UiO-66/Palygorskite/TiO2 Ternary Composites as Adsorbents and Photocatalysts for Methyl Orange Removal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of UiO-66
2.2. Synthesis of TiO2
2.3. Synthesis of Binary Palygorskite/UiO-66 Composite
2.4. Synthesis of Palygorskite/UiO-66/TiO2 Composite
2.5. Materials Characterization
2.6. Adsorption Experiments
2.7. Photocatalytic Reactions
2.8. Recycling of the Materials
3. Results and Discussion
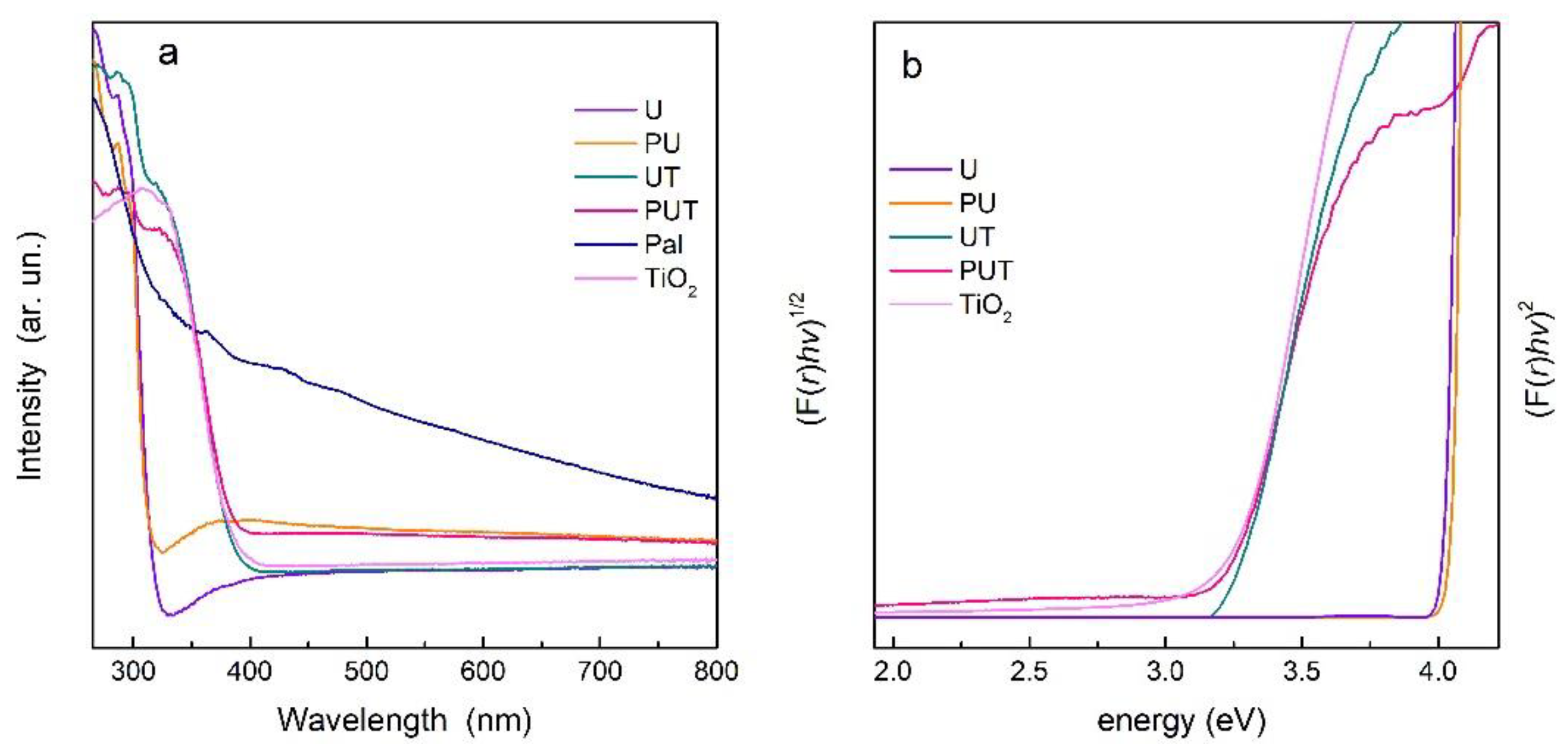
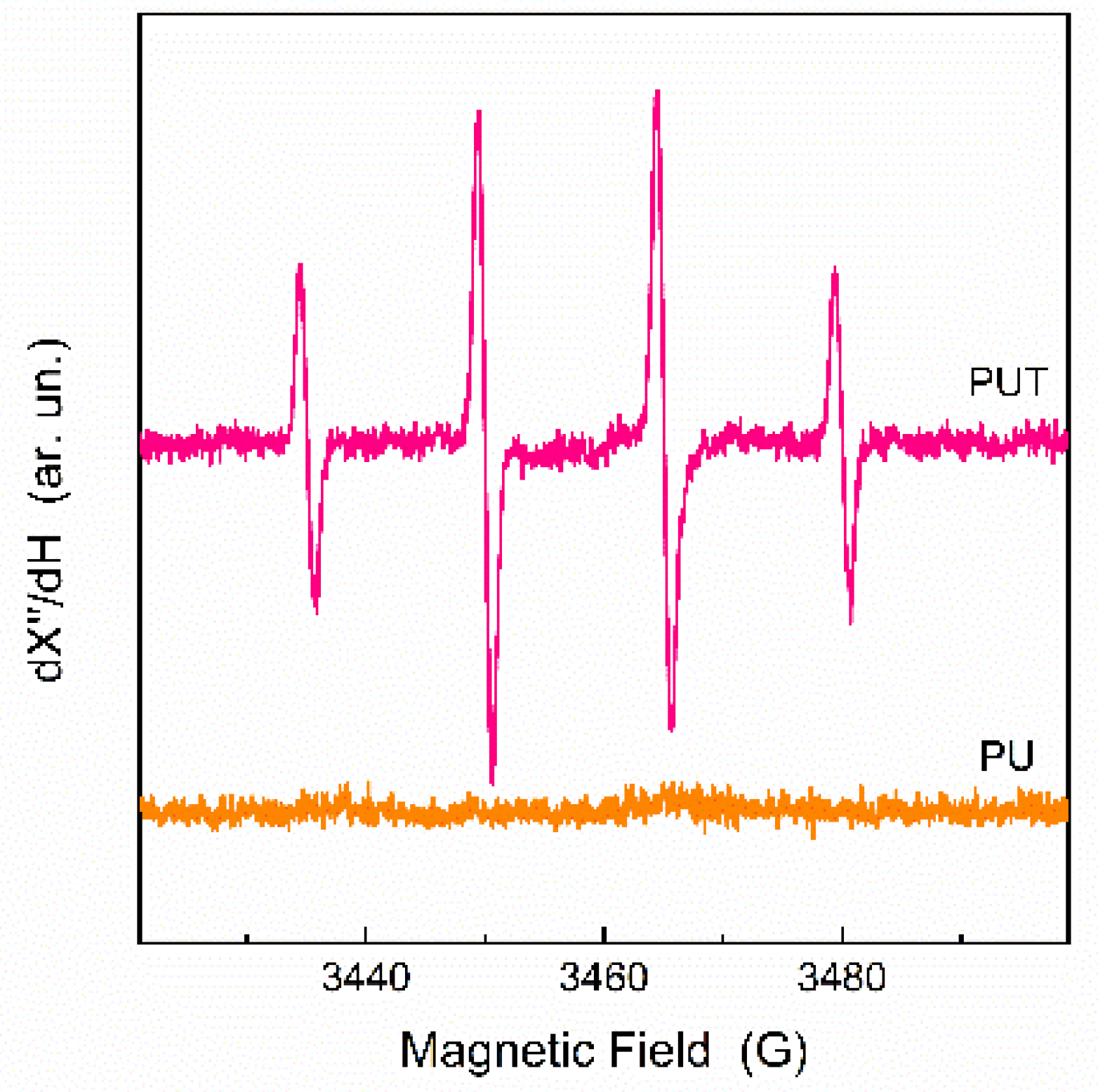
3.1. Materials Characterization
3.2. Dye Adsorption Evaluation
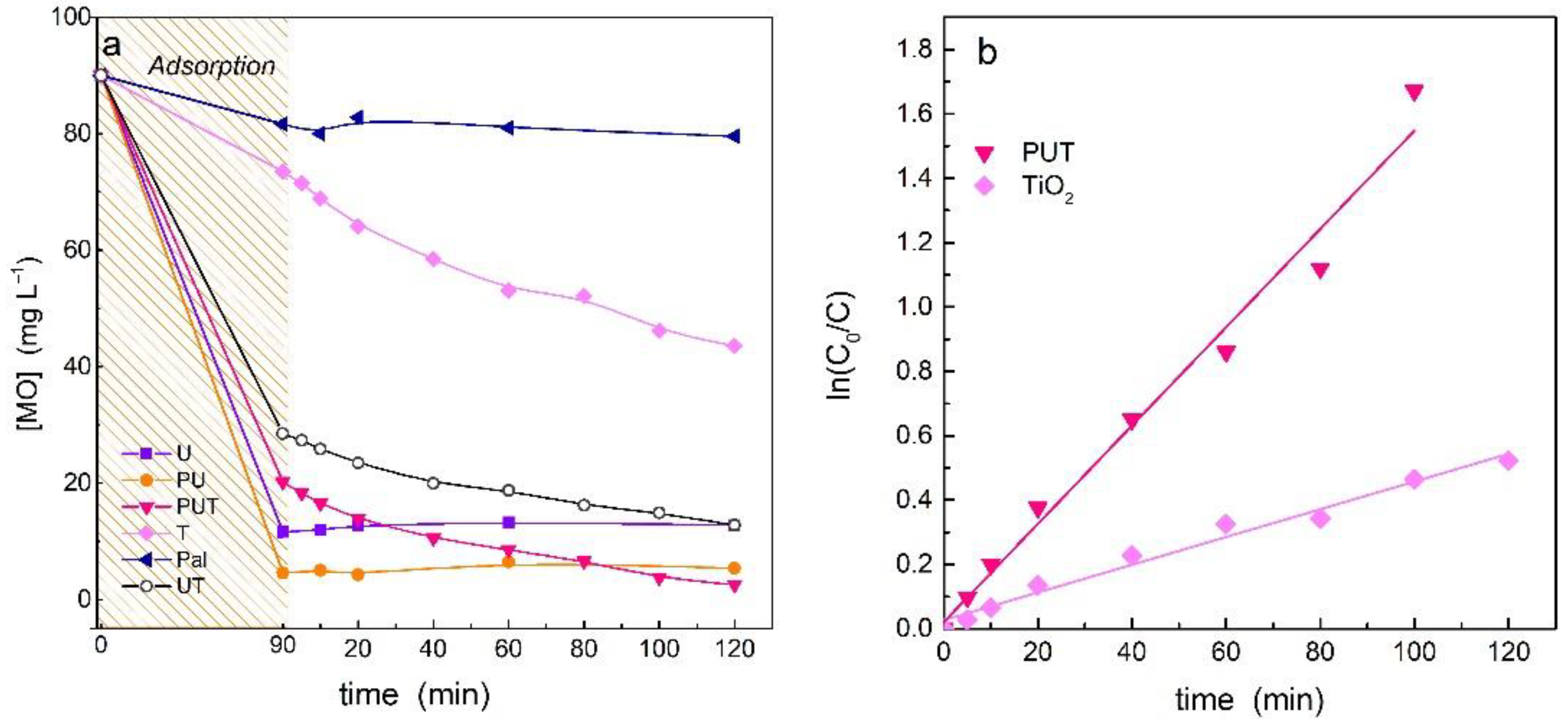
3.3. Photocatalytic Activity Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Saravanan, A.; Kumar, P.S.; Yaashikaa, P.R.; Karishma, S.; Jeevanantham, S.; Swetha, S. Mixed Biosorbent of Agro Waste and Bacterial Biomass for the Separation of Pb(II) Ions from Water System. Chemosphere 2021, 277, 130236. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.J.; Ampiaw, R.E.; Lee, W. Adsorptive Removal of Dyes from Wastewater Using a Metal-Organic Framework: A Review. Chemosphere 2021, 284, 131314. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, A.; Loganathan, M.; Senthil Kumar, P.; Vo, D.-V.N. Cobalt and Nickel Oxides Supported Activated Carbon as an Effective Photocatalysts for the Degradation Methylene Blue Dye from Aquatic Environment. Sustain. Chem. Pharm. 2021, 21, 100406. [Google Scholar] [CrossRef]
- Renita, A.A.; Vardhan, K.H.; Kumar, P.S.; Ngueagni, P.T.; Abilarasu, A.; Nath, S.; Kumari, P.; Saravanan, R. Effective Removal of Malachite Green Dye from Aqueous Solution in Hybrid System Utilizing Agricultural Waste as Particle Electrodes. Chemosphere 2021, 273, 129634. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Chen, M.; Wang, H.; Zeng, G.; Huang, D.; Cheng, M.; Liu, Y.; Xue, W.; Wang, Z. The Application of Different Typological and Structural MOFs-Based Materials for the Dyes Adsorption. Coord. Chem. Rev. 2019, 380, 471–483. [Google Scholar] [CrossRef]
- Abhinaya, M.; Parthiban, R.; Kumar, P.S.; Vo, D.-V.N. A Review on Cleaner Strategies for Extraction of Chitosan and Its Application in Toxic Pollutant Removal. Environ. Res. 2021, 196, 110996. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; AlGarni, T.S.; Kumar, P.S.; Bhogal, S.; Kumar, A.; Sharma, S.; Naushad, M.; ALOthman, Z.A.; Stadler, F.J. Utilization of Ag2O–Al2O3–ZrO2 Decorated onto RGO as Adsorbent for the Removal of Congo Red from Aqueous Solution. Environ. Res. 2021, 197, 111179. [Google Scholar] [CrossRef]
- Ullah, S.; Al-Sehemi, A.G.; Mubashir, M.; Mukhtar, A.; Saqib, S.; Bustam, M.A.; Cheng, C.K.; Ibrahim, M.; Show, P.L. Adsorption Behavior of Mercury over Hydrated Lime: Experimental Investigation and Adsorption Process Characteristic Study. Chemosphere 2021, 271, 129504. [Google Scholar] [CrossRef]
- Yang, R.; Li, D.; Li, A.; Yang, H. Adsorption Properties and Mechanisms of Palygorskite for Removal of Various Ionic Dyes from Water. Appl. Clay Sci. 2018, 151, 20–28. [Google Scholar] [CrossRef]
- Christoforidis, K.C.; Montini, T.; Bontempi, E.; Zafeiratos, S.; Jaén, J.J.D.; Fornasiero, P. Synthesis and Photocatalytic Application of Visible-Light Active β-Fe2O3/g-C3N4 Hybrid Nanocomposites. Appl. Catal. B Environ. 2016, 187, 171–180. [Google Scholar] [CrossRef]
- Dias, E.M.; Petit, C. Towards the Use of Metal–Organic Frameworks for Water Reuse: A Review of the Recent Advances in the Field of Organic Pollutants Removal and Degradation and the next Steps in the Field. J. Mater. Chem. A 2015, 3, 22484–22506. [Google Scholar] [CrossRef]
- Akpan, U.G.; Hameed, B.H. Parameters Affecting the Photocatalytic Degradation of Dyes Using TiO2-Based Photocatalysts: A Review. J. Hazard. Mater. 2009, 170, 520–529. [Google Scholar] [CrossRef]
- Salleh, M.A.M.; Mahmoud, D.K.; Karim, W.A.W.A.; Idris, A. Cationic and Anionic Dye Adsorption by Agricultural Solid Wastes: A Comprehensive Review. Desalination 2011, 280, 1–13. [Google Scholar] [CrossRef]
- Cheung, W.H.; Szeto, Y.S.; McKay, G. Enhancing the Adsorption Capacities of Acid Dyes by Chitosan Nano Particles. Bioresour. Technol. 2009, 100, 1143–1148. [Google Scholar] [CrossRef] [PubMed]
- Belaid, K.D.; Kacha, S.; Kameche, M.; Derriche, Z. Adsorption Kinetics of Some Textile Dyes onto Granular Activated Carbon. J. Environ. Chem. Eng. 2013, 1, 496–503. [Google Scholar] [CrossRef]
- Crake, A.; Christoforidis, K.C.; Gregg, A.; Moss, B.; Kafizas, A.; Petit, C. The Effect of Materials Architecture in TiO2 /MOF Composites on CO2 Photoreduction and Charge Transfer. Small 2019, 15, 1805473. [Google Scholar] [CrossRef]
- Crake, A.; Christoforidis, K.C.; Kafizas, A.; Zafeiratos, S.; Petit, C. CO2 Capture and Photocatalytic Reduction Using Bifunctional TiO2/MOF Nanocomposites under UV–Vis Irradiation. Appl. Catal. B Environ. 2017, 210, 131–140. [Google Scholar] [CrossRef]
- Au, V.K.-M. Recent Advances in the Use of Metal-Organic Frameworks for Dye Adsorption. Front. Chem. 2020, 8, 708. [Google Scholar] [CrossRef]
- Ramírez, D.J.; Alfonso Herrera, L.A.; Colorado-Peralta, R.; Rodríguez, R.P.; Camarillo Reyes, P.K.; Chiñas, L.E.; Sánchez, M.; Rivera, J.M. Highly Efficient Methyl Orange Adsorption by UV-012, a New Crystalline Coii MOF. CrystEngComm 2021, 23, 3537–3548. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, K.; Li, X.; Huang, L.; Liang, J.; Zheng, G.; Shan, G. Nickel-Metal-Organic Framework Nanobelt Based Composite Membranes for Efficient Sr2+ Removal from Aqueous Solution. Environ. Sci. Ecotechnology 2020, 3, 100035. [Google Scholar] [CrossRef]
- Cheng, J.; Liang, J.; Dong, L.; Chai, J.; Zhao, N.; Ullah, S.; Wang, H.; Zhang, D.; Imtiaz, S.; Shan, G.; et al. Self-Assembly of 2D-Metal–Organic Framework/Graphene Oxide Membranes as Highly Efficient Adsorbents for the Removal of Cs+ from Aqueous Solutions. RSC Adv. 2018, 8, 40813–40822. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, M.; Sadeghian, S.; Mansouri, G.; Setareshenas, N. Enhanced Photocatalytic Performance of UiO-66-NH2/TiO2 Composite for Dye Degradation. Environ. Sci. Pollut. Res. 2021, 28, 25552–25565. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhuang, Y.; Dong, L.; Mu, H.; Tian, S.; Wang, L.; Huang, A. Preparation of CeO2/UiO-66-NH2 Heterojunction and Study on a Photocatalytic Degradation Mechanism. Materials 2022, 15, 2564. [Google Scholar] [CrossRef] [PubMed]
- Man, Z.; Meng, Y.; Lin, X.; Dai, X.; Wang, L.; Liu, D. Assembling UiO-66@TiO2 Nanocomposites for Efficient Photocatalytic Degradation of Dimethyl Sulfide. Chem. Eng. J. 2022, 431, 133952. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Zhang, M.; Duan, W.; Liu, B. A Dual-Functional UiO-66/TiO2 Composite for Water Treatment and CO 2 Capture. RSC Adv. 2017, 7, 16232–16237. [Google Scholar] [CrossRef]
- Abidi, N.; Errais, E.; Duplay, J.; Berez, A.; Jrad, A.; Schäfer, G.; Ghazi, M.; Semhi, K.; Trabelsi-Ayadi, M. Treatment of Dye-Containing Effluent by Natural Clay. J. Clean. Prod. 2015, 86, 432–440. [Google Scholar] [CrossRef]
- Lagaly, G. Colloid Clay Science. In Developments in Clay Science; Bergaya, F., Theng, B.K.G., Lagaly, G., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2006; Volume 1, pp. 141–245. ISBN 978-0-08-044183-2. [Google Scholar]
- Moreira, M.A.; Ciuffi, K.J.; Rives, V.; Vicente, M.A.; Trujillano, R.; Gil, A.; Korili, S.A.; de Faria, E.H. Effect of Chemical Modification of Palygorskite and Sepiolite by 3-Aminopropyltriethoxisilane on Adsorption of Cationic and Anionic Dyes. Appl. Clay Sci. 2017, 135, 394–404. [Google Scholar] [CrossRef]
- Middea, A.; Spinelli, L.S.; Souza Jr, F.G.; Neumann, R.; Fernandes, T.L.A.P.; Gomes, O. da F.M. Preparation and Characterization of an Organo-Palygorskite-Fe3O4 Nanomaterial for Removal of Anionic Dyes from Wastewater. Appl. Clay Sci. 2017, 139, 45–53. [Google Scholar] [CrossRef]
- Chen, H.; Zhong, A.; Wu, J.; Zhao, J.; Yan, H. Adsorption Behaviors and Mechanisms of Methyl Orange on Heat-Treated Palygorskite Clays. Ind. Eng. Chem. Res. 2012, 51, 14026–14036. [Google Scholar] [CrossRef]
- Zhao, G.; Shi, L.; Feng, X.; Yu, W.; Zhang, D.; Fu, J. Palygorskite-Cerium Oxide Filled Rubber Nanocomposites. Appl. Clay Sci. 2012, 67–68, 44–49. [Google Scholar] [CrossRef]
- Christoforidis, K.C.; Montini, T.; Fittipaldi, M.; Jaén, J.J.D.; Fornasiero, P. Photocatalytic Hydrogen Production by Boron Modified TiO2/Carbon Nitride Heterojunctions. ChemCatChem 2019, 11, 6408–6416. [Google Scholar] [CrossRef]
- Christoforidis, K.C.; Fornasiero, P. Photocatalytic Hydrogen Production: A Rift into the Future Energy Supply. ChemCatChem 2017, 9, 1523–1544. [Google Scholar] [CrossRef]
- Gao, Y.; Zheng, Y.; Chai, J.; Tian, J.; Jing, T.; Zhang, D.; Cheng, J.; Peng, H.; Liu, B.; Zheng, G. Highly Effective Photocatalytic Performance of {001}-TiO2/MoS2/RGO Hybrid Heterostructures for the Reduction of Rh B. RSC Adv. 2019, 9, 15033–15041. [Google Scholar] [CrossRef] [PubMed]
- Reza, K.M.; Kurny, A.; Gulshan, F. Parameters Affecting the Photocatalytic Degradation of Dyes Using TiO2: A Review. Appl. Water Sci. 2017, 7, 1569–1578. [Google Scholar] [CrossRef]
- Ling, L.; Wang, Y.; Zhang, W.; Ge, Z.; Duan, W.; Liu, B. Preparation of a Novel Ternary Composite of TiO2/UiO-66-NH2/Graphene Oxide with Enhanced Photocatalytic Activities. Catal. Lett. 2018, 148, 1978–1984. [Google Scholar] [CrossRef]
- Papoulis, D.; Panagiotaras, D.; Tsigrou, P.; Christoforidis, K.C.; Petit, C.; Apostolopoulou, A.; Stathatos, E.; Komarneni, S.; Koukouvelas, I. Halloysite and Sepiolite–TiO2 nanocomposites: Synthesis Characterization and Photocatalytic Activity in Three Aquatic Wastes. Mater. Sci. Semicond. Processing 2018, 85, 1–8. [Google Scholar] [CrossRef]
- Papoulis, D.; Komarneni, S.; Panagiotaras, D.; Nikolopoulou, A.; Christoforidis, K.C.; Fernández-Garcia, M.; Li, H.; Shu, Y.; Sato, T. Palygorskite-TiO2 Nanocomposites: Part 2. Photocatalytic Activities in Decomposing Air and Organic Pollutants. Appl. Clay Sci. 2013, 83–84, 198–202. [Google Scholar] [CrossRef]
- Papoulis, D.; Komarneni, S.; Panagiotaras, D.; Stathatos, E.; Christoforidis, K.C.; Fernández-García, M.; Li, H.; Shu, Y.; Sato, T.; Katsuki, H. Three-Phase Nanocomposites of Two Nanoclays and TiO2: Synthesis, Characterization and Photacatalytic Activities. Appl. Catal. B Environ. 2014, 147, 526–533. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, Q.; Li, J.; Feng, R.; Xu, H.; Xue, C. Adsorption of Methyl Orange on Magnetically Separable Mesoporous Titania Nanocomposite. Chin. J. Chem. Eng. 2014, 22, 1168–1173. [Google Scholar] [CrossRef]
- Jafari, S.; Sillanpää, M. Adsorption of Dyes onto Modified Titanium Dioxide. In Advanced Water Treatment; Elsevier: Amsterdam, The Netherlands, 2020; pp. 85–160. [Google Scholar]
- Wu, W.; Yao, T.; Xiang, Y.; Zou, H.; Zhou, Y. Efficient Removal of Methyl Orange by a Flower-like TiO2 /MIL-101(Cr) Composite Nanomaterial. Dalton Trans. 2020, 49, 5722–5729. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Q.; Li, Y.; Zhang, R.; Lu, G. Large-Scale Synthesis of Monodisperse UiO-66 Crystals with Tunable Sizes and Missing Linker Defects via Acid/Base Co-Modulation. ACS Appl. Mater. Interfaces 2017, 9, 15079–15085. [Google Scholar] [CrossRef] [PubMed]
- Christoforidis, K.C.; Figueroa, S.J.A.; Fernández-García, M. Iron-Sulfur Codoped TiO2 Anatase Nano-Materials: UV and Sunlight Activity for Toluene Degradation. Appl. Catal. B Environ. 2012, 117–118, 310–316. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, Z.; Yang, Z.; Wang, J.; Xie, J.; Fu, M.; Hu, Y. TiO 2 @UiO-66 Composites with Efficient Adsorption and Photocatalytic Oxidation of VOCs: Investigation of Synergistic Effects and Reaction Mechanism. ChemCatChem 2021, 13, 581–591. [Google Scholar] [CrossRef]
- Han, Y.; Liu, M.; Li, K.; Zuo, Y.; Wei, Y.; Xu, S.; Zhang, G.; Song, C.; Zhang, Z.; Guo, X. Facile Synthesis of Morphology and Size-Controlled Zirconium Metal–Organic Framework UiO-66: The Role of Hydrofluoric Acid in Crystallization. CrystEngComm 2015, 17, 6434–6440. [Google Scholar] [CrossRef]
- Tang, J.; Dong, W.; Wang, G.; Yao, Y.; Cai, L.; Liu, Y.; Zhao, X.; Xu, J.; Tan, L. Efficient Molybdenumvi Modified Zr-MOF Catalysts for Epoxidation of Olefins. RSC Adv. 2014, 4, 42977–42982. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, T.; Liu, X.; Yue, C.; Ao, L.; Deng, T.; Zhang, Y. Heteropoly Acid-Encapsulated Metal–Organic Framework as a Stable and Highly Efficient Nanocatalyst for Esterification Reaction. RSC Adv. 2019, 9, 16357–16365. [Google Scholar] [CrossRef]
- Christoforidis, K.C.; Iglesias-Juez, A.; Figueroa, S.J.A.; Newton, M.A.; Michiel, M.D.; Fernández-García, M. A Structural and Surface Approach to Size and Shape Control of Sulfur-Modified Undoped and Fe-Doped TiO2 Anatase Nano-Materials. Phys. Chem. Chem. Phys. 2012, 14, 5628–5634. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, P.; Wang, Y.; Wen, K.; Su, X.; Zhu, R.; He, H.; Xi, Y. Effect of Acid Activation of Palygorskite on Their Toluene Adsorption Behaviors. Appl. Clay Sci. 2018, 159, 60–67. [Google Scholar] [CrossRef]
- Giustetto, R.; Chiari, G. Crystal Structure Refinement of Palygorskite from Neutron Powder Diffraction. Eur. J. Mineral. 2004, 16, 521–532. [Google Scholar] [CrossRef]
- Giustetto, R.; Seenivasan, K.; Pellerej, D.; Ricchiardi, G.; Bordiga, S. Spectroscopic Characterization and Photo/Thermal Resistance of a Hybrid Palygorskite/Methyl Red Mayan Pigment. Microporous Mesoporous Mater. 2012, 155, 167–176. [Google Scholar] [CrossRef]
- Christoforidis, K.C.; Melchionna, M.; Montini, T.; Papoulis, D.; Stathatos, E.; Zafeiratos, S.; Kordouli, E.; Fornasiero, P. Solar and Visible Light Photocatalytic Enhancement of Halloysite Nanotubes/g-C3N4 Heteroarchitectures. RSC Adv. 2016, 6, 86617–86626. [Google Scholar] [CrossRef]
- Shahriyari Far, H.; Hasanzadeh, M.; Najafi, M.; Rahimi, R. In-Situ Self-Assembly of Mono- and Bi-Metal Organic Frameworks onto Clay Mineral for Highly Efficient Adsorption of Pollutants from Wastewater. Chem. Phys. Lett. 2022, 799, 139626. [Google Scholar] [CrossRef]
- Hidayat, A.R.P.; Sulistiono, D.O.; Murwani, I.K.; Endrawati, B.F.; Fansuri, H.; Zulfa, L.L.; Ediati, R. Linear and Nonlinear Isotherm, Kinetic and Thermodynamic Behavior of Methyl Orange Adsorption Using Modulated Al2O3@UiO-66 via Acetic Acid. J. Environ. Chem. Eng. 2021, 9, 106675. [Google Scholar] [CrossRef]
- Vasiliadou, I.A.; Ioannidou, T.; Anagnostopoulou, M.; Polyzotou, A.; Papoulis, D.; Christoforidis, K.C. Adsorption of Methyl Orange on a Novel Palygorskite/UiO-66 Nanocomposite. Appl. Sci. 2022, 12, 7468. [Google Scholar] [CrossRef]
- Yang, J.-M. A Facile Approach to Fabricate an Immobilized-Phosphate Zirconium-Based Metal-Organic Framework Composite (UiO-66-P) and Its Activity in the Adsorption and Separation of Organic Dyes. J. Colloid Interface Sci. 2017, 505, 178–185. [Google Scholar] [CrossRef]
- Chen, Q.; He, Q.; Lv, M.; Xu, Y.; Yang, H.; Liu, X.; Wei, F. Selective Adsorption of Cationic Dyes by UiO-66-NH2. Appl. Surf. Sci. 2015, 327, 77–85. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-Second Order Model for Sorption Processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Yao, P.; Liu, H.; Wang, D.; Chen, J.; Li, G.; An, T. Enhanced Visible-Light Photocatalytic Activity to Volatile Organic Compounds Degradation and Deactivation Resistance Mechanism of Titania Confined inside a Metal-Organic Framework. J. Colloid Interface Sci. 2018, 522, 174–182. [Google Scholar] [CrossRef]
- Li, F.; Sun, S.; Jiang, Y.; Xia, M.; Sun, M.; Xue, B. Photodegradation of an Azo Dye Using Immobilized Nanoparticles of TiO2 Supported by Natural Porous Mineral. J. Hazard. Mater. 2008, 152, 1037–1044. [Google Scholar] [CrossRef]
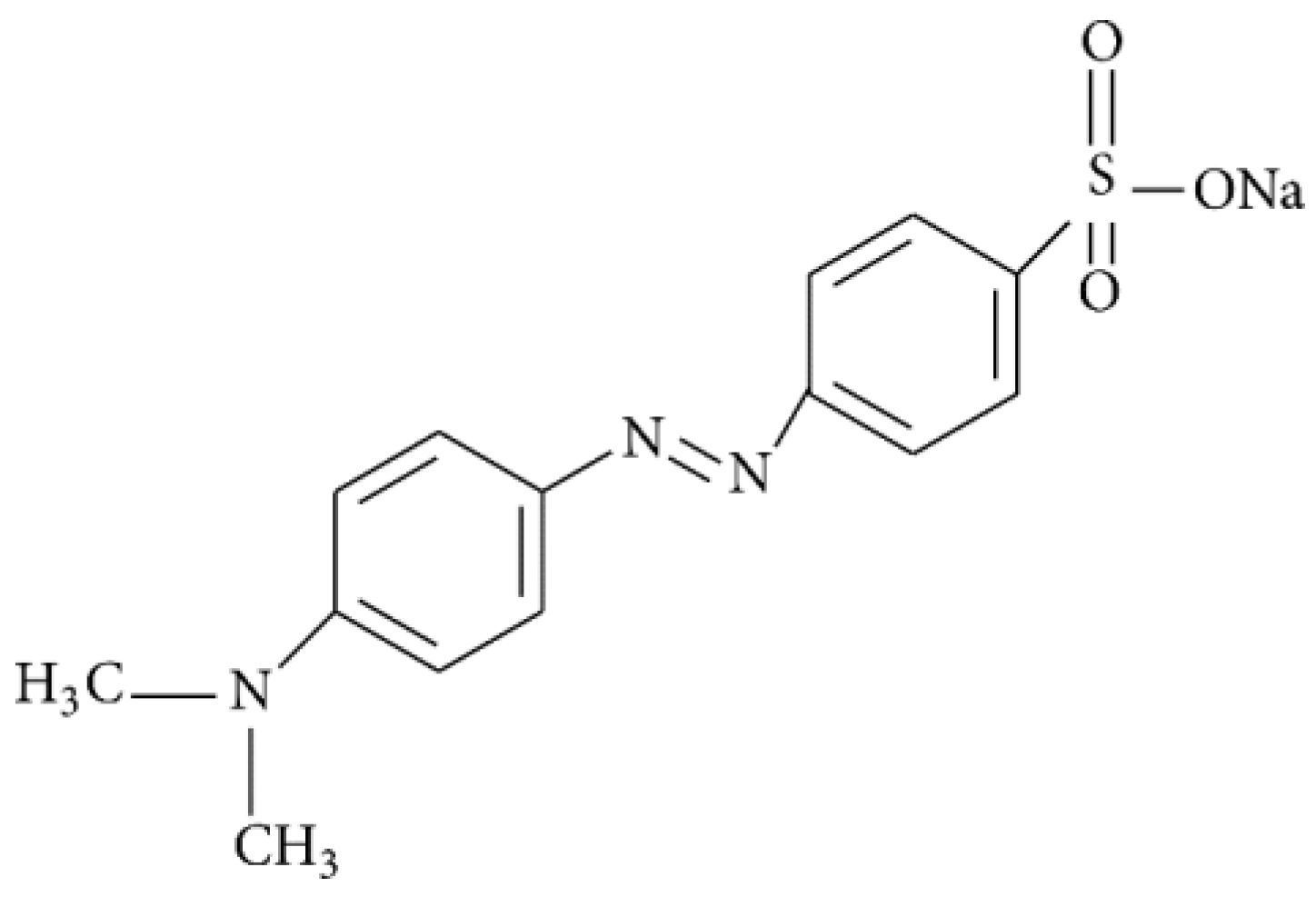



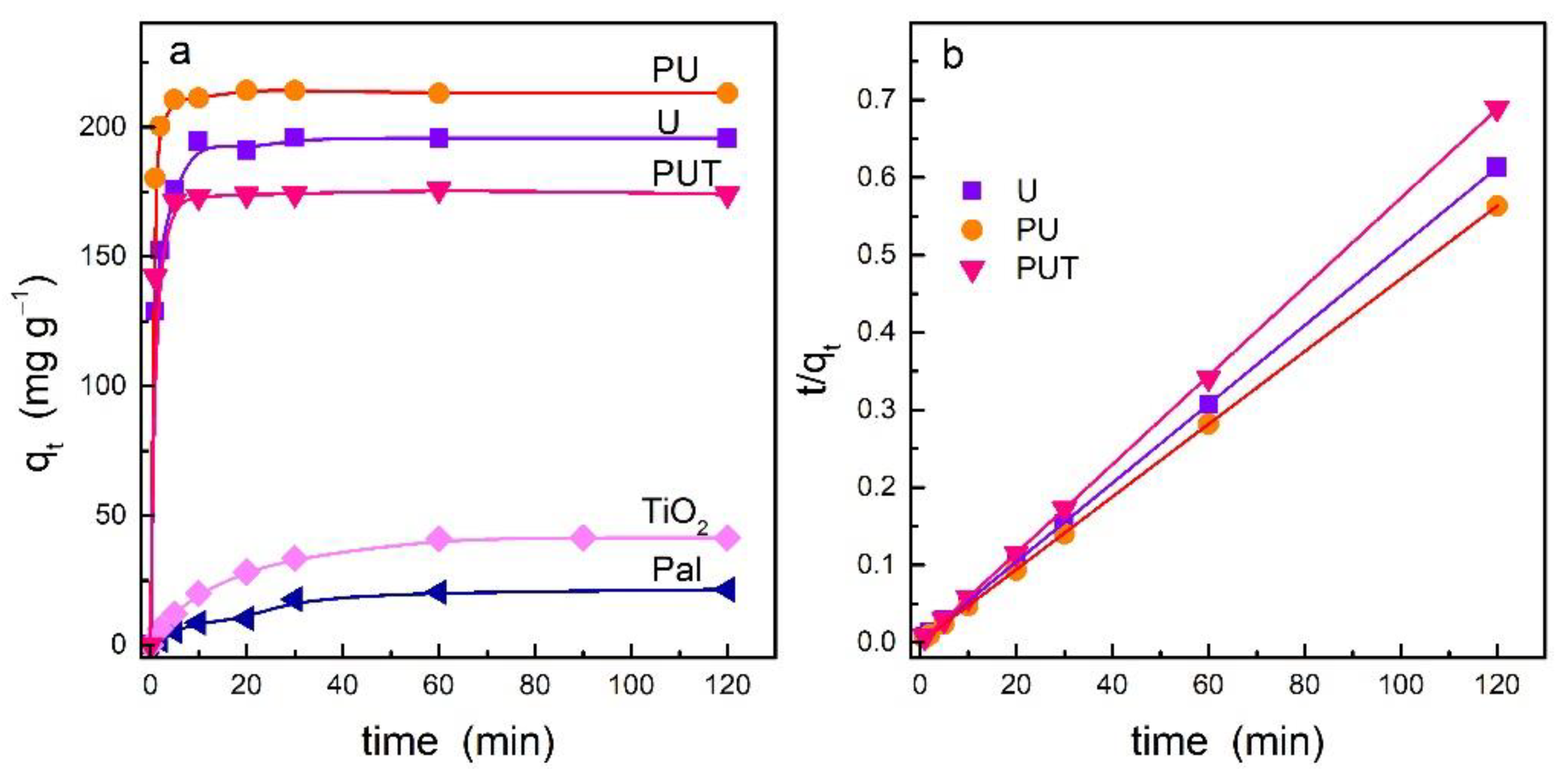
| Sample | qe,exp (mg·g−1) | RE (%) 1 | qe,cal (mg·g−1) | k2 (g·mg−1·min−1) 2 | R2 | k (min−1) 3 |
|---|---|---|---|---|---|---|
| U | 195.7 | 87.1 | 196.1 | 0.012 | 0.999 | |
| PU | 213.3 | 94.9 | 212.8 | 0.074 | 0.999 | |
| PUT | 174.7 | 77.5 | 175.4 | 0.108 | 0.999 | 0.0153 |
| TiO2 | 41.3 | 18.3 | 0.0043 | |||
| Pal | 20.9 | 9.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ioannidou, T.; Anagnostopoulou, M.; Papoulis, D.; Christoforidis, K.C.; Vasiliadou, I.A. UiO-66/Palygorskite/TiO2 Ternary Composites as Adsorbents and Photocatalysts for Methyl Orange Removal. Appl. Sci. 2022, 12, 8223. https://doi.org/10.3390/app12168223
Ioannidou T, Anagnostopoulou M, Papoulis D, Christoforidis KC, Vasiliadou IA. UiO-66/Palygorskite/TiO2 Ternary Composites as Adsorbents and Photocatalysts for Methyl Orange Removal. Applied Sciences. 2022; 12(16):8223. https://doi.org/10.3390/app12168223
Chicago/Turabian StyleIoannidou, Thaleia, Maria Anagnostopoulou, Dimitrios Papoulis, Konstantinos C. Christoforidis, and Ioanna A. Vasiliadou. 2022. "UiO-66/Palygorskite/TiO2 Ternary Composites as Adsorbents and Photocatalysts for Methyl Orange Removal" Applied Sciences 12, no. 16: 8223. https://doi.org/10.3390/app12168223
APA StyleIoannidou, T., Anagnostopoulou, M., Papoulis, D., Christoforidis, K. C., & Vasiliadou, I. A. (2022). UiO-66/Palygorskite/TiO2 Ternary Composites as Adsorbents and Photocatalysts for Methyl Orange Removal. Applied Sciences, 12(16), 8223. https://doi.org/10.3390/app12168223









