Biomechanical Comparison of Salvage Pedicle Screw Augmentations Using Different Biomaterials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Blocks and Pilot Hole Sizes
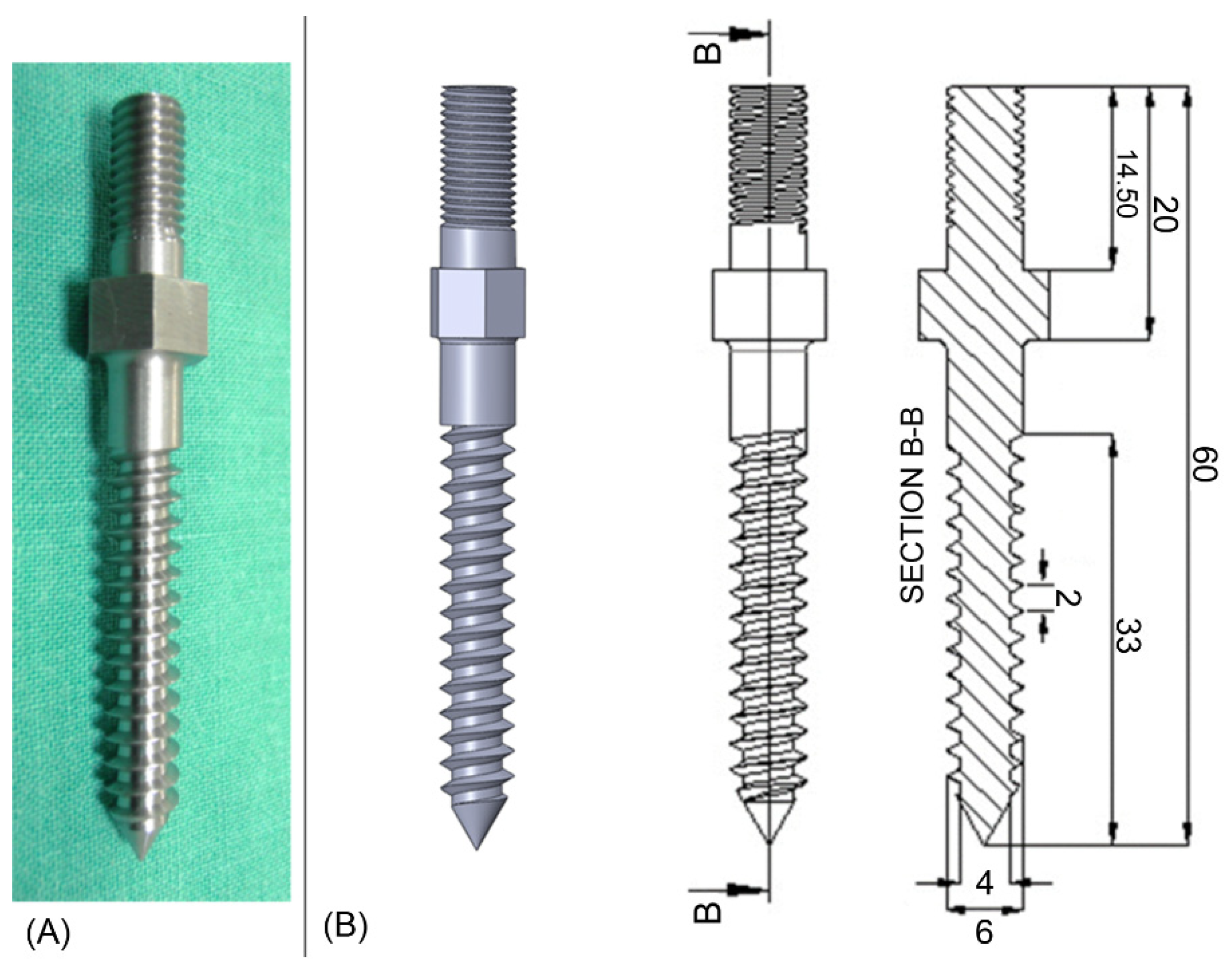
2.2. Pedicle Screw Geometries
2.3. Filling Biomaterials and Preparation
2.4. Experimental Grouping: Primary and Revision
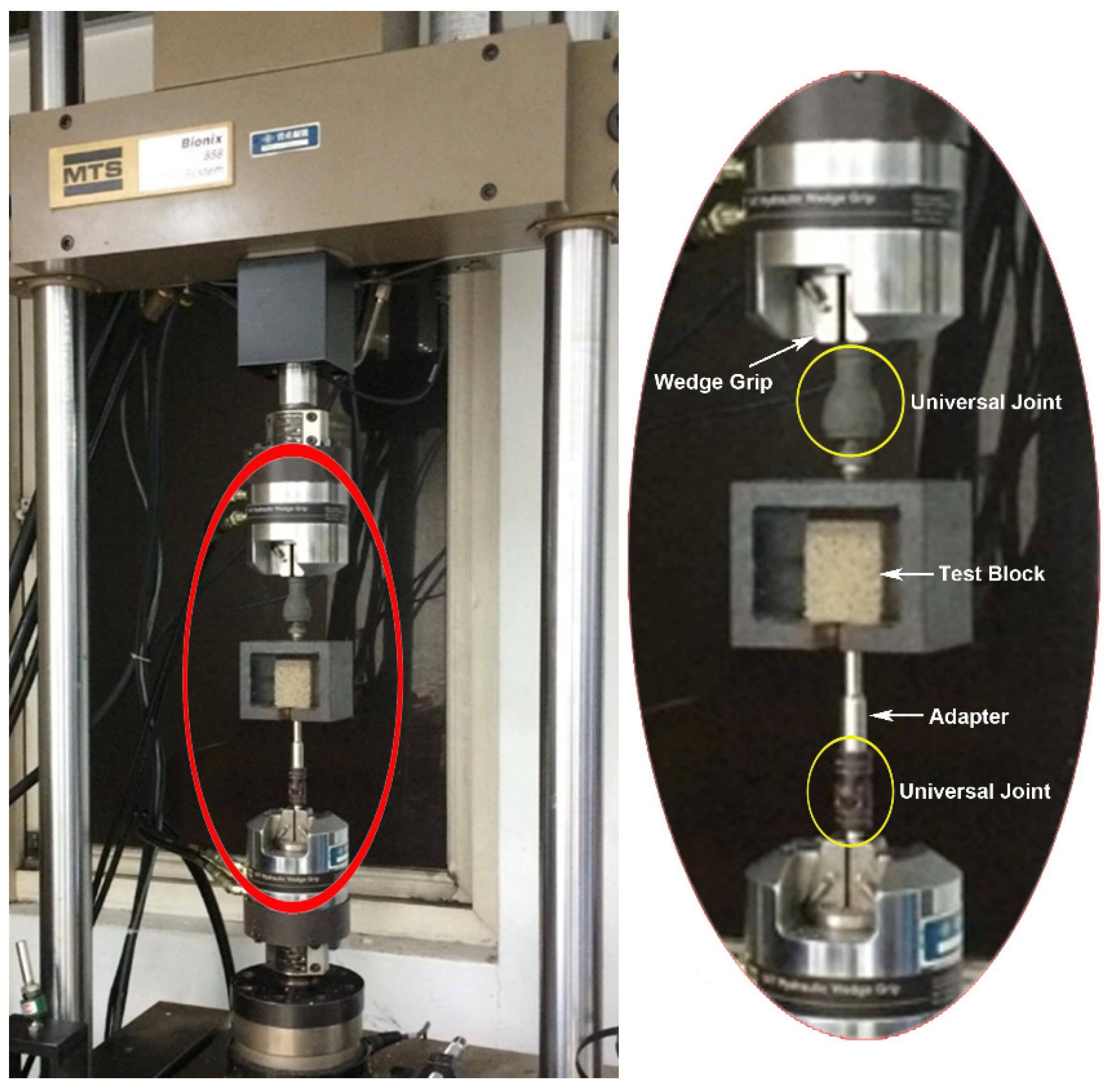
2.5. Biomechanical Pullout Testing
2.6. Statistical Analysis
3. Results
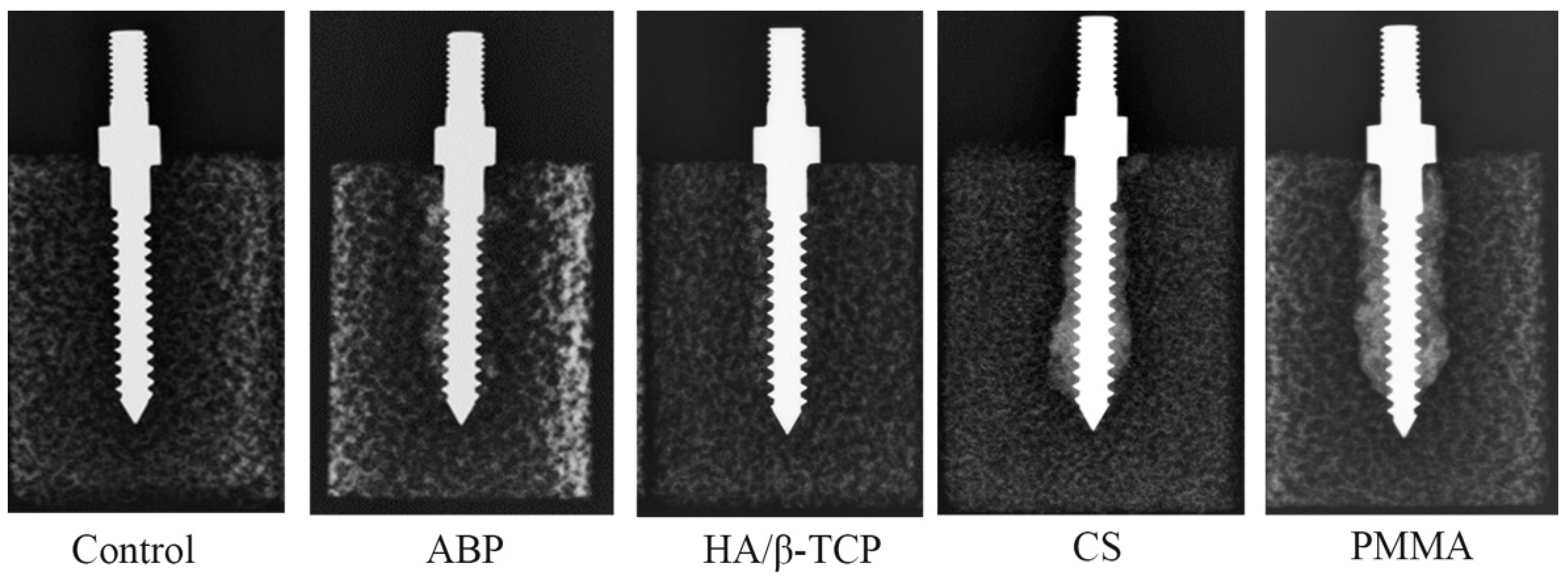
3.1. Radiological Images after the Insertion of Screws with Various Materials
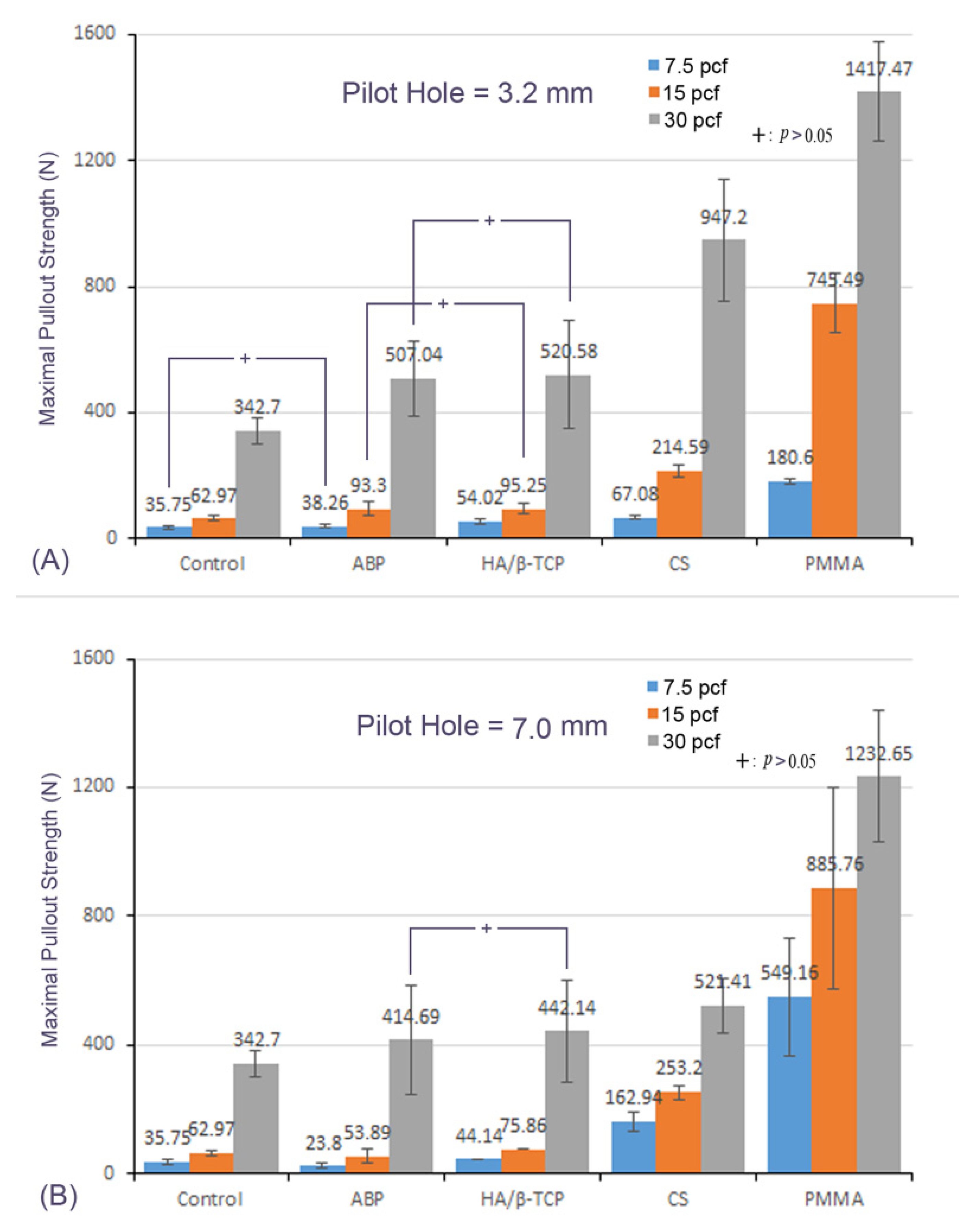
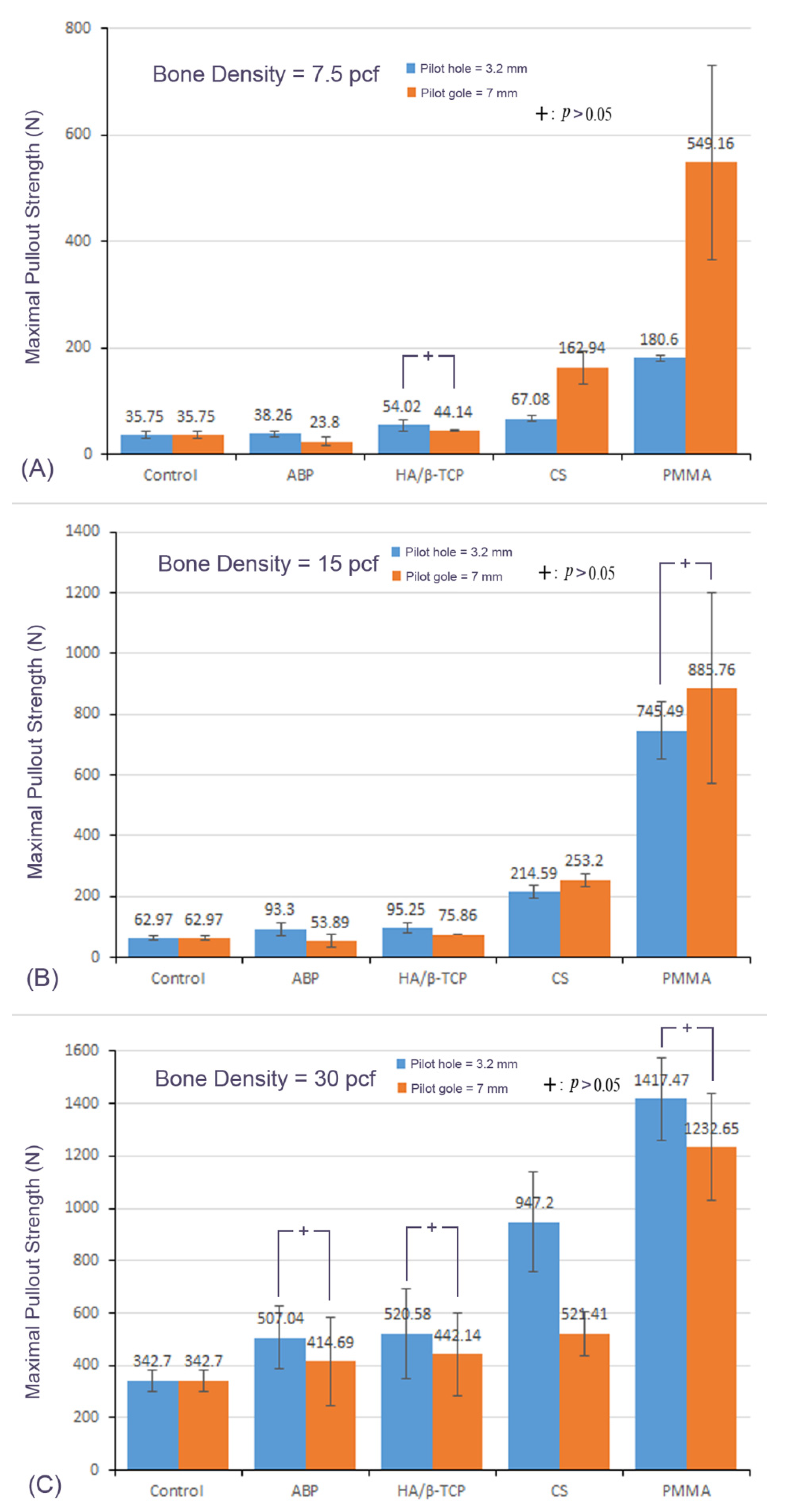
3.2. Mean Maximal Pullout Strength of Different Materials with the Different Pilot-Hole Sizes and Bone Densities
3.3. Effect of Bone Density
3.4. Effect of the Pilot-Hole Size
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boucher, H.H. A method of spinal fusion. J. Bone Joint Surg. Br. 1959, 41, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Elder, B.D.; Lo, S.-F.L.; Holmes, C.; Goodwin, C.R.; Kosztowski, T.A.; Lina, I.A.; Locke, J.E.; Witham, T.F. The biomechanics of pedicle screw augmentation with cement. Spine J. 2015, 15, 1432–1445. [Google Scholar] [CrossRef] [PubMed]
- Gaines, R.W., Jr. The use of pedicle screw internal fixation for the operative treatment of spinal disorders. J. Bone Joint Surg. Am. 2000, 82, 1458–1476. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, H.; Yukawa, Y.; Imagama, S.; Kanemura, T.; Kamiya, M.; Yanase, M.; Ito, K.; Machino, M.; Yoshida, G.; Ishikawa, Y.; et al. Complications of cervical pedicle screw fixation for nontraumatic lesions: A multicenter study of 84 patients. J. Neurosurg. Spine 2012, 16, 238–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, B.I.; Deyo, R.A.; Mirza, S.K.; Turner, J.A.; Comstock, B.A.; Hollingworth, W.; Sullivan, S.D. Expenditures and health status among adults with back and neck problems. JAMA 2008, 299, 656–664. [Google Scholar] [CrossRef] [Green Version]
- Deyo, R.A.; Gray, D.T.; Kreuter, W.; Mirza, S.; Martin, B.I. United States Trends in Lumbar Fusion Surgery for Degenerative Conditions. Spine 2005, 30, 1441–1445. [Google Scholar] [CrossRef]
- Szpalski, M.; Gunzburg, R.; Aebi, M. The aging of the population: A growing concern for spine care in the twenty-first century. Eur. Spine J. 2003, 12, S81–S83. [Google Scholar] [CrossRef] [Green Version]
- Esses, S.I.; Sachs, B.L.; Dreyzin, V. Complications Associated with the Technique of Pedicle Screw Fixation A Selected Survey of ABS Members. Spine 1993, 18, 2231–2239. [Google Scholar] [CrossRef]
- Mirovsky, Y.; Floman, Y.; Smorgick, Y.; Ashkenazi, E.; Anekstein, Y.; Millgram, M.A.; Giladi, M. Management of Deep Wound Infection After Posterior Lumbar Interbody Fusion with Cages. J. Spinal Disord. Tech. 2007, 20, 127–131. [Google Scholar] [CrossRef]
- Kobayashi, K.; Ando, K.; Kato, F.; Kanemura, T.; Sato, K.; Hachiya, Y.; Matsubara, Y.; Kamiya, M.; Sakai, Y.; Yagi, H.; et al. Reoperation within 2 years after lumbar interbody fusion: A multicenter study. Eur. Spine J. 2018, 27, 1972–1980. [Google Scholar] [CrossRef]
- Li, Y.-D.; Chi, J.-E.; Chiu, P.-Y.; Kao, F.-C.; Lai, P.-L.; Tsai, T.-T. The comparison between anterior and posterior approaches for removal of infected lumbar interbody cages and a proposal regarding the use of endoscope-assisted technique. J. Orthop. Surg. Res. 2021, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Talu, U.; Kaya, I.; Dikici, F.; Sar, C. Pedicle screw salvage: The effect of depth and diameter on pullout strength: A biomechanical study. Acta Orthop Traumatol Turc. 2004, 34, 300–307. [Google Scholar]
- Hsieh, M.-K.; Liu, M.-Y.; Chen, J.-K.; Tsai, T.-T.; Lai, P.-L.; Niu, C.-C.; Tai, C.-L. Use of longer sized screws is a salvage method for broken pedicles in osteoporotic vertebrae. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kaye, I.D.; Prasad, S.K.; Vaccaro, A.R.; Hilibrand, A.S. The Cortical Bone Trajectory for Pedicle Screw Insertion. JBJS Rev. 2017, 5, e13. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Fernandez, J.; García-Pallero, M.; Blasco, G.; Pulido-Rivas, P.; Rafael, G.S. Review of Cortical Bone Trajectory: Evidence of a New Technique. Asian Spine J. 2017, 11, 817–831. [Google Scholar] [CrossRef] [Green Version]
- Hashemi, A.; Bednar, D.; Ziada, S. Pullout strength of pedicle screws augmented with particulate calcium phosphate: An experimental study. Spine J. 2009, 9, 404–410. [Google Scholar] [CrossRef]
- Yi, S.; Rim, D.-C.; Park, S.W.; Murovic, J.A.; Lim, J.; Park, J. Biomechanical Comparisons of Pull Out Strengths After Pedicle Screw Augmentation with Hydroxyapatite, Calcium Phosphate, or Polymethylmethacrylate in the Cadaveric Spine. World Neurosurg. 2015, 83, 976–981. [Google Scholar] [CrossRef]
- Liu, M.-Y.; Tsai, T.-T.; Lai, P.-L.; Hsieh, M.-K.; Chen, L.-H.; Tai, C.-L. Biomechanical comparison of pedicle screw fixation strength in synthetic bones: Effects of screw shape, core/thread profile and cement augmentation. PLoS ONE 2020, 15, e0229328. [Google Scholar] [CrossRef]
- Agrawal, M.; Katti, D.; Boyan, B.; McMillan, J.; Lohmann, C.; Ranly, D.; Schwartz, Z. Chapter 13—Bone Graft Substitutes: Basic Information for Successful Clinical Use with Special Focus on Synthetic Graft Substitutes; ASTM International: West Conshohocken, PA, USA, 2009; p. 231. [Google Scholar] [CrossRef]
- Campana, V.; Milano, G.; Pagano, E.; Barba, M.; Cicione, C.; Salonna, G.; Lattanzi, W.; Logroscino, G. Bone substitutes in orthopaedic surgery: From basic science to clinical practice. J. Mater. Sci. Mater. Med. 2014, 25, 2445–2461. [Google Scholar] [CrossRef]
- Peltier, L.F.; Bickel, E.Y.; Lillo, R.; Thein, M.S. The Use of Plaster of Paris to Fill Defects in Bone. Ann. Surg. 1957, 146, 61–69. [Google Scholar] [CrossRef]
- Kumar, Y.; Nalini, K.B.; Menon, J.; Patro, D.K.; Banerji, B.H. Calcium sulfate as bone graft substitute in the treatment of osseous bone defects, a prospective study. J. Clin. Diagn. Res. 2013, 7, 2926–2928. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-H.; Tai, C.-L.; Lee, D.-M.; Lai, P.-L.; Lee, Y.-C.; Niu, C.-C.; Chen, W.-J. Pullout strength of pedicle screws with cement augmentation in severe osteoporosis: A comparative study between cannulated screws with cement injection and solid screws with cement pre-filling. BMC Musculoskelet Disord. 2011, 12, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.; Wu, Z.-X.; Pan, X.-M.; Fu, S.-C.; Gao, M.-X.; Shi, L.; Lei, W. Biomechanical comparison of different techniques in primary spinal surgery in osteoporotic cadaveric lumbar vertebrae: Expansive pedicle screw versus polymethylmethacrylate-augmented pedicle screw. Arch. Orthop. Trauma. Surg. 2011, 131, 1227–1232. [Google Scholar] [CrossRef] [PubMed]
- Stürup, J.; Nimb, L.; Kramhøft, M.; Jensen, J.S. Effects of polymerization heat and monomers from acrylic cement on canine bone. Acta Orthop. Scand. 1994, 65, 20–23. [Google Scholar] [CrossRef]
- Lai, P.-L.; Tai, C.-L.; Chen, L.-H.; Nien, N.-Y. Cement leakage causes potential thermal injury in vertebroplasty. BMC Musculoskelet. Disord. 2011, 12, 116. [Google Scholar] [CrossRef] [Green Version]
- Lai, P.-L.; Tai, C.-L.; Chu, I.-M.; Fu, T.-S.; Chen, L.-H.; Chen, W.-J. Hypothermic manipulation of bone cement can extend the handling time during vertebroplasty. BMC Musculoskelet. Disord. 2012, 13, 198. [Google Scholar] [CrossRef] [Green Version]
- Tai, C.-L.; Lai, P.-L.; Lin, W.-D.; Tsai, T.-T.; Lee, Y.-C.; Liu, M.-Y.; Chen, L.-H. Modification of Mechanical Properties, Polymerization Temperature, and Handling Time of Polymethylmethacrylate Cement for Enhancing Applicability in Vertebroplasty. BioMed Res. Int. 2016, 2016, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Gibson, L.; Ashby, M. Cancellous bone. Cellular Solids: Structure & Properties; Pergamon Press: New York, NY, USA, 1988; pp. 316–331. [Google Scholar]
- Amirouche, F.; Solitro, G.F.; Magnan, B.P. Stability and Spine Pedicle Screws Fixation Strength—A Comparative Study of Bone Density and Insertion Angle. Spine Deform. 2016, 4, 261–267. [Google Scholar] [CrossRef]
- Weiser, L.; Huber, G.; Sellenschloh, K.; Viezens, L.; Püschel, K.; Morlock, M.M.; Lehmann, W. Insufficient stability of pedicle screws in osteoporotic vertebrae: Biomechanical correlation of bone mineral density and pedicle screw fixation strength. Eur. Spine J. 2017, 26, 2891–2897. [Google Scholar] [CrossRef]
- Jia, C.; Zhang, R.; Xing, T.; Gao, H.; Li, H.; Dong, F.; Zhang, J.; Ge, P.; Song, P.; Xu, P.; et al. Biomechanical properties of pedicle screw fixation augmented with allograft bone particles in osteoporotic vertebrae: Different sizes and amounts. Spine J. 2019, 19, 1443–1452. [Google Scholar] [CrossRef]
- Fan, H.T.; Zhang, R.J.; Shen, C.L.; Dong, F.L.; Li, Y.; Song, P.W.; Gong, C.; Wang, Y.J. The Biomechanical Properties of Pedicle Screw Fixation Combined with Trajectory Bone Cement Augmentation in Osteoporotic Vertebrae. J. Spinal Disord. Tech. 2016, 29, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Weiser, L.; Huber, G.; Sellenschloh, K.; Püschel, K.; Morlock, M.M.; Viezens, L.; Lehmann, W. Rescue Augmentation: Increased Stability in Augmentation After Initial Loosening of Pedicle Screws. Glob. Spine J. 2021, 11, 679–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.-D.; Hsieh, M.-K.; Lee, D.-M.; Lin, Y.-J.; Tsai, T.-T.; Lai, P.-L.; Tai, C.-L. Biomechanical Comparison of Salvage Pedicle Screw Augmentations Using Different Biomaterials. Appl. Sci. 2022, 12, 7792. https://doi.org/10.3390/app12157792
Li Y-D, Hsieh M-K, Lee D-M, Lin Y-J, Tsai T-T, Lai P-L, Tai C-L. Biomechanical Comparison of Salvage Pedicle Screw Augmentations Using Different Biomaterials. Applied Sciences. 2022; 12(15):7792. https://doi.org/10.3390/app12157792
Chicago/Turabian StyleLi, Yun-Da, Ming-Kai Hsieh, De-Mei Lee, Yi-Jiun Lin, Tsung-Ting Tsai, Po-Liang Lai, and Ching-Lung Tai. 2022. "Biomechanical Comparison of Salvage Pedicle Screw Augmentations Using Different Biomaterials" Applied Sciences 12, no. 15: 7792. https://doi.org/10.3390/app12157792
APA StyleLi, Y.-D., Hsieh, M.-K., Lee, D.-M., Lin, Y.-J., Tsai, T.-T., Lai, P.-L., & Tai, C.-L. (2022). Biomechanical Comparison of Salvage Pedicle Screw Augmentations Using Different Biomaterials. Applied Sciences, 12(15), 7792. https://doi.org/10.3390/app12157792





