Abstract
There is an urgent need to establish new agro-technical practices that require the delivery of effective, natural, ecological, and verified solutions. The evaluation of possible applications in the field of cropping and farming in recent years has resulted in numerous products and approaches, which may potentially reduce our dependence on artificial pesticides. A major requirement to help establish these new agro-technical practices is to determine their efficiency. Here we present a study that investigates the relationship between volatile organic compounds (VOCs) released by Triticum sp. plants under two artificial stress conditions. We discuss their effectiveness in natural pest management and for use in monitoring crop health. Two varieties of spring wheat, “Kandela” and “Serenada”, were exposed to either mechanical (deliberate) wounding, to imitate the stress caused by insect attack, or exposure to methyl jasmonate, a defence volatile used by plants. Both stress factors caused an increased release of green leaf volatiles (C6 aldehydes and alcohols) and other volatile compounds, such as (E)-β-ocimene, linalool, β-caryophyllene, and (E)-β-farnesene. VOC emission rates are reported at three time points (24, 48, and 72 h) following a stress factor. Correlation coefficients between the emitted plant’s VOCs indicate the potential of monitoring just one single compound from the combination of volatiles emitted by plants to predict the overall condition of a crop. This has major implications for the development of a chemically specific and fieldable analytical sensor that could be used to provide an array of volatile monitoring stations delivering information continuously and in real-time. Finally, we demonstrate the effectiveness of the volatiles released by damaged spring wheat for pest management by exposing a shield bug (Bishop’s Mitre (Aelia acuminata L.), Hemiptera: Pentatomidae), to them.
1. Introduction
A major goal of the European Commission’s 2020 Farm to Fork Strategy, which is at the heart of the European Green Deal [1], is to have, by 2030, a 50% reduction in agricultural pesticide use. To help achieve this, effective natural pest control strategies need to be investigated.
In recent years, natural and environmentally safe practices for farming have been investigated extensively, and a key one is the use of plant-emitted volatile organic compounds (VOCs) as elicitors of natural defences against pests [2,3,4,5].
The plant VOCs, which are linked to efficient defence strategies against various pests, include hexanal, (Z)-3-hexenal, (E)-2-hexenal, (Z)-3-hexen-1-ol, (E)-2-hexen-1-ol, β-caryophyllene, (E)-β-farnesene, and (Z)-3-hexen-1-yl acetate [6,7,8,9]. The possibility of using induced VOCs to enhance the natural defence mechanisms of plants and hence protect crops is a particularly attractive and reasonable strategy, which could help to reduce our dependence on commercial pesticides. This could be achieved, for example, by the deliberate release of plant volatiles that are associated with signalling and defence methods in order to prepare a whole crop against pest invasion.
A number of biotic and abiotic stress factors induce plant VOC emissions. An obvious biotic stress is that resulting from insect feeding, with signalling volatiles being released by the damaged plant informing other plants to prepare for a likely attack [7]. Physical damage by deliberate (mechanical) plant tissue injury will also induce the release of VOCs, as too can the exposure of plants to exogenous volatile compounds, such as methyl jasmonate (MeJA) or (Z)-11-hexadecenal, which elicit stress responses. These can be used to influence plant VOC synthesis and secretion pathways [5,10]. Finally, the specific use of genetically modified plants to emit particular VOCs has the potential to provide indicators for other plants to increase defence VOC emissions [2].
The introduction of natural and efficient pest control strategies into the farming practice would bring huge societal benefits, but this can only be fully realised if we have ways to monitor their effectiveness and their effects on the environment. This is particularly crucial if the natural pest control strategies are to be used on an industrial scale, for which it needs to be shown that they are reliable, safe, and easy to use.
To further our understanding of the use of plant volatiles for use as natural pest control, we present here studies of the release of volatiles from spring wheat (Triticum L.) following either mechanical wounding or exposure to MeJA (either as a vapour or directly sprayed onto the plants). We also describe the effects of exposing the identified volatiles on a shield bug, the Bishop’s Mitre. The Bishop’s Mitre (Aelia acuminata L., Hemiptera: Pentatomidae) is a common and dominant species of shield bug that causes kernel damage in wheat across Europe [11]. The threat they present is currently limited, but with increasing global warming the impact of Bishop’s Mitre shieldbugs, and sunbugs in general, on wheat cultivation is significantly increasing [12]. A key hypothesis of the study is that the common biosynthetic pathway for most VOCs released by Triticum L. will result in correlated levels of their emission, which could be used to predict the health of plants and the presence of pests or diseases in cultivation. A major objective of this study is to demonstrate that MeJa can be used to activate the natural defence system of spring wheat prior to an insect attack.
2. Materials and Methods
2.1. Plant Material
“Kandela” and “Serenada” varieties of certified seeds (free of pathogens and spoiling factors) of spring wheat (Triticum L.) were purchased from the Seed Company ROLNAS (Bydgoszcz, Poland). The selection of these two particular varieties was based on the 2020 Centralny Ośrodek Badania Odmian Roślin Uprawnych (COBORU) [13] register, using the following attributes of the seeds: (i) quality of crop (commonly used by farmers), (ii) high crop yield and (iii) popularity amongst farmers. Both varieties have been listed in the Polish National Register of Varieties.
2.2. Wheat Cultivation
Plants were initially grown in pots in a greenhouse at the Plant Growth Centre of Bydgoszcz University of Science and Technology in 2019 and later delivered to the laboratory. Sowing was carried out on four dates, (1, 5, 9, and 11 March 2019), and each sowing involved fourteen replications (each pot was replicated). In each pot (of diameter 12 cm), six seeds were sown into a universal soil (pH 5.5–6.5). The plants were kept at a constant temperature of 22 ± 2 °C, exposed to 15 h of light per day, and watered every day. After 3.5 weeks of growth, the cultivation was screened in terms of health, size, and overall condition. One plant from each pot was then selected according to the above screening for further growing.
2.3. Plant Artificial Stress Generation
After 6 weeks of cultivation, by which time the wheat plants were in their 32-growth phase, according to the BBCH-scale [14] (Biologische Bundesanstalt, Bundessortenamt und Chemische Industrie), the plants were subjected to artificial stress induction procedures. Two methods of artificial stress were adopted: (i) mechanical damage to imitate insects’ feeding on wheat, and (ii) chemical exposure to MeJA (Sigma-Aldrich, Steinheim, Germany, purity 95%), either by vaporization or by direct spraying onto the plants. The codes for the particular types of stress to induce VOC emission are given in Table 1. As a control, plants that were not subjected to any artificial stress, apart from placement in nalophan bag during VOC collection and olfactory tests, were used.

Table 1.
Artificial stress codes used to identify the volatile samples collected.
Mechanical damage was accomplished by scraping the plants’ leaves with a scalpel, either with just one 5 cm scraping per leaf (1SL) or with three 5 cm scrapings per leaf (3SL). For MeJA exposure, the compound was first diluted to give a quantity of 250 µg of MeJA per 100 mL of distilled water. For the vapour exposure experiments (MJ-V), 50 µL of the solution was absorbed onto filter paper. The filter paper was then placed into a plastic centrifuge tube (2 mL) that was closed with a plastic lid. To facilitate constant MeJA vaporization, the lid was perforated with a 0.5 mm diameter needle. The aerial parts of the wheat were placed individually into nalophan sleeves along with one plastic tube per plant. In the second chemical treatment (MJ-S), the surfaces of the plants were directly sprayed (1 mL of application mixture) with the stock solution, where the whole plant was covered by the mixture. The plants were then placed into nalophan sleeves.
2.4. Collection of Volatile Organic Compounds
VOCs were collected using “Super-Q traps”, consisting of glass tubes having an outside diameter of 6.35 mm and a length of 76 mm (Analytical Research System, Inc., Gainesville, FL, USA). Each trap contained 30 mg of an adsorbent (Altech Associates, Inc., Deerfield, IL, USA). The glass tubes were connected on one side with a sleeve (~50 cm high × 30 cm dia.) containing a plant, and on the other side with flexible tubes that were connected to a suction pump (Thermo Fisher Scientific, Waltham, MA, USA) to produce an airstream. Purified and humidified air was delivered to the plants from the bottom of the sleeve at a rate of 1.0 L min−1. To avoid confounding volatiles entering the system from outside of the system, positive pressure was maintained by having the suction pump operating at 0.8 L min−1. The VOCs being released by the plants were collected over a period of 2 h at three fixed time points, 24, 48 and 72 h, following a given artificial stress, and then analysed. For each kind of artificial stress exposure and for each time collection, three repetitions were performed.
2.5. Extraction of Volatile Organic Compounds and Subsequent GC/MS Analysis
The “Super-Q traps” were subjected to an extraction procedure prior to the GC/MS analysis. Briefly, 30 mg of the adsorbent was washed in three 500 µL portions of n-hexane (Sigma-Aldrich, Steinheim, Germany). 7 ng of n-decane (Sigma-Aldrich, Steinheim, Germany) was added to 1 µL of the sample that was injected into the GC in order to provide an internal standard for the GC/MS analysis. Thereafter, the extract was placed into a chromatographical vial with a glass insert. The chromatographical vials were tightly sealed with parafilm and stored at 4 °C until analysis.
The qualitative and quantitative analysis of the plant-emitted VOCs was carried out on a GC/MS AutoSystem XL system (Perkin Elmer, Shelton, CT, USA), fitted with a Restek (Bellefonte, PA, USA) DB-5MS column (30 m long, 0.25 mm internal diameter, and 0.25 μm film thickness). The inlet temperature of the GC was set at 250 °C. The injection volume was 1 µL using a carrier gas (helium) flow of 1.0 mL min−1 and a split ratio of 1:10. A temperature program was used to ramp the chromatography oven temperature from 40 °C to 200 °C at a rate of 5 °C min−1.
To record the mass spectra, the following mass spectrometric operational conditions were used: ion source temperature 250 °C, interface temperature 200 °C, and scan range 35-350 m/z at a rate of 250 amu s−1. To identify the VOCs, the mass spectra and the volatiles’ retention times were compared with data available in mass spectral libraries (NIST 17 Mass Spectral and Retention Index Libraries, and The National Institute of Standards and Technology, Gaithersburg, MD, USA). To provide a semi-quantitative measure of a VOC emitted, its associated peak area in the chromatograph was compared to that associated with the internal standard. The values obtained are expressed as an emission rate in units of μg h−1.
2.6. Insects
Using a sweep net, adult Aelia acuminata L. were collected directly from fields (Bydgoszcz vicinity, Poland; geographical coordinates 53°7′24.5352″ N, 18°0′30.3768″ E). They were reared on potted wheat plants (kept in isolators) under continuous light and maintained at a temperature of 22 ± 2 °C and relative humidity of 60 ± 5%. Only newly emerged adults of both sexes were used in this study.
2.7. Four-Way Olfactometer and Insect Behaviour
A four-way olfactometer (SYNTECH GmbH, Oldenburg, Germany) was used to evaluate the influence of the VOCs emitted from wheat after MeJa exposure on insect behaviour, using the Bishop’s Mitre as a test insect. Purified and humidified air with a known combination of plant VOCs was introduced into two arms of the olfactometer at a rate of 0.3 L min−1. In the two opposite arms, purified humidified air with hexane was introduced at the same rate. A vacuum pump was used to create an airflow of 1.2 L min−1 from the bottom of the olfactometer to prevent the accumulation of air. Between the airflow meters and the four inlet ports, the air passed through a set of two gas wash bottles, one to capture the insects moving into one of the ports, and the other to load the air with the test volatiles. The system was washed and rotated after measurements involving each concentration to limit the effects of chemical residues from the previous bioassay. The insects were introduced either by lifting the glass cover or by placing them into the central aperture using the introduction trap. At each dose level of the VOC combination, 20 young adults of each sex of Aelia acuminata L. were tested individually. The duration of a run was 10 min (dosages at rates of 10 or 100 ng min−1 were measured by CS-55 equipment). Insects were scored in the olfactometer by accumulation in one of the olfactometer arms. For each MeJA dosage, three replications were undertaken.
2.8. Statistical Analysis
The normality of the distribution of the VOCs was tested using the Shapiro-Wilk normality test [15]. Two-way analysis of variance (ANOVA) was carried out to determine the effects of cultivars and times as well as cultivar × time interaction on the variability of particular VOCs, independently for mechanical leaf damage and for MeJA exposure (vaporization and aerial spraying). The mean values for VOC emission rates were calculated. The Fisher’s least significant differences (LSDs) were calculated for cultivars and times as well as cultivar × time interaction and on this basis, homogeneous groups were determined. The relationships between particular VOCs were assessed using Pearson’s correlation, independently for each artificial stress factor [16]. These relationships are represented in the results section as heatmaps. All analyses were conducted using the GenStat 18th edition statistical software package.
To analyse insect behaviour, chi-squared goodness-of-fit tests with Yates correction for small sample sizes were applied. These were used to determine whether the insects in the four-way olfactometer were attracted or repelled by a given exposure dose of volatiles.
3. Results
3.1. VOC Identification
The empirical distribution of observations of all VOCs was normal. In total nine volatiles were identified, namely (Z)-3-hexenal (retention time: 5.59 min), (E)-2-hexenal (8.32 min), (Z)-3-hexen-1-ol (9.02 min), (E)-2-hexen-1-ol (9.16 min), (Z)-3-hexen-1-yl acetate (13.45 min), (E)-β-ocimene (15.36 min), linalool (17.35 min), β-caryophyllene (26.02 min) and (E)-β-farnesene (26.25 min). However, not all were necessarily detectable at the same time depending on the type of artificial stress. For example, β-caryophyllene, and β-farnesene were not observed (i.e., either no emissions or below the limits of detection) at any of the sampling time points following mechanical leaf damage to the “Kandela” variety. In addition, for the “Kandela” variety, no (E)-2-hexenal and (Z)-3-hexen-1-ol were detected at any collection time points from plants that were sprayed with MeJA. Similarly, both varieties of wheat sprayed with MeJA showed no detectable emissions of (E)-2-hexen-1-ol.
Mean values for the volatile emission rates and combinations of cultivars and times for the observed VOCs are presented in Table 2. The first and the most important observation is that the level of VOC emissions from controls is significantly lower than those coming from the plants that are exposed to artificial stress. Results of ANOVA indicate that all factors (i.e., cultivars, time, and cultivar × time interaction) were significant for all VOCs in all four treatments: 1SL, 3SL, MJ-V, and MJ-S (see Table 3).

Table 2.
Mean values for two wheat cultivars corresponding to the fixed three volatile sample collection times following a given artificial stress (n = 6).

Table 3.
Mean squares from two-way analysis of variance for a particular VOC.
3.2. VOC Correlation Coefficients
The correlation coefficients for particular VOCs released from wheat plants have been determined. All correlation coefficients were found to be positive (Figure 1, Figure 2, Figure 3 and Figure 4). For the 1SL treatment, we observed statistically significant results between all pairs of VOCs, except for (Z)-3-hexen-1-yl acetate and linalool, and (Z)-3-hexene-1-yl acetate and (E)-β-farnesene (Figure 1). For the 3SL treatment, we observed statistically significant results between all pairs of VOCs except for (Z)-3-hexen-1-yl acetate and linalool, (Z)-3-hexen-1-yl acetate and β-caryophyllene, and (Z)-3-hexen-1-yl acetate and (E)-β-farnesene (Figure 2). For the MJ-V treatment, no significant correlations were observed between (Z)-3-hexen-1-yl acetate and the following four VOCs: (Z)-β-ocimene, linalool, β-caryophyllene, and (E)-β-farnesene (Figure 3). For the MJ-S treatment, all pairs of VOCs were found to be correlated (Figure 4).
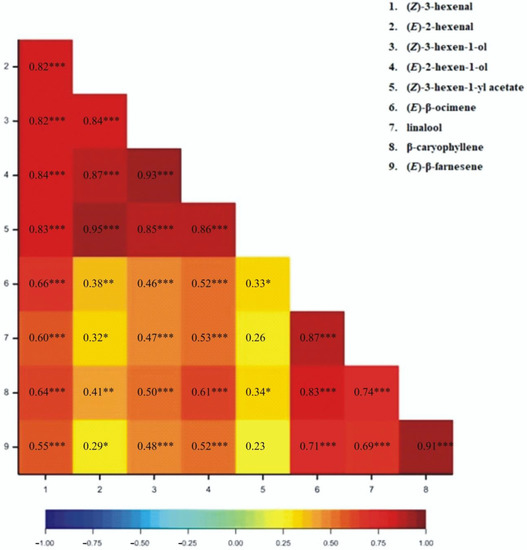
Figure 1.
Heatmap for Pearson’s correlation coefficients between observed VOCs resulting from 1SL treatment (* p < 0.05; ** p < 0.01; *** p < 0.001).
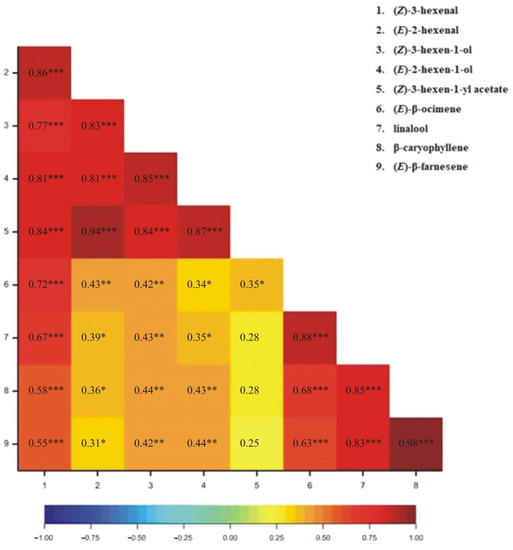
Figure 2.
Heatmap for Pearson’s correlation coefficients between observed VOCs resulting from 3SL treatment (* p < 0.05; ** p < 0.01; *** p < 0.001).
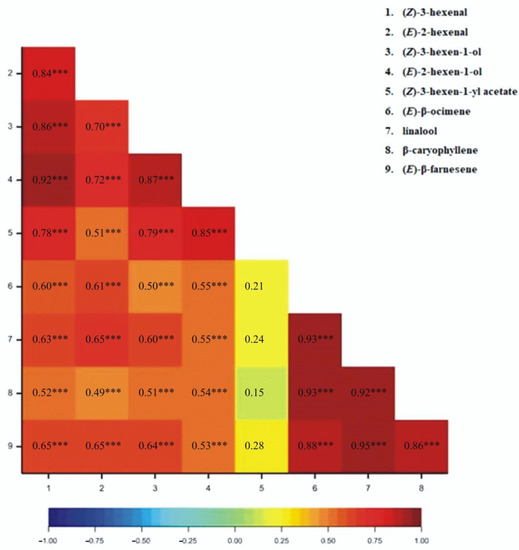
Figure 3.
Heatmap for Pearson’s correlation coefficients between observed VOCs resulting from MJ-V treatment (*** p < 0.001).
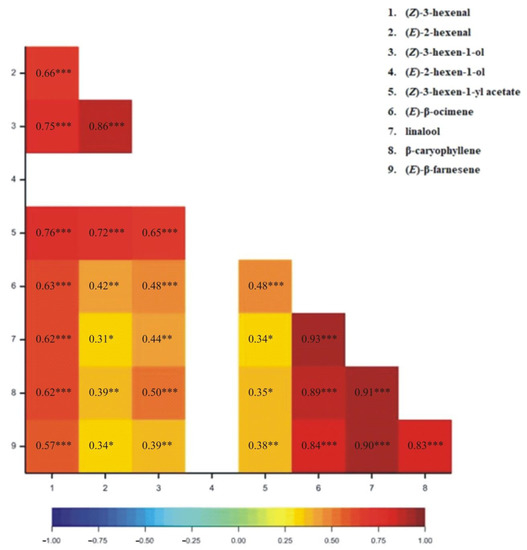
Figure 4.
Heatmap for Pearson’s correlation coefficients between observed VOCs resulting from MJ-S treatment (* p < 0.05; ** p < 0.01; *** p < 0.001).
Regarding the intensities (emission rates) of particular VOCs at the three time points chosen, the majority of the VOCs showed the highest release at 48 h after the stress induction. The clearest trend was observed for the “Serenada” variety with 3SL and MJ-S treatment. In addition, for the “Serenada” variety, and with MJ-V treatment, the highest released levels for C6 aldehydes and the corresponding alcohols were observed.
The correlation coefficients demonstrate that the emission levels of the C6 aldehydes and alcohols, classified as Green Leaf Volatiles (GLVs), are well correlated, and especially so for those associated with mechanical wounding that imitated Bishop’s Mitre shieldbugs feeding on wheat. Of note is that the monitoring of MeJA application efficiency may be done by observing a single compound from (Z)-β-ocimene, linalool, β-caryophyllene, and (E)-β-farnesene group.
3.3. Four-Way Olfactometer Results
During the insect behaviour studies, no non-responders were observed. All insects chose one arm of the 4-way olfactometer, and usually within 4- or 5 min. Chi-squared tests revealed that females and males were attracted only by 10 ng min−1 combinations of VOCs released from cv. Kandela (spraying); χ2 = 4.05 and χ2 = 6.05, respectively (Table 4). Both sexes exhibited significant negative (repellent) responses for both VOCs dose rates (Table 5). An especially large negative reaction was observed for cv. Serenada (vaporization) for females and males; χ2 = 18.5 and χ2 = 14.45, respectively.

Table 4.
Effect of volatile odours released by wheat plants cv. Kandela following methyl jasmonate exposure at two levels (10 ng min−1 and 100 ng min−1) on the number of Aelia acuminata L. adult females and males choosing to enter an arm of a four-way olfactometer. One arm contained the combination of odours, whereas the other arm contained purified, humidified air and hexane solvent (no odour) to act as the control.

Table 5.
Effect of volatile odours released by wheat plants cv. Serenada following methyl jasmonate exposure at two levels (10 ng min−1 and 100 ng min−1) on the number of Aelia acuminata L. adult females and males choosing to enter an arm of a four-way olfactometer. One arm contained the combination of odours, whereas the other arm contained purified, humidified air and hexane solvent (no odour) to act as the control.
4. Discussion
Two earlier studies that investigated volatiles associated with wheat plants have been reported [17,18]. In contrast to this study, these two investigations identified many more volatiles; 105 in the case of the Shibamoto et al. [18] study and 25 by Buttery et al. [17]. However, Shibamoto et al. [18] used another method for VOC extraction, and Buttery et al. [17] investigated undamaged leaves. Shibamoto et al. [18]. used hydrodistillation of wheat plants followed by direct organic solvent extraction of crude distillate. Our approach, which is focused on in vivo monitoring of wheat VOC emissions, gives information on volatiles released from plant tissue, and importantly we monitored their intensities at three fixed times (24, 48, and 72 h following a given artificial stress).
In a study by Engelberth and Engelberth [4], which investigated the behaviour of plants during abiotic stress exposure, they focused their analysis on three known major plant volatiles, namely, (E)-2-hexenal, (Z)-3-hexenal, and hexanal. The major difference between our current study and that of Engelberth and Engelberth [4] is that we observed no hexanal release. In agreement with our study, other investigations have reported the stress-induced release of (E)-β-ocimene, linalool, β-caryophyllene, and (E)-β-farnesene as signalling and defensive volatiles of plants [2,6,19].
The correlation of increased levels of C6 aldehydes and corresponding to alcohol emission may be caused by upregulation of the biosynthetic pathway originating in linolenic acid [6], which is common for all of those compounds. It is of note that whilst a high (Z)-3-hexen-1-yl acetate emission was found for all treatments, it was especially so for those that did not include any physical damage to the leaves. This result may be a confirmation of Pare and Tumlinson’s [20] suggestion that the conversion of C6 compounds into an acetate form is promoted while crossing the undamaged plant tissue barrier. Moreover, using GC/MS, Sendel et al. [21] found that Aelia acuminata L. (1 or 2 adult pairs) significantly induced VOC emissions from wheat plants, where, as a result of biotic stress, larger amounts of the following VOCs were released: (Z)-3-hexenal, (E)-2-hexenal, (Z)-3-hexen-1-ol, (E)-2-hexen-1-ol, β-pinene, β-myrcene, (Z)-3-hexen-1-yl acetate, 1-hexyl acetate, 4-heptanone, (Z)-ocimene, linalool, linalool oxide, benzyl acetate, methyl salicylate, indole, β-caryophyllene, and (E)-β-farnesene. It was also reported by them that two pairs of insects caused a significantly stronger plant reaction.
Many of the studies that report VOCs emitted by plants are often carried out by harvesting the plant or cutting a representative fragment of plant tissue and then subjecting that material to some form of extraction (organic solvent, SPME) [4,22,23]. Such an approach is divorced from the reality of the various harmful situations plants face in nature, and the procedure adopted may strongly influence the behaviour of the plant material, resulting in volatiles that may not be present under real-world conditions, such as during harvesting, which enhances GLVs owing to this additional abiotic stress (actually terminal) source. Therefore, the results from solvent extraction to monitor VOC release have to be conducted with care, because the contents of phytochemicals inside the plant tissue do not necessarily translate directly to the VOC emissions [24,25]. Thus, our in vivo approach to monitor VOC release is especially valuable. Nevertheless, other approaches, including solid-phase microextraction (SPME) applied in vivo, as presented by Rice et al. [26] for volatiles emitted from grapes during ripening, may also be considered.
With regards to VOC emission correlation coefficients, the above discussion explains why our results are contrary to those found in the study by Engelberth and Engelberth [4], which reported no correlations for any of the GLVs. The crucial difference in our study is the VOC collection method. Engelberth and Engelberth [4] used a sample preparation method that consisted of plant tissue cutting and homogenizing. As mentioned above, this can cause significant differences in VOC release, because the structure of the whole plant tissue is destroyed and hence the VOCs have no barriers to overcome to limit their emission. Using our experimental design, VOC correlations are more reliably evaluated, as illustrated in this work and our earlier works [9,27,28]. The correlation coefficients show how mutually related the emitted VOCs are to a plant’s natural defence strategies.
Ameye et al. [29] found that plants respond to stress by releasing biogenic volatile organic compounds (BVOCs). They used a meta-analysis approach and found that there is less variation in the GLV profile than we presumed and that pathogens induce more GLVs than insects and wounding.
To prove that the identified and investigated GLVs and other VOCs play a significant role in pest management, experiments with Bishops’ Mitre shieldbugs are reported in this paper. Using a four-way olfactometer, both sexes of the insects were found to respond negatively to the VOCs released at a rate of 100 ng min−1. Attraction was observed only for release rates of 10 ng min−1 and then only for the “Kandela” (spraying) wheat plants. Piesik et al. [30] reported that Cephus cinctus Norton females are attracted to some dose levels of (Z)-3-hexenyl acetate, (Z)-3-hexenol, and the terpene, (Z)-β-ocimene, but are repelled by the highest release rates of (Z)-3-hexenyl acetate they tested (8400 ng h−1). Piesik et al. [31] found that Fusarium infection induces VOC emissions in maize and that adult cereal leaf beetles (Oulema melanopus L.) are attracted to a synthetic blend of volatiles containing two common GLVs, namely (Z)-3-hexenal and (Z)-3-hexenyl acetate, and two terpenes linalool and β-caryophyllene. Ukeh and Umoetok [32] reported repellent effects of five monoterpenoids (including R-linalool, which is a natural component of the essential oils of Aframomum melegueta K. Schum and Zingiber officinale Roscoe) against Tribolium castaneum erbst. and Rhyzopertha dominica (Fabr.) in a four-way olfactometer study. Zoubiri and Baaliouamer [33] showed that linalool, a major volatile constituent in C. sativum seed essential oil, provided some fumigant potential for food grain protection. The above examples amply demonstrate that insects may respond to plant volatiles in various ways, depending on the chemical types and combinations of volatiles and/or their rates of emission (and hence concentrations in the ambient environment).
5. Conclusions
In comparison to spraying and wounding, vaporization resulted in the largest VOC emissions. Of the two tested cultivars, “Serenada” released larger amounts of VOCs. Taking into consideration the olfactory tests, an especially large negative reaction of Aelia acuminata was observed for cv. Serenada (vaporization).
The significance of plant volatiles for use in pest management has been successfully evaluated in this study for Aelia acuminata L., and the most significant findings are the clear patterns in correlation coefficients associated with volatiles related to a plant’s natural defence strategies, which can be used to indicate if the crops are under insect attack and possibly to monitor the presence of disease.
The positive correlations between particular GLVs and other volatile compounds emitted from the wheat plants during stressful conditions have significant consequences. Importantly, these correlations show that only one volatile needs to be monitored in order to provide information that a crop is under attack. This has major implications for the development of an analytical tool for field use. For example, cheap electronic sensors with appropriate filters could be developed that provide a high chemical specificity to select a particular volatile for monitoring.
Author Contributions
Conceptualization, D.P. and J.A.; methodology, D.P., J.Ł. and B.B.; software, J.B.; validation, B.B.; formal analysis, J.B. and M.P.; investigation, J.A.; resources, J.A. and J.Ł.; data curation, D.P., J.A. and J.Ł.; writing—original draft preparation, J.Ł.; writing—review and editing, D.P., J.Ł. and C.A.M.; visualization, J.B.; supervision, D.P.; project administration, D.P.; funding acquisition, D.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Farm to Fork Strategy. For a Fair, Healthy and Environmentally-Friendly FOOD System. 2020. Available online: https://ec.europa.eu/food/horizontal-topics/farm-fork-strategy_en (accessed on 18 March 2022).
- Cascone, P.; Iodice, L.; Maffei, M.E.; Bossi, S.; Arimura, G.I.; Guerrieri, E. Tobacco overexpressing β-ocimene induces direct and indirect responses against aphids in receiver tomato plants. J. Plant Physiol. 2015, 173, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Wenda-Piesik, A.; Piesik, D.; Nowak, A.; Wawrzyniak, M. Tribolium confusum responses to blends of cereal kernels and plant volatiles. J. Appl. Entomol. 2016, 140, 558–563. [Google Scholar] [CrossRef]
- Engelberth, J.; Engelberth, M. Variability in the capacity to produce damage-induced aldehyde green leaf volatiles among different plant species provides novel insights into biosynthetic diversity. Plants 2020, 9, 213. [Google Scholar] [CrossRef]
- Brosset, A.; Islam, M.; Bonzano, S.; Maffei, M.E.; Blande, J.D. Exposure to (Z)-11-hexadecenal [(Z)-11-16:Ald] increases Brassica nigra susceptibility to subsequent herbivory. Sci. Rep. 2021, 11, 13532. [Google Scholar] [CrossRef] [PubMed]
- Paré, P.W.; Tumlinson, J.H. Plant Volatiles as a Defense against Insect Herbivores. Plant Physiol. 1999, 121, 325–331. [Google Scholar] [CrossRef]
- Maffei, M.E.; Gertsch, J.; Appendino, G. Plant volatiles: Production, function and pharmacology. Nat. Prod. Rep. 2011, 28, 1359–1380. [Google Scholar] [CrossRef]
- Piesik, D.; Wenda-Piesik, A. Sitophilus granarius responses to blends of five groups of cereal kernels and one group of plant volatiles. J. Stored Prod. Res. 2015, 62, 36–39. [Google Scholar] [CrossRef]
- Piesik, D.; Wenda-Piesik, A.; Krasińska, A.; Wrzesińska, D.; Delaney, K.J. Volatile organic compounds released by Rumex confertus following Hypera rumicis herbivory and weevil responses to volatiles. J. Appl. Entomol. 2016, 140, 308–316. [Google Scholar] [CrossRef]
- Rahnamaie-Tajadod, R.; Goh, H.H.; Mohd Noor, N. Methyl jasmonate-induced compositional changes of volatile organic compounds in Polygonum minus leaves. J. Plant Physiol. 2019, 240, 152994. [Google Scholar] [CrossRef]
- Vaccino, P.; Ingegno, B.L.; Pansa, M.G.; Coppa, T.; Tavella, L. Common wheat and cereal bug interactions: Kernel quality depletion and immunodetection of damage. J. Agric. Sci. 2017, 155, 193–204. [Google Scholar] [CrossRef]
- Dragoş, I.; Malschi, D.; Vălean, A.-M.; Tărău, A.D.; Cheţan, C.; Oltean, I. Preliminary Research on the Wheat Pests and on Their Integrated Control during. Bull. USAMV Ser. Agric. 2017, 74, 1–14. [Google Scholar] [CrossRef]
- Centralny Ośrodek Badania Odmian Roślin Uprawnych. 2020. Available online: https://www.coboru.gov.pl/ (accessed on 27 June 2022).
- Hess, M.; Barraust, G.; Blefholderj, H.; Buhrg, L.; Hack, H.; Nu, R.A. Use of the extended BBCH scale-general for the descriptions of the growth stages of mono-and dicotyledonous weed species. Weed Res. 1997, 37, 433–441. [Google Scholar] [CrossRef]
- Shapiro, S.S.; Wilk, A.M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Warzecha, T.; Skrzypek, E.; Bocianowski, J.; Sutkowska, A. Impact of Selected PSII Parameters on Barley DH Lines Biomass and Yield Elements. Agronomy 2021, 11, 1705. [Google Scholar] [CrossRef]
- Buttery, R.G.; Xu, C.; Ling, L.C. Volatile Components of Wheat Leaves (and Stems): Possible Insect Attractants. J. Agric. Food Chem. 1985, 33, 115–117. [Google Scholar] [CrossRef]
- Shibamoto, T.; Horiuchi, M.; Umano, K. Composition of the young green barley and wheat leaves. J. Essent. Oil Res. 2007, 19, 134–137. [Google Scholar] [CrossRef]
- Scandiffio, R.; Geddo, F.; Cottone, E.; Querio, G.; Antoniotti, S.; Pia Gallo, M.; Maffei, M.E.; Bovolin, P. Protective Effects of (E)-β-Caryophyllene (BCP) in Chronic Inflammation. Nutrients 2020, 12, 3273. [Google Scholar] [CrossRef] [PubMed]
- Pare, P.W.; Tumlinson, J.H. Cotton Volatiles Synthesized And Released Distal To The Site Of Insect Damage. Phytochemistry 1998, 47, 521–526. [Google Scholar] [CrossRef]
- Sendel, S.; Bocianowski, J.; Buszewski, B.; Piesik, M.; Mayhew, C.A.; Piesik, D. Volatile organic compounds released by wheat as a result of striped shieldbug feeding and insect behaviour. J. Appl. Entomol. 2022, 146, 710–724. [Google Scholar] [CrossRef]
- Tamogami, S.; Noge, K.; Abe, M.; Agrawal, G.K.; Rakwal, R. Methyl jasmonate is transported to distal leaves via vascular process metabolizing itself into JA-Ile and triggering VOCs emission as defensive metabolites. Plant Signal. Behav. 2012, 7, 1378–1381. [Google Scholar] [CrossRef][Green Version]
- Tamogami, S.; Rakwal, R.; Agrawal, G.K. Interplant communication: Airborne methyl jasmonate is essentially converted into JA and JA-Ile activating jasmonate signaling pathway and VOCs emission. Biochem. Biophys. Res. Commun. 2008, 376, 723–727. [Google Scholar] [CrossRef]
- Łyczko, J.; Jałoszyński, K.; Surma, M.; García-Garví, J.-M.; Carbonell-Barrachina, A.A.; Szumny, A. Determination of Various Drying Methods’ Impact on Odour Quality of True Lavender (Lavandula angustifolia Mill.) Flowers. Molecules 2019, 24, 2900. [Google Scholar] [CrossRef] [PubMed]
- Łyczko, J.; Masztalerz, K.; Lipan, L.; Lech, K.; Carbonell-Barrachina, Á.A.; Szumny, A. Chemical determinants of dried Thai basil (O. basilicum var. thyrsiflora) aroma quality. Ind. Crops Prod. 2020, 155, 112769. [Google Scholar] [CrossRef]
- Rice, S.; Maurer, D.L.; Fennell, A.; Dharmadhikari, M.; Koziel, J.A. Evaluation of volatile metabolites emitted in-vivo from cold-hardy grapes during ripening using SPME and GC-MS: A proof-of-concept. Molecules 2019, 24, 536. [Google Scholar] [CrossRef] [PubMed]
- Piesik, D.; Bocianowski, J.; Sendel, S.; Krawczyk, K.; Kotwica, K. Beetle orientation responses of Gastrophysa viridula and Gastrophysa polygoni (Coleoptera: Chrysomelidae) to a blend of synthetic volatile organic compounds. Environ. Entomol. 2020, 49, 1071–1076. [Google Scholar] [CrossRef]
- Skoczek, A.; Piesik, D.; Wenda-Piesik, A.; Buszewski, B.; Bocianowski, J.; Wawrzyniak, M. Volatile organic compounds released by maize following herbivory or insect extract application and communication between plants. J. Appl. Entomol. 2017, 141, 630–643. [Google Scholar] [CrossRef]
- Ameye, M.; Allmann, S.; Verwaeren, J.; Smagghe, G.; Haesaert, G.; Schuurink, R.C.; Audenaert, K. Green leaf volatile production by plants: Ameta-analysis. New Phytol. 2018, 220, 666–683. [Google Scholar] [CrossRef]
- Piesik, D.; Weaver, D.K.; Runyon, J.B.; Buteler, M.; Peck, G.E.; Morrill, W.L. Behavioural responses of wheat stem sawflies to wheat volatiles. Agric. For. Entomol. 2008, 10, 245–253. [Google Scholar] [CrossRef]
- Piesik, D.; Lemńczyk, G.; Skoczek, A.; Lamparski, R.; Bocianowski, J.; Kotwica, K.; Delaney, K.J. Fusarium infection in maize: Volatile induction of infected and neighboring uninfected plants has the potential to attract a pest cereal leaf beetle, Oulema melanopus. J. Plant Physiol. 2011, 168, 1534–1542. [Google Scholar] [CrossRef]
- Ukeh, D.A.; Umoetok, S.B.A. Repellent effects of five monoterpenoid odours against Tribolium castaneum (Herbst) and Rhyzopertha dominica (F.) in Calabar, Nigeria. Crop Prot. 2011, 30, 1351–1355. [Google Scholar] [CrossRef]
- Zoubiri, S.; Baaliouamer, A. Essential oil composition of Coriandrum sativum seed cultivated in Algeria as food grains protectant. Food Chem. 2010, 122, 1226–1228. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).