Poly (β-Cyclodextrin-co-citric Acid) Functionalized Natural Nanozeolite: An Eco-Friendly Platform for IB Delivery
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Characterization
2.3. Experiment
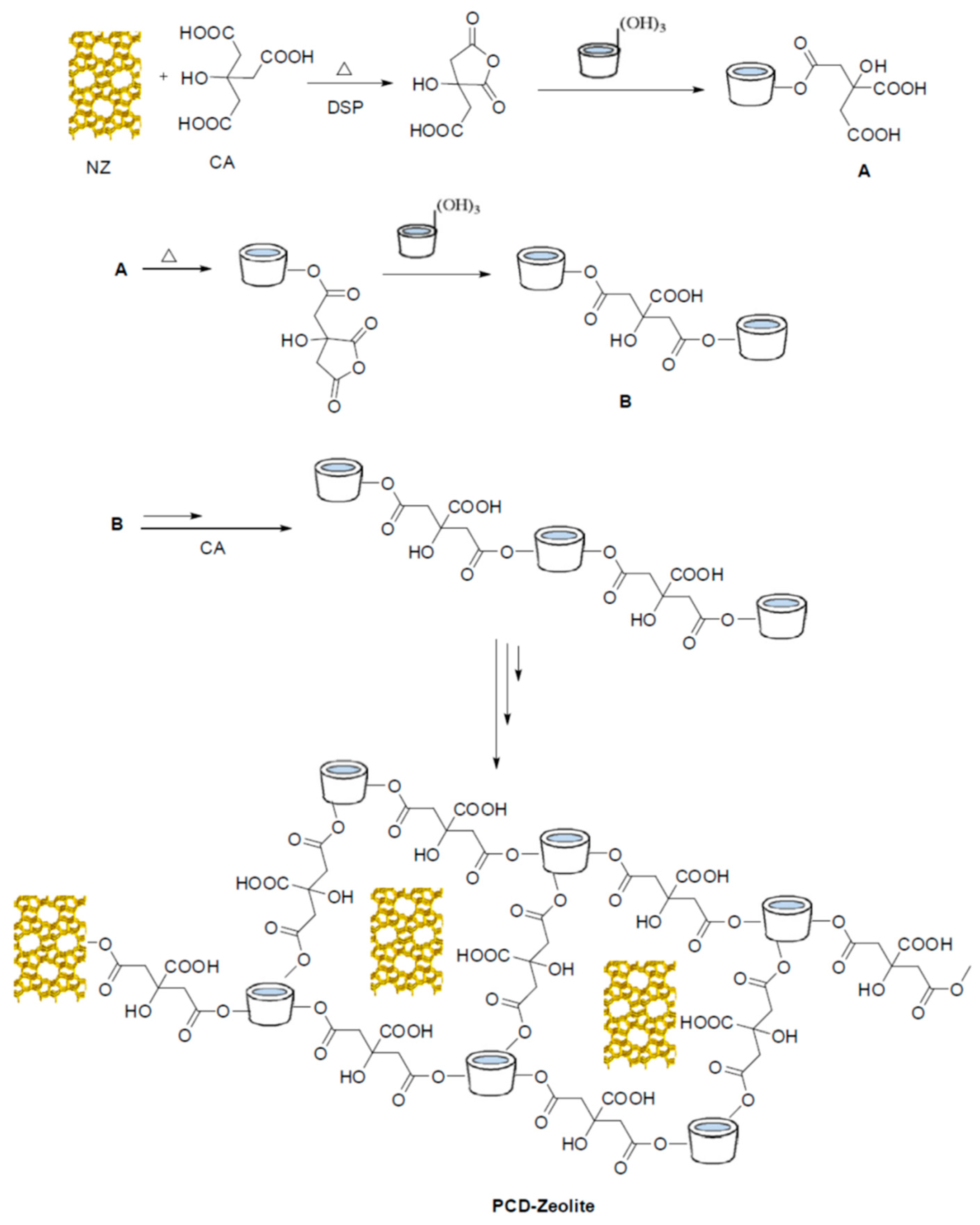
2.3.1. Preparation of Polybetacyclodextrin-co-Citric Acid/NZ Clinoptilolite (PCD-Zeolite)
2.3.2. Preparation of PCD-Zeolite-IB
2.3.3. Drug Release Studies
3. Results and Discussion
3.1. Nanostructure Characterizations
3.1.1. FTIR Analysis
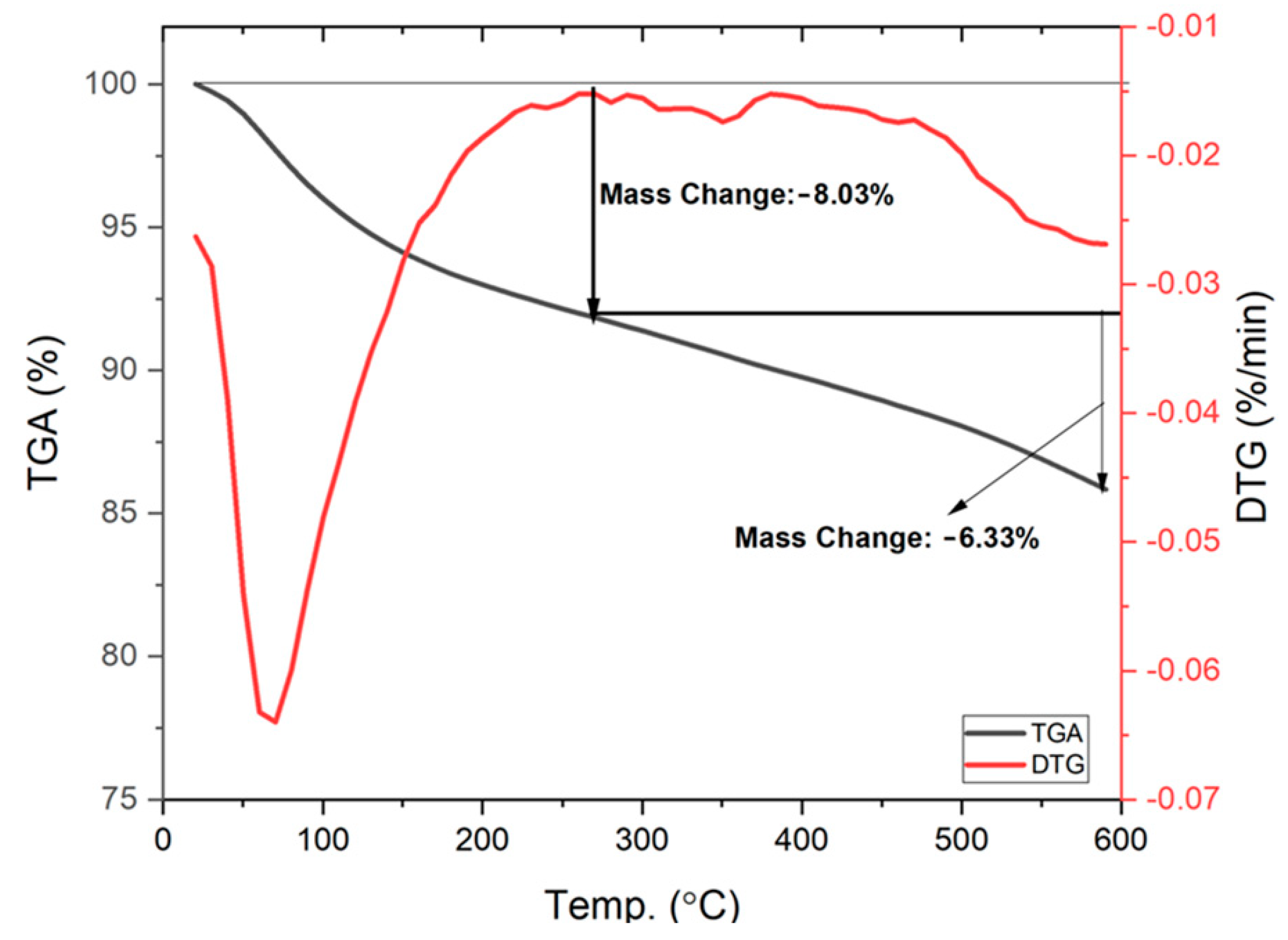
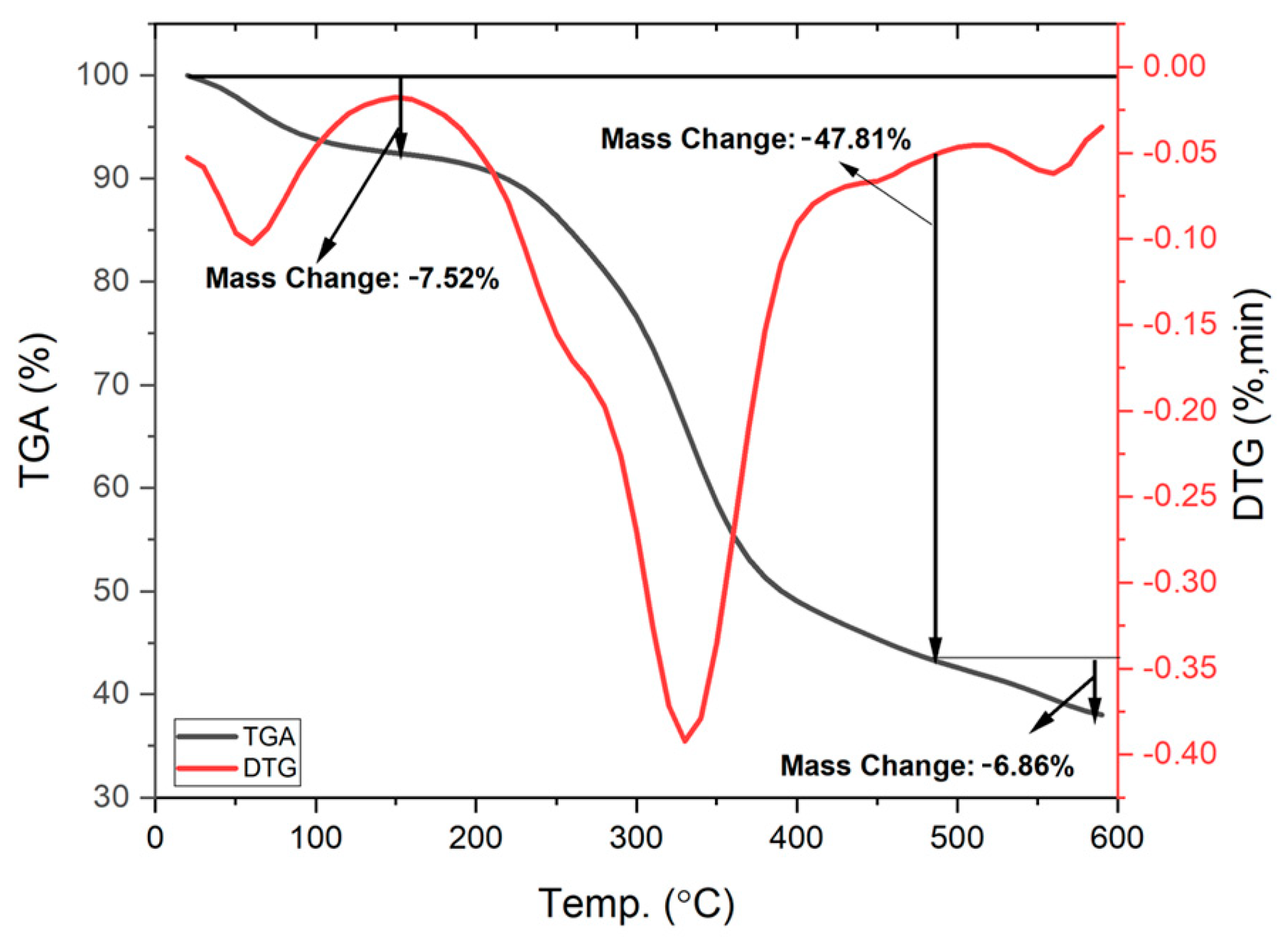
3.1.2. TGA Analysis
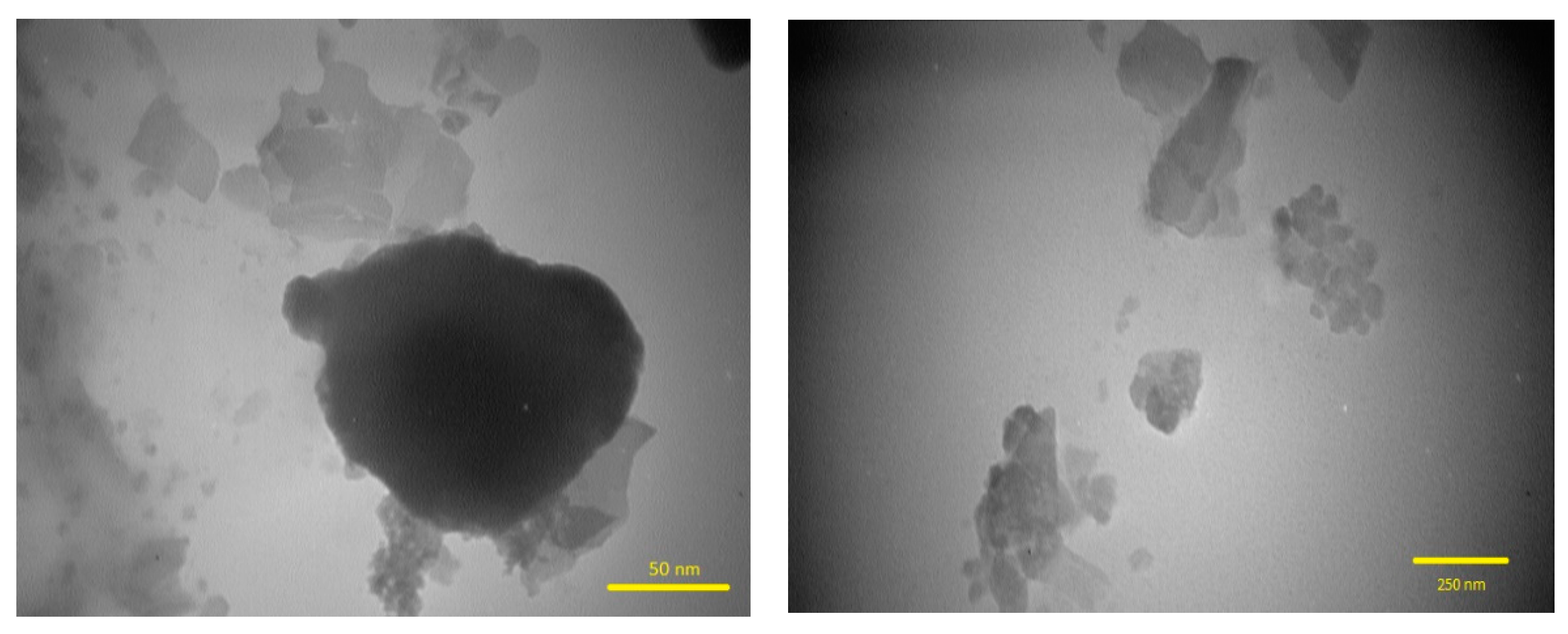
3.1.3. TEM Analysis
3.2. Studies on the Uptake and Release of IB
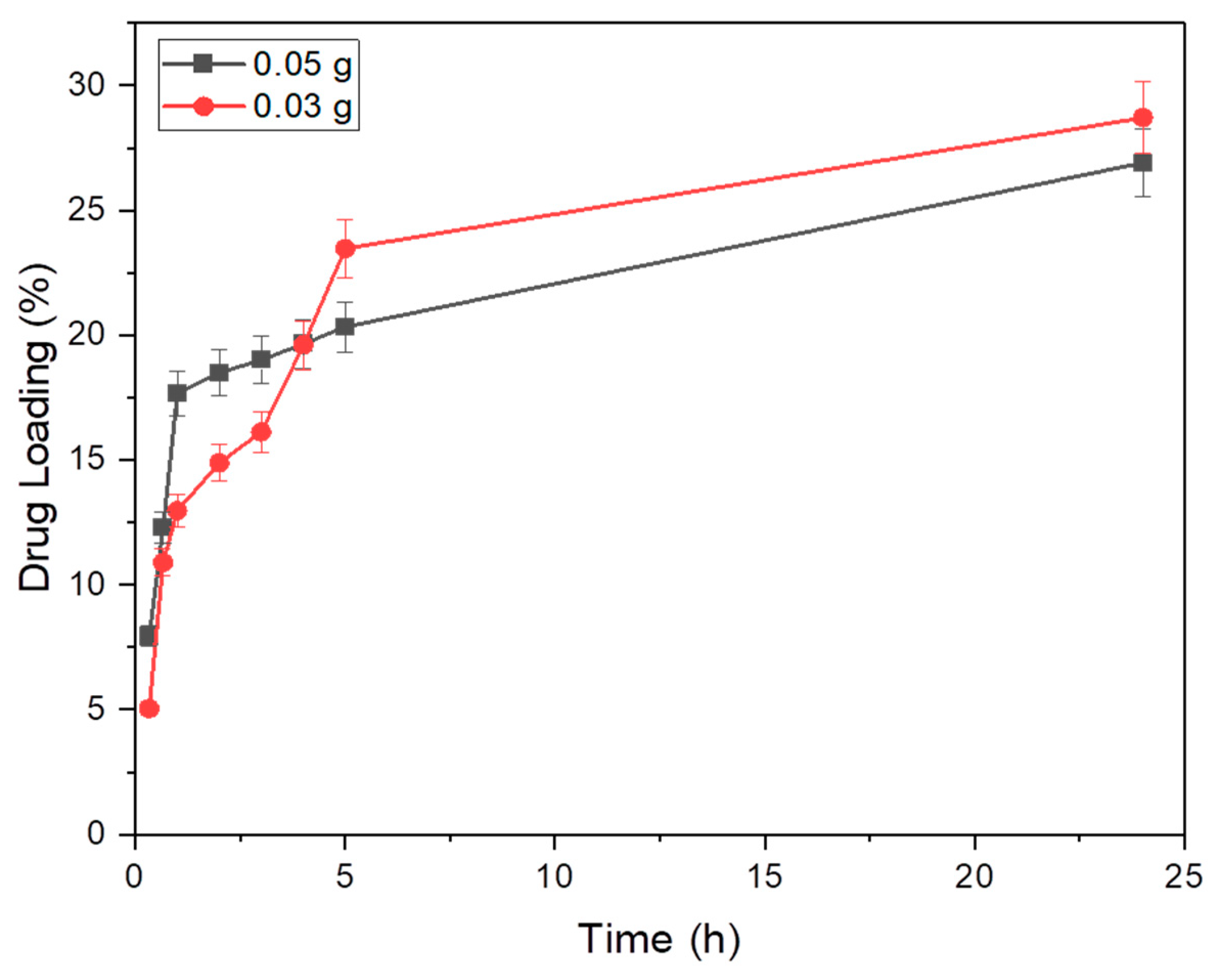
3.2.1. General Method to Optimize the Amount of the PCD-Zeolite for the Preparation of PCD-Zeolite-IB
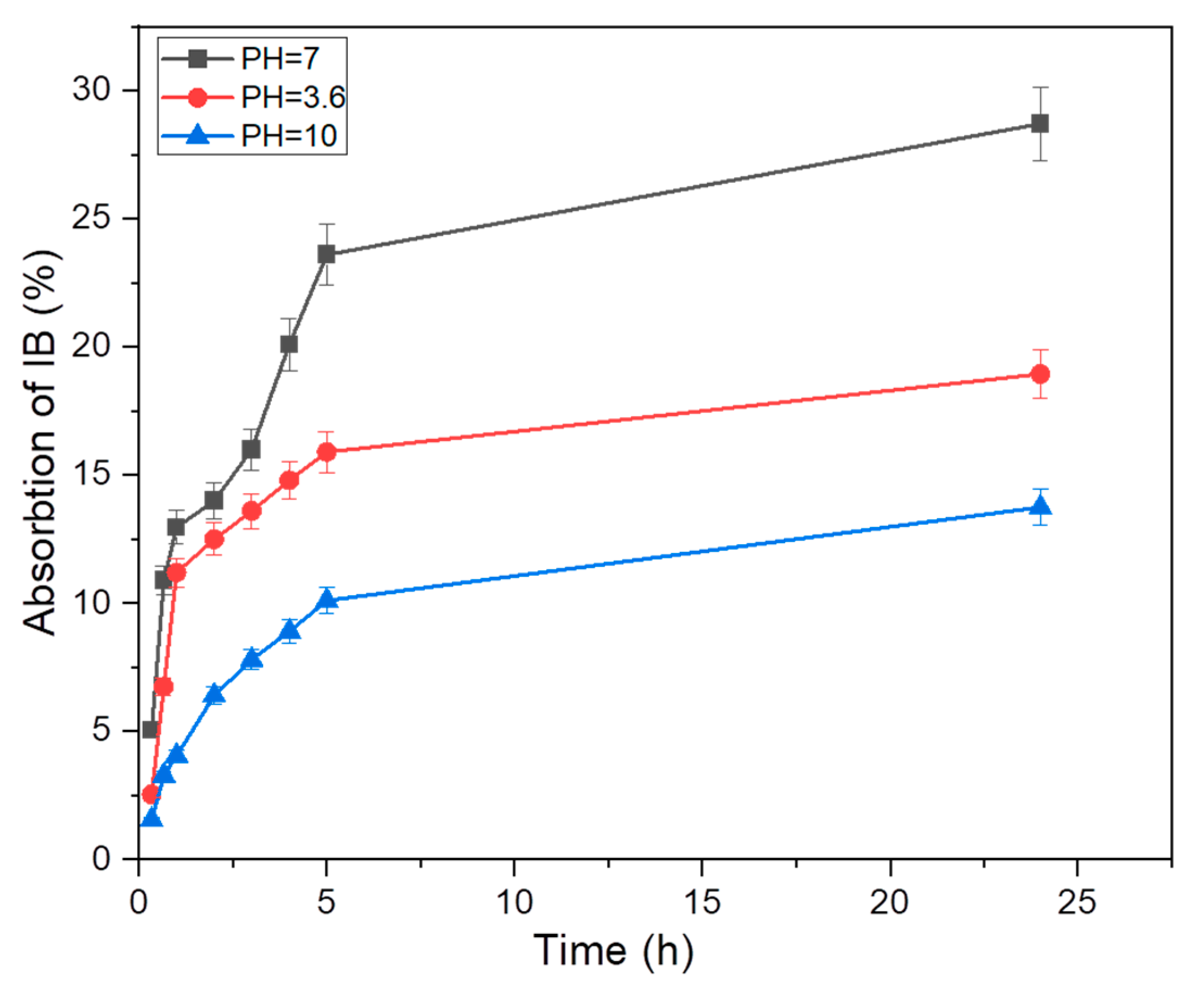
3.2.2. General Method to Optimization of pH
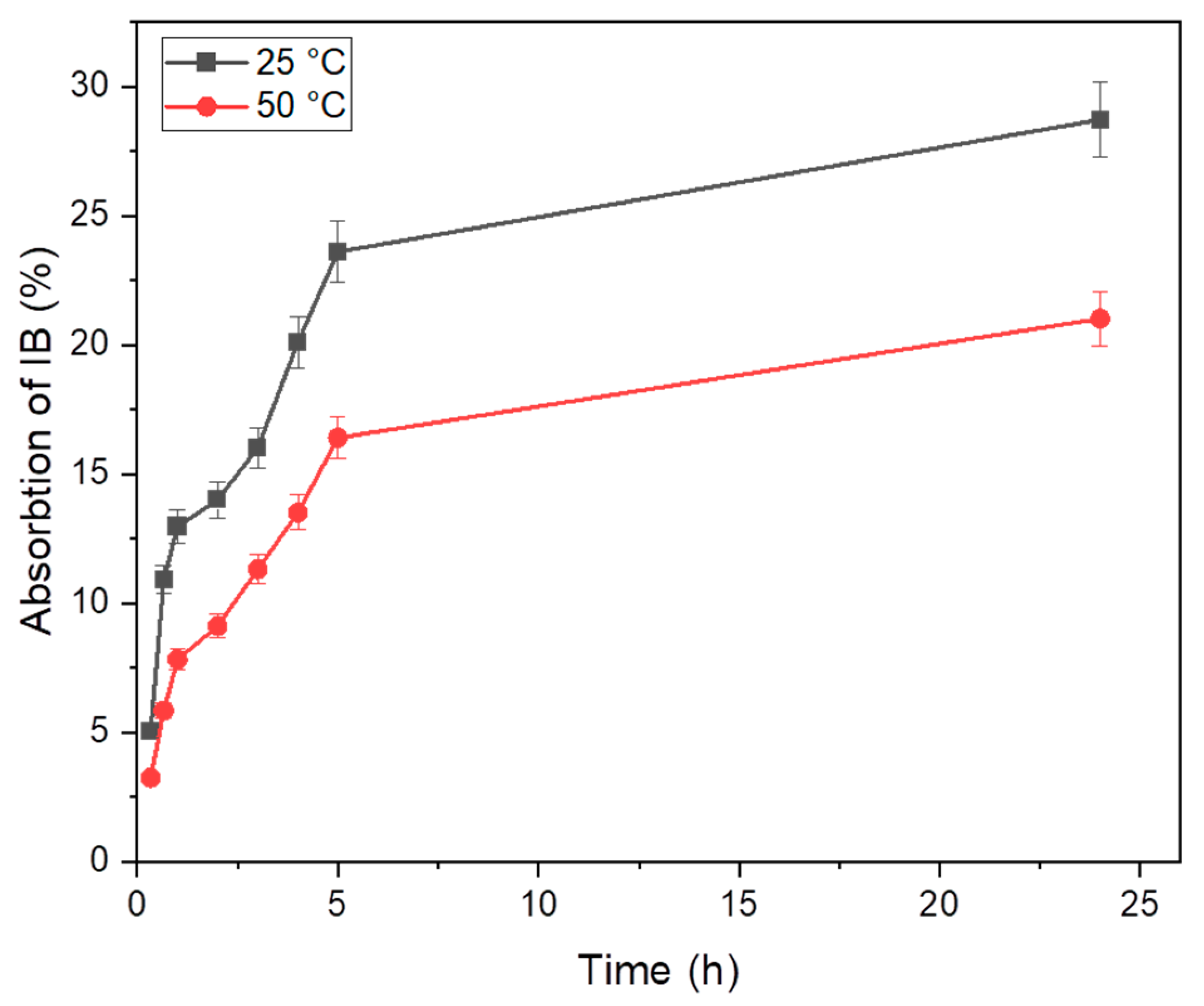
3.2.3. General Method for the Optimization of Temperature
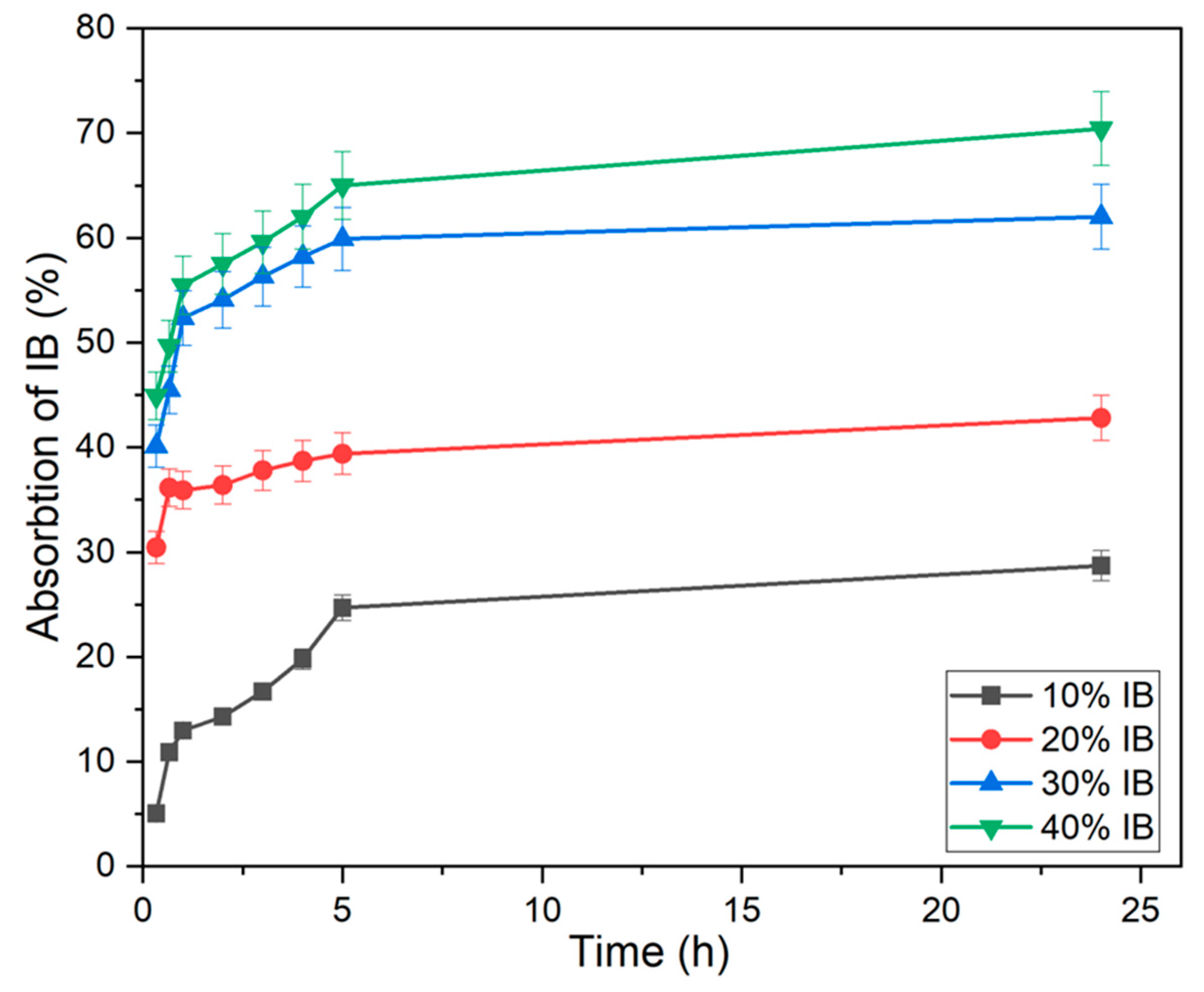
3.2.4. General Method to Optimize the Amount of IB
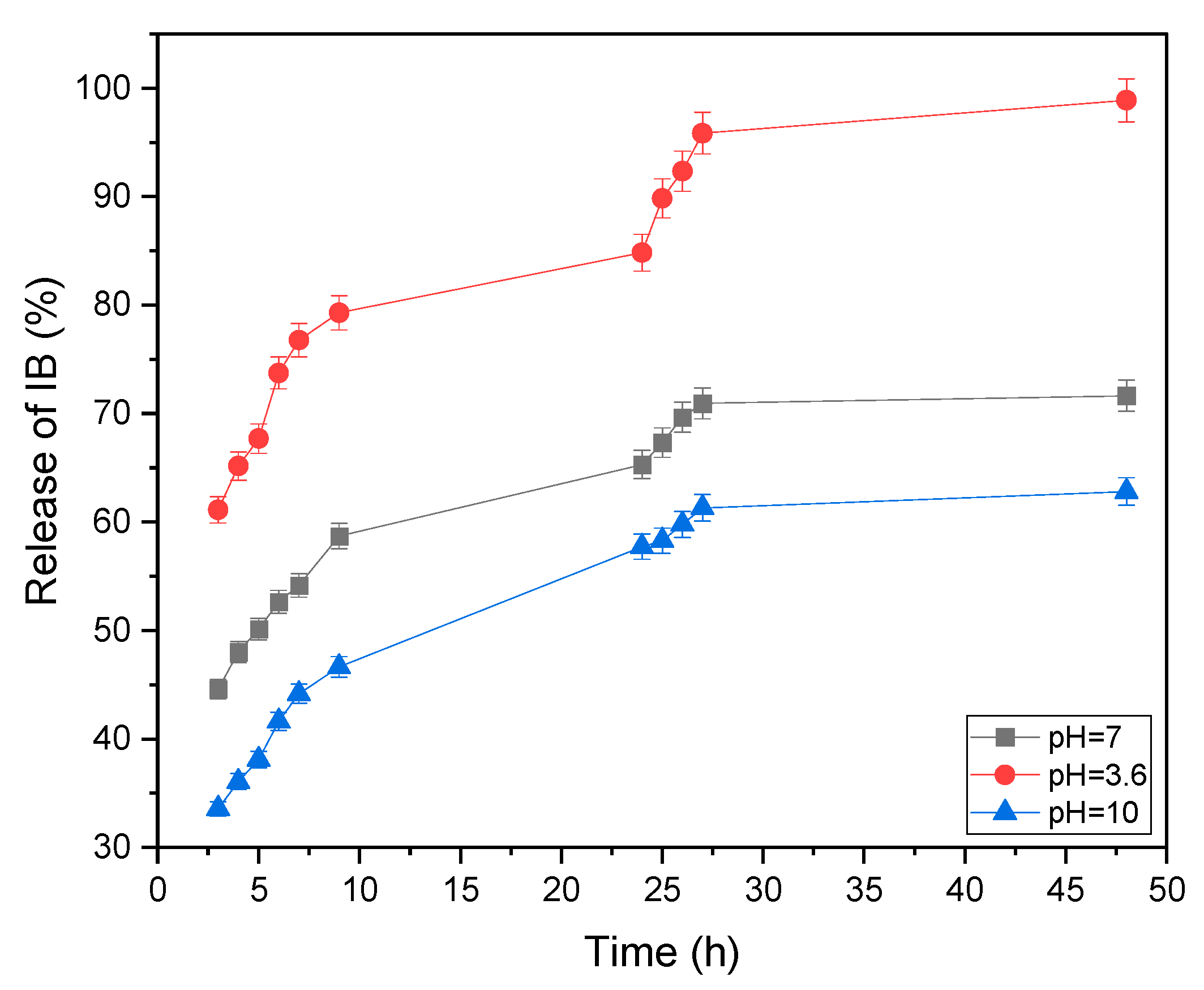
3.3. In Vitro Reduction-Triggered Release of IB from the PCD-Zeolite in a Phosphate Buffer
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ebrahimi, H.; Afshar Najafi, F.S.; Shahabadi, S.I.S.; Garmabi, H. A response surface study on microstructure and mechanical properties of poly(lactic acid)/thermoplastic starch/nanoclay nanocomposites. J. Compos. Mater. 2015, 50, 269–278. [Google Scholar] [CrossRef]
- Ghiyasi, S.; Sari, M.G.; Shabanian, M.; Hajibeygi, M.; Zarrintaj, P.; Rallini, M.; Torre, L.; Puglia, D.; Vahabi, H.; Jouyandeh, M.; et al. Hyperbranched poly(ethyleneimine) physically attached to silica nanoparticles to facilitate curing of epoxy nanocomposite coatings. Prog. Org. Coat. 2018, 120, 100–109. [Google Scholar] [CrossRef]
- Mercurio, M.; Sarkar, B.; Langella, A. Modified Clay and Zeolite Nanocomposite Materials: Environmental and Pharmaceutical Applications; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Servatan, M.; Ghadiri, M.; Damanabi, A.T.; Bahadori, F.; Zarrintaj, P.; Ahmadi, Z.; Vahabi, H.; Saeb, M.R. Zeolite-based catalysts for exergy efficiency enhancement: The insights gained from nanotechnology. Mater. Today: Proc. 2018, 5, 15868–15876. [Google Scholar] [CrossRef]
- Nemati, A.; Saghafi, M.; Khamseh, S.; Alibakhshi, E.; Zarrintaj, P.; Saeb, M.R. Magnetron-sputtered TixNy thin films applied on titanium-based alloys for biomedical applications: Composition-microstructure-property relationships. Surf. Coat. Technol. 2018, 349, 251–259. [Google Scholar] [CrossRef]
- Mousa, S.K.; Sara, K.; Akbar, R.; Fatemi, T.S.M.; Payam, Z.; Reza, S.M. Thermally stable antibacterial wool fabrics surface-decorated by TiON and TiON/Cu thin films. Surf. Innov. 2018, 6, 258–265. [Google Scholar] [CrossRef]
- Geraili, A.; Xing, M.; Mequanint, K. Design and fabrication of drug-delivery systems toward adjustable release profiles for personalized treatment. View 2021, 2, 20200126. [Google Scholar] [CrossRef]
- Borandeh, S.; van Bochove, B.; Teotia, A.; Seppälä, J. Polymeric drug delivery systems by additive manufacturing. Adv. Drug Deliv. Rev. 2021, 173, 349–373. [Google Scholar] [CrossRef]
- Coelho, J.F.; Ferreira, P.C.; Alves, P.; Cordeiro, R.; Fonseca, A.C.; Góis, J.R.; Gil, M.H. Drug delivery systems: Advanced technologies potentially applicable in personalized treatments. Epma J. 2010, 1, 164–209. [Google Scholar] [CrossRef]
- Tanaka, M.; Sato, K.; Kitakami, E.; Kobayashi, S.; Hoshiba, T.; Fukushima, K. Design of biocompatible and biodegradable polymers based on intermediate water concept. Polym. J. 2015, 47, 114–121. [Google Scholar] [CrossRef]
- Sung, Y.K.; Kim, S.W. Recent advances in polymeric drug delivery systems. Biomater. Res. 2020, 24, 12. [Google Scholar] [CrossRef]
- Gagliardi, A.; Giuliano, E.; Venkateswararao, E.; Fresta, M.; Bulotta, S.; Awasthi, V.; Cosco, D. Biodegradable Polymeric Nanoparticles for Drug Delivery to Solid Tumors. Front. Pharmacol. 2021, 12, 601626. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef]
- Liechty, W.B.; Kryscio, D.R.; Slaughter, B.V.; Peppas, N.A. Polymers for drug delivery systems. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 149–173. [Google Scholar] [CrossRef]
- Begines, B.; Ortiz, T.; Perez-Aranda, M.; Martinez, G.; Merinero, M.; Arguelles-Arias, F.; Alcudia, A. Polymeric Nanoparticles for Drug Delivery: Recent Developments and Future Prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef]
- Jana, P.; Shyam, M.; Singh, S.; Jayaprakash, V.; Dev, A. Biodegradable polymers in drug delivery and oral vaccination. Eur. Polym. J. 2021, 142, 110155. [Google Scholar] [CrossRef]
- Tong, X.; Pan, W.; Su, T.; Zhang, M.; Dong, W.; Qi, X. Recent advances in natural polymer-based drug delivery systems. React. Funct. Polym. 2020, 148, 104501. [Google Scholar] [CrossRef]
- Szejtli, J. Introduction and General Overview of Cyclodextrin Chemistry. Chem. Rev. 1998, 98, 1743–1754. [Google Scholar] [CrossRef]
- Hedges, A.R. Industrial Applications of Cyclodextrins. Chem. Rev. 1998, 98, 2035–2044. [Google Scholar] [CrossRef]
- Del Valle, E.M.M. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Amass, W.; Amass, A.; Tighe, B. A review of biodegradable polymers: Uses, current developments in the synthesis and characterization of biodegradable polyesters, blends of biodegradable polymers and recent advances in biodegradation studies. Polym. Int. 1998, 47, 89–144. [Google Scholar] [CrossRef]
- Zhong, Y.; Godwin, P.; Jin, Y.; Xiao, H. Biodegradable polymers and green-based antimicrobial packaging materials: A mini-review. Adv. Ind. Eng. Polym. Res. 2020, 3, 27–35. [Google Scholar] [CrossRef]
- Maraveas, C. Production of Sustainable and Biodegradable Polymers from Agricultural Waste. Polymers 2020, 12, 1127. [Google Scholar] [CrossRef]
- Salihu, R.; Abd Razak, S.I.; Ahmad Zawawi, N.; Rafiq Abdul Kadir, M.; Izzah Ismail, N.; Jusoh, N.; Riduan Mohamad, M.; Hasraf Mat Nayan, N. Citric acid: A green cross-linker of biomaterials for biomedical applications. Eur. Polym. J. 2021, 146, 110271. [Google Scholar] [CrossRef]
- Nangare, S.; Vispute, Y.; Tade, R.; Dugam, S.; Patil, P. Pharmaceutical applications of citric acid. Future J. Pharm. Sci. 2021, 7, 54. [Google Scholar] [CrossRef]
- Hernandez-Montelongo, J.; Naveas, N.; Degoutin, S.; Tabary, N.; Chai, F.; Spampinato, V.; Ceccone, G.; Rossi, F.; Torres-Costa, V.; Manso-Silvan, M.; et al. Porous silicon-cyclodextrin based polymer composites for drug delivery applications. Carbohydr. Polym. 2014, 110, 238–252. [Google Scholar] [CrossRef]
- Anand, R.; Malanga, M.; Manet, I.; Manoli, F.; Tuza, K.; Aykac, A.; Ladaviere, C.; Fenyvesi, E.; Vargas-Berenguel, A.; Gref, R.; et al. Citric acid-gamma-cyclodextrin crosslinked oligomers as carriers for doxorubicin delivery. Photochem. Photobiol. Sci. 2013, 12, 1841–1854. [Google Scholar] [CrossRef]
- Jayaprabha, K.N.; Joy, P.A. Citrate modified β-cyclodextrin functionalized magnetite nanoparticles: A biocompatible platform for hydrophobic drug delivery. RSC Adv. 2015, 5, 22117–22125. [Google Scholar] [CrossRef]
- Baghbanian, S.M.; Rezaei, N.; Tashakkorian, H. Nanozeolite clinoptilolite as a highly efficient heterogeneous catalyst for the synthesis of various 2-amino-4H-chromene derivatives in aqueous media. Green Chem. 2013, 15, 3446–3458. [Google Scholar] [CrossRef]
- Li, D.; Wang, M.; Song, W.L.; Yu, D.G.; Bligh, S.W.A. Electrospun Janus Beads-On-A-String Structures for Different Types of Controlled Release Profiles of Double Drugs. Biomolecules 2021, 11, 635. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gao, H.; Jin, Y.; Chen, X.; Wang, F.; Yang, H.; Fang, L.; Chen, X.; Tang, S.; Li, D. Defect engineering in novel broad-band gap hexaaluminate MAl12O19 (M = Ca, sr, Ba)-Based photocatalysts Boosts Near ultraviolet and visible light-driven photocatalytic performance. Mater. Today Chem. 2022, 24, 100942. [Google Scholar] [CrossRef]
- Nikolov, A.; Nugteren, H.; Rostovsky, I. Optimization of geopolymers based on natural zeolite clinoptilolite by calcination and use of aluminate activators. Constr. Build. Mater. 2020, 243, 118257. [Google Scholar] [CrossRef]
- Mukhtar, N.Z.F.; Borhan, M.Z.; Rusop, M.; Abdullah, S. Nanozeolite Produced by Wet Milling at Different Milling Time. In Recent Trends in Nanotechnology and Materials Science: Selected Review Papers from the 2013 International Conference on Manufacturing, Optimization, Industrial and Material Engineering (MOIME 2013); Gaol, F.L., Webb, J., Eds.; Springer International Publishing: Cham, Switzerland, 2014; pp. 41–47. [Google Scholar]
- Mansouri, N.; Rikhtegar, N.; Panahi, H.A.; Atabi, F.; Shahraki, B.K. Porosity, characterization and structural properties of natural zeolite-clinoptilolite-as a sorbent. Environ. Prot. Eng. 2013, 39, 139–152. [Google Scholar]
- Heydari, A.; Sheibani, H. Fabrication of poly(β-cyclodextrin-co-citric acid)/bentonite clay nanocomposite hydrogel: Thermal and absorption properties. RSC Adv. 2015, 5, 82438–82449. [Google Scholar] [CrossRef]
- Bednarz, S.; Lukasiewicz, M.; Mazela, W.; Pajda, M.; Kasprzyk, W. Chemical structure of poly(β-cyclodextrin-co-citric acid). J. Appl. Polym. Sci. 2011, 119, 3511–3520. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jahangard, N.; Baghbanian, S.M.; Khaksarmaghami, S. Poly (β-Cyclodextrin-co-citric Acid) Functionalized Natural Nanozeolite: An Eco-Friendly Platform for IB Delivery. Appl. Sci. 2022, 12, 8241. https://doi.org/10.3390/app12168241
Jahangard N, Baghbanian SM, Khaksarmaghami S. Poly (β-Cyclodextrin-co-citric Acid) Functionalized Natural Nanozeolite: An Eco-Friendly Platform for IB Delivery. Applied Sciences. 2022; 12(16):8241. https://doi.org/10.3390/app12168241
Chicago/Turabian StyleJahangard, Novin, Seyed Meysam Baghbanian, and Samad Khaksarmaghami. 2022. "Poly (β-Cyclodextrin-co-citric Acid) Functionalized Natural Nanozeolite: An Eco-Friendly Platform for IB Delivery" Applied Sciences 12, no. 16: 8241. https://doi.org/10.3390/app12168241
APA StyleJahangard, N., Baghbanian, S. M., & Khaksarmaghami, S. (2022). Poly (β-Cyclodextrin-co-citric Acid) Functionalized Natural Nanozeolite: An Eco-Friendly Platform for IB Delivery. Applied Sciences, 12(16), 8241. https://doi.org/10.3390/app12168241







