Abstract
This review summarizes the current knowledge on current and future applications of carbon nanoparticles in medicine. The carbon nanoparticle family has a large number of representatives with unique physicochemical properties that make them good candidates for use in clinical medicine. The best-known (and most researched) carbon nanoparticles include graphene, graphene oxide, and carbon nanotubes. The main direction of use involves medical diagnostics, which includes bioimaging and the detection of chemicals or metabolites present in the body. Since the question of nanoparticle toxicity has not been fully answered, the use of nanoparticles in the fields of therapeutics (drug delivery), regenerative medicine (cell scaffolding, tissue engineering), and vaccine production is still under research and many in vivo studies are ongoing. These preclinical studies suggest that carbon nanoparticles have great potential for diagnosis and treatment; the results show that the nanoparticles used do not have significant toxic effects; however, great caution is needed before nanoparticles are introduced into routine clinical practice.
1. Introduction
Carbon is one of the most abundant minerals on Earth. It is one component of a large number of macromolecules that are essential for life, including sugars, proteins, DNA, etc.
Pure carbon exists in various forms, such as allotropes. The most well-known natural crystalline carbon allotropes are diamonds and graphite; amorphous carbon allotropes represent coal, charcoal, and lampblack. The extraordinary properties of carbon are exploited in carbon-based nanomaterials. The first carbon nanoparticles (CNPs) were discovered in the 1980s [1,2]. CNPs represent a wide range of carbon individuals, including amorphous carbon nanoparticles (ultrafine carbon particles, carbon nanoparticles, and carbon dots), sp2 carbon nanomaterials (fullerene, carbon nanotubes, carbon nanohorns, graphene, graphene quantum dots), and nanodiamonds [3].
CNPs are formed from pure carbon; therefore, they have high stability, outstanding electrical and heat conductivity, mechanical properties (extreme stiffness, strength, and toughness), and are highly biocompatible with low toxicity; furthermore, they are highly hydrophobic due to their sp2 hybridization [4]. One of the most studied CNPs is graphene, which is a structural parent of several carbon allotropes (graphenoids), including nanorings, single-, double-, and multi-walled nanotubes, graphite, carbon fibers, and graphyne (Figure 1) [5,6].
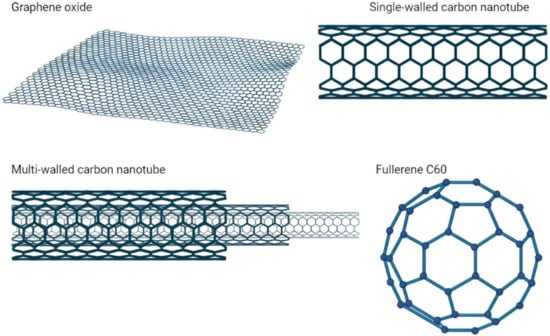
Figure 1.
Main types of carbon nanoparticles.
CNPs have been studied in depth, moved out of laboratories, and produced at large scales. The industrial production of nanoparticles, such as graphene, nanotubes, or fullerenes, is performed via various processes. The main processes for the mass production of graphene include the chemical or plasma exfoliation of raw graphite, mechanical cleavage from natural graphite, chemical vapor deposition, or the epitaxial growth of graphene on the silicon face of silicon carbide [7]. It is possible to manufacture graphene from its derivates, by unzipping carbon nanotubes or via the evaporation of fullerene [8]. The most commonly used graphene derivatives, graphene oxide (GO) and reduced GO (rGO), are prepared by the oxidation of graphene. In these processes, graphene is treated with oxidizing agents: protonated solvents (nitric, sulfuric, or phosphoric acid, or their mixtures) and KMnO4, resulting in the formation of a covalent bond of carboxyl and hydroxyl groups to graphene. To form rGO, a reduction is then carried out in which most of the oxygen functional groups are removed by various processes, including treatment with vitamin C or hydrazine hydrate [9,10].
Carbon nanotubes (CNTs) are nanoparticles that have cylindrical tubular structures with a diameter of nanometers and are formed by rolling graphene sheets. CNTs are divided into single-walled CNTs and multi-walled CNTs according to the number of layers of graphene sheets [11].
The most frequent methods of synthesizing carbon nanotubes involve chemical vapor deposition (CVD), arc discharge, and pulsed laser ablation. The CVD is performed using carbon-containing precursor gases, such as CO2 or C2H2, C2H4, and other hydrocarbons. The temperature during CVD moves around 350–1000 °C. Various parameters, such as the reaction time, temperature, catalyst particle size, or type/velocity of the reaction gas affect the growth of CNTs [12]. The arc discharge method is based on the use of two graphite rods, which are used as cathode and anode electrodes. The direct current arc voltage is subsequently applied across the electrodes immersed in an inert gas (helium). During this method, fullerenes and CNTs are synthesized. Fullerenes are deposited as soot inside the chamber and multi-walled carbon nanotubes are deposited on the cathode [13]. Pulsed laser ablation of graphite is a very promising method. It is fast and inexpensive, does not require the use of a catalyst or vacuum, and produces a high purity product of the desired size and morphology [14].
CNPs have found widespread use in various industries, including electronics, agriculture, food, pharmaceuticals, cosmetics, and medicine. In particular, carbon nanomaterials have many potential biomedical applications, including imaging, biosensors, anticancer drugs, drug delivery vehicles, and bioengineering [15,16]. In this review, we focused on the use of various types of CNPs in different fields of medicine (Figure 2).
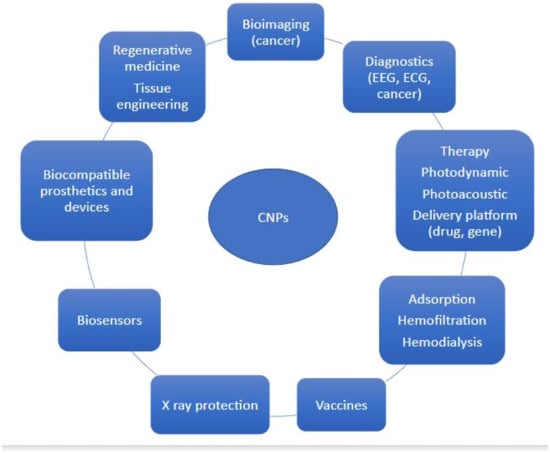
Figure 2.
Application of CNPs.
2. Bioimaging, Detection, and Diagnostics of Various Pathologies
Early and accurate diagnoses are essential for treatment outcomes, whether it is the detection of damaged tissue or cancer. CNPs can significantly improve the quality of medical imaging.
Most CNPs have the natural ability to emit fluorescence upon photoexcitation [17,18]. Carbon dots, graphene quantum dots, and carbon nanotubes are widely used. Lastly, the ‘named’ has an ultra-large surface and can carry multiple molecules and ensure multiplex sensing; on the other hand, CNPs with fast excretion via the kidney or hepatobiliary system (carbon dots under 10 nm) are suitable for bioimaging [19].
Based on the different nanostructures and functionalization of CNPs, the individual organelles and cells can be imaged and the fluorescence can be targeted.
After intravenous application in mice, the functionalized carbon dots rapidly move into the tissue and accumulate in the lung, liver, and kidney, but also cross the blood–brain barrier [20]. The M13 virus coated with single-wall carbon nanotubes can especially target the tumor cells. Additionally, radionuclide-labeled particles (Technetium-99 m Tc) target tumors and are detectable by positron emission tomography (PET) [21,22]. These methods can combine the fluorescence effect of CNPs with medical imaging techniques, such as computed tomography (CT), magnetic resonance imaging (MRI), or PET. This combination enhances the quality and sensitivity of imaging and displays more details with better contrast [23].
The use of CNPs also allows deep-tissue optical imaging by near-infrared optical imaging. The near-infrared system (NIR-II; 1000–1700 nm) has shown a deeper penetration depth and, therefore, can explore deep tissue information with higher resolution and no autofluorescence. The benefits include whole body and brain angiography (visualization of blood flow, microcirculation), visualization of organs, and imaging-guided cancer therapy or surgery [24,25].
The practical intraoperative use of CNPs is well documented. Li et al. studied the transport and accumulation of CNPs (mean 150 nm) into the lymph nodes of patients with advanced gastric cancer during gastrectomy with lymph node dissection. Identification/staining of metastatic lymph nodes is essential to reduce the risk of cancer recurrence. Thirty patients participated in the study. No serious adverse reactions were documented after intravenous application. CNPs accumulated selectively in the draining lymph nodes and colored them black. This method could detect the smaller lymph nodes; thus, the number of potentially dangerous lymph nodes was higher [26].
The same effect has been documented in the preoperative and perioperative detection of lymph nodes in patients with thyroid, colorectal, and breast cancer [27,28,29]. In thyroid cancer, CNPs help discriminate lymph nodes and parathyroid glands [30]. Importantly, CNPs used for endoscopic tattooing of colorectal cancer allow the detection and precise localization of tumors and precancerous lesions before and during surgery. This allows an accurate resection to be performed and, thus, reduces surgical trauma [31].
Interestingly, nanodiamond nanoparticles with silicon-vacancy centers can easily be up-taken by cells and, thus, can be used for live-cell dual-color imaging and intracellular tracking. They have shown greater photostability and narrow emissions in the near-infrared region [32].
Graphene and graphene-based nanomaterials can be used to detect the neural interface. Garcia-Cortadella et al. created a sensing system composed of a flexible 64-channel graphene solution-gated field-effect transistor array and a wireless headstage. In vivo, they performed 24-h quasi-continuous monitoring of brain activity to assess the sensitivity of the method. They recorded epicortical brain activity in spontaneously behaving rats, allowing them to investigate associations between patterns of neural activity and behavioral events. The results suggest that graphene-based sensor arrays can be used at least in neuroscience research [33].
Ko et al. described the development of electroencephalography electrodes using graphene/GO. These electrodes can be applied without conductive gel. Although the electroencephalogram (EEG) examination is painless for patients, the use of conductive gel is very uncomfortable [34]. The advantages of using graphene-based dry electrodes have also been described by Faisal et al. [35].
Finally, it should be mentioned that graphene electrodes can stimulate and record neuronal activity, which can be used in neural stimulation techniques (deep brain stimulation; retinal or cochlear implants) [36].
The CNP-based material has high electrical conductivity and the CNP transistor allows high amplification of cardiac-electrical signals and, thus, can be used as part of the electrodes for recording cardiac electrophysiological signals.
Graphene or multi-walled carbon nanotubes (MWCNTs) are often used, not only for short-term monitoring in the hospital but also for long-term monitoring as wearable devices to monitor the electroencephalogram (ECG) changes in real-time during daily activities [37,38].
3. Photoacoustic Diagnostic
Photoacoustic imaging (photoacoustic microscopy and photoacoustic computed tomography; PA) is non-invasive biomedical imaging based on the photoacoustic effect, where the light/laser pulses are absorbed by exogenous or endogenous contrast agents and converted into thermal energy that is emitted as an ultrasound signal. PA has a high spatial/temporal resolution with relatively deep tissue imaging ability that allows detecting or treating a wide variety of cancers (breast, colorectal, and skin).
CNPs serve as good contrast agents, especially carbon nanotubes, nanodots, graphene, or graphene oxide conjugated with organic polymers, metallic NPs, or dyes [39,40]. These contrast agents can target specific tissues; cyclic Arg-Gly-Asp peptides loaded on single-walled carbon nanotubes are preferentially up-taken in various types of tumors [41] Han et al. described ultra-small near-infrared-II photothermal carbon dots that can easily penetrate tumor tissue and showed multicolor fluorescence and emit photoacoustic signals in vitro and in vivo [42].
4. Adsorption (Hemosorbents)/Hemofiltration/Hemodialysis
Carbon materials have high adsorption capacities and are widely used in clinical practice. CNPs can be used to adsorb ions, drugs, toxins, proinflammatory proteins, metabolites, and lipids from blood circulation and, thereby, reduce toxicity.
The study by Zheng et al. showed that graphene nanoplatelets can adsorb proinflammatory cytokines (IL-6, IL-8, and TNF-α) that can drive sepsis and cause multiorgan failure. Their incorporation into a flexible polytetrafluoroethylene (PTFE) film could improve the result of hemoperfusion [43]. Seredych et al. confirmed the absorption of proinflammatory cytokines by graphene-based nanomaterials. They used granulated graphene nanoplates and granulated graphene nanoplatelets with PTFE. Both types of CPNs have high removal efficiencies, in some cases even up to 100% [44]. Nanodiamonds also have high absorption capacities for pro-inflammatory cytokines. Yoo et al. showed that nanodiamonds can absorb inflammatory cytokines (IL-1β, IL-4, IL-6, IFN-γ, and TNF-α) and, thus, alleviate inflammation and decrease the risk of multiorgan failure and death due to sepsis [45].
Wu et al. fabricated lysine-immobilized chitin/carbon nanotube microspheres and attempted to verify their capacity to remove bilirubin from human blood. The level in plasma reflects the dysfunction of the hepatobiliary system or hemolysis. Bilirubin is toxic to the central nervous system and causes bilirubin encephalopathy. The prepared CNPs exhibited a high bilirubin adsorption capacity and increased bilirubin clearance [46].
Ifran et al. exploited the extreme efficiency of CNPs in the absorption of chemicals from the blood. They constructed a hemodialysis membrane from polyethersulfone and carboxyl functionalized multi-walled carbon nanotubes (MWCNT). This membrane had excellent blood compatibility; membrane fouling was reduced by 30%, while urea and creatinine removal significantly increased [47]. CNPs can also absorb cholesterol, which is a risk factor for several diseases (cardiovascular and liver disorders, and cancer). The most effective are carbon nanotubes that bind cholesterol to both external and internal surfaces [48].
5. X-ray Protection
Radiology and nuclear medicine (as well as patients with cancer who undergo radiotherapy) can expose patients to radiation that can endanger their health. It is necessary to protect them adequately and effectively. Studies suggest that CNP-based materials could help to achieve this goal. Hashemi et al. tested a material (polyaniline) decorated with GO as an X-ray radiation shielding. These shields have shown superior performance in blocking X-ray beams compared to non-GO shields [49]. Silva et al. also confirmed that poly(vinylidene) fluoride homopolymer (PVDF)/barium sulfate attenuated 9.14% of a 20 kV X-ray beam while the addition of 4.0 wt% of a GO nanosheet increased the attenuation efficiency to 24.65% [50]. Fujimori et al. described that carbon nanotubes (CNTs) are more effective at attenuating X-rays than pyrolytic graphite and fullerenes. The coating of textiles with a thickness of 25 mm, with CNTs, enhanced X-ray attenuation by 70% [51]. Viegas et al. compared the X-ray shielding effect among GO, pyrolytic graphite, MWCNTs, and amorphous carbon loaded on PVDF. They found that GO film with a thickness of 0.1 mm attenuated 82.9 and 48.5% of X-ray beams with energies of 6.9 and 8.1 keV [52]. Zarei et al. fabricated an X-ray radiation shield that combined polyaniline and hybrid GO nanoflakes with tungsten–bismuth-tin. They confirmed the effectiveness of this shield and suggested that this material could be used to make radiation-protective clothing, such as aprons, gonadal, and thyroid shields to protect against X-rays. Standard protective equipment and clothing are made of lead-based material, which is relatively heavy and difficult to handle [53]. Türkaslan et al. also attempted to replace conventional lead-based material with more lightweight and flexible material. They used a commonly available fabric, combined it with GO, and produced a shielding material that is wearable in clinical practice; it is effective, lightweight, flexible, and permeable to water and air [54].
Study results suggest that CNPs are ideal candidates for X-ray protection.
6. Photodynamic Therapy
Photodynamic therapy (PDT) is a treatment method that can destroy cells, which is why it is used in the treatment of cancer.
PDT is based on the toxic effects of reactive oxygen species (ROS; OCl−, OH−, H2O2, O2, 1O2, ·OH, ·O2−, ·O2−2) whose local production is induced by the presence of CNPs. The formed ROS display toxic effects, especially toward cancer cells. Distinct CNPs generate different types of ROS. Light-independent, single-walled carbon nanotubes generate superoxide anions (·O2−), while graphene quantum dots generate singlet oxygen (1O2) [55,56]. This method is non-invasive and very effective with low side effects and minimal systemic toxicity. To enhance the effect of PDT, the application of CNPs is combined with light (light-mediated PDT), anticancer drugs, or photothermal therapy. CNPs are most often activated with NIR, which penetrates deeper into the tissue than visible light or ultraviolet light [57,58].
This type of treatment can also be used to treat bacterial and fungal infections. Cuadrado et al. produced nanocomposites based on GO and MWCNTs. MWCNTs eliminated Escherichia coli, Staphylococcus aureus, and Candida albicans in vitro, while GO only eliminated bacteria [59]. Chen et al. showed that indocyanine green-loaded GO effectively eliminated methicillin-resistant S. aureus [60].
7. Photothermal Therapy
Photothermal therapy is based on the photothermal capacity of CPNs (especially nanotubes and nanohorns) to convert light energy into heat under near-infrared light irradiation and kill targeted tumor cells (Figure 3) [61,62]. The therapy is minimally invasive, spatiotemporally-controlled, selective, and combinable with other types of anticancer therapy, especially systemic and local chemotherapy. The anticancer drug is loaded on the particle and NIR irradiation accelerates the release of the drug, which significantly increases the effectiveness of the therapy (systemic chemotherapy or loaded on nanoparticles). In vitro and in vivo studies have proven the effectiveness of photothermal therapy in breast, lung, and bladder cancer and melanoma [63,64]. This therapy not only kills cancer cells but also induces an anticancer immune response. Apoptotic and necroptotic cells increased the release of tumor-associated antigens, alarmins, and other damage-associated molecular patterns (DAMPS) stimulating both innate and specific immunity [65].
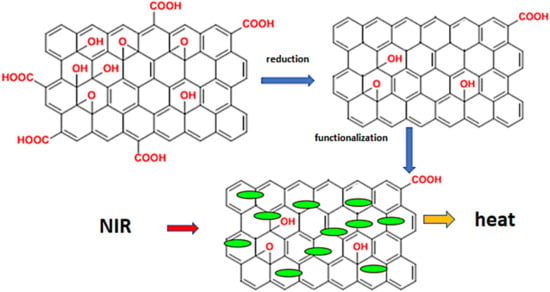
Figure 3.
The principal of phototherapy. Legend: When using GO, it must first be reduced to rGO and functionalized with various substances (human serum proteins, hyaluronic acid, and pharmaceuticals). The functionalized rGO is then exposed to NIR and releases heat.
Cheng et al. used multifunctional CNPs as lymph node tracers for the treatment of lymph node metastases in mice. The CNPs they used were loaded with docetaxel. The laser irradiation potentiated the release of the drug and the photothermal effect was achieved, which destroyed the cancer cells [66].
Li et al. described the effectivity of the nanodiamond-based multifunctional platform in the treatment of colon cancer. Orally delivered CNPs showed long intestinal retention times and were localized mainly in the colon and tumor, which led to tumor growth inhibition in vivo [67]. We cannot omit mesoporous carbon nanoparticles (MCNs). Zhou et al. confirmed that MCNs have a higher absorption coefficient than graphene and SWCNTs in the near-infrared region of NIR-I and NIR-II and, therefore, generate stronger photothermal and photoacoustic signals, which can enhance the efficiency of diagnosis and treatment [68].
8. Vaccine Production
Vaccines must induce an immune response against the selected antigen to prevent transmission or, at least, a severe course of the disease. Nowadays, vaccines are more widely used, not only against pathogens but also to treat cancer or autoimmune diseases [69]. CNPs can be loaded with target microbial or tumor antigens and immunogenic structures that enhance the immune system response, antibody production, and memory T and B cell formation, so CNPs can function as antigen carriers and adjuvants [70].
In animal models, carbon nanotube-binding antigens induce the production of neutralizing specific IgGs against a pathogen or improve cytolytic T cell activity against cancer cells [71]. The interesting work by Dong et al. described the production of the polyethyleneimine-functionalized graphene oxide (GO) influenza vaccine nanoplatform that binds recombinant influenza hemagglutinin. In mice, intranasally-administered vaccines elicited an exceptional humoral and cellular immune response and protected against infection after the viral challenge [72]. Nanodiamonds can also serve as an influenza virus antigen carrier. In mice, they can enhance the production of neutralizing antibodies three-fold [73].
9. Therapy—Delivery Platform, Treatment
Drug treatment is systemic, affecting the whole body; to achieve the necessary concentration at the site of the effect (e.g., at the site of a tumor), it is often necessary to administer high doses that damage the body. The ability to target therapy can greatly increase efficacy and reduce systemic inflammation.
CNPs are (almost) an ideal platform for drug delivery to various tissues (Figure 4). They have large loading capacities for different drug substances, including combinations of them. The functionalization of CNPs enhances the penetration and retention effects, allows targeting of specific tissues or molecules, and controls drug release. Released stimuli include pH, enzymatic activity, light, or temperature [74].
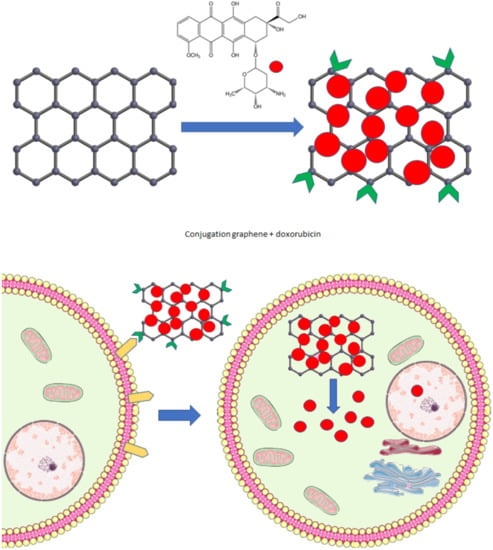
Figure 4.
NCP drug delivery system. Graphene conjugated with doxorubicin and a receptor specific for antigens expressed primarily on the target cell. The loaded graphene interacts with the cell membrane, enters the cell, and releases the drug (parts of the figure were drawn using pictures from Servier Medical Art. Servier, licensed under a Creative Commons Attribution 3.0 Unported License.).
Functionalized carbon nanotubes, graphene, fullerene, and other CNPs can bind anticancer, antimicrobial drugs, or proteins (monoclonal and polyclonal antibodies). Targeted therapy reduces the systemic toxicity of drugs and allows lower doses to be used to achieve the same therapeutic effect [75]. CNPs can be loaded, for example, with cisplatin, oxaliplatin, doxorubicin, methotrexate, gemcitabine, paclitaxel, tamoxifen, and rituximab. After administration, CNPs are attracted and retained in the tissues and the drug is released over several days [76].
Lay et al. loaded paclitaxel onto polyethylene glycol (PEG)-graft-carbon nanotubes. Multi-walled nanotubes had a higher loading capacity and showed lower toxicity and higher efficacy in killing cancer cells (HeLa and MCF-7 cells) than free paclitaxel [77]. The same effect was documented with multiloaded carbon nanotubes with β-estradiol, PEG, and lobaplatin [78]. Graphene oxide (GO)-PEG (GOP) loaded with protocatechuic acid (PA), chlorogenic acid (PC), and folic acid (FA) effectively killed hepatocellular carcinoma cells. The half-maximum inhibitory concentration (IC50) was for PC 40.78 ± 1.92, CA 43.61 ± 1.74, GOP-PC/CA 34.73 ± 1.04, and for GOP-PC/CA-FA 26.79 ± 1.63 [79].
CNPs can be functionalized with a wide range of molecules to target a specific type of cancer. One of these is folic acid because cancer cells overexpress the folic acid receptors. Other options are, for example, monoclonal antibodies with an affinity for tumor markers, such as anti-HER2 in breast cancer or rituximab (anti-CD20) in lymphomas, and antibodies targeting the prostate-specific membrane antigen [80,81,82]. Nanoscale GOs loaded with doxorubicin and HN-1 (specific antigen) have been successfully used to treat oral squamous cell carcinoma. The particles were easily absorbed by cancer cells and, thus, showed significantly higher toxicity compared to free doxorubicin [83]. MWCNTs loaded with bromocriptine or doxorubicin can induce apoptosis or necrosis in lung and prostate cancer cells [84,85].
Fullerene can also serve as a drug carrier. Conjugation with 5-aminolevulinic acid or doxorubicin may help to treat various types of cancers. Importantly, fullerene C60-β-cyclodextrin conjugated with doxorubicin improves drug delivery into the nucleus [86,87]. Mesoporous carbon nanoparticles (MCNs) have great potential as a platform for anticancer drug delivery. Zhou et al. showed that MCNs are more efficient in the delivery and release of doxycycline than mesoporous silica nanoparticles [88].
Furthermore, CNPs can deliver anticancer drugs to the central nervous system to treat gliomas. Their functionalization will allow them to penetrate the blood–brain barrier [89].
CNPs can not only carry anticancer drugs but also growth factors, including VEGF, to support angiogenesis in ischemic cardiac tissue and antimicrobial drugs (antibiotics, antifungal, antiparasitic, and antiviral drugs) [90,91,92]. Even without loading with antimicrobial drugs, CNPs can eliminate viral (especially bacterial or fungal) infections and bypass bacteria resistant to antibiotics (e.g., Escherichia coli, Staphylococcus aureus, Streptococcus mutants, Porphyromonas gingivalis, Pseudomonas aeruginosa, Klebsiella pneumoniae, Salmonella typhimurium). Their antimicrobial effects are enhanced by the formation of nanocomposites with Ag [93].
Importantly, CNPs can be used to treat inflammatory diseases, such as ulcerative colitis. MCNs loaded with Musca domestica cecropin can accumulate in the damaged intestine and suppress inflammation, restoring the homeostasis of the intestinal microflora and improving the intestinal barrier function [94].
CNPs enable the nonviral delivery of genetic material, including plasmid DNA, miRNA, siRNA into cells, and the nucleus [74,95]. They can regulate gene expression and induce silencing of target genes. By silencing distinct genes (selective blocking), suppression of cancer cell growth can be achieved. Andersen et al. prepared single-walled carbon nanotubes conjugated with siRNA. The nanocomplex was internalized by pancreatic cancer cells (PANC-1). The transfection of naked siRNA was very low, on the other hand, the transfection of the nanoparticle complex was higher than 92%. After internalization, siRNA was released and interacted with the targeted gene mutant K-Ras, which led to the downregulation of its expression. The expression of K-Ras mRNA was suppressed by 66.88 ± 5.14% [96].
Using multidrug therapy and siRNA single-walled carbon nanotubes targeting the CCR5 gene, Hasan et al. inhibited inflammatory processes in nonalcoholic steatohepatitis and prevented hepatocellular carcinoma [97].
CNPs have enormous therapeutic potential. They can make treatments more targeted and effective, using lower doses of drugs, which will reduce the cost of treatment while reducing risks to patients.
10. Detection, Electrochemical Biosensors
Biosensors are used to detect biomarkers associated with certain diseases. They allow the diagnosis of diseases and monitoring of their progress. Biomarkers are biologically important molecules, such as metabolites, proteins, nucleic acids, hormones, antigens, glucose, uric acid, cholesterol, acetone, hydrogen sulfide, glutathione, and vitamin B12, which can be detected in blood, saliva, urine, sputum, or breath [98,99]. The CNPs can be used to analyze the levels of hydrogen sulfide, toluene, acetone, benzene, ammonia, and nitrous oxide levels in the breath to diagnose distinct diseases, such as diabetes, lung or gastric cancer, halitosis, asthma, poor liver, and kidney function [100]. Specifically, CNP-detected substances associated with lung cancer include benzene, styrene (ethenylbenzene), hexanal, 2,2,4,6,6-pentamethyl heptanes, propyl benzene, 2-methyl heptanes, decane, 1,4-dimethyl benzene, 3-methyl nonane, 1-hexene, heptanal, undecane, 1-methyl-2-pentylcyclopropane, methyl cyclopentane, trichloro-fluorobenzene, 1-methylethenyl benzene, cyclohexane, 1-heptene, 3-methyl octane, 1,2,4-trimethyl benzene, 2-methyl-(isoprene)-1,3-butadiene, and 2,4-dimethyl heptanes [101]. For the detection of myocardial damage, graphene and its derivatives and carbon nanotubes can be used. They detect myoglobin, creatinine kinase, troponins, and B-type natriuretic peptides [90,102].
Glucose monitoring is the standard practice for the diagnosis and control of diabetes. CNPs (nanotubes, graphene, graphene dots) with attached glucose oxidase detect glucose with high sensitivity and selectivity (carbon nanosensors were tested in the presence of interferents, such as acetaminophen and uric and ascorbic acid) [103]. Kang et al. prepared a single-walled carbon nanotube biosensor with a detection limit of 50 μM and an ultrafast response of less than 5 s. They designed wearable glucose sensors that can be combined with a smartphone to serve diabetic patients [103]. Glucose can also be measured using non-enzymatic detection with distinct graphene-based electrodes. These electrodes can be functionalized with a wide range of nanoparticles to improve their detection limits, for example, the detection limit of copper nanocubes with reduced GO is very low at 250 nM, while reduced GO (in combination with SnO2) has a detection limit of 13.35 µM [104].
Monitoring cholesterol levels is clinically essential. For this purpose, both enzymatic and nonenzymatic methods can be used. The enzymatic method uses CNP electrodes enriched with cholesterol oxidase enzymes [105]. For nonenzymatic detection, graphene functionalized with β-cyclodextrin is often used. Electrochemical detection is mediated by methylene blue (MB) (redox indicator), which forms complexes with the nanomaterial. MB is replaced by cholesterol and released into the buffer solution [106].
Nanoparticles can also be used to detect compounds associated with neurodegenerative processes. Aminabad measured the levels of the α-synuclein protein (which is associated with Parkinson’s disease) in human plasma by graphene conjugated with gold nanoparticles. The dynamic range and lower limits of quantification were determined to be from 4 to 128 ng/mL and 4 ng/mL, respectively [107].
Diagnoses of bacterial diseases can be prolonged and complicated. CNPs can greatly simplify and speed up the diagnostic process. Graphene loaded with specific antibodies can detect many types of pathogens, such as E. coli, Salmonella typhimurium, and Zika virus. GO might be used, for example, for the detection of dengue virus and rotavirus [108]. Recently, scientists have shown that CNPs can be used for SARS-CoV-2 detection from body fluid or exhaled breath. These methods are very effective, fast, and relatively inexpensive compared to commonly used methods [109,110,111].
CNP can also be designed for real-time monitoring, e.g., to monitor the release of neurochemicals from the nervous system. In a study by Wu et al., the authors presented an implantable aptamer–graphene microtransistor probe that selectively (and with high sensitivity) detected dopamine release ex vivo and in vivo in a mouse model. This type of device can make a significant contribution to the understanding of the functioning of the nervous system, as well as to its treatment [112].
11. Stents, Biocompatible Coating of Prosthetics, Special Cardiac Devices, Tooth Reparation
Biocompatibility is an important factor in materials developed for medical applications and can be improved by coating stents, cardiovascular grafts, and prosthetics with nanomaterials. This modification can change the physiochemical properties of materials, improve mechanical strength, hemocompatibility, and anti-thrombogenic properties (reduce platelet adhesion), enhance reepithelization and regeneration, protect against restenosis of stents, and reduce the risk of infection and biofilm formation [113].
The 316 L stainless steel material has good properties for use in cardiovascular medicine (stent production). However, there is a risk of thrombosis and restenosis. Coating the 316 L stent with graphene layers markedly improves its properties [113]. The 316 L stents coated with graphene were not toxic to endothelial cells, supporting their adhesion, proliferation, and metabolic activity; additionally, graphene reduced platelet adhesion and, thus, may inhibit thrombus formation and support vessel wall regeneration [114].
CNPs can also be used for the fabrication of heart valves. Hastalex is a material made from functionalized graphene oxide and poly(carbonate-urea)urethane, which, in studies, showed superior mechanical, hemocompatibility, and calcific resistance properties compared with GOTE-TEX. Modification of valves made of polyurethane and nanotubes with heparin further increases their usability by reducing coagulation [115,116].
In addition to cardiac stents and grafts, orthopedic replacements or implants can also be coated with carbon nanoparticles. They might improve regeneration, osteoblast attachment, and proliferation. They also protect implants against damage and in the years after surgery [117]. Interestingly, GOs, in combination with poly(vinylidene fluoride), can be used as polymer-based nanogenerators in self-powered cardiac pacemakers. Azimi et al. implanted this pacemaker in dogs and found that PNG harvested 0.487 μJ from every heartbeat. This value is higher than the threshold energy required to stimulate the human heart [118].
Nanodiamond-like CNPs are also suitable for medical applications. Nanodiamond-like layers reduce the risk of thrombus formation (limit platelet adhesion and activation) and inhibit the formation of fibrin deposits. The antimicrobial effects were also confirmed [119].
CNPs can also be used as part of the material to repair damaged teeth. The addition of nanodiamonds to the repair resin increased the quality of the material, its flexural strength, elastic modulus, and surface roughness. Nanodiamonds incorporated into resin reduced the adherence of Candida albicans, which is important for the prevention of denture stomatitis [120,121,122].
12. Scaffolds, Tissue Engineering, and Healing
CNPs exhibit excellent biocompatibility and can be easily functionalized to enhance the viability of specific cell types and control their behavior (shape, adhesion, proliferation, differentiation, and dedifferentiation toward multipotency). Therefore, CNPs can serve as material for coating scaffolds (3D scaffolds enable the formation of tissue from stem cells). The results of the following studies suggest that CNPs have great potential for use in regenerative medicine. Studies especially show their regenerative effects on the nervous system, bones, and cartilage (Figure 5) [123,124].
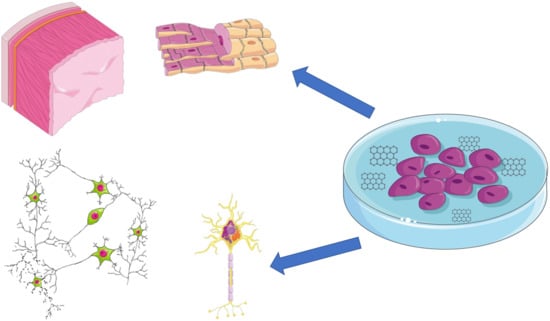
Figure 5.
Regenerative potential of CNPs. Stem cell cultivation with graphene. Induction of the cell and differentiation and tissue formation (nervous tissue and myocardium) (parts of the figure were drawn using pictures from Servier Medical Art. Servier, licensed under a Creative Commons Attribution 3.0 Unported License.).
Graphene has been proven to support the growth of mouse hippocampal neurons, enhance the number of neurites and their lengths, and modulate synaptogenesis as well as neural activity [125,126]. Importantly, graphene, GO, and carbon nanotubes can induce the differentiation of mouse embryonic stem cells into dopaminergic neurons, which decline in patients with Parkinson’s disease [127]. The implantation of an electrospun microfiber scaffold coated with self-assembled colloidal graphene into the striatum or subventricular zone of adult rats has led to the suppression of microglial and astrocyte activation, inhibiting inflammation, and significantly reducing glial scarring in the brain after a brain injury [128].
The 3D porous scaffold is an excellent microenvironment for the growth, activity, and proliferation of neurons to form neuronal tissue. It mimics the in vivo situation during neural development [128]. Hippocampal neurons cultured on a 3D graphene scaffold show more extensive connectivity and a higher degree of synchronicity than neurons cultured on a 2D scaffold. Moreover, the two regimes of synchronicity were detected as highly synchronized and moderately synchronized. The first have never been detected in 2D scaffolds [129]. Lopéz-Dolado et al. implanted a 3D scaffold with GO for 30 days into the injured spinal cords of rats. It potentiated the healing process and promoted angiogenesis around and within the scaffold structure. New functional blood vessels and neural axons were found in the damaged tissue [130].
CNPs not only help to regenerate the central nervous system but also the peripheral. Zhao et al. created an electrospun composite scaffold of graphene and silk fibroin that promotes Schwann cell growth and could be used in the treatment of peripheral nervous system injuries [131]. Similar results were described by Pi et al., who cultured Schwann cells with a scaffold fabricated from polycaprolactone and CNT modified with brain-derived neurotrophic factors that promote cell proliferation and myelin production. The in vivo part of this study proved the regenerative properties of the scaffold. The rat sciatic nerve defect model improved the functional recovery of the damaged sciatic nerve [132].
Nanoparticles can also promote bone, cartilage, skin, and muscle formation and regeneration [133]. Bone injuries, fractures, and bone-affecting diseases are often associated with poor healing and the necessity of the use of bone grafts. A major problem is the limited availability of tissues; the solution would be tissue engineering [133]. In vitro, graphene potentiates the differentiation of stem cells into osteoblasts and enhances osteogenesis. In vivo studies showed that CNPs potentiate the repair of bone defects and ectopic bone formation [134]. Nie et al. prepared a 3D porous nanohydroxyapatite scaffold with GOs and found that (in vivo, in a rabbit model) a circular calvarial defect (4 mm in diameter) was fully healed by this scaffold. They performed CT imaging and analyzed bone tissue samples. The scaffold improved osteogenesis, cell proliferation, and collagen deposition compared to rabbits treated with standard therapy [134]. The results of Daneshmandi et al. also confirmed the osteogenic potential of CNP. They used a 3D scaffold made of graphene functionalized with calcium phosphate, which induced osteogenic differentiation in vitro in human mesenchymal stem cells and bone regeneration in vivo in a mouse calvarial defect model [135].
Li et al. also confirmed the therapeutic potential of CNP materials. They coated a titanium alloy implant with graphene and used it in the treatment of femoral condyle defects in rabbits. The nanomaterial promoted and accelerated osteogenesis and osseointegration. The quality of newly formed bone, bone mineralization, and implant attachment was better in the graphene group than in the non-graphene group [136].
CNPs can help to promote the regeneration of other types of tissue, including sensory, cardiac, and vascular tissue.
Retinal damage often leads to an irreversible loss of sight. To regenerate this important tissue is crucial to protect vision. Carbon nanotubes and nanofibers were used by Chemla et al. to promote the differentiation of human embryonic stem cells into photoreceptor precursor cells. In clinical practice, this could be applied to the treatment of damaged retinas [137].
The incidence of cardiovascular disease continues to rise. Ischemic heart diseases irreversibly damage the myocardium, which has a very limited regenerative capacity and loses its function. In the most severe cases, the only treatment is a heart transplant, but there is a severe shortage of organs. A therapeutic option could be to induce regeneration of the heart muscle and blood vessels or even to create a new functional organ. Scaffolds or injectable hydrogels with CNPs alone or seeded with stem cells might help to induce the regeneration of damaged tissue. They must mimic the extracellular matrix, which provides mechanical support as well as crucial signals required for stem cell maturation and differentiation. They have good biocompatibility, mechanical properties, and electrical conductivity that cardiac cells need to form new functional tissue. The scaffold of PEG with GO cultured with neonatal rat ventricular myocytes facilitated the growth of cardiac cells, their conductivity, and contractility more than PEG [138].
Saravanan et al. fabricated a biodegradable chitosan scaffold with GO-gold nanosheets. They implanted and tested its properties both in vitro in an isolated heart and in vivo in a rat model of myocardial infarction. After 5 weeks, the ECG results showed that the scaffold improved the conduction velocity and the cardiac contraction in the infarcted cardiac tissue. ECHO proved that the implant increased the ejection fraction. These positive effects were not associated with immune system activation and inflammation [139].
The delivery of stem cells to scaffolds with CNPs can promote neoangiogenesis and, thus, restore blood flow in the damaged area [90]. Mukherjee et al. confirmed that different concentrations of GO and reduced GO promote angiogenesis [140]. Neoangiogenesis is also crucial for the regeneration of peripheral nerves. Quian et al. fabricated a polycaprolactone scaffold with GO that supported not only Schwann cells and neurite growth but also angiogenesis, which is necessary for nerve regeneration. The presence of GO enhances the activity of the AKT–eNOS–VEGF axis [141].
We cannot omit the involvement of CNPs in the healing of skin wounds, including chronic and infected wounds. MWCNTs can control infection and accelerate the regenerative processes (fibroblast proliferation, re-epithelialization) even in difficult-to-treat diabetic wounds and burns [142]. Khalid et al. described the effect of bacterial cellulose with MWCNTs, which induced faster closure of diabetic wounds, re-epithelialization, and the formation of healthy granulation tissue. The molecular analysis confirmed a decrease in the levels of proinflammatory cytokines, including IL-1α and TNF-α, while the expression of VEGF increased [143]. Du et al. confirmed the antibacterial properties of graphene. They used a nitrocellulose membrane doped with nanoporous graphene. The membrane reduced the risk of wound infection with Escherichia coli and Staphylococcus aureus and accelerated wound closure and skin recovery [144].
Similar results were obtained by Gupta et al., who tested the nanofibrous film with graphene oxide, silver, and magnesium. In the wound model, the wounds covered with a nanofibrous film showed faster wound contraction and re-epithelialization [145].
The results of the studies show that CNPs have great potential for use in regenerative medicine. They promote tissue neogenesis, remodeling, and regeneration of damaged tissue, and have a low cytotoxicity rate.
13. Nanotoxicity
When we discuss the possibility of using CNPs in medicine, we must not forget the potentially toxic effects of individual nanoparticles. The toxicity of CNPs depends on numerous factors, including physiochemical properties, such as size, shape, charge, functionalization (functionalized nanoparticles may exhibit significantly lower toxicity than pristine ones), corona formation, the duration of exposure, and the dose of CNPs. Nanotoxicity is often time- and dose-dependent.
Experimental and epidemiological studies have shown that CNPs have cytotoxic and genotoxic potential against numerous tissue types and systems (respiratory, cardiovascular, nervous, gastro-hepatic, and reproductive systems). They also show toxicity against the kidneys, immune system, skin, and eyes; in addition, developmental nanotoxicity was confirmed (Figure 6) [3,146]. Damage is often induced by the interaction of CNPs with the cell membrane, nucleus, or mitochondria that is associated with increased production of reactive oxygen species (ROS) and the activation of signal pathways responsible for inflammation, accompanied by increased production of proinflammatory cytokines (TNF-α, IL-6) [147]. Inflammation, ROS, and direct interaction of CNPs with the nucleus can damage DNA and disrupt DNA reparative processes, which may lead to the accumulation of mutations and malignant transformation [148].
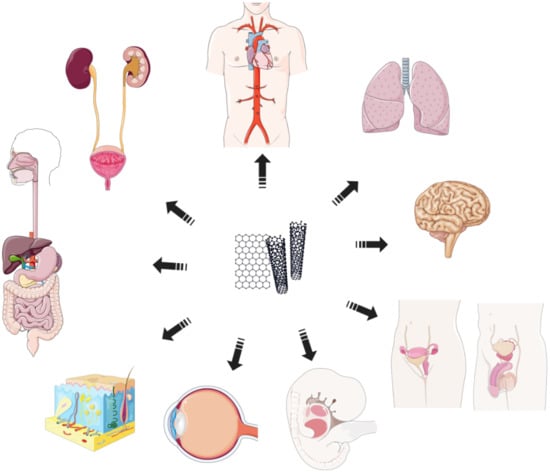
Figure 6.
Nanotoxicity. CNPs exhibit nano-toxic effects on various tissue types and systems: cardiovascular, respiratory, nervous, reproductive system, fetal and developmental toxicity, eye and skin toxicity, and digestive and urinary systems (parts of the figure were drawn using pictures from Servier Medical Art. Servier, licensed under a Creative Commons Attribution 3.0 Unported License.).
The presence of CNPs can also induce cell death (apoptosis, pyroptosis, or necrosis); in particular, necrosis and pyroptosis induce an inflammatory response [149]. In vivo studies detected changes in various tissues, inflammation with the accumulation of immune cells, and fibrotic changes. Importantly, reproductive and developmental changes are documented; the alteration of hormonal levels, decreased sperm production and functions (malformation of sperm and decreased mobility), developmental delays, damage to the nervous or cardiovascular systems, inflammation, and skeletal malformation of the fetus [146]. Toxicity also depends on the route of administration of the CNPs. However, they can also penetrate tissues, enter the bloodstream, and reach distant tissues. Intratracheal instillation results mainly in damage to the lung tissue and the cardiovascular system, whereas per os administration is mainly associated with the accumulation of CNPs in the gastrointestinal tract [150].
Epidemiological studies in workplaces where workers have been exposed to inhaled CNPs for long periods of time also have demonstrated potential health risks of exposure to CNPs. In these persons (workers), changes in the expressions of genes associated with various biological pathways (cell cycle control, carcinogenesis, apoptosis, and cell proliferation), increases in inflammatory cytokines, profibrotic factors, oxidative stress (decreased activity of antioxidant enzymes, such as superoxide dismutase, glutathione peroxidase, and increased ROS production), endothelial activation, and associations with hypersensitivity reactions (allergies) have been described [151].
Single- or short-term exposure to nanoparticles usually does not cause temporary or irreversible tissue damage (as opposed to long-term chronic exposure). When CNPs are used in medical investigation methods, the exposure is short-term, usually a one-off, and targeted with a well-defined dose and route of administration, which can significantly eliminate health risks to the patient. However, these medical procedures (exposures) may increase the health risks for staff exposed long-term to CNPs. The toxicity of nanoparticles can be modulated by their functionalization (polyethylene glycol, poly-l-glutamic acid, etc.), which could reduce the risk of toxicity; on the other hand, the medical functions of CNPs, their distribution in the body, penetration into tissues, response to illumination, etc., could be negatively affected [152].
The risk of toxicity of CNPs in medicine appears to be low, especially for patients; however, it is important to keep it in mind and weigh the benefits and risks.
14. Conclusions
In the presented review, we mentioned only the most important carbon nanoparticles and their possible applications in medicine. Due to their wide applicability, effectiveness, and relatively low price-to-performance ratio, it is possible that carbon nanoparticles will become a common part of clinical practice. The benefits of CNPs are unexceptionable; however, we should note that the use of CNPs also raises concerns about their biosafety. Nanoparticles are a relatively new phenomenon and we still do not know all the factors that can affect their behavior in an environment and the human body. Therefore, in addition to the research, development, and production of new nanomaterials, it is necessary to pay attention to toxicological and biomedical research on their (possible) adverse effects.
Author Contributions
Conceptualization, D.H. and Z.F.; collection of articles and discussion: D.H., T.S. and L.B.; writing—original draft preparation, D.H., T.S. and P.B.; writing—review and editing, Z.F., L.B. and P.B.; schemes design, D.H.; supervision, Z.F. and L.B.; funding acquisition, Z.F. All authors have read and agreed to the published version of the manuscript.
Funding
The work was supported by the ERDF-Project “NANOBIO” no. CZ.02.1.01/0.0/0.0/17_048/0007421, Charles University project SVV 260543/2020, and by the Cooperation Program, research area HEAS.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sharma, M. Understanding the mechanism of toxicity of carbon nanoparticles in humans in the new millennium: A systemic review. Indian J. Occup. Environ. Med. 2010, 14, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Kroto, H.W.; Heath, J.R.; Brien, S.C.O.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Chen, C.; Haifang, W. Biomedical Applications and Toxicology of Carbon Nanomaterials; Wiley: Hoboken, NJ, USA, 2016. [Google Scholar] [CrossRef]
- Choudhary, N.; Hwang, S.; Choi, W. Carbon Nanomaterials: A Review. In Handbook of Nanomaterials Properties; Springer: Berlin/Heidelberg, Germany, 2014; pp. 709–769. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Kumar, V.; Huczko, A.; Oraon, R.; De Adhikari, A.; Nayak, G.C. Magical Allotropes of Carbon: Prospects and Applications. Crit. Rev. Solid State Mater. Sci. 2016, 41, 257–317. [Google Scholar] [CrossRef]
- Lu, H.; Li, S.-D. Two-dimensional carbon allotropes from graphene to graphyne. J. Mater. Chem. C 2013, 1, 3677–3680. [Google Scholar] [CrossRef]
- Lin, L.; Peng, H.; Liu, Z. Synthesis challenges for graphene industry. Nat. Mater. 2019, 18, 520–524. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, S.; Komvopoulos, K. A review of graphene synthesis by indirect and direct deposition methods. J. Mater. Res. 2020, 35, 76–89. [Google Scholar] [CrossRef]
- Smith, A.T.; LaChance, A.M.; Zeng, S.; Liu, B.; Sun, L. Synthesis, properties, and applications of graphene oxide/reduced graphene oxide and their nanocomposites. Nano Mater. Sci. 2019, 1, 31–47. [Google Scholar] [CrossRef]
- Habte, A.T.; Ayele, D.W. Synthesis and Characterization of Reduced Graphene Oxide (rGO) Started from Graphene Oxide (GO) Using the Tour Method with Different Parameters. Adv. Mater. Sci. Eng. 2019, 2019, 5058163. [Google Scholar] [CrossRef]
- Eatemadi, A.; Daraee, H.; Karimkhanloo, H.; Kouhi, M.; Zarghami, N.; Akbarzadeh, A.; Abasi, M.; Hanifehpour, Y.; Joo, S.W. Carbon nanotubes: Properties, synthesis, purification, and medical applications. Nanoscale Res. Lett. 2014, 9, 393. [Google Scholar] [CrossRef]
- Wang, X.-D.; Vinodgopal, K.; Dai, G.-P. Synthesis of Carbon Nanotubes by Catalytic Chemical Vapor Deposition. In Perspective of Carbon Nanotubes; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Arora, N.; Sharma, N. Arc discharge synthesis of carbon nanotubes: Comprehensive review. Diam. Relat. Mater. 2014, 50, 135–150. [Google Scholar] [CrossRef]
- Ismail, R.A.; Mohsin, M.H.; Ali, A.K.; Hassoon, K.I.; Erten-Ela, S. Preparation and characterization of carbon nanotubes by pulsed laser ablation in water for optoelectronic application. Phys. E Low Dimens. Syst. Nanostruct. 2020, 119, 113997. [Google Scholar] [CrossRef]
- Zielińska, A.; Costa, B.; Ferreira, M.V.; Miguéis, D.; Louros, J.M.S.; Durazzo, A.; Lucarini, M.; Eder, P.; Chaud, M.V.; Morsink, M.; et al. Nanotoxicology and Nanosafety: Safety-By-Design and Testing at a Glance. Int. J. Environ. Res. Public Health 2020, 17, 4657. [Google Scholar] [CrossRef]
- Griger, S.; Sands, I.; Chen, Y. Comparison between Janus-Base Nanotubes and Carbon Nanotubes: A Review on Synthesis, Physicochemical Properties, and Applications. Int. J. Mol. Sci. 2022, 23, 2640. [Google Scholar] [CrossRef]
- Liu, F.; Gao, Y.; Li, H.; Sun, S. Interaction of propidium iodide with graphene oxide and its application for live cell staining. Carbon 2014, 71, 190–195. [Google Scholar] [CrossRef]
- Liu, J.; Li, D.; Zhang, K.; Yang, M.; Sun, H.; Yang, B. One-Step Hydrothermal Synthesis of Nitrogen-Doped Conjugated Carbonized Polymer Dots with 31% Efficient Red Emission for In Vivo Imaging. Small 2018, 14, e1703919. [Google Scholar] [CrossRef]
- Liu, J.; Li, R.; Yang, B. Carbon Dots: A New Type of Carbon-Based Nanomaterial with Wide Applications. ACS Cent. Sci. 2020, 6, 2179–2195. [Google Scholar] [CrossRef]
- Patel, K.D.; Singh, R.K.; Kim, H.-W. Carbon-based nanomaterials as an emerging platform for theranostics. Mater. Horiz. 2019, 6, 434–469. [Google Scholar] [CrossRef]
- Ghosh, D.; Bagley, A.F.; Na, Y.J.; Birrer, M.J.; Bhatia, S.N.; Belcher, A.M. Deep, noninvasive imaging and surgical guidance of submillimeter tumors using targeted M13-stabilized single-walled carbon nanotubes. Proc. Natl. Acad. Sci. USA 2014, 111, 13948–13953. [Google Scholar] [CrossRef]
- Bayoumi, N.A.; Emam, A.N. 99mTc radiolabeling of polyethylenimine capped carbon dots for tumor targeting: Synthesis, characterization and biodistribution. Int. J. Radiat. Biol. 2021, 97, 977–985. [Google Scholar] [CrossRef]
- Cherukula, K.; Manickavasagam Lekshmi, K.; Uthaman, S.; Cho, K.; Cho, C.-S.; Park, I.-K. Multifunctional Inorganic Nanoparticles: Recent Progress in Thermal Therapy and Imaging. Nanomaterials 2016, 6, 76. [Google Scholar] [CrossRef]
- Hu, Z.; Fang, C.; Li, B.; Zhang, Z.; Cao, C.; Cai, M.; Su, S.; Sun, X.; Shi, X.; Li, C.; et al. First-in-human liver-tumour surgery guided by multispectral fluorescence imaging in the visible and near-infrared-I/II windows. Nat. Biomed. Eng. 2020, 4, 259–271. [Google Scholar] [CrossRef]
- Wan, H.; Yue, J.; Zhu, S.; Uno, T.; Zhang, X.; Yang, Q.; Yu, K.; Hong, G.; Wang, J.; Li, L.; et al. A bright organic NIR-II nanofluorophore for three-dimensional imaging into biological tissues. Nat. Commun. 2018, 9, 1171. [Google Scholar] [CrossRef]
- Li, Z.; Zhaode, B.; Bu, Z.; Wu, A.; Wu, X.; Shan, F.; Ji, X.; Zhang, Y.; Xing, Z.; Ji, J. Clinical study of harvesting lymph nodes with carbon nanoparticles in advanced gastric cancer: A prospective randomized trial. World J. Surg. Oncol. 2016, 14, 88. [Google Scholar] [CrossRef]
- Ma, J.-J.; Zhang, D.-B.; Zhang, W.-F.; Wang, X. Application of Nanocarbon in Breast Approach Endoscopic Thyroidectomy Thyroid Cancer Surgery. J. Laparoendosc. Adv. Surg. Tech. 2020, 30, 547–552. [Google Scholar] [CrossRef]
- Liu, P.; Tan, J.; Tan, Q.; Xu, L.; He, T.; Lv, Q. Application of Carbon Nanoparticles in Tracing Lymph Nodes and Locating Tumors in Colorectal Cancer: A Concise Review. Int. J. Nanomed. 2020, 15, 9671–9681. [Google Scholar] [CrossRef]
- Du, J.; Zhang, Y.; Ming, J.; Liu, J.; Zhong, L.; Liang, Q.; Fan, L.; Jiang, J. Evaluation of the tracing effect of carbon nanoparticle and carbon nanoparticle-epirubicin suspension in axillary lymph node dissection for breast cancer treatment. World J. Surg. Oncol. 2016, 14, 164. [Google Scholar] [CrossRef]
- Xu, S.-W.; Li, Z.-F.; Xu, M.-B.; Peng, H.-W. The Role of Carbon Nanoparticle in Lymph Node Detection and Parathyroid Gland Protection during Thyroidectomy—A Meta Analysis. bioRxiv 2019, 783993. [Google Scholar] [CrossRef]
- Wang, R.; Zhan, H.L.; Li, D.Z.; Li, H.T.; Yu, L.; Wang, W. [Application of endoscopic tattooing with carbon nanoparticlet in the treatment for advanced colorectal cancer]. Zhonghua Wei Chang Wai Ke Za Zhi 2020, 23, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Alam, N.A.; Liu, Y.; Agafonov, V.N.; Qi, H.; Koynov, K.; Davydov, V.A.; Uzbekov, R.; Kaiser, U.; Lasser, T.; et al. Silicon-Vacancy Nanodiamonds as High Performance Near-Infrared Emitters for Live-Cell Dual-Color Imaging and Thermometry. Nano Lett. 2022, 22, 2881–2888. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Cortadella, R.; Schwesig, G.; Jeschke, C.; Illa, X.; Gray, A.L.; Savage, S.; Stamatidou, E.; Schiessl, I.; Masvidal-Codina, E.; Kostarelos, K.; et al. Graphene active sensor arrays for long-term and wireless mapping of wide frequency band epicortical brain activity. Nat. Commun. 2021, 12, 211. [Google Scholar] [CrossRef] [PubMed]
- Ko, L.-W.; Su, C.-H.; Liao, P.-L.; Liang, J.-T.; Tseng, Y.-H.; Chen, S.-H. Flexible graphene/GO electrode for gel-free EEG. J. Neural Eng. 2021, 18, 046060. [Google Scholar] [CrossRef]
- Faisal, S.N.; Amjadipour, M.; Izzo, K.; Singer, J.A.; Bendavid, A.; Lin, C.-T.; Iacopi, F. Non-invasive on-skin sensors for brain machine interfaces with epitaxial graphene. J. Neural Eng. 2021, 18, 066035. [Google Scholar] [CrossRef]
- Bramini, M.; Alberini, G.; Colombo, E.; Chiacchiaretta, M.; DiFrancesco, M.L.; Maya-Vetencourt, J.F.; Maragliano, L.; Benfenati, F.; Cesca, F. Interfacing Graphene-Based Materials With Neural Cells. Front. Syst. Neurosci. 2018, 12, 12. [Google Scholar] [CrossRef]
- Cui, T.-R.; Li, D.; Huang, X.-R.; Yan, A.-Z.; Dong, Y.; Xu, J.-D.; Guo, Y.-Z.; Wang, Y.; Chen, Z.-K.; Shao, W.-C.; et al. Graphene-Based Flexible Electrode for Electrocardiogram Signal Monitoring. Appl. Sci. 2022, 12, 4526. [Google Scholar] [CrossRef]
- Tasneem, N.T.; Pullano, S.A.; Critello, C.D.; Fiorillo, A.S.; Mahbub, I. A Low-Power On-Chip ECG Monitoring System Based on MWCNT/PDMS Dry Electrodes. IEEE Sensors J. 2020, 20, 12799–12806. [Google Scholar] [CrossRef]
- Lee, C.; Jeon, M.; Kim, C. 3—Photoacoustic imaging in nanomedicine. In Applications of Nanoscience in Photomedicine; Elsevier: Amsterdam, The Netherlands, 2015; pp. 31–47. [Google Scholar] [CrossRef]
- Jia, Z.; Dai, R.; Zheng, Z.; Qin, Y.; Duan, A.; Peng, X.; Xie, X.; Zhang, R. Hollow carbon-based nanosystem for photoacoustic imaging-guided hydrogenothermal therapy in the second near-infrared window. RSC Adv. 2021, 11, 12022–12029. [Google Scholar] [CrossRef]
- De La Zerda, A.; Zavaleta, C.; Keren, S.; Vaithilingam, S.; Bodapati, S.; Liu, Z.; Levi, J.; Smith, B.; Ma, T.-J.; Oralkan, O.; et al. Carbon nanotubes as photoacoustic molecular imaging agents in living mice. Nat. Nanotechnol. 2008, 3, 557–562. [Google Scholar] [CrossRef]
- Han, Y.; Liu, H.; Fan, M.; Gao, S.; Fan, D.; Wang, Z.; Chang, J.; Zhang, J.; Ge, K. Near-infrared-II photothermal ultra-small carbon dots promoting anticancer efficiency by enhancing tumor penetration. J. Colloid Interface Sci. 2022, 616, 595–604. [Google Scholar] [CrossRef]
- Zheng, Y.; Pescatore, N.; Gogotsi, Y.; Dyatkin, B.; Ingavle, G.; Mochalin, V.; Ozulumba, T.; Mikhalovsky, S.; Sandeman, S. Rapid Adsorption of Proinflammatory Cytokines by Graphene Nanoplatelets and Their Composites for Extracorporeal Detoxification. J. Nanomater. 2018, 2018, 6274072. [Google Scholar] [CrossRef]
- Seredych, M.; Haines, B.; Sokolova, V.; Cheung, P.; Meng, F.; Stone, L.; Mikhalovska, L.; Mikhalovsky, S.; Mochalin, V.N.; Gogotsi, Y. Graphene-Based Materials for the Fast Removal of Cytokines from Blood Plasma. ACS Appl. Bio Mater. 2018, 1, 436–443. [Google Scholar] [CrossRef]
- Yoo, W.; Lee, W.; Kim, H.N.; Jeong, J.; Park, H.H.; Ahn, J.H.; Jung, D.; Lee, J.; Kim, J.-S.; Lee, S.W.; et al. Nanodiamond as a Cytokine Sponge in Infectious Diseases. Front. Bioeng. Biotechnol. 2022, 10, 504. [Google Scholar] [CrossRef]
- Wu, S.; Duan, B.; Zeng, X.; Lu, A.; Xu, X.; Wang, Y.; Ye, Q.; Zhang, L. Construction of blood compatible lysine-immobilized chitin/carbon nanotube microspheres and potential applications for blood purified therapy. J. Mater. Chem. B 2017, 5, 2952–2963. [Google Scholar] [CrossRef]
- Irfan, M.; Irfan, M.; Idris, A.; Baig, N.; Saleh, T.A.; Nasiri, R.; Iqbal, Y.; Muhammad, N.; Rehman, F.; Khalid, H. Fabrication and performance evaluation of blood compatible hemodialysis membrane using carboxylic multiwall carbon nanotubes and low molecular weight polyvinylpyrrolidone based nanocomposites. J. Biomed. Mater. Res. Part A 2019, 107, 513–525. [Google Scholar] [CrossRef]
- Vakhrushev, A.V.; Chuckova, N.N.; Cherenkov, I.A.; Cormilina, N.V.; Vakhrushev, A.A. Adsorption of Cholesterol by Carbon Nanotubes. In Carbon Nanotubes and Nanoparticles; Apple Academic Press: Palm Bay, FL, USA, 2019; pp. 65–80. [Google Scholar] [CrossRef]
- Hashemi, S.A.; Mousavi, S.M.; Faghihi, R.; Arjmand, M.; Rahsepar, M.; Bahrani, S.; Ramakrishna, S.; Lai, C.W. Superior X-ray Radiation Shielding Effectiveness of Biocompatible Polyaniline Reinforced with Hybrid Graphene Oxide-Iron Tungsten Nitride Flakes. Polymers 2020, 12, 1407. [Google Scholar] [CrossRef]
- Silva, L.A.; Batista, A.D.S.M.; Serodre, T.; Neto, A.T.B.; Furtado, C.A.; Faria, L.O. Enhancement of X-ray Shielding Properties of PVDF/BaSO4 Nanocomposites Filled with Graphene Oxide. MRS Adv. 2019, 4, 169–175. [Google Scholar] [CrossRef]
- Fujimori, T.; Tsuruoka, S.; Fugetsu, B.; Maruyama, S.; Tanioka, A.; Terrones, M.; Dresselhaus, M.S.; Endo, M.; Kaneko, K. Enhanced X-Ray Shielding Effects of Carbon Nanotubes. Mater. Express 2011, 1, 273–278. [Google Scholar] [CrossRef]
- Viegas, J.; Silva, L.A.; Batista, A.M.S.; Furtado, C.A.; Nascimento, J.P.; Faria, L.O. Increased X-ray Attenuation Efficiency of Graphene-Based Nanocomposite. Ind. Eng. Chem. Res. 2017, 56, 11782–11790. [Google Scholar] [CrossRef]
- Zarei, M.; Sina, S.; Hashemi, S.A. Superior X-ray radiation shielding of biocompatible platform based on reinforced polyaniline by decorated graphene oxide with interconnected tungsten–bismuth–tin complex. Radiat. Phys. Chem. 2021, 188, 109588. [Google Scholar] [CrossRef]
- Türkaslan, S.S.; Ugur, S.; Türkaslan, B.E.; Fantuzzi, N. Evaluating the X-ray-Shielding Performance of Graphene-Oxide-Coated Nanocomposite Fabric. Materials 2022, 15, 1441. [Google Scholar] [CrossRef]
- Hsieh, H.-S.; Wu, R.; Jafvert, C.T. Light-Independent Reactive Oxygen Species (ROS) Formation through Electron Transfer from Carboxylated Single-Walled Carbon Nanotubes in Water. Environ. Sci. Technol. 2014, 48, 11330–11336. [Google Scholar] [CrossRef]
- Ge, J.; Lan, M.; Zhou, B.; Liu, W.; Guo, L.; Wang, H.; Jia, Q.; Niu, G.; Huang, X.; Zhou, H.; et al. A graphene quantum dot photodynamic therapy agent with high singlet oxygen generation. Nat. Commun. 2014, 5, 4596. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, M.; Zhou, Y.; Ye, C.; Liu, R. NIR Photosensitizer for Two-Photon Fluorescent Imaging and Photodynamic Therapy of Tumor. Front. Chem. 2021, 9, 629062. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, L.F. Nanomaterials for Enhanced Photodynamic Therapy. In Photodynamic Therapy—From Basic Science to Clinical Research; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Cuadrado, C.F.; Díaz-Barrios, A.; Campaña, K.O.; Romani, E.C.; Quiroz, F.; Nardecchia, S.; Debut, A.; Vizuete, K.; Niebieskikwiat, D.; Ávila, C.E.; et al. Broad-Spectrum Antimicrobial ZnMintPc Encapsulated in Magnetic-Nanocomposites with Graphene Oxide/MWCNTs Based on Bimodal Action of Photodynamic and Photothermal Effects. Pharmaceutics 2022, 14, 705. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, W.; Dong, Y.; Yu, X.; Mo, A.; Peng, Q. Enhanced Antibacterial Activity of Indocyanine Green-Loaded Graphene Oxide via Synergistic Contact Killing, Photothermal and Photodynamic Therapy. J. Biomed. Nanotechnol. 2022, 18, 185–192. [Google Scholar] [CrossRef]
- Lu, H.; Zhao, Q.; Wang, X.; Mao, Y.; Chen, C.; Gao, Y.; Sun, C.; Wang, S. Multi-stimuli responsive mesoporous silica-coated carbon nanoparticles for chemo-photothermal therapy of tumor. Colloids Surfaces B Biointerfaces 2020, 190, 110941. [Google Scholar] [CrossRef]
- Yu, J.; Yang, L.; Yan, J.; Wang, W.-C.; Chen, Y.-C.; Chen, H.-H.; Lin, C.-H. Carbon Nanomaterials for Photothermal Therapies. In Carbon Nanomaterials for Bioimaging, Bioanalysis, and Therapy; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018; pp. 309–340. [Google Scholar] [CrossRef]
- McKernan, P.; Virani, N.A.; Faria, G.N.F.; Karch, C.G.; Silvy, R.P.; Resasco, D.E.; Thompson, L.F.; Harrison, R.G. Targeted Single-Walled Carbon Nanotubes for Photothermal Therapy Combined with Immune Checkpoint Inhibition for the Treatment of Metastatic Breast Cancer. Nanoscale Res. Lett. 2021, 16, 9. [Google Scholar] [CrossRef]
- Wang, S.; Jin, S.; Li, G.; Xu, M.; Deng, D.; Xiao, Z.; Sun, H.; Zhang, S.; Zhang, E.; Xie, L.; et al. Transmucosal Delivery of Self-Assembling Photosensitizer–Nitazoxanide Nanocomplexes with Fluorinated Chitosan for Instillation-Based Photodynamic Therapy of Orthotopic Bladder Tumors. ACS Biomater. Sci. Eng. 2021, 7, 1485–1495. [Google Scholar] [CrossRef]
- Xu, P.; Liang, F. Nanomaterial-Based Tumor Photothermal Immunotherapy. Int. J. Nanomed. 2020, 15, 9159–9180. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, Y.; He, L.; Liu, W.; Chen, Y.; Liu, F.; Guo, Y.; Ran, H.; Yang, L. Novel Multifunctional Nanoagent for Visual Chemo/Photothermal Therapy of Metastatic Lymph Nodes via Lymphatic Delivery. ACS Omega 2020, 5, 3194–3206. [Google Scholar] [CrossRef]
- Li, Y.; Su, Y.; Pan, H.; Deng, W.; Wang, J.; Liu, D.; Pan, W. Nanodiamond-based Multifunctional Platform for Oral Chemo-photothermal Combinational Therapy of Orthotopic Colon Cancer. Pharmacol. Res. 2022, 176, 106080. [Google Scholar] [CrossRef]
- Zhou, L.; Jing, Y.; Liu, Y.; Liu, Z.; Gao, D.; Chen, H.; Song, W.; Wang, T.; Fang, X.; Qin, W.; et al. Mesoporous Carbon Nanospheres as a Multifunctional Carrier for Cancer Theranostics. Theranostics 2018, 8, 663–675. [Google Scholar] [CrossRef]
- Van Eden, W. Vaccination against autoimmune diseases moves closer to the clinic. Hum. Vaccines Immunother. 2020, 16, 228–232. [Google Scholar] [CrossRef]
- Gottardi, R.; Douradinha, B. Carbon nanotubes as a novel tool for vaccination against infectious diseases and cancer. J. Nanobiotechnol. 2013, 11, 30. [Google Scholar] [CrossRef]
- Scheinberg, D.A.; McDevitt, M.R.; Dao, T.; Mulvey, J.J.; Feinberg, E.; Alidori, S. Carbon nanotubes as vaccine scaffolds. Adv. Drug Deliv. Rev. 2013, 65, 2016–2022. [Google Scholar] [CrossRef]
- Dong, C.; Wang, Y.; Gonzalez, G.X.; Ma, Y.; Song, Y.; Wang, S.; Kang, S.-M.; Compans, R.W.; Wang, B.-Z. Intranasal vaccination with influenza HA/GO-PEI nanoparticles provides immune protection against homo- and heterologous strains. Proc. Natl. Acad. Sci. USA 2021, 118, e2024998118. [Google Scholar] [CrossRef]
- Ho, T.; Pham, V.; Nguyen, T.; Trinh, V.; Vi, T.; Lin, H.-H.; Nguyen, P.; Bui, H.; Pham, N.; Le, T.; et al. Effects of Size and Surface Properties of Nanodiamonds on the Immunogenicity of Plant-Based H5 Protein of A/H5N1 Virus in Mice. Nanomaterials 2021, 11, 1597. [Google Scholar] [CrossRef]
- Zare, H.; Ahmadi, S.; Ghasemi, A.; Ghanbari, M.; Rabiee, N.; Bagherzadeh, M.; Karimi, M.; Webster, T.J.; Hamblin, M.R.; Mostafavi, E. Carbon Nanotubes: Smart Drug/Gene Delivery Carriers. Int. J. Nanomed. 2021, 16, 1681–1706. [Google Scholar] [CrossRef]
- Liu, B.; Wang, W. Comprehensive Review on Graphene Oxide for Use in Drug Delivery System. Curr. Med. Chem. 2020, 27, 3665–3685. [Google Scholar] [CrossRef]
- Sequeira, C.A.C. Carbon Nanotubes in Cancer Research and Therapy. Biomed. J. Sci. Tech. Res. 2020, 25, 19437–19442. [Google Scholar] [CrossRef]
- Lay, C.L.; Liu, H.Q.; Tan, H.R.; Liu, Y. Delivery of paclitaxel by physically loading onto poly(ethylene glycol) (PEG)-graftcarbon nanotubes for potent cancer therapeutics. Nanotechnology 2010, 21, 065101. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, Y.; Chen, L.; Li, Q.; Du, J.; Gao, Y.; Zhang, L.; Yang, Y. Antitumor effects of carbon nanotube-drug complex against human breast cancer cells. Exp. Ther. Med. 2018, 16, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Buskaran, K.; Hussein, M.; Moklas, M.; Masarudin, M.; Fakurazi, S. Graphene Oxide Loaded with Protocatechuic Acid and Chlorogenic Acid Dual Drug Nanodelivery System for Human Hepatocellular Carcinoma Therapeutic Application. Int. J. Mol. Sci. 2021, 22, 5786. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, V.; Yadav, P.; Bhattacharya, S.S.; Mishra, A.K.; Verma, N.; Verma, A.; Pandit, J.K. Carbon Nanotubes: An Emerging Drug Carrier for Targeting Cancer Cells. J. Drug Deliv. 2014, 2014, 670815. [Google Scholar] [CrossRef] [PubMed]
- Perepelytsina, O.M.; Yakymchuk, O.M.; Sydorenko, M.V.; Bakalinska, O.N.; Bloisi, F.; Vicari, L.R.M. Functionalization of Carbon Nanomaterial Surface by Doxorubicin and Antibodies to Tumor Markers. Nanoscale Res. Lett. 2016, 11, 314. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Gao, X.; Taratula, O.; Treado, S.; Urbas, A.; Holbrook, R.D.; Cavicchi, R.E.; Avedisian, C.T.; Mitra, S.; Savla, R.; et al. Anti-HER2 IgY antibody-functionalized single-walled carbon nanotubes for detection and selective destruction of breast cancer cells. BMC Cancer 2009, 9, 351. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, Y.; Du, J.; Wang, X.; Duan, A.; Gao, R.; Liu, J.; Li, B. Graphene oxide loaded with tumor-targeted peptide and anti-cancer drugs for cancer target therapy. Sci. Rep. 2021, 11, 1725. [Google Scholar] [CrossRef]
- Kamazani, F.M.; Nematalahi, F.S.; Siadat, S.D.; Pornour, M.; Sheikhpour, M. A success targeted nano delivery to lung cancer cells with multi-walled carbon nanotubes conjugated to bromocriptine. Sci. Rep. 2021, 11, 24419. [Google Scholar] [CrossRef]
- Alisani, R.; Rakhshani, N.; Abolhallaj, M.; Motevallid, F.; Abadi, P.G.-S.; Akrami, M.; Shahrousvand, M.; Sharifianjazi, F.; Irani, M. Adsorption, and controlled release of doxorubicin from cellulose acetate/polyurethane/multi-walled carbon nanotubes composite nanofibers. Nanotechnology 2022, 33, 155102. [Google Scholar] [CrossRef]
- Serda, M.; Gawecki, R.; Dulski, M.; Sajewicz, M.; Talik, E.; Szubka, M.; Zubko, M.; Malarz, K.; Mrozek-Wilczkiewicz, A.; Musioł, R. Synthesis and applications of [60]fullerene nanoconjugate with 5-aminolevulinic acid and its glycoconjugate as drug delivery vehicles. RSC Adv. 2022, 12, 6377–6388. [Google Scholar] [CrossRef]
- Biswas, R.; Yang, S.; Crichton, R.A.; Adly-Gendi, P.; Chen, T.K.; Kopcha, W.P.; Shi, Z.; Zhang, J. C60-β-cyclodextrin conjugates for enhanced nucleus delivery of doxorubicin. Nanoscale 2022, 14, 4456–4462. [Google Scholar] [CrossRef]
- Zhou, M.; Zhao, Q.; Wu, Y.; Feng, S.; Wang, D.; Zhang, Y.; Wang, S. Mesoporous Carbon Nanoparticles as Multi-functional Carriers for Cancer Therapy Compared with Mesoporous Silica Nanoparticles. AAPS PharmSciTech 2020, 21, 42. [Google Scholar] [CrossRef]
- Wang, B.; Guo, H.; Xu, H.; Chen, Y.; Zhao, G.; Yu, H. The Role of Graphene Oxide Nanocarriers in Treating Gliomas. Front. Oncol. 2022, 12, 736177. [Google Scholar] [CrossRef]
- Alagarsamy, K.N.; Mathan, S.; Yan, W.; Rafieerad, A.; Sekaran, S.; Manego, H.; Dhingra, S. Carbon nanomaterials for cardiovascular theranostics: Promises and challenges. Bioact. Mater. 2021, 6, 2261–2280. [Google Scholar] [CrossRef]
- Teixeira-Santos, R.; Gomes, M.; Gomes, L.C.; Mergulhão, F.J. Antimicrobial and anti-adhesive properties of carbon nanotube-based surfaces for medical applications: A systematic review. iScience 2021, 24, 102001. [Google Scholar] [CrossRef]
- Sengupta, J.; Hussain, C.M. Carbon nanomaterials to combat virus: A perspective in view of COVID-19. Carbon Trends 2020, 2, 100019. [Google Scholar] [CrossRef]
- Xia, M.-Y.; Xie, Y.; Yu, C.-H.; Chen, G.-Y.; Li, Y.-H.; Zhang, T.; Peng, Q. Graphene-based nanomaterials: The promising active agents for antibiotics-independent antibacterial applications. J. Control. Release 2019, 307, 16–31. [Google Scholar] [CrossRef]
- Zhang, L.; Gui, S.; Xu, Y.; Zeng, J.; Wang, J.; Chen, Q.; Su, L.; Wang, Z.; Deng, R.; Chu, F.; et al. Colon tissue-accumulating mesoporous carbon nanoparticles loaded with Musca domestica cecropin for ulcerative colitis therapy. Theranostics 2021, 11, 3417–3438. [Google Scholar] [CrossRef]
- Bates, K.; Kostarelos, K. Carbon nanotubes as vectors for gene therapy: Past achievements, present challenges and future goals. Adv. Drug Deliv. Rev. 2013, 65, 2023–2033. [Google Scholar] [CrossRef]
- Anderson, T.; Hu, R.; Yang, C.; Yoon, H.S.; Yong, K.-T. Pancreatic cancer gene therapy using an siRNA-functionalized single walled carbon nanotubes (SWNTs) nanoplex. Biomater. Sci. 2014, 2, 1244–1253. [Google Scholar] [CrossRef]
- Hasan, T.; Campbell, E.; Sizova, O.; Lyle, V.; Akkaraju, G.; Kirkpatrick, D.L.; Naumov, A.V. Multi-Drug/Gene NASH Therapy Delivery and Selective Hyperspectral NIR Imaging Using Chirality-Sorted Single-Walled Carbon Nanotubes. Cancers 2019, 11, 1175. [Google Scholar] [CrossRef]
- Shahdeo, D.; Roberts, A.; Abbineni, N.; Gandhi, S. Graphene based sensors. Compr. Anal. Chem. 2020, 91, 175–199. [Google Scholar] [CrossRef]
- Cheraghi, S.; Taher, M.A.; Karimi-Maleh, H.; Karimi, F.; Shabani-Nooshabadi, M.; Alizadeh, M.; Al-Othman, A.; Erk, N.; Raman, P.K.Y.; Karaman, C. Novel enzymatic graphene oxide based biosensor for the detection of glutathione in biological body fluids. Chemosphere 2022, 287, 132187. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Pan, F.; Liu, J.; Wang, K.; Zhang, C.; Cheng, S.; Lu, L.-G.; Zhang, W.; Zhang, Z.; et al. Breath Analysis Based on Surface-Enhanced Raman Scattering Sensors Distinguishes Early and Advanced Gastric Cancer Patients from Healthy Persons. ACS Nano 2016, 10, 8169–8179. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Trock, E.; Haick, H. Detecting Simulated Patterns of Lung Cancer Biomarkers by Random Network of Single-Walled Carbon Nanotubes Coated with Nonpolymeric Organic Materials. Nano Lett. 2008, 8, 3631–3635. [Google Scholar] [CrossRef] [PubMed]
- Demirbakan, B.; Sezgintürk, M.K. A novel ultrasensitive immunosensor based on disposable graphite paper electrodes for troponin T detection in cardiovascular disease. Talanta 2020, 213, 120779. [Google Scholar] [CrossRef]
- Lin, Y.; Lu, F.; Tu, A.Y.; Ren, Z. Glucose Biosensors Based on Carbon Nanotube Nanoelectrode Ensembles. Nano Lett. 2004, 4, 191–195. [Google Scholar] [CrossRef]
- Reghunath, R.; Devi, K.; Singh, K. Recent advances in graphene based electrochemical glucose sensor. Nano Struct. Nano Objects 2021, 26, 100750. [Google Scholar] [CrossRef]
- Alagappan, M.; Immanuel, S.; Sivasubramanian, R.; Kandaswamy, A. Development of cholesterol biosensor using Au nanoparticles decorated f-MWCNT covered with polypyrrole network. Arab. J. Chem. 2020, 13, 2001–2010. [Google Scholar] [CrossRef]
- Agnihotri, N.; Chowdhury, A.D.; De, A. Non-enzymatic electrochemical detection of cholesterol using β-cyclodextrin functionalized graphene. Biosens. Bioelectron. 2015, 63, 212–217. [Google Scholar] [CrossRef]
- Aminabad, E.D.; Mobed, A.; Hasanzadeh, M.; Feizi, M.A.H.; Safaralizadeh, R.; Seidi, F. Sensitive immunosensing of α-synuclein protein in human plasma samples using gold nanoparticles conjugated with graphene: An innovative immuno-platform towards early stage identification of Parkinson’s disease using point of care (POC) analysis. RSC Adv. 2022, 12, 4346–4357. [Google Scholar] [CrossRef]
- Peña-Bahamonde, J.; Nguyen, H.N.; Fanourakis, S.K.; Rodrigues, D.F. Recent advances in graphene-based biosensor technology with applications in life sciences. J. Nanobiotechnol. 2018, 16, 75. [Google Scholar] [CrossRef]
- Kim, S.; Ryu, H.; Tai, S.; Pedowitz, M.; Rzasa, J.R.; Pennachio, D.J.; Hajzus, J.R.; Milton, D.K.; Myers-Ward, R.; Daniels, K.M. Real-time ultra-sensitive detection of SARS-CoV-2 by quasi-freestanding epitaxial graphene-based biosensor. Biosens. Bioelectron. 2022, 197, 113803. [Google Scholar] [CrossRef]
- Zhu, A.; Luo, X. Detection of Covid-19 through a Heptanal Biomarker Using Transition Metal Doped Graphene. J. Phys. Chem. B 2022, 126, 151–160. [Google Scholar] [CrossRef]
- Jia, H.; Zhang, A.; Yang, Y.; Cui, Y.; Xu, J.; Jiang, H.; Tao, S.; Zhang, D.; Zeng, H.; Hou, Z.; et al. A graphene oxide coated tapered microfiber acting as a super-sensor for rapid detection of SARS-CoV-2. Lab Chip 2021, 21, 2398–2406. [Google Scholar] [CrossRef]
- Wu, G.; Zhang, N.; Matarasso, A.; Heck, I.; Li, H.; Lu, W.; Phaup, J.G.; Schneider, M.J.; Wu, Y.; Weng, Z.; et al. Implantable Aptamer-Graphene Microtransistors for Real-Time Monitoring of Neurochemical Release in Vivo. Nano Lett. 2022, 22, 3668–3677. [Google Scholar] [CrossRef]
- Jaganathan, S.K.; Vellayappan, M.V.; Balaji, A.; Subramanian, A.; John, A.A.; Murugesan, S.; Supriyanto, E.; Yusof, M. Multifaceted prospects of nanocomposites for cardiovascular grafts and stents. Int. J. Nanomed. 2015, 10, 2785–2803. [Google Scholar] [CrossRef]
- Wawrzyńska, M.; Bil-Lula, I.; Krzywonos-Zawadzka, A.; Arkowski, J.; Łukaszewicz, M.; Hreniak, D.; Stręk, W.; Sawicki, G.; Woźniak, M.; Drab, M.; et al. Biocompatible Carbon-Based Coating as Potential Endovascular Material for Stent Surface. BioMed Res. Int. 2018, 2018, 2758347. [Google Scholar] [CrossRef]
- Ovcharenko, E.A.; Seifalian, A.; Rezvova, M.A.; Klyshnikov, K.; Glushkova, T.V.; Akenteva, T.N.; Antonova, L.V.; Velikanova, E.A.; Chernonosova, V.S.; Shevelev, G.Y.; et al. A New Nanocomposite Copolymer Based On Functionalised Graphene Oxide for Development of Heart Valves. Sci. Rep. 2020, 10, 5271. [Google Scholar] [CrossRef]
- Dehghani, F.; Khorasani, M.T.; Movahedi, M. Fabrication of polyurethane—Heparinized carbon nanotubes composite for heart valves application. Mater. Chem. Phys. 2022, 280, 125819. [Google Scholar] [CrossRef]
- Bhong, S.Y.; More, N.; Choppadandi, M.; Kapusetti, G. Review on carbon nanomaterials as typical candidates for orthopaedic coatings. SN Appl. Sci. 2019, 1, 76. [Google Scholar] [CrossRef]
- Azimi, S.; Golabchi, A.; Nekookar, A.; Rabbani, S.; Amiri, M.H.; Asadi, K.; Abolhasani, M.M. Self-powered cardiac pacemaker by piezoelectric polymer nanogenerator implant. Nano Energy 2021, 83, 105781. [Google Scholar] [CrossRef]
- Beshchasna, N.; Saqib, M.; Kraskiewicz, H.; Wasyluk, Ł.; Kuzmin, O.; Duta, O.C.; Ficai, D.; Ghizdavet, Z.; Marin, A.; Ficai, A.; et al. Recent Advances in Manufacturing Innovative Stents. Pharmaceutics 2020, 12, 349. [Google Scholar] [CrossRef] [PubMed]
- Gad, M.M.A.; Ali, M.S.; Al-Thobity, A.M.; Al-Dulaijan, Y.A.; El Zayat, M.; Emam, A.-N.M.; Akhtar, S.; Khan, S.Q.; Al-Harbi, F.A.; Fouda, S.M. Polymethylmethacrylate Incorporating Nanodiamonds for Denture Repair: In Vitro Study on the Mechanical Properties. Eur. J. Dent. 2022, 16, 286–295. [Google Scholar] [CrossRef]
- Al-Harbi, F.A.; Abdel-Halim, M.S.; Gad, M.M.; Fouda, S.M.; Baba, N.Z.; AlRumaih, H.S.; Akhtar, S. Effect of Nanodiamond Addition on Flexural Strength, Impact Strength, and Surface Roughness of PMMA Denture Base. J. Prosthodont. 2019, 28, e417–e425. [Google Scholar] [CrossRef]
- Fouda, S.M.; Gad, M.M.; Ellakany, P.; Al Ghamdi, M.A.; Khan, S.Q.; Akhtar, S.; Al Eraky, D.M.; Al-Harbi, F.A. Effect of Low Nanodiamond Concentrations and Polymerization Techniques on Physical Properties and Antifungal Activities of Denture Base Resin. Polymers 2021, 13, 4331. [Google Scholar] [CrossRef]
- Ghosh, S.; Chatterjee, K. Poly(Ethylene Glycol) Functionalized Graphene Oxide in Tissue Engineering: A Review on Recent Advances. Int. J. Nanomed. 2020, 15, 5991–6006. [Google Scholar] [CrossRef]
- Weidong, L.; Li, S.; Le, W. Nanomaterials modulate stem cell differentiation: Biological interaction and underlying mechanisms. J. Nanobiotechnol. 2017, 15, 75. [Google Scholar] [CrossRef]
- Li, N.; Zhang, X.; Song, Q.; Su, R.; Zhang, Q.; Kong, T.; Liu, L.; Jin, G.; Tang, M.; Cheng, G. The promotion of neurite sprouting and outgrowth of mouse hippocampal cells in culture by graphene substrates. Biomaterials 2011, 32, 9374–9382. [Google Scholar] [CrossRef]
- Rauti, R.; Musto, M.; Bosi, S.; Prato, M.; Ballerini, L. Properties and behavior of carbon nanomaterials when interfacing neuronal cells: How far have we come? Carbon 2019, 143, 430–446. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.; Yang, P. Effects of Graphene-Based Materials on the Behavior of Neural Stem Cells. J. Nanomater. 2020, 2020, 2519105. [Google Scholar] [CrossRef]
- Zhou, K.; Motamed, S.; Thouas, G.A.; Bernard, C.C.A.; Li, D.; Parkington, H.C.; Coleman, H.A.; Finkelstein, D.; Forsythe, J.S. Graphene Functionalized Scaffolds Reduce the Inflammatory Response and Supports Endogenous Neuroblast Migration when Implanted in the Adult Brain. PLoS ONE 2016, 11, e0151589. [Google Scholar] [CrossRef]
- Severino, F.P.U.; Ban, J.; Song, Q.; Tang, M.; Bianconi, G.; Cheng, G.; Torre, V. The role of dimensionality in neuronal network dynamics. Sci. Rep. 2016, 6, 29640. [Google Scholar] [CrossRef]
- López-Dolado, E.; González-Mayorga, A.; Gutiérrez, M.C.; Serrano, M.C. Immunomodulatory and angiogenic responses induced by graphene oxide scaffolds in chronic spinal hemisected rats. Biomaterials 2016, 99, 72–81. [Google Scholar] [CrossRef]
- Zhao, Y.; Gong, J.; Niu, C.; Wei, Z.; Shi, J.; Li, G.; Yang, Y.; Wang, H. A new electrospun graphene-silk fibroin composite scaffolds for guiding Schwann cells. J. Biomater. Sci. Polym. Ed. 2017, 28, 2171–2185. [Google Scholar] [CrossRef]
- Pi, W.; Zhang, Y.; Li, L.; Li, C.; Zhang, M.; Zhang, W.; Cai, Q.; Zhang, P. Polydopamine-coated polycaprolactone/carbon nanotube fibrous scaffolds loaded with brain-derived neurotrophic factor for peripheral nerve regeneration. Biofabrication 2022, 14, 035006. [Google Scholar] [CrossRef]
- Du, Z.; Wang, C.; Zhang, R.; Wang, X.; Li, X. Applications of Graphene and Its Derivatives in Bone Repair: Advantages for Promoting Bone Formation and Providing Real-Time Detection, Challenges and Future Prospects. Int. J. Nanomed. 2020, 15, 7523–7551. [Google Scholar] [CrossRef]
- Nie, W.; Peng, C.; Zhou, X.; Chen, L.; Wang, W.; Zhang, Y.; Ma, P.X.; He, C. Three-dimensional porous scaffold by self-assembly of reduced graphene oxide and nano-hydroxyapatite composites for bone tissue engineering. Carbon 2017, 116, 325–337. [Google Scholar] [CrossRef]
- Daneshmandi, L.; Holt, B.D.; Arnold, A.M.; Laurencin, C.T.; Sydlik, S.A. Ultra-low binder content 3D printed calcium phosphate graphene scaffolds as resorbable, osteoinductive matrices that support bone formation in vivo. Sci. Rep. 2022, 12, 6960. [Google Scholar] [CrossRef]
- Li, K.; Wang, C.; Yan, J.; Zhang, Q.; Dang, B.; Wang, Z.; Yao, Y.; Lin, K.; Guo, Z.; Bi, L.; et al. Evaluation of the osteogenesis and osseointegration of titanium alloys coated with graphene: An in vivo study. Sci. Rep. 2018, 8, 1843. [Google Scholar] [CrossRef]
- Chemla, Y.; Avraham, E.S.; Markus, A.; Teblum, E.; Slotky, A.; Kostikov, Y.; Farah, N.; Telkhozhayeva, M.; Shoval, I.; Nessim, G.D.; et al. Carbon nanostructures as a scaffold for human embryonic stem cell differentiation toward photoreceptor precursors. Nanoscale 2020, 12, 18918–18930. [Google Scholar] [CrossRef]
- Smith, A.S.T.; Yoo, H.; Yi, H.; Ahn, E.H.; Lee, J.H.; Shao, G.; Nagornyak, E.; Laflamme, M.A.; Murry, C.E.; Kim, D.-H. Micro- and nano-patterned conductive graphene–PEG hybrid scaffolds for cardiac tissue engineering. Chem. Commun. 2017, 53, 7412–7415. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, S.; Sareen, N.; Abu-El-Rub, E.; Ashour, H.; Sequiera, G.L.; Ammar, H.I.; Gopinath, V.; Shamaa, A.A.; Sayed, S.; Moudgil, M.; et al. Graphene Oxide-Gold Nanosheets Containing Chitosan Scaffold Improves Ventricular Contractility and Function After Implantation into Infarcted Heart. Sci. Rep. 2018, 8, 15069. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Sriram, P.; Barui, A.K.; Nethi, S.K.; Veeriah, V.; Chatterjee, S.; Suresh, K.I.; Patra, C.R. Graphene Oxides Show Angiogenic Properties. Adv. Health Mater. 2015, 4, 1722–1732. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Song, J.; Zhao, X.; Chen, W.; Ouyang, Y.; Yuan, W.; Fan, C. 3D Fabrication with Integration Molding of a Graphene Oxide/Polycaprolactone Nanoscaffold for Neurite Regeneration and Angiogenesis. Adv. Sci. 2018, 5, 1700499. [Google Scholar] [CrossRef]
- Kaur, G.; Narayanan, G.; Garg, D.; Sachdev, A.; Matai, I. Biomaterials-Based Regenerative Strategies for Skin Tissue Wound Healing. ACS Appl. Bio Mater. 2022, 5, 2069–2106. [Google Scholar] [CrossRef]
- Khalid, A.; Madni, A.; Raza, B.; Islam, M.U.; Hassan, A.; Ahmad, F.; Ali, H.; Khan, T.; Wahid, F. Multiwalled carbon nanotubes functionalized bacterial cellulose as an efficient healing material for diabetic wounds. Int. J. Biol. Macromol. 2022, 203, 256–267. [Google Scholar] [CrossRef]
- Du, S.; Liu, B.; Li, Z.; Tan, H.; Qi, W.; Liu, T.; Qiang, S.; Zhang, T.; Song, F.; Chen, X.; et al. A Nanoporous Graphene/Nitrocellulose Membrane Beneficial to Wound Healing. ACS Appl. Bio Mater. 2021, 4, 4522–4531. [Google Scholar] [CrossRef]
- Gupta, S.; Prasad, P.; Roy, A.; Alam, M.M.; Ahmad, I.; Bit, A. Metallic ion-based graphene oxide functionalized silk fibroin-based dressing promotes wound healing via improved bactericidal outcomes and faster re-epithelization. Biomed. Mater. 2022, 17, 035010. [Google Scholar] [CrossRef]
- Holmannova, D.; Borsky, P.; Svadlakova, T.; Borska, L.; Fiala, Z. Reproductive and Developmental Nanotoxicity of Carbon Nanoparticles. Nanomaterials 2022, 12, 1716. [Google Scholar] [CrossRef]
- Svadlakova, T.; Kolackova, M.; Vankova, R.; Karakale, R.; Malkova, A.; Kulich, P.; Hubatka, F.; Turanek-Knotigova, P.; Kratochvilova, I.; Raska, M.; et al. Carbon-Based Nanomaterials Increase Reactivity of Primary Monocytes towards Various Bacteria and Modulate Their Differentiation into Macrophages. Nanomaterials 2021, 11, 2510. [Google Scholar] [CrossRef]
- Malkova, A.; Svadlakova, T.; Singh, A.; Kolackova, M.; Vankova, R.; Borsky, P.; Holmannova, D.; Karas, A.; Borska, L.; Fiala, Z. In Vitro Assessment of the Genotoxic Potential of Pristine Graphene Platelets. Nanomaterials 2021, 11, 2210. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, G.; Zhang, P.; Fan, J. Programmed cell death and its role in inflammation. Mil. Med. Res. 2015, 2, 12. [Google Scholar] [CrossRef]
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.H.; Qoronfleh, M.W. Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 2019, 23, 20. [Google Scholar] [CrossRef]
- Bergamaschi, E.; Garzaro, G.; Jones, G.W.; Buglisi, M.; Caniglia, M.; Godono, A.; Bosio, D.; Fenoglio, I.; Canu, I.G. Occupational Exposure to Carbon Nanotubes and Carbon Nanofibres: More Than a Cobweb. Nanomaterials 2021, 11, 745. [Google Scholar] [CrossRef]
- Zhang, T.; Tang, M.; Zhang, S.; Hu, Y.; Li, H.; Zhang, T.; Xue, Y.; Pu, Y. Systemic and immunotoxicity of pristine and PEGylated multi-walled carbon nanotubes in an intravenous 28 days repeated dose toxicity study. Int. J. Nanomed. 2017, 12, 1539–1554. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).