core-Shell Structured Magnetic Graphene Oxide@MIL-101(Fe): Photocatalysis under Shell to Remove Diazinon and Atrazine Pesticides. Sol. Energy 2020, 208, 990–1000. [Google Scholar] [CrossRef]
- Zhong, Z.; Li, M.; Fu, J.; Wang, Y.; Muhammad, Y.; Li, S. Construction of Cu-Bridged Cu2O/MIL (Fe/Cu) Catalyst with Enhanced Interfacial Contact for the Synergistic Photo-Fenton Degradation of Thiacloprid. Chem. Eng. J. 2020, 395, 125184. [Google Scholar] [CrossRef]
- Liang, H.; Liu, R.; An, X.; Hu, C.; Zhang, X.; Liu, H. Bimetal-Organic Frameworks with Coordinatively Unsaturated Metal Sites for Highly Efficient Fenton-like Catalysis. Chem. Eng. J. 2021, 414, 128669. [Google Scholar] [CrossRef]
- Fdez-Sanromán, A.; Pazos, M.; Sanroman, A. Peroxymonosulphate Activation by Basolite F-300 for Escherichia coli Disinfection and Antipyrine Degradation. Int. J. Environ. Res. Public Health 2022, 19, 6852. [Google Scholar] [CrossRef]
- He, W.; Li, Z.; Lv, S.; Niu, M.; Zhou, W.; Li, J.; Lu, R.; Gao, H.; Pan, C.; Zhang, S. Facile Synthesis of Fe3O4@MIL-100(Fe) towards Enhancing Photo-Fenton like Degradation of Levofloxacin via a Synergistic Effect between Fe3O4 and MIL-100(Fe). Chem. Eng. J. 2021, 409, 128274. [Google Scholar] [CrossRef]
- Yang, T.; Yu, D.; Wang, D.; Yang, T.; Li, Z.; Wu, M.; Petru, M.; Crittenden, J. Accelerating Fe(III)/Fe(II) Cycle via Fe(II) Substitution for Enhancing Fenton-like Performance of Fe-MOFs. Appl. Catal. B Environ. 2021, 286, 119859. [Google Scholar] [CrossRef]
- Yang, W.; Hong, P.; Yang, D.; Yang, Y.; Wu, Z.; Xie, C.; He, J.; Zhang, K.; Kong, L.; Liu, J. Enhanced Fenton-like Degradation of Sulfadiazine by Single Atom Iron Materials Fixed on Nitrogen-Doped Porous Carbon. J. Colloid Interface Sci. 2021, 597, 56–65. [Google Scholar] [CrossRef]
- Ping, C.; Yi, C.; Wei, Z.; Yu, S.; Yan, X.; Zhi, C.; Fang, Y.; Jun, S. Facile Synthesis of Porphyrin-MOFs with High Photo-Fenton Activity to Efficiently Degrade Ciprofloxacin. J. Colloid Interface Sci. 2022, 622, 690–699. [Google Scholar] [CrossRef]
- Tian, H.; Peng, J.; Du, Q.; Hui, X.; He, H. One-Pot Sustainable Synthesis of Magnetic MIL-100(Fe) with Novel Fe3O4 Morphology and Its Application in Heterogeneous Degradation. Dalton Trans. 2018, 47, 3417–3424. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, C.; Wang, P. Effective Norfloxacin Elimination via Photo-Fenton Process over the MIL-101(Fe)-NH2 Immobilized on α-Al2O3 Shee. Chin. Chem. Lett. 2022, 33, 4828–4833. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, X.; Tan, Y.; Jiang, C.; Wang, H.; Zhang, S. Preparation of Millimeter-Scale MIL-53 (Fe)@ Polyethersulfone Balls to Optimize Photo-Fenton Process. Chem. Eng. J. 2022, 441, 135881. [Google Scholar] [CrossRef]
- Liu, N.; Zheng, Y.; Jing, C.; Gao, B.; Huang, W.; Li, Z.; Lei, J.; Zhang, X.; Cui, L.; Tang, L. Boosting Catalytic Degradation Efficiency by Incorporation of MIL-53 (Fe) with Ti3C2Tx Nanosheets. J. Mol. Liq. 2020, 311, 113201. [Google Scholar] [CrossRef]
- Tan, C.; Lu, X.; Cui, X.; Jian, X.; Hu, Z.; Dong, Y.; Liu, X.; Huang, J.; Deng, L. Novel Activation of Peroxymonosulfate by an Easily Recyclable VC@Fe3O4 Nanoparticles for Enhanced Degradation of Sulfadiazine. Chem. Eng. J. 2019, 363, 318–328. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, D.; Chen, Y.; Hu, Y. Enhanced Degradation of Triclosan in Heterogeneous E-Fenton Process with MOF-Derived Hierarchical Mn/Fe@PC Modified Cathode. Chem. Eng. J. 2020, 384, 123324. [Google Scholar] [CrossRef]
- Liu, M.; Feng, Z.; Luan, X.; Chu, W.; Zhao, H.; Zhao, G. Accelerated Fe2+ Regeneration in an Effective Electro-Fenton Process by Boosting Internal Electron Transfer to a Nitrogen-Conjugated Fe(III) Complex. Environ. Sci. Technol. 2021, 55, 6042–6051. [Google Scholar] [CrossRef] [PubMed]










| UiO | Pollutant | Processes | Reference |
|---|---|---|---|
| Acid-modulated UiO-66 | 2,4-dichlorophenoxyacetic acid, ciprofloxacin and naproxen | Adsorption | [62] |
| C3N4-TE@TiO2/UiO-66 | TC | Photocatalytic | [65] |
| IN2S3/UiO-66 | Methyl orange and TC | Photocatalytic | [66] |
| MWCNT/N-TiO2/UiO-66-NH2 | Ketoprofen | Photocatalytic | [67] |
| NiCo2O4/HP-UiO-66 | TC | Photocatalytic with PMS | [68] |
| UiO-66(Zr) and NH2-UiO-66(Zr) | Pyrene | Adsorption | [69] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fdez-Sanromán, A.; Rosales, E.; Pazos, M.; Sanroman, A. Metal–Organic Frameworks as Powerful Heterogeneous Catalysts in Advanced Oxidation Processes for Wastewater Treatment. Appl. Sci. 2022, 12, 8240. https://doi.org/10.3390/app12168240
Fdez-Sanromán A, Rosales E, Pazos M, Sanroman A. Metal–Organic Frameworks as Powerful Heterogeneous Catalysts in Advanced Oxidation Processes for Wastewater Treatment. Applied Sciences. 2022; 12(16):8240. https://doi.org/10.3390/app12168240
Chicago/Turabian StyleFdez-Sanromán, Antía, Emilio Rosales, Marta Pazos, and Angeles Sanroman. 2022. "Metal–Organic Frameworks as Powerful Heterogeneous Catalysts in Advanced Oxidation Processes for Wastewater Treatment" Applied Sciences 12, no. 16: 8240. https://doi.org/10.3390/app12168240
APA StyleFdez-Sanromán, A., Rosales, E., Pazos, M., & Sanroman, A. (2022). Metal–Organic Frameworks as Powerful Heterogeneous Catalysts in Advanced Oxidation Processes for Wastewater Treatment. Applied Sciences, 12(16), 8240. https://doi.org/10.3390/app12168240









