Featured Application
The application of this work is related to the potential use of lactic acid bacteria as a biopreparation for seed potatoes against the development of phytopathogens.
Abstract
Biological control offers an alternative to chemical pesticides, which are inconsistent with the global trend of “going green”. Biological control includes various approaches, from natural predators to biologically produced molecules. This article focuses on the selection of lactic acid bacteria (LAB) as biological control agents against potato pathogens. The scope included evaluating the antimicrobial activity of 100 LAB strains against ten phytopatogens (Pectobacterium carotovorum, Streptomyces scabiei, Fusarium oxysporum, Fusarium sambucinum, Alternaria solani, Alternaria, tenuissima, Alternaria alternata, Phoma exigua, Rhizoctonia solani, Colletotrichum coccodes) by cross-streak plate method. HPLC determined the metabolic profiles for the most active LAB strains, and lactic acid, acetic acid, propionic acid and ethanol were found in the largest quantities. The strain Lactiplantibacillus plantarum KB2 LAB 03 was finally selected and cultured on supplemented acid whey. After the selection in laboratory tests, the strain KB2 LAB 03 was assessed in situ on seed potatoes against phytopathogens. The test showed a 40–90% reduction of eight potato pathogens infestation; only F. sambucinum and F. oxysporum were not inhibited at all. L. plantarum KB2 LAB 03 was proposed as the potential biocontrol agent for the potato protection against phytopathogens.
1. Introduction
The potato (Solanum tuberosum L.) is cultivated in 160 countries. These tubers are so prominent among consumers and farmers due to their remarkable yield per unit area, versatility and high nutritional value [1]. In European Union (EU), potato cultivation acreage in 2020 was 1.66 million ha. In Poland, the area of potato cultivation, according to the estimates of Statistics Poland, amounted to approx. 0.2 million ha in 2021. Around 55.3 Mt of tubers are harvested in the EU, with Belgium, France, the Netherlands and Germany being the largest producers. The potato harvest in 2021 in Poland amounted to approx. 7.10 Mt [2,3]. However, the annual production of potatoes in many countries is slightly decreasing; in the leading ones (China and India), a steady increase is observed [4].
Since healthy and robust plants produce good crops, proper attention is required in the context of preventing infections. Diseases caused by phytopathogens are the source of severe losses for farmers due to lower yield and disposal of crops [5]. In order to prevent it, there are facilities designed to maintain temperature and humidity during potatoes storage on large farms specialised in cultivation. Annual losses during potato storage average about 7.5% in the United States, the fifth largest producer in the world [4,6] and importantly, some of the tuber defects emerge only after the farmer has invested an entire season of inputs in cultivating the crops [7].
The source of infection is often seed potatoes or contaminated crop residues in the field after previous cultivation. Diseases caused by bacterial phytopathogens are particularly hindering potato production agents, and they can lead to severe damage to crops and tubers—the most important parts from an economic point of view. The bacterial genera Pectobacterium have been used as a model pathogen for phytobacteriology research and currently include 12 species with a wide range of host plants, including potatoes. In Europe, it is associated with soft rot in storage or blackleg disease [7,8]. Another economically important bacterial disease is common scab caused by Streptomyces species. It causes necrosis in all underground potato parts and can reduce roots’ growth from seed tubers [9]. In turn, fungi belonging to the genera: Fusarium, Alternaria, Rhizoctonia and Phoma are fungal pathogens of potato, causing gangrene, dry rot of stored tubers or other rot symptoms, and they are responsible for significant crop losses [10,11]. The phytopathogens may act as primary or secondary invaders [10].
Considering the multitude and the threat of phytopathogens, farmers use a variety of pesticides such as metalaxyl, fluopicolide or mancozeb. Such chemically synthesised substances exhibit a strong antimicrobial effect but pose a potential environmental threat. While in use, they tend to migrate deep into the soil, destabilise the populations of microorganisms, and contaminate groundwater [12,13,14]. Currently, in the EU, there is a trend to limit the use of this type of chemical plant protection products, especially in organic farms, which constitute more than 13% of all potato holdings [2]. This agricultural production places the highest emphasis on environmental and wildlife protection and avoids synthetic chemicals such as fertilisers and pesticides, replacing them with biological and mechanical methods, along with cultural techniques, for instance, hand weeding, pruning or mulching [15]. In order to achieve it, biological control is often applied, which refers to the use of microbial antagonists to suppress phytopathogens causing plant diseases. A high antimicrobial effect characterises multiple biological control agents against specific pathogens, and currently, different types of interactions are sought, such as the production of antimicrobials, lytic enzymes, unregulated waste products, physical or chemical interference or competition for space [16]. The simplicity of the multiplication of biocontrol agents on various available organic media is also important [17].
Potential candidates for biocontrol can be lactic acid bacteria (LAB), especially facultative heterofermentative lactic acid bacteria belonging to genera Lactiplantibacillus, Lactobacillus, Levilactobacillus, Lacticaseibacillus, Leuconostoc and Lactococcus that is often found in dairy products, meat, vegetables, wine, silage and vaginal, urogenital and gastrointestinal tracts. The omnipresence of the species suggests an imminent potential for adaptation to a wide variety of environments and a metabolic pathway diversity [18,19]. The LAB has the Generally Recognised As Safe status (GRAS) and the Qualified Presumption of Safety status (QPS). For this reason, great attention has been given to the species in the case of probiotics and biological control agents [19]. LAB strains are able to produce multiple antimicrobial compounds, such as organic acids (including lactic and phenyllactic acids), diacetyl, hydrogen peroxide and bacteriocins which create a relatively harsh environment for any pathogens. However, acids play the most significant role in biocontrol abilities [20,21].
The study aimed to investigate the possibility of using a biological preparation containing LAB as a biocontrol agent against seed potato pathogens. The selection of an appropriate bacterial strain was developed, and the chemical composition of the culture medium based on a waste product-acid whey, was optimised. After the selection in laboratory tests, the biocontrol ability of the selected LAB was assessed in situ on seed potatoes. Our hypothesis is that some strains of lactic acid bacteria can inhibit the growth of potato pathogens, and that the selection of the optimal growth medium increases antimicrobial activity.
2. Materials and Methods
2.1. Microorganisms Used in the Research
2.1.1. Lactic Acid Bacteria (LAB)
In this case, 100 strains of lactic acid bacteria, including 42 strains acquired from the Collection of Pure Cultures of Industrial Microorganisms ŁOCK at the Lodz University of Technology (Łódź, Poland) and 58 isolates from pickled vegetables and milk environment, were assessed in terms of antimicrobial activity against potato phytopathogens. Bacteria were cultivated in De Man, Rogosa and Sharpe medium (MRS; Merck, Darmstadt, Germany) at 30 °C for 48 h and stored at 4 °C. All of the species are listed in Table S1.
2.1.2. Potato Phytopathogens
Eight fungal and two bacterial phytopathogens were used to assess the antimicrobial activities of lactic acid bacteria. All of the species are presented in Table 1.

Table 1.
Origin of the phytopathogen strains used in the research.
Bacterial strains were cultivated on Tryptic Soy Agar—TSA (Merck, Darmstadt, Germany) and fungi strains on Potato Dextrose Agar—PDA (Merck, Darmstadt, Germany). They were stored at 4 °C. Bacteria suspensions were prepared from the pure culture on the TSA agar plate and adjusted in 0.85% NaCl to a final concentration of 106 CFU/mL. Fungal suspensions were prepared by washing out spores from the surface of pure cultures on the PDA agar plate into the 0.85% NaCl with Tween (0.02%) and adjusted to a final concentration of 106 CFU/mL.
2.2. Agar-Plug Diffusion Method for In Vitro Antimicrobial Assay
The antimicrobial activity of 100 strains of lactic acid bacteria against potato phytopathogens was determined by the agar-plug diffusion method [22]. Lactic acid bacteria were cultured for 48 h at 30 °C on MRS (Merck, Darmstadt, Germany) agar plates using the pour method to obtain agar plugs, cut with sterile cork bore (Φ = 10 mm). Freshly prepared pathogens’ suspensions of 100 µL were inoculated onto TSA (Merck, Darmstadt, Germany) for bacteria and PDA (Merck, Darmstadt, Germany) for fungi. Then, lactic acid bacteria agar plugs were transferred onto inoculated plates. Pure MRS agar plugs were used as a control sample. The plates were incubated for 2–5 days (depending on the pathogen) at 25 °C. After incubation, the width of the phytopathogen growth inhibition zones was measured, excluding the agar plug diameter. The results were expressed as the average of three independent repetitions for each LAB strain—pathogen combination with a standards deviation value.
2.3. High-Performance Liquid Chromatography (HPLC) for Lactic Acid Bacteria Fermentation by-Products Determination
Lactic and acetic, propionic, isobutyric and valeric acids were procured from Sigma-Aldrich (St. Louis, MO, USA). Fermentation media with investigating strains were filtered using a 0.45 mm filter before HPLC analysis. HPLC analysis was performed using the Finnigan Surveyor HPLC system (Thermo Fisher Scientific Inc, Waltham, MA, USA) equipped with an autosampler, a refractive index detector (Finnigan Surveyor-RI Plus), a diode array detector (Finnigan Surveyor-PDA Plus) and thermostated column compartment, controlled by ChromQuest 5.0 chromatography software (Thermo Fisher Scientific Inc, Waltham, MA, USA). The investigated compounds were separated on an Aminex HPX87H column (300 × 7.8 mm, Bio-Rad, Hercules, CA, USA) protected by a guard column and thermostated at 60 °C. The separation was carried out by isocratic elution with 0.005 M H2SO4 and a flow rate of 0.6 mL/min; the column temperature was maintained at a constant 60 °C. The standard of the individual acid was prepared and chromatographed separately to determine each acid’s retention time. The calibration curves of individual acids were obtained using five levels of concentration of standard mixtures and three injections per level [23].
2.4. Optimisation of the Acid Whey-Based Culture Media for Cultivation of L. plantarum KB2 LAB 03
Optimisation of the acid whey-based culture media for cultivating L. plantarum KB2 LAB 03 was performed microscale using Multiskan GO Microplate Spectrophotometer (Thermo Scientific, Waltham, MA, USA) equipped with SkanIt RE for Multiskan GO 3.2 software (Thermo Scientific, Waltham, MA, USA). 96-well plates were filled with 200 µL of 16 different variants of supplemented acid whey-based media listed in Section 2.4.1 and inoculated with 50 µL of L. plantarum culture. The control samples were MRS (Merck, Darmstadt, Germany) and MRS with no glucose (BTL, Łódź, Poland). The plates were incubated for 72 h at 30 °C, while the turbidity was measured every hour at a wavelength of 600 nm. The experiment was performed in triplicate. Biomass yield (Yx) was calculated according to Equation (1):
where Amax is the maximum absorbance value of inoculated culture medium and Amin is the minimum absorbance value of inoculated culture medium. The maximum specific growth rate (µmax) was calculated according to Equation (2):
where Amax is the maximum absorbance value of inoculated culture medium, Amin is the minimum absorbance value of inoculated culture medium, and Δt is the time difference.
Yx = Amax − Amin
µmax = (lnAmax − lnAmin)/∆tlog
2.4.1. Acid Whey-Based Culture MEDIA
The acid whey was obtained from JOGO—Łódź Dairy Cooperative (Kraszewo, Poland). The supplements were combined into two groups: A—containing Yeast extract (BTL, Łódź, Poland) with a base concentration (BC) of 8 g/L and 14 g/L of Peptone K (BTL, Łódź, Poland); B—containing 2 g/L of Ammonium citrate (Chempur, Piekary Śląskie, Poland), 2 g/L of K2HPO4 (Chempur, Piekary Śląskie, Poland), 5 g/L of NaCH3COO (Chempur, Piekary Śląskie, Poland), 0.2 g/L of MgSO4 × 7 H20 (Chempur, Piekary Śląskie, Poland) and 0.05 g/L of MgSO4 × 4 H20 (Chempur, Piekary Śląskie, Poland). The acid whey-based media were supplemented with different concentrations (100, 50, 25 and 12.5% of BC) of each group in order to obtain all possible combinations: A100 M100 (group A—100% of BC and group B—100% of BC), A100 M50 (group A—100% of BC and group B—50% of BC), A100 M25 (group A—100% of BC and group B—25% of BC), A100 M12.5 (group A—100% of BC and group B—12.5% of BC), A50 M100 (group A—50% of BC and group B—100% of BC), A50 M25 (group A—50% of BC and group B—25% of BC) etc. The detailed composition of each variant is presented in Table S2.
2.5. Agar-Well Diffusion Method for In Vitro Antimicrobial Assay
The agar well diffusion method [22] determined the antimicrobial activity of L. plantarum KB2 LAB 03 cultured on different variants of supplemented acid-whey-based media against potato phytopathogens. L. plantarum KB2 LAB 03 was cultured for 48 h at 30 °C in 4 selected variants of supplemented acid-whey-based media. Freshly prepared pathogens’ suspensions of 100 µL were inoculated onto TSA (Merck, Darmstadt, Germany) for bacteria and PDA (Merck, Darmstadt, Germany) for fungi. Then, 10 mm diameter wells were cut by sterile cork bore, and 250 µL of LAB cultures were introduced into the wells. Pure media were used as a control. The plates were incubated for 2–5 days (depending on the pathogen) at 25 °C. Then, the width of the phytopathogen growth inhibition zones was measured, excluding the agar well diameter. The results were expressed as the average of 3 independent repetitions for each LAB strain-pathogen combination with a standard deviation value.
2.6. Analytical Methods for Culture Media Characteristics
Pure acid whey, MRS medium and selected A100 M100 acid whey-based medium were chemically analysed. The analysis of ammonium nitrogen (test no. 8038, modified Nessler method), iron (test no. 8008, FerroVer®), ortophosphates (test no. 8048, PhosVer®), potassium (tests no. LCK228) and chlorides (method no. 8113) were conducted using DR6000 spectrophotometer (HACH-LANGE, Loveland, CO, USA), according to the manufacturer’s instructions. Sodium and calcium were measured with flame photometers (BWB-XP, BWB Technologies, UK). Lactic acid and lactose were determined using a UV-spectrophotometer (Thermo Scientific Multiskan Go, Thermo Fisher Scientific, Munich, Germany) and enzymatic kits (Megazyme Ltd., Bray, Ireland) according to the manufacturer’s procedure. Samples for phosphorus and nitrogen were mineralised using the LT200 thermoreactor (HACH-LANGE, Loveland, CO, USA). Total solids and volatile solids were determined in accordance with the standard methods [24]. Protein content was evaluated by the Kjeldahl method [25].
2.7. In Situ Antimicrobial Assay on Seed Potato
Seed potatoes cv. Impresja were obtained from Zamarte Potato Breeding (Zamarte, Poland). In situ antimicrobial tests on seed potatoes were performed according to the methodology of our previous research [26]. Briefly, five mm deep and five mm in diameter cuts were made of potatoes. Potatoes were immersed in the 48-h cultures of L. plantarum KB2 LAB 03 and left to dry. Then, 20 µL of each bacterial or fungal phytopathogens’ suspension was introduced into the cuts. Controlled potatoes were only inoculated with phytopathogens. Potatoes were incubated at 25 °C for 14 days. The percentage of phytopathogen infestation was measured in comparison to the controls according to the Equation (3):
where C is the % of phytopathogen infestation on control potatoes, and T is the % of phytopathogen infestation on potatoes treated with bacteria culture. The percentage of the infested area was measured after cutting the seed potatoes in half. The assay was performed in triplicate.
Inhibition (%) = (C − T)/C × 100
2.8. Statistical Analysis
Statistical analysis was carried out with Statistica 13.1 (Statsoft, Tulsa, OK, USA). The antimicrobial activity (phytopathogen growth inhibition zones) of LAB and the production of fermentation by-products was compared within the sample using a one-way analysis of variance (ANOVA) at a significance level of 0.05. When a statistical difference was detected (p < 0.05), the means were compared using Tukey’s post hoc procedure at a significance level of 0.05.
3. Results and Discussion
3.1. Antimicrobial Activity of Lactic Acid Bacteria In Vitro
The antimicrobial activity of lactic acid bacteria has already been widely described for foodborne bacterial pathogens such as Escherichia coli, Salmonella spp., Listeria monocytogenes and Bacillus cereus [27] as well as for fungal foodborne pathogens, e.g., Aspergillus niger, Fusarium culmorum, Penicillium chrysogenum [28]. In addition, the biocontrol of mycotoxins is of great interest to researchers since certain LAB strains demonstrated the ability to convert mycotoxins into fewer or non-toxic compounds [29,30,31,32]. LAB activity against many potato pathogens is, however, underreported. This work is the first extensive study on potato biocontrol using lactic acid bacteria.
The antimicrobial activity of 42 isolates and 58 collection strains of lactic acid bacteria (LAB) from the genera Lactococcus, Leuconostoc, Lacticaseibacillus, Lactiplantibacillus, Levilactobacillus and Lactobacillus were assessed against ten potato phytopathogens. A summary of the antimicrobial activity of LAB species is presented in Table 1. Detailed results of growth inhibition zones for each LAB strain and pathogen combination are listed in Table S1.
The antimicrobial activity of LAB species varied depending on the pathogen and examined strain relationship. Lactiplantibacillus plantarum and Lactobacillus acidophilus were the most widely represented a group of LAB species (47 and 19 strains, respectively). They exhibited a wide range of antimicrobial activity spectrum, including inhibition of both bacterial and fungi potato pathogens. Several studies reported antifungal activity of both L. plantarum and L. acidophilus species against Fusarium oxysporum [33,34,35,36] and Rhizoctonia solani [33,35,37]. Alternaria alternata was inhibited by L. acidophilus [38]. In our work, more than 90% of L. plantarum strains inhibited the growth of Alternaria solani, Alternaria tenuissima, Colletotrichum coccodes and Pectobacterium carotovorum while the remaining pathogens were inhibited by at least 30% of the tested strains. All of the L. acidodphilus strains used in our research were active against A. solani, C. coccodes, P. carotovorum and Streptomyces scabiei, and at least 35% of them inhibited the growth of the other selected potato pathogens (Table 2).

Table 2.
The antimicrobial activity of different species of lactic acid bacteria (LAB) cultivated on MRS medium against potato phytopathogens.
Each of the Lactococcus lactis strains was inactive against all fungal pathogens in our research. This LAB species has a homofermentative metabolism—lactic acid is the main product of sugar fermentation [39], while antifungal properties are most often described as the synergistic effect of acetic, propionic, phenyllactic and lactic acids [40,41]. Khalaf and Raizada [42] also reported no activity of L. lactis against R. solani. Significant growth inhibition was, however, obtained in another study for F. oxysporum [43]. Antifungal activity was previously indicated for Leuconostoc mesenteroides against R. solani [42] and F. oxysporum [43]. Our study confirmed these results only for a single strain of L. mesenteroides (out of nine tested). Most of the tested L. mesenteroides strains did not exhibit any antifungal activity against potato pathogens. However, a significant antibacterial activity against potato pathogens (P. carotovorum and S. scabiei) was observed for both L. lactis and L. mesenteroides strains. The remaining species of investigated LAB demonstrated a wide spectrum of antibacterial and antifungal activity, yet they were represented by a small number of strains (Table 2, Table S1).
Ten most active strains (Figure 1, Table S1), selected based on both the widest spectrum of activity and growth inhibition zone of pathogens, were further assessed.
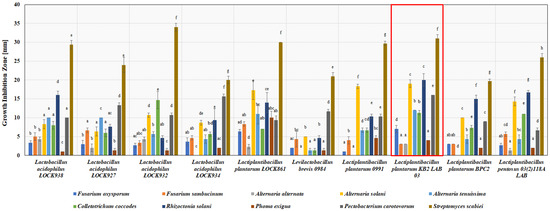
Figure 1.
Antimicrobial activity of selected ten most active lactic acid bacteria strains cultivated on MRS medium (about 108 CFU/mL) against potato phytopathogens after 2–5 days of incubation (depending on the pathogen), measured as growth inhibition zones in the agar-plug diffusion method. a–h—results with different letters within the sample are significantly different (Tukey’s test, α = 0.05).
Streptomyces scabiei was the most susceptible pathogen to selected LAB strains. Growth inhibition zones for this pathogen ranged from 19.7 ± 0.6 to 34.0 ± 1.0 mm for L. plantarum BPC2 and L. acidophilus ŁOCK932, respectively. In turn, F. oxysporum and Phoma exigua showed the highest resistance among all inhibited pathogens by selected LAB (Figure 1). Their growth inhibition zones did not exceed 7.0 ± 1.2 and 10 ± 1.7 mm in diameter, respectively, by any of the selected LAB strains. F. oxysporum was similarly indicated as the most resistant fungi in a study concerning the application of LAB as biocontrol agents of tomato diseases [35].
3.2. Determination of Metabolites Production by Selected Strains of Lactic Acid Bacteria by HPLC
The antimicrobial efficacy of LAB has previously been well documented in the literature as a result of the production of several compounds with low (<1000 Da) and high (>1000 Da; bacteriocins) molecular mass [40,44,45]. The most significant antimicrobial agent produced by LAB in the highest amounts is lactic acid [45]. Although, acetic acid was reported to be far more effective for fungi inhibition than lactic acid in several studies [46,47]. In our research, lactic acid was determined to be the major fermentation by-product of selected LAB. The highest values in the range from 1680 up to 1750 mg/100 mL were obtained for six strains—L. acidophilus ŁOCK938, L. acidophilus ŁOCK927, L. acidophilus ŁOCK934, L. plantarum ŁOCK861, L. plantarum KB2 LAB 03 and L. plantarum BPC2 (Figure 2). The mode of action of lactic acid is associated with cytoplasmic pH reduction of pathogen cells and, consequently, inhibition of metabolic activity. Another hypothesis suggests that organic acids neutralise the electrochemical potential of the plasmic membrane, which increases its permeability and eventually leads to the death of the susceptible microorganism. These mechanisms were also demonstrated for acetic and propionic acids [44]. The importance of organic acid’s role in the antimicrobial spectrum of LAB activity was confirmed in Gerez et al. [20] study, where the pH neutralisation treatment resulted in a loss of antimicrobial activity. In addition to lactic acid, the chromatographic assay also revealed a smaller presence of another four organic acids in the tested samples—acetic, propionic, isobutyric, and valeric acid (Figure 2). Acetic acid reached the second highest concentration for eight strains, from 11.15 up to 37.31 mg/mL. For other L. pentosus 03(2)118A LAB and L. acidophilus ŁOCK932, it was propionic acid instead (11.65 and 16.72 mg/mL, respectively). Small amounts of isobutyric acid (3.01–5.32 mg/mL) and valeric acid (1.09–2.45 mg/mL) were produced by all ten or nine selected strains, respectively (Figure 2). Additionally, ethanol (19.51 mg/mL) was observed in the post-culture fluid of L. plantarum BPC2 (Figure 2). Broad antimicrobial activity is often related to other low molecular weight components, such as hydrogen peroxidase, diacetyl, acetaldehyde, reuterin, cyclic dipeptides and 3-hydroxy fatty acids [45,48]. Bacteriocins, in turn, demonstrate antimicrobial activity mainly against closely related G—positive bacteria, although reports are confirming a wider spectrum of activity for certain bacteriocins [49].
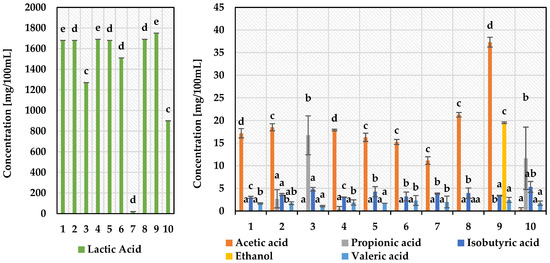
Figure 2.
Fermentation by-products of 10 most active lactic acid bacteria strains cultivated on MRS medium. 1—Lactobacillus acidophilus ŁOCK938; 2—Lactobacillus acidophilus ŁOCK927; 3—Lactobacillus acidophilus ŁOCK932; 4—Lactobacillus acidophilus ŁOCK934; 5—Lactiplantibacillus plantarum ŁOCK861; 6—Levilactobacillus brevis 0984; 7—Lactiplantibacillus plantarum 0991; 8—Lactiplantibacillus plantarum KB2 LAB 03; 9—Lactiplantibacillus plantarum BPC2; 10—Lactiplantibacillus pentosus 03(2)118A LAB; a–e—results with different letters within the sample are significantly different (Tukey’s test, α = 0.05).
Lactiplantibacillus plantarum KB2 LAB 03 was selected as the most effective agent against potato pathogens based on antimicrobial activity in the agar-plug diffusion method and metabolites production.
3.3. Growth Medium Selection for L. plantarum KB2 LAB 03 Cultivation
The willingness to find sustainable management of acid whey, a waste product of cheese manufacture [50], as well as the search for a more economically profitable medium for LAB growth than laboratory medium (MRS), was the reason to select this component as the basis for the development of an optimal medium for L. plantarum KB2 LAB 03 growth. Acid whey was previously described as a medium for LAB culture [51] and also specifically for lactic acid production by LAB [52]. Lactic acid bacteria have complex nutritional requirements; however, some studies suggest that the need for supplementation is strain-dependent [51]. In our research, acid whey was supplemented with nitrogen sources (Yeast Extract and peptone K) and mineral sources (ammonium citrate, dipotassium phosphate, sodium acetate, magnesium sulphate heptahydrate, magnesium sulphate tetrahydrate) in 16 different variants of concentrations. The acid whey itself was a carbon source in the form of lactose. The results of biomass yield (Yx) and maximum specific growth rate (µmax) are presented in Figure 3.
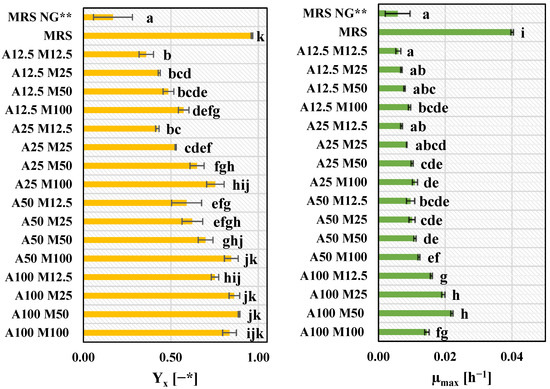
Figure 3.
Biomass yield (Yx) and maximum specific growth rate (µmax) for L. plantarum KB2 LAB 03 cultivated on different variants of acid whey-based media. *—Optical Density; **—MRS water-based medium without glucose; A—group of nitrogen sources (yeast extract, peptone K); M—group of mineral sources (ammonium citrate, dipotassium phosphate, sodium acetate, magnesium sulphate heptahydrate, magnesium sulphate tetrahydrate). A100 M100—acid whey medium supplemented with 100% base concentration of group A supplements and 100% base concentration of group M supplements. The following numbers (50, 25, 12.5) indicate supplementation with a certain percentage of the base concentration of group A and M supplements. MRS, MRS no glucose—control, water-based media. The specific concentrations of each component are presented in Section 2.4.1 point of Materials and Methodology section. a–k—results with different letters are significantly different (Tukey’s test, α = 0.05).
Out of all variants of the acid whey-based medium, the highest values of biomass yield were achieved for A100 M100, A100 M50, A100 M25 and A50 M100 acid whey-based medium (in the range of 0.8354–0.8900). A high concentration of nitrogen sources played a more significant role in obtaining satisfactory results of Yx than a group of mineral sources. However, within variants with an equal concentration of nitrogen sources, the higher concentration of supplied minerals, the higher Yx and µmax were obtained (with the exception of A100 variants). The highest level of µmax, 41% higher than for the A100 M50 variant, was overall observed for the MRS control medium due to the previous adaptation of L. plantarum KB2 LAB 03 to this medium. Nevertheless, the Yx was only 7% higher than the A100 M50 variant Figure 3.
L. plantarum KB2 LAB 03 cultures on four variants of the acid whey-based medium with the highest Yx were selected for the antimicrobial activity evaluation against potato pathogens. The results are presented in Figure 4 and Figure 5.
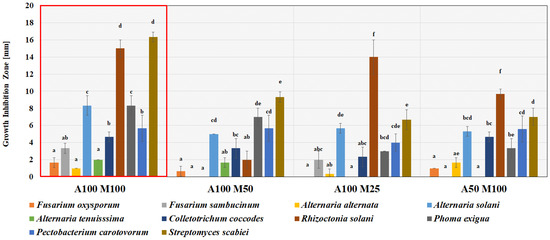
Figure 4.
Antimicrobial activity of L. plantarum KB2 LAB 03 cultivated on different variants of acid whey-based media against potato phytopathogens measured as growth inhibition zones in the agar-well diffusion method. A—group of nitrogen sources (yeast extract, peptone K); M—group of mineral sources (ammonium citrate, dipotassium phosphate, sodium acetate, magnesium sulphate heptahydrate, magnesium sulphate tetrahydrate). A100 M100—acid whey medium supplemented with 100% base concentration of group A supplements and 100% base concentration of group M supplements. The following numbers (50, 25, 12.5) indicate supplementation with a certain percentage of the initial concentration of group A and M supplements. The specific concentrations of each component are presented in Section 2.4.1 point of Materials and Methodology section. a–f—results with different letters within the sample are significantly different (Tukey’s test, α = 0.05).
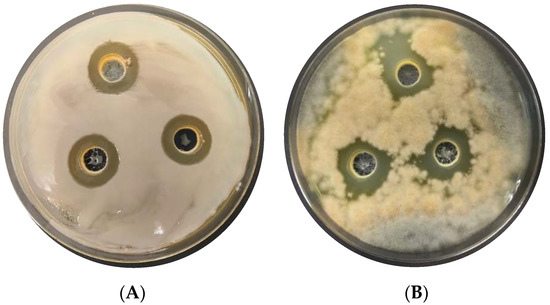
Figure 5.
Growth inhibition zones of P. carotovorum (A) and A. solani (B) by L. plantarum KB2 LAB 03 cultured on an A100 M100 acid whey-based medium.
The growth of both bacterial pathogens was inhibited by L. plantarum KB2 LAB 03 culture in all tested variants of the acid whey-based medium. S. scabiei was found to be more susceptible than P. carotovorum (Figure 4). A broad spectrum of antifungal activity for the A100 M100 variant (all eight fungi inhibited) was achieved. The most resistant fungi were the F. oxysporum, F. sambucinum, A. alternata and A. tenuissima. Among fungi, growth inhibition zones were the widest for R. solani (9.7–15 mm) in all four variants (A100 M100, A100 M25, A50 M100). Similar dependencies were observed regarding pathogens’ sensitivity compared to the L. plantarum KB2 LAB 03 culture in the MRS medium. A100 M100 acid whey-based medium was selected for further cultivation of L. plantarum KB2 LAB 03 due to its wide range of antimicrobial activity, a satisfactory level of biomass yields, and maximum specific growth rate.
The chemical composition of the selected A100 M100 acid whey-based medium was analysed. The results are presented in Table 3. The selected variant of supplemented acid whey-based medium contained 10 g/L of lactose, which replaced glucose used as a carbon source in laboratory MRS medium. Acid whey supplementation allowed to achieve higher level of nitrogen (183.65 mg/L), iron (11 mg/L), potassium (101.8 mg/L) and calcium (461 mg/L).

Table 3.
Characteristics of acid whey-based medium selected for the cultivation of L. plantarum KB2 LAB 03.
The use of A100 M100 acid whey-based medium significantly modulated the metabolism of L. plantarum KB2 LAB 03 compared to MRS cultures. It allowed us to achieve almost 6-times higher concentrations of acetic acid—121 mg/100 mL (Figure 6 and Figure 7). Content of lactic acid, however, slightly decreased to 1630 g/100 mL in comparison to 1690 g/100 mL for MRS culture. While cultured on an acid whey-based medium, L. plantarum KB2 LAB 03 produced 25.36 mg/100 mL of propionic acid and also 17.65 mg/100 mL of ethanol. Both of these compounds were absent in the MRS culture of this strain (Figure 2). In turn, isobutyric acid was observed only in the MRS culture of L. plantarum KB2 LAB 03 (Figure 6 and Figure 7).
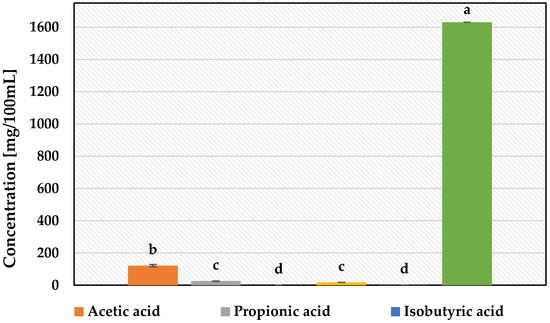
Figure 6.
Fermentation by-products of the L. plantarum KB2 LAB 03 cultured on A100 M100 acid whey-based medium. a–d—results with different letters are significantly different (Tukey’s test, α = 0.05).
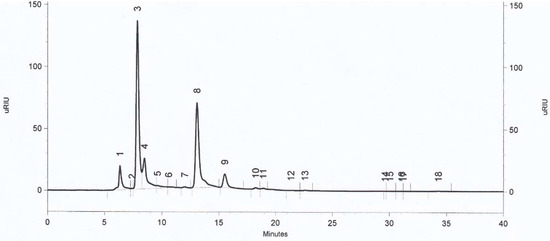
Figure 7.
RI chromatogram of investigated by-products of the L. plantarum KB2 LAB 03 cultured on A100 M100 acid whey-based medium. 8-lactic acid; 9-acetic acid; 10-propionic acid; 13-ethanol; 15-valeric acid.
3.4. In Situ Test of Biocontrol Potential of L. plantarum KB2 LAB 03 against Pathogens on Potatoes
The LAB antimicrobial activity against plant pathogens is underreported during vegetable and fruit storage. L. plantarum prolonged the shelf-life of kiwifruits contaminated with Botrytis cinerea for two weeks [53]. This species was also effectively used to delay the development of Fusarium graminearum, B. cinerea and Rhizopus stolonifer in storage cucumbers [37]. More reports concerning pot and field experiments reveal the influence of LAB on plant growth. Canpolat et al. [54] described the antifungal effect of Lactobacillus sp., Pediococcus acidilactici and Pediococcus pentosaceus treatment in a pot experiment for strawberries inoculated with Fusarium sp., Rhizoctonia sp., Botrytis sp. And other moulds. Several studies reported plant growth-promoting properties of lactic acid bacteria for tomatoes [55] and spring wheat [56] while facing pathogen infection. The in situ antimicrobial activities of L. plantarum KB2 LAB 03 cultured on A100 M100 acid whey-based medium and laboratory MRS medium against the ten potato pathogens were assessed on seed potatoes. The results are presented in Table 4 and Figure 8.

Table 4.
Inhibition of potato seed cv. Impresja infestation treated with L. plantarum KB2 LAB 03 cultured on A100 M100 acid whey-based medium against potato phytopathogens.
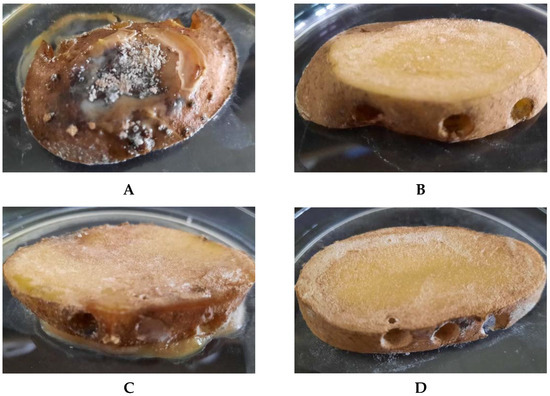
Figure 8.
Inhibition of Streptomyces scabiei (A,B) and Pectobacterium carotovorum (C,D) infestation of potatoes cv. Impresja treated with L. plantarum KB2 LAB 03 cultured on A100 M100 acid whey-based medium. (B,D)—control samples; (A,C)—tested samples.
To the best of our knowledge, this is the first report of in situ experiments on potatoes that confirms the antimicrobial activities of lactic acid bacteria against Fusarium, Alternaria, Phoma, Colletotrichum, Rhizoctonia, Pectobacterium and Streptomyces phytopathogens. It was revealed that L. plantarum KB2 LAB 03 cultured on an A100 M100 acid whey-based medium reduced the development of both bacterial pathogens as well as six out of eight fungal species (Table 4). F. oxysporum and F. sambucinum were found to be resistant, even though the in vitro assay showed the inhibition of these fungi by tested L. plantarum KB2 LAB 03 culture (Table 3 and Table 4). Hamed et al. [33] and Abdel-Aziz et al. [35] research reported plant growth promoting activities of L. plantarum on tomato seeds in pot trials, while the seeds were infected with F. oxysporum. However, these studies did not assess the reduction of pathogen development. Cultivation of LAB selected strain on acid whey-based medium ensured the same or more effective inhibition of potato pathogens than on MRS medium (with the exception of P. exigua). These results correlated with characteristics of fermentation by-products (Figure 2 and Figure 6), where A100 M100 acid whey-based medium post-culture fluid of L. plantarum KB2 LAB 03 contained a significantly higher concentration of acetic, propionic acid and ethanol than MRS post-culture fluid od selected LAB strain. The most satisfactory results were achieved for bacterial inhibition—85 and 90% for S. scabiei and P. carotovorum, respectively (Table 4, Figure 8). A. solani and C. coccodes development was inhibited by 75%. In addition, plant growth-promoting properties of L. plantarum were confirmed for chilli seeds contaminated with Colletotrichum gloeosporioides, another Colletotrichum strain responsible for anthracnose and black dot disease of several plants [57]. The lowest reduction of potato infestation by L. plantarum KB2 LAB 03 was observed for R. solani, the most susceptible mould in the in vitro assay (Table 4, Figure 8). The potential of L. plantarum application as a biocontrol agent was also previously described for Pseudomonas syringae (kiwifruit disease), Xanthomonas fragariae (strawberry disease) and Xanthomonas arboricola (plum disease) [58].
Our work demonstrated that the mechanism of the antimicrobial action of the LAB treatment on potato pathogens is related to the production of organic acids. The determined lactic and acetic acids exhibit antimicrobial properties confirmed in numerous studies [46]. Proteinaceous antifungal compounds, the release of reuterin and hydroxy fatty acids may also be responsible for antimicrobial efficacy of LAB [59]. Other possible mode of action our treatment work is competition for nutrients [44]. Our suggestion is that the effectiveness of LAB as a biocontrol agent against potato pathogens is the result of the synergistic action of the described mechanisms.
4. Conclusions
The primary method of control of potato seeds is the use of chemical compounds. However, growing public concern about the use of pesticides, together with the development of resistance by phytopathogens and the high cost of the development of new chemicals, promote the search for alternative strategies. Among the bacteria used as biocontrol agents, LAB strains may be used as suitable biocontrol agents. The selected strain Lactiplantibacillus plantarum KB2 LAB 03 proved to be effective as preventative treatment to control potato pathogens representing different groups and genera. It has been shown that the antagonism of this strain is based on the production of antimicrobial metabolites, mostly organic acids and ethanol. To reach the population required for biocontrol, acid whey can be used as an appropriate medium to ensure good growth and antimicrobial activities of the LAB strain. In situ experiment on seed potatoes showed that culturing L. plantarum KB2 LAB 03 on the selected A100 M100 acid whey-based medium increased overall antimicrobial activity in comparison to MRS culture. However, more research is needed under different agricultural and climatic conditions to confirm the effectiveness of the strain KB2 LAB 03 against potato pathogens in the field.
5. Patents
No. P.441896, Method of bio-protection of seed potato tubers against the development of bacterial and fungal phytopathogens, Patent Application at the Patent Office in Poland, 1 August 2022.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app12157763/s1, Table S1: Antimicrobial activity of lactic acid bacteria against potato pathogens. Table S2: Groups of components for preparation of acid whey-based culture media for cultivation of L. plantarum KB2 LAB 03. Table S3: Compositions of acid whey-based culture media for cultivation of L. plantarum KB2 LAB 03.
Author Contributions
Conceptualisation, A.S., J.B., D.K. and B.G.; formal analysis, A.S., A.C. and M.O.; funding acquisition, B.G.; investigation, A.S., A.K., I.M., A.C. and W.C.-W.; methodology, A.S., I.M., J.B., A.C. and W.C.-W.; visualization, A.S.; writing—original draft, A.S. and A.K.; writing—review and editing, D.K. and B.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Agency for Restructuring and Modernization of Agriculture, Poland, grant number 00010.DDD.6509.00016.2018.05. The Lodz University of Technology funded the APC.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors express their gratitude to Jadwiga Sliwka from the Plant Breeding and Acclimatization Institute–National Research Institute, Poland, for kindly providing strains of potato pathogens. We also acknowledge the help of Dawid Dygas with culture media analysis. This article was completed while the first author was a Doctoral Candidate in the Interdisciplinary Doctoral School at the Lodz University of Technology, Poland.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
- Camire, M.E.; Kubow, S.; Donnelly, D.J. Potatoes and Human Health. Crit. Rev. Food Sci. Nutr. 2009, 49, 823–840. [Google Scholar] [CrossRef]
- Eurostat. The EU Potato Sector—Statistics on Production, Prices and Trade. 2021. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=The_EU_potato_sector_-_statistics_on_production,_prices_and_trade (accessed on 30 April 2022).
- Statistics Poland. Wynikowy Szacunek Głównych Ziemiopłodów Rolnych I Ogrodniczych W 2021 R (The Resulting Estimate of the Main Agricultural and Horticultural Crops in 2021). Available online: https://stat.gov.pl/obszary-tematyczne/rolnictwo-lesnictwo/uprawy-rolne-i-ogrodnicze/wynikowy-szacunek-glownych-ziemioplodow-rolnych-i-ogrodniczych-w-2021-roku,5,20.html (accessed on 30 April 2022).
- FAO. Top Countries in Potatoes Production. Potatoes Production—Food and Agriculture Organization of the United Nations; FAO: Rome, Italy, 2019. [Google Scholar]
- Smith, I.M. European Handbook of Plant Diseases; Smith, I.M., Dunez, J., Phillips, D.H., Lelliott, R.A., Archer, S.A., Eds.; Blackwell Scientific Publications: Oxford, UK, 2009. [Google Scholar]
- Olsen, N.; Miller, J.; Nolte, P. Diagnosis & Management of Potato Storage Diseases; University of Idaho, College of Agricultural and Life Sciences: Moscow, Idaho, 2006. [Google Scholar]
- Charkowski, A.; Sharma, K.; Parker, M.L.; Secor, G.A.; Elphinstone, J. The Potato Crop; Springer International Publishing: Berlin, Germany, 2020; pp. 351–388. [Google Scholar]
- Khayi, S.; Cigna, J.; Chong, T.M.; Quêtu-Laurent, A.; Chan, K.-G.; Hélias, V.; Faure, D. Transfer of the potato plant isolates of Pectobacterium wasabiae to Pectobacterium parmentieri sp. nov. Int. J. Syst. Evol. Microbiol. 2016, 66, 5379–5383. [Google Scholar] [CrossRef]
- Han, L.; Dutilleul, P.; Prasher, S.O.; Beaulieu, C.; Smith, D.L. Assessment of Common Scab-Inducing Pathogen Effects on Potato Underground Organs Via Computed Tomography Scanning. Phytopathology 2008, 98, 1118–1125. [Google Scholar] [CrossRef] [Green Version]
- Stammler, G.; Bohme, F.; Philippi, J.; Miessner, S.; Tegge, V. Pathogenicity of Alternaria-species on potatoes and tomatoes. Spec. Rep. Appl. Plant R. 2014, 16, 85–96. [Google Scholar]
- A’Hara, D. Detection and Identification of Phoma Pathogens of Potato. In Plant Pathology; Springer: Berlin/Heidelberg, Germany, 2015; pp. 17–27. [Google Scholar]
- Sukul, P.; Spiteller, M. Metalaxyl: Persistence, degradation, metabolism, and analytical methods. Rev. Environ. Contam. Toxicol. 2000, 164, 1–26. [Google Scholar]
- Huang, Z.; Wang, P.; Pu, Z.; Lu, L.; Chen, G.; Hu, X.; Fayyaz, A.; Gai, Y. Effects of mancozeb on citrus rhizosphere bacterial community. Microb. Pathog. 2021, 154, 104845. [Google Scholar] [CrossRef]
- OuYang, M.-N.; Liu, X.; Guo, H.-M.; Lu, Z.-H.; Zhou, D.-D.; Yang, Z.-H. The different toxic effects of metalaxyl and metalaxyl-M on Tubifex tubifex. Ecotoxicol. Environ. Saf. 2021, 208, 111587. [Google Scholar] [CrossRef]
- Eurostat, 2013: Glossary: Organic Farming. Statistics Explained. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Glossary:Organic_farming&oldid=122448 (accessed on 30 April 2022).
- Pal, K.K.; McSpadden Gardener, B. Biological Control of Plant Pathogens. Plant Health Instr. 2006, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Devakumar, N.; Shubha, S.; Rao, G.G.; Gowda, S.B. Multiplication of bio-control agents on locally available organic media. Build. Org. Bridg. 2014, 20, 643–646. [Google Scholar]
- Fiocco, D.; Capozzi, V.; Collins, M.; Gallone, A.; Hols, P.; Guzzo, J.; Weidmann, S.; Rieu, A.; Msadek, T.; Spano, G. Characterization of the CtsR Stress Response Regulon in Lactobacillus plantarum. J. Bacteriol. 2010, 192, 896–900. [Google Scholar] [CrossRef] [Green Version]
- Seddik, H.A.; Bendali, F.; Gancel, F.; Fliss, I.; Spano, G.; Drider, D. Lactobacillus plantarum and Its Probiotic and Food Potentialities. Probiotics Antimicrob. Proteins 2017, 9, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Gerez, C.L.; Torres, M.J.; Font de Valdez, G.; Rollán, G. Control of spoilage fungi by lactic acid bacteria. Biol. Control 2013, 64, 231–237. [Google Scholar] [CrossRef]
- Arena, M.P.; Silvain, A.; Normanno, G.; Grieco, F.; Drider, D.; Spano, G.; Fiocco, D. Use of Lactobacillus plantarum Strains as a Bio-Control Strategy against Food-Borne Pathogenic Microorganisms. Front. Microbiol. 2016, 7, 464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czyżowska, A.; Kucharska, A.Z.; Nowak, A.; Sokół-Łętowska, A.; Motyl, I.; Piórecki, N. Suitability of the probiotic lactic acid bacteria strains as the starter cultures in unripe cornelian cherry (Cornus mas L.) fermentation. J. Food Sci. Technol. 2017, 54, 2936–2946. [Google Scholar] [CrossRef] [PubMed]
- Rice, E.W.; Baird, R.B.; Eaton, A.D.; Clesceri, L.S. (Eds.) Standard Methods for the Examination of Water and Wastewater; American Public Health Association (APHA), American Water Works Association (AWWA) and Water Environment Federation (WEF): Washington, DC, USA, 2012. [Google Scholar]
- EN ISO 20483:2013; Cereals and Pulses—Determination of the Nitrogen Content and Calculation of the Crude Protein Content—Kjeldahl Method. ISO: Geneva, Switzerland, 2013.
- Steglińska, A.; Bekhter, A.; Wawrzyniak, P.; Kunicka-Styczyńska, A.; Jastrząbek, K.; Fidler, M.; Śmigielski, K.; Gutarowska, B. Antimicrobial Activities of Plant Extracts against Solanum tuberosum L. Phytopathogens. Molecules 2022, 27, 1579. [Google Scholar] [CrossRef]
- Mokoena, M.P.; Omatola, C.A.; Olaniran, A.O. Applications of lactic acid bacteria and their bacteriocins against food spoilage microorganisms and foodborne pathogens. Molecules 2021, 26, 7055. [Google Scholar] [CrossRef]
- Nasrollahzadeh, A.; Mokhtari, S.; Khomeiri, M.; Saris, P.E.J. Antifungal Preservation of Food by Lactic Acid Bacteria. Foods 2022, 11, 395. [Google Scholar] [CrossRef]
- Perczak, A.; Goliński, P.; Bryła, M.; Waśkiewicz, A. The efficiency of lactic acid bacteria against pathogenic fungi and mycotoxins. Arh. Hig. Rada Toksikol. 2018, 69, 32–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muhialdin, B.J.; Saari, N.; Meor Hussin, A.S. Review on the Biological Detoxification of Mycotoxins Using Lactic Acid Bacteria to Enhance the Sustainability of Foods Supply. Molecules 2020, 25, 2655. [Google Scholar] [CrossRef]
- Liu, Y.; Galani Yamdeu, J.H.; Gong, Y.Y.; Orfila, C. A review of postharvest approaches to reduce fungal and mycotoxin contamination of foods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1521–1560. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, W.; Chen, S.; Shao, T.; Tao, X.; Yuan, X. Effect of Lactic Acid Bacteria on the Fermentation Quality and Mycotoxins Concentrations of Corn Silage Infested with Mycotoxigenic Fungi. Toxins 2021, 13, 699. [Google Scholar] [CrossRef] [PubMed]
- Hamed, H.A.; Moustafa, Y.A.; Abdel-Aziz, S.M. In vivo efficacy of lactic acid bacteria in biological control against Fusarium oxysporum for protection of tomato plant. Life Sci. J. 2011, 8, 462–468. [Google Scholar]
- Wang, H.K.; Yan, Y.H.; Wang, J.M.; Zhang, H.P.; Qi, W. Production and characterization of antifungal compounds produced by Lactobacillus plantarum IMAU10014. PLoS ONE 2012, 7, e29452. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Aziz, S.M.; Moustafa, Y.A.; Hamed, H.A. Lactic Acid Bacteria in the Green Biocontrol against some Phytopathogenic Fungi: Treatment of Tomato Seeds. J. Basic. Appl. Sci. Res 2014, 4, 1–9. [Google Scholar]
- Husain, A.; Hassan, Z.; Huda-Faujan, N.; Nizam Lani, M. Antifungal Activity of Lactic Acid Bacteria Isolated from Soil Rhizosphere on Fusarium Species Infected Chilli Seeds. Technol. Sci. Am. Sci. Res. J. Eng. 2017, 29, 182–202. [Google Scholar]
- Sathe, S.J.; Nawani, N.N.; Dhakephalkar, P.K.; Kapadnis, B.P. Antifungal lactic acid bacteria with potential to prolong shelf-life of fresh vegetables. J. Appl. Microbiol. 2007, 103, 2622–2628. [Google Scholar] [CrossRef]
- Lipińska, L.; Klewicki, R.; Klewicka, E.; Kołodziejczyk, K.; Sójka, M.; Nowak, A. Antifungal activity of lactobacillus sp. Bacteria in the presence of xylitol and galactosyl-xylitol. BioMed Res. Int. 2016, 2016, 5897486. [Google Scholar] [CrossRef] [Green Version]
- Cavanagh, D.; Fitzgerald, G.F.; McAuliffe, O. From field to fermentation: The origins of Lactococcus lactis and its domestication to the dairy environment. Food Microbiol. 2015, 47, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Schnürer, J.; Magnusson, J. Antifungal lactic acid bacteria as biopreservatives. Trends Food Sci. Technol. 2005, 16, 70–78. [Google Scholar] [CrossRef]
- Cortés-Zavaleta, O.; López-Malo, A.; Hernández-Mendoza, A.; García, H.S. Antifungal activity of lactobacilli and its relationship with 3-phenyllactic acid production. Int. J. Food Microbiol. 2014, 173, 30–35. [Google Scholar] [CrossRef]
- Khalaf, E.M.; Raizada, M.N. Bacterial seed endophytes of domesticated cucurbits antagonize fungal and oomycete pathogens including powdery mildew. Front. Microbiol. 2018, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Zebboudj, N.; Yezli, W.; Hamini-kadar, N.; Mebrouk, K. Antifungal activity of lactic acid bacteria against Fusarium oxysporum f. sp. albedinis isolated from diseased date palm in south Algeria. Int. J. Biosci. 2014, 5, 99–106. [Google Scholar] [CrossRef]
- Dalié, D.K.D.; Deschamps, A.M.; Richard-Forget, F. Lactic acid bacteria—Potential for control of mould growth and mycotoxins: A review. Food Control 2010, 21, 370–380. [Google Scholar] [CrossRef]
- Šušković, J.; Kos, B.; Beganović, J.; Pavunc, A.L.; Habjanič, K.; Matoć, S. Antimicrobial activity—The most important property of probiotic and starter lactic acid bacteria. Food Technol. Biotechnol. 2010, 48, 296–307. [Google Scholar]
- Batish, V.K.; Roy, U.; Lal, R.; Grower, S. Antifungal Attributes of Lactic Acid Bacteria—A Review. Crit. Rev. Biotechnol. 1997, 17, 209–225. [Google Scholar] [CrossRef]
- Le Lay, C.; Coton, E.; Le Blay, G.; Chobert, J.-M.; Haertlé, T.; Choiset, Y.; Van Long, N.N.; Meslet-Cladière, L.; Mounier, J. Identification and quantification of antifungal compounds produced by lactic acid bacteria and propionibacteria. Int. J. Food Microbiol. 2016, 239, 79–85. [Google Scholar] [CrossRef]
- Sjögren, J.; Magnusson, J.; Broberg, A.; Schnürer, J.; Kenne, L. Antifungal 3-Hydroxy Fatty Acids from Lactobacillus plantarum MiLAB14. Appl. Environ. Microbiol. 2003, 69, 7554–7557. [Google Scholar] [CrossRef] [Green Version]
- Zhou, F.; Zhao, H.; Bai, F.; Piotr, D.; Liu, Y.; Zhang, B. Purification and characterisation of the bacteriocin produced by Lactobacillus plantarum, isolated from Chinese pickle. Czech J. Food Sci. 2014, 32, 430–436. [Google Scholar] [CrossRef] [Green Version]
- Rocha-Mendoza, D.; Kosmerl, E.; Krentz, A.; Zhang, L.; Badiger, S.; Miyagusuku-Cruzado, G.; Mayta-Apaza, A.; Giusti, M.; Jiménez-Flores, R.; García-Cano, I. Invited review: Acid whey trends and health benefits. J. Dairy Sci. 2021, 104, 1262–1275. [Google Scholar] [CrossRef]
- Rama, G.R.; Kuhn, D.; Beux, S.; Maciel, M.J.; Volken de Souza, C.F. Potential applications of dairy whey for the production of lactic acid bacteria cultures. Int. Dairy J. 2019, 98, 25–37. [Google Scholar] [CrossRef]
- Catone, M.V.; Palomino, M.M.; Legisa, D.M.; Fina Martin, J.; Monedero García, V.; Ruzal, S.M.; Allievi, M.C. Lactic acid production using cheese whey based medium in a stirred tank reactor by a ccpA mutant of Lacticaseibacillus casei. World J. Microbiol. Biotechnol. 2021, 37, 1–13. [Google Scholar] [CrossRef]
- De Simone, N.; Capozzi, V.; de Chiara, M.L.V.; Amodio, M.L.; Brahimi, S.; Colelli, G.; Drider, D.; Spano, G.; Russo, P. Screening of Lactic Acid Bacteria for the Bio-Control of Botrytis cinerea and the Potential of Lactiplantibacillus plantarum for Eco-Friendly Preservation of Fresh-Cut Kiwifruit. Microorganisms 2021, 9, 773. [Google Scholar] [CrossRef] [PubMed]
- Canpolat, E.; Doğaner, M.M.; Derviş, S.; Ulubaş Serçe, Ç. Antifungal Activity of Some Lactic Acid Bacteria Against Several Soil-borne Fungal Pathogens Isolated from Strawberry Plants. Turkish J. Agric. Food Sci. Technol. 2018, 6, 1163. [Google Scholar] [CrossRef]
- Murthy, K.N.; Malini, M.; Savitha, J.; Srinivas, C. Lactic acid bacteria (LAB) as plant growth promoting bacteria (PGPB) for the control of wilt of tomato caused by Ralstonia solanacearum. Pest Manag. Hortic. Ecosyst. 2012, 18, 60–65. [Google Scholar]
- Suproniene, S.; Semaskiene, R.; Juodeikiene, G.; Mankeviciene, A.; Cizeikiene, D.; Vidmantiene, D.; Basinskiene, L.; Sakalauskas, S. Seed treatment with lactic acid bacteria against seed-borne pathogens of spring wheat. Biocontrol Sci. Technol. 2015, 25, 144–154. [Google Scholar] [CrossRef]
- El-Mabrok, A.S.W.; Hassan, Z.; Mokhtar, A.M.; Hussin, K.M.A. Antifungal activity of Lactobacillus plantarum LAB-C5 and LAB-G7 isolated from Malaysian fruits. Acta Biol. Malaysiana 2013, 2, 22–30. [Google Scholar] [CrossRef]
- Daranas, N.; Roselló, G.; Cabrefiga, J.; Donati, I.; Francés, J.; Badosa, E.; Spinelli, F.; Montesinos, E.; Bonaterra, A. Biological control of bacterial plant diseases with Lactobacillus plantarum strains selected for their broad-spectrum activity. Ann. Appl. Biol. 2019, 174, 92–105. [Google Scholar] [CrossRef] [Green Version]
- Magnusson, J.; Ström, K.; Roos, S.; Sjögren, J.; Schnürer, J. Broad and complex antifungal activity among environmental isolates of lactic acid bacteria. FEMS Microbiol. Lett. 2003, 219, 129–135. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).