Correlation Mapping of Perfusion Patterns in Cutaneous Tissue
Abstract
:1. Introduction
2. Materials and Methods
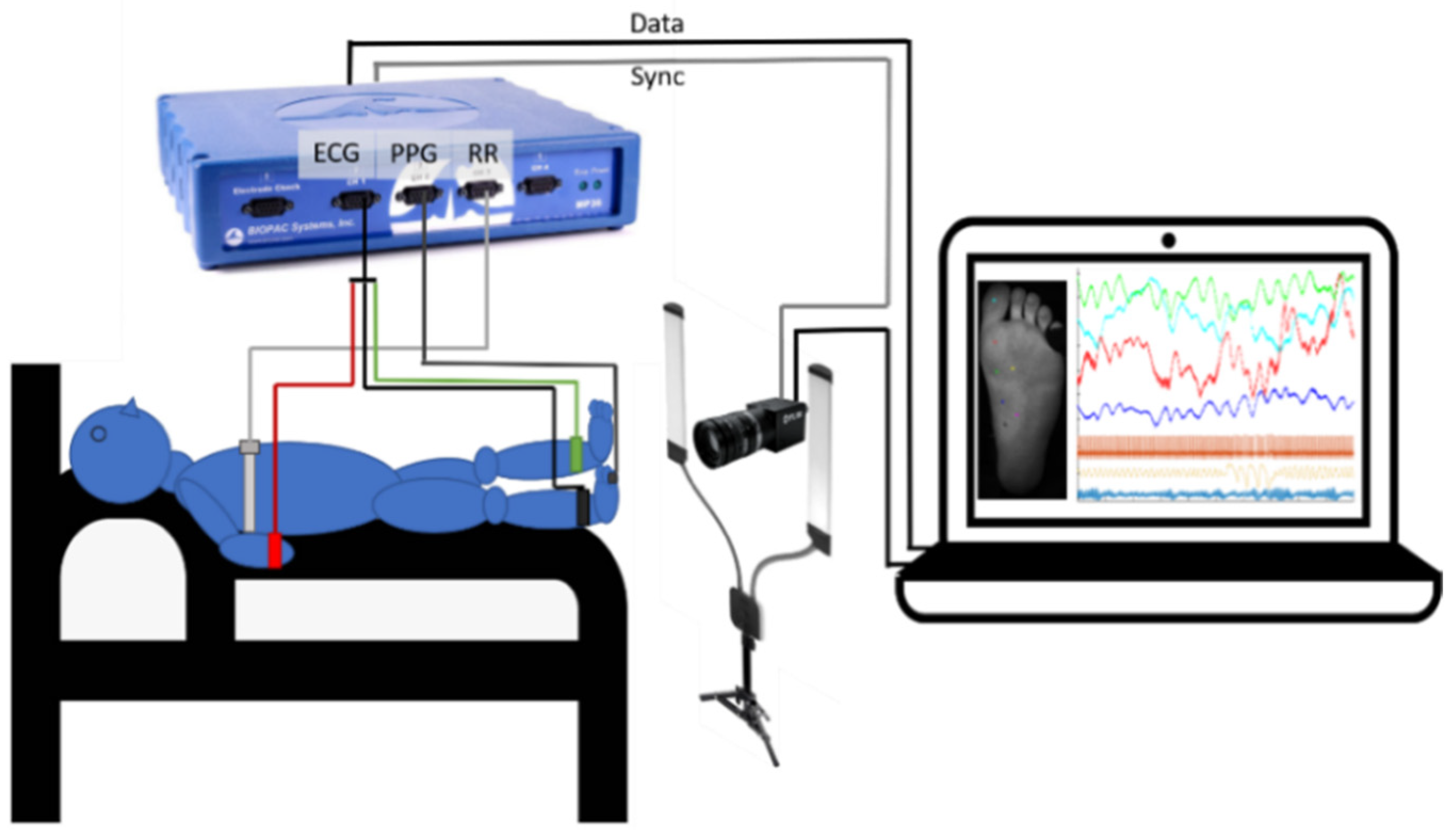
2.1. Measurement Setup
2.2. Experimental Protocol
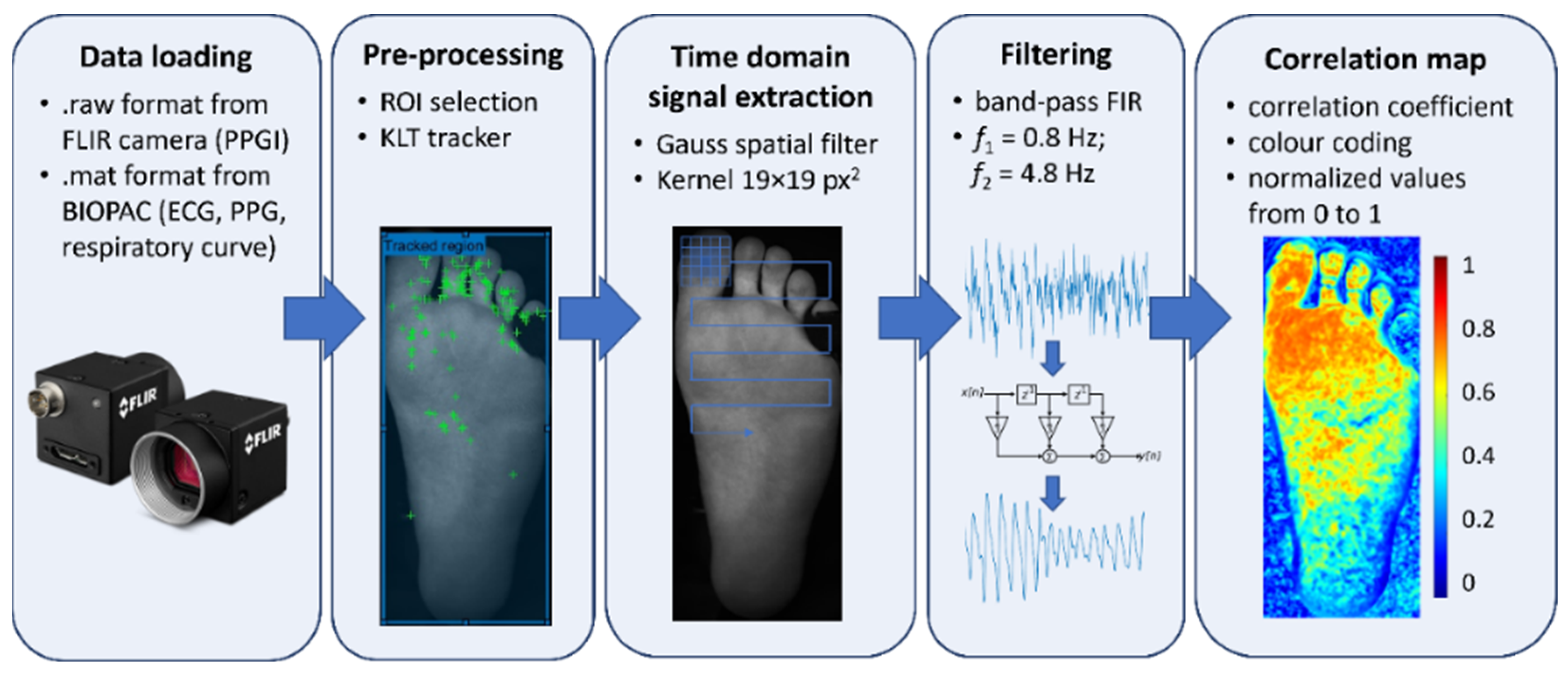
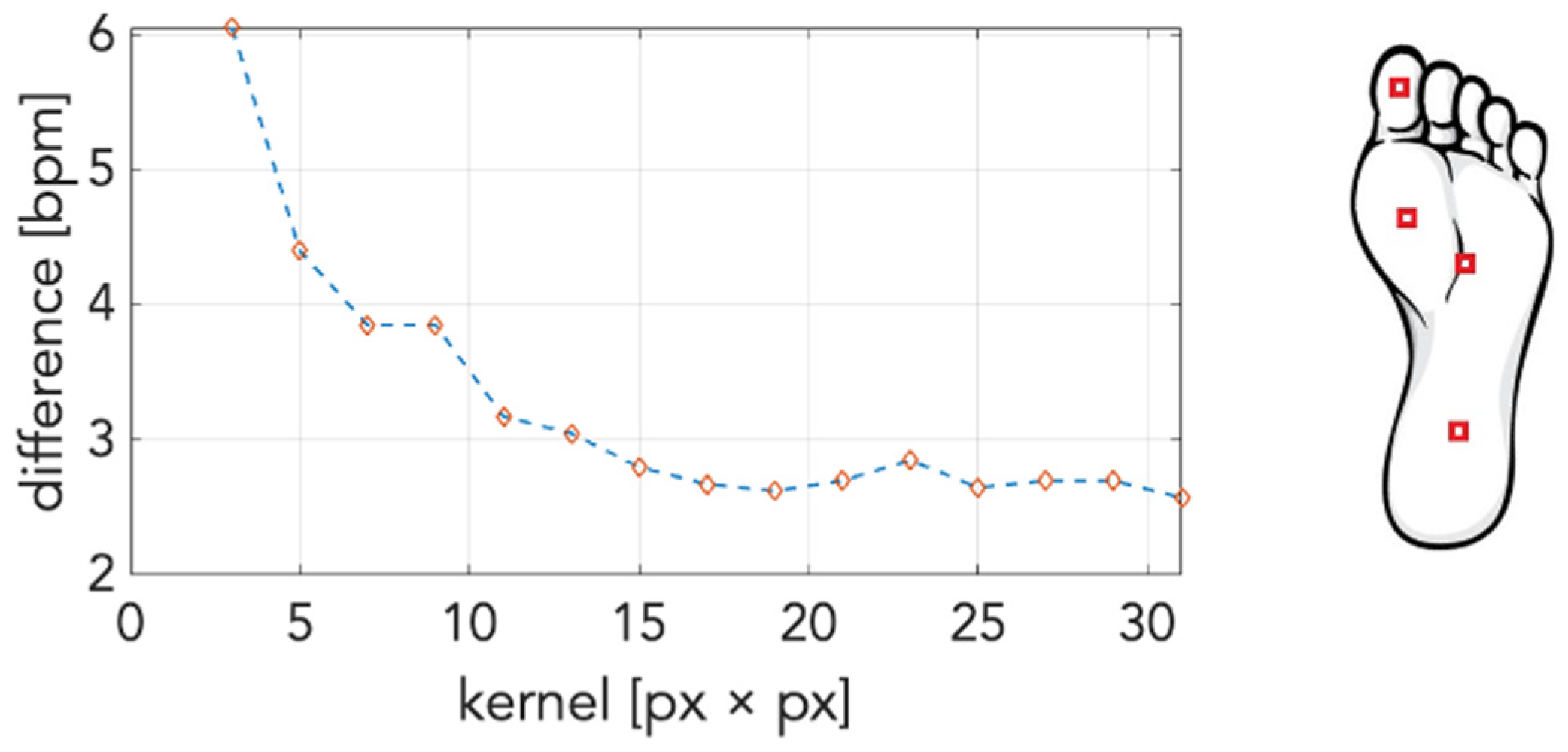
2.3. Data Processing
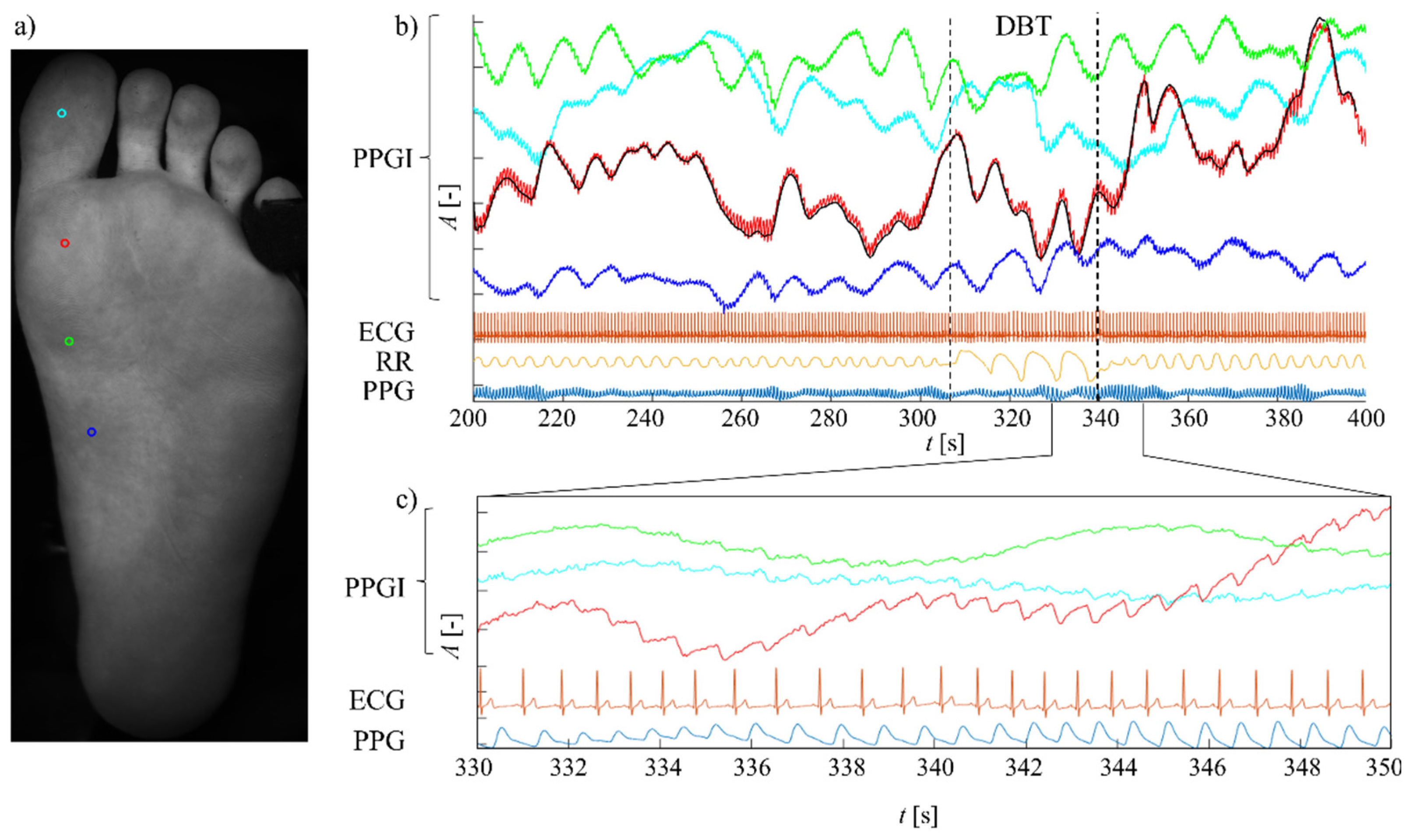
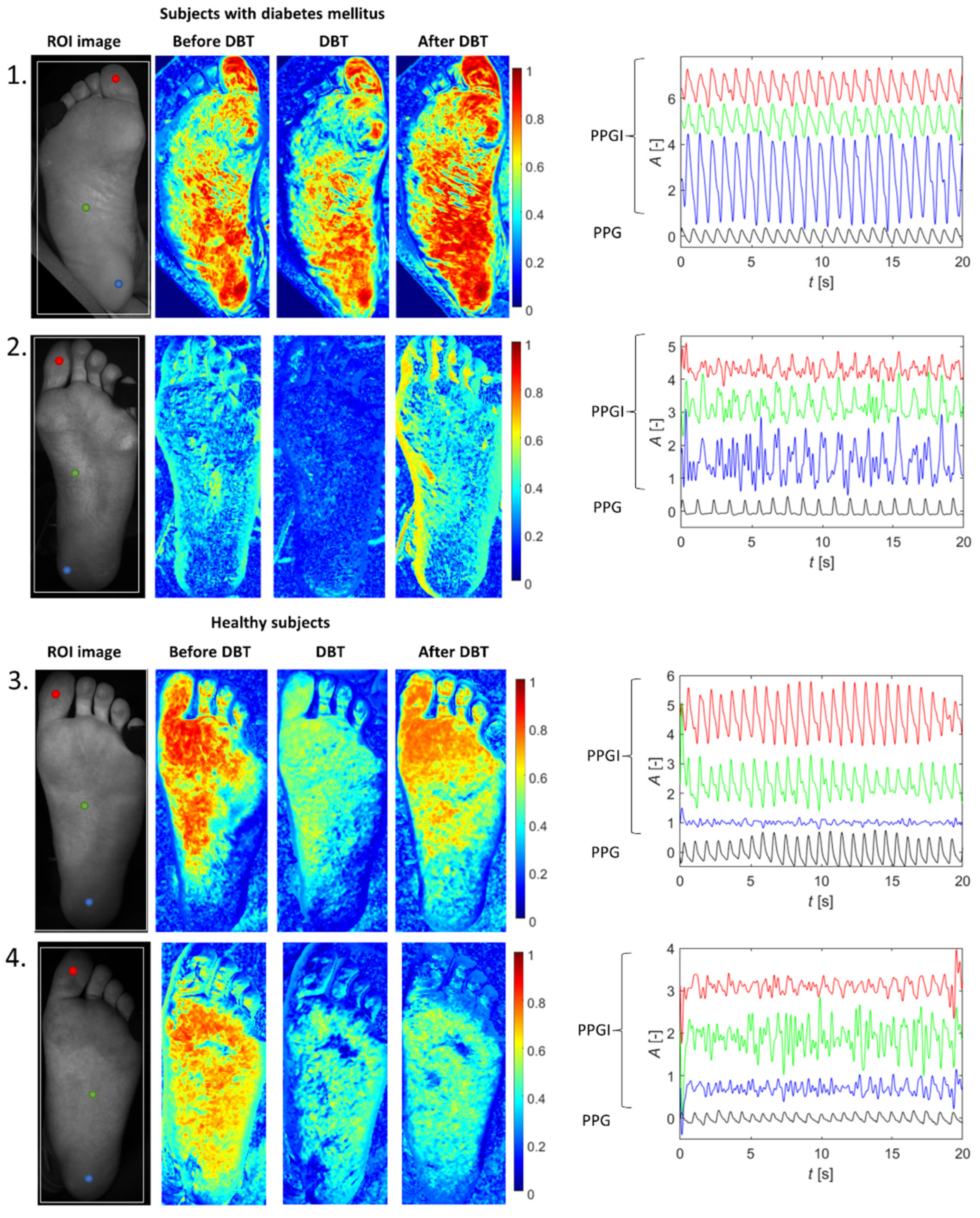
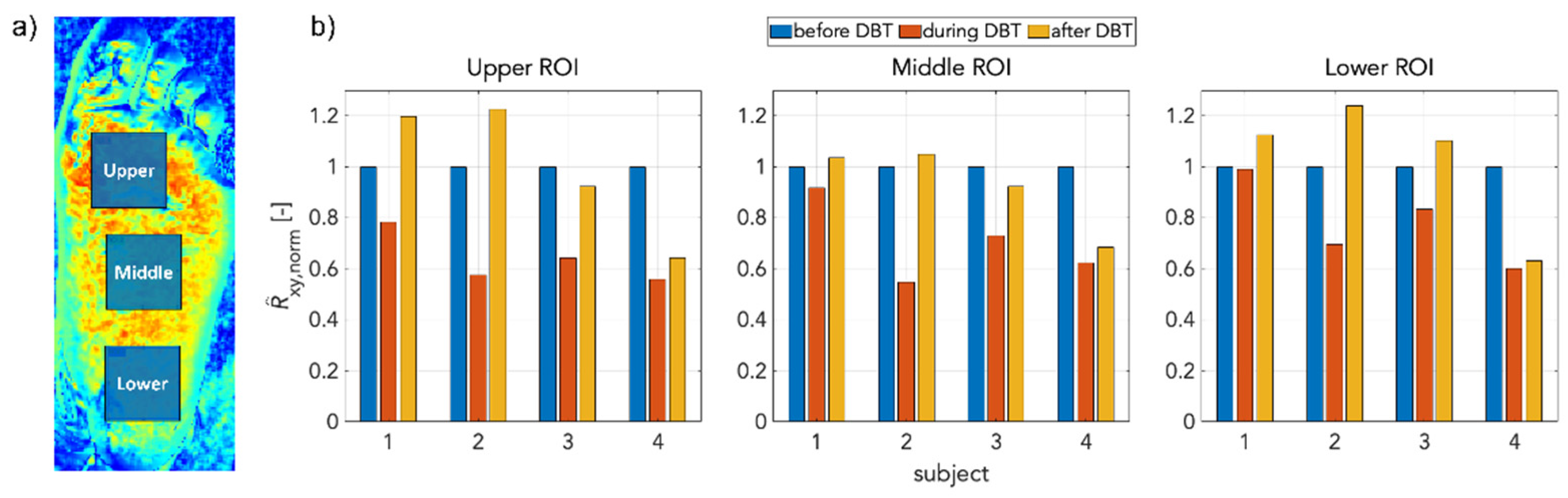
3. Results
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Diabetes. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 10 May 2021).
- Leonhardt, S. Concluding Remarks and New Horizons in Skin Perfusion Studies. In Studies in Skin Perfusion Dynamics; Springer: Berlin/Heidelberg, Germany, 2021; pp. 223–232. [Google Scholar]
- Hertzman, A.B. Photoelectric plethysmography of the fingers and toes in man. Proc. Soc. Exp. Biol. Med. 1937, 37, 529–534. [Google Scholar] [CrossRef]
- Such, O.; Acker, S.; Blazek, V. Mapped hemodynamic data acquisition by near infrared CCD imaging. In Proceedings of the 19th Annual International Conference of the IEEE Engineering in Medicine and Biology Society ’Magnificent Milestones and Emerging Opportunities in Medical Engineering’ (Cat. No. 97CH36136); IEEE, Chicago, IL, USA, 30 October–2 November 1997; Volume 2, pp. 637–639. [Google Scholar]
- Wu, T.; Blazek, V.; Schmitt, H.J. Photoplethysmography imaging: A New noninvasive and noncontact method for mapping of the dermal perfusion changes. In Proceedings of the Optical Techniques and Instrumentation for the Measurement of Blood Composition, Structure, and Dynamics; International Society for Optics and Photonics, Amsterdam, The Netherlands, 7–8 August 2000; Volume 4163, pp. 62–70. [Google Scholar]
- Blazek, V.; Schultz-Ehrenburg, U. Frontiers in computer-aided visualization of vascular functions. In Proceedings of the Seventh International Symposium CNVD’97, Paris, France, 10–12 January 1997; VDI Verlag: Düsseldorf, Germany, 1998. ISBN 3183263203. [Google Scholar]
- Verkruysse, W.; Svaasand, L.O.; Nelson, J.S. Remote plethysmographic imaging using ambient light. Opt. Express 2008, 16, 21434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarassenko, L.; Villarroel, M.; Guazzi, A.; Jorge, J.; Clifton, D.A.; Pugh, C. Non-contact video-based vital sign monitoring using ambient light and auto-regressive models. Physiol. Meas. 2014, 35, 807–831. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Veeraraghavan, A.; Sabharwal, A. DistancePPG: Robust non-contact vital signs monitoring using a camera. Biomed. Opt. Express 2015, 6, 1565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanik, N.; Heimann, K.; Pereira, C.; Paul, M.; Blazek, V.; Venema, B.; Orlikowsky, T.; Leonhardt, S. Remote vital parameter monitoring in neonatology—Robust, unobtrusive heart rate detection in a realistic clinical scenario. Biomed. Tech. 2016, 61, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Den Brinker, A.C.; Stuijk, S.; De Haan, G. Robust heart rate from fitness videos. Physiol. Meas. 2017, 38, 1023–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno, J.; Ramos-Castro, J.; Movellan, J.; Parrado, E.; Rodas, G.; Capdevila, L. Facial video-based photoplethysmography to detect HRV at rest. Int. J. Sports Med. 2015, 36, 474–480. [Google Scholar] [CrossRef]
- Verkruysse, W.; Bartula, M.; Bresch, E.; Rocque, M.; Meftah, M.; Kirenko, I. Calibration of contactless pulse oximetry. Anesth. Analg. 2017, 124, 136–145. [Google Scholar] [CrossRef] [Green Version]
- Murakami, K.; Yoshioka, M.; Ozawa, J. Non-contact pulse transit time measurement using imaging camera, and its relation to blood pressure. In Proceedings of the MVA 2015—14th IAPR International Conference on Machine Vision Applications, Tokyo, Japan, 18–22 May 2015; pp. 414–417. [Google Scholar] [CrossRef]
- Huelsbusch, M.; Blazek, V. Contactless Mapping of Rhythmical Phenomena in Tissue Perfusion Using PPGI. In Proceedings of the Medical Imaging 2002: Physiology and Function from Multidimensional Images, San Diego, CA, USA, 24–26 February 2002; Volume 4683, p. 110. [Google Scholar] [CrossRef]
- Thatcher, J.E.; Li, W.; Rodriguez-Vaqueiro, Y.; Squiers, J.J.; Mo, W.; Lu, Y.; Plant, K.D.; Sellke, E.; King, D.R.; Fan, W.; et al. Multispectral and photoplethysmography optical imaging techniques identify important tissue characteristics in an animal model of tangential burn excision. J. Burn Care Res. 2016, 37, 38–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, J.; Koppel, S.; Charlton, J.L.; Rudin-Brown, C.M. Evaluation of a video-based measure of driver heart rate. J. Safety Res. 2015, 54, 55.e29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilz, C.S.; Zaunseder, S.; Canzler, U.; Krajewski, J. Heart rate from face videos under realistic conditions for advanced driver monitoring. Curr. Dir. Biomed. Eng. 2017, 3, 483–487. [Google Scholar] [CrossRef] [Green Version]
- Leonhardt, S.; Leicht, L.; Teichmann, D. Unobtrusive vital sign monitoring in automotive environments—A review. Sensors 2018, 18, 3080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koeny, M.; Blanik, N.; Yu, X.; Czaplik, M.; Walter, M.; Rossaint, R.; Blazek, V.; Leonhardt, S. Using photoplethysmography imaging for objective contactless pain assessment. Acta Polytech. 2014, 54, 275–280. [Google Scholar] [CrossRef]
- Nitzan, M.; de Boer, H.; Turivnenko, S.; Babchenko, A.; Sapoznikov, D. Power spectrum analysis of spontaneous fluctuations in the photoplethysmographic signal. J. Basic Clin. Physiol. Pharmacol. 1994, 5, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, V.; Liu, H.; Chen, F.; Qiu, Q.; Hughes, S.; Zheng, D. Quantitative comparison of photoplethysmographic waveform characteristics: Effect of measurement site. Front. Physiol. 2019, 10, 198. [Google Scholar] [CrossRef]
- Liu, H.; Chen, F.; Hartmann, V.; Khalid, S.G.; Hughes, S.; Zheng, D. Comparison of different modulations of photoplethysmography in extracting respiratory rate: From a physiological perspective. Physiol. Meas. 2020, 41, 094001. [Google Scholar] [CrossRef]
- Nilsson, L.M. Respiration signals from photoplethysmography. Anesth. Analg. 2013, 117, 859–865. [Google Scholar] [CrossRef] [Green Version]
- Allen, J. Quantifying the delays between multi-site photoplethysmography pulse and electrocardiogram r-r interval changes under slow-paced breathing. Front. Physiol. 2019, 10, 1190. [Google Scholar] [CrossRef] [Green Version]
- Shields, J.W. Heart rate variability with deep breathing as a clinical test of cardiovagal function. Cleve. Clin. J. Med. 2009, 76, 37–40. [Google Scholar] [CrossRef]
- Hynynen, E.; Konttinen, N.; Kinnunen, U.; Kyröläinen, H.; Rusko, H. The incidence of stress symptoms and heart rate variability during sleep and orthostatic test. Eur. J. Appl. Physiol. 2011, 111, 733–741. [Google Scholar] [CrossRef]
- Levin, A.B. A simple test of cardiac function based upon the heart rate changes induced by the valsalva maneuver. Am. J. Cardiol. 1966, 18, 90–99. [Google Scholar] [CrossRef]
- Ewing, D.J.; Martyn, C.N.; Young, R.J.; Clarke, B.F. The Value of Cardiovascular Autonomic Function Tests: 10 Years Experience in Diabetes. Diabetes Care 1985, 8, 491–498. [Google Scholar] [CrossRef]
- Hu, S.; Azorin-Peris, V.; Zheng, J. Opto-Physiological Modeling Applied to Photoplethysmographic Cardiovascular Assessment. J. Healthc. Eng. 2013, 4, 505–528. [Google Scholar] [CrossRef] [Green Version]
- Lucas, B.D.; Kanade, T. An iterative image registration technique with an application to stereo vision. In Proceedings of the 7th International Joint Conference on Artificial Intelligence, Vancouver, BC, Canada, 24–28 August 1981. [Google Scholar]
- Tomasi, C.; Kanade, T. Detection and Tracking of Point Features; (Technical Report CMUCS-91-132); Carnegie Mellon University: Pittsburgh, PA, USA, 1991. [Google Scholar]
- Zheng, J.; Hu, S.; Azorin-Peris, V.; Echiadis, A.; Chouliaras, V.; Summers, R. Remote simultaneous dual wavelength imaging photoplethysmography: A further step towards 3-D mapping of skin blood microcirculation. In Proceedings of the Multimodal Biomedical Imaging III, San Jose, CA, USA, 19–24 January 2008; Volume 6850, p. 68500S. [Google Scholar]
- Pan, J.; Tompkins, W.J. A real-time QRS detection algorithm. IEEE Trans. Biomed. Eng. 1985, BME-32, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Perlitz, V.; Cotuk, B.; Lambertz, M.; Grebe, R.; Schiepek, G.; Petzold, E.R.; Schmid-Schönbein, H.; Flatten, G. Coordination dynamics of circulatory and respiratory rhythms during psychomotor drive reduction. Auton. Neurosci. Basic Clin. 2004, 115, 82–93. [Google Scholar] [CrossRef]
- Schwerdtfeger, A.R.; Schwarz, G.; Pfurtscheller, K.; Thayer, J.F.; Jarczok, M.N.; Pfurtscheller, G. Heart rate variability (HRV): From brain death to resonance breathing at 6 breaths per minute. Clin. Neurophysiol. 2020, 131, 676–693. [Google Scholar] [CrossRef] [PubMed]
- Pfurtscheller, G.; Schwerdtfeger, A.R.; Rassler, B.; Andrade, A.; Schwarz, G.; Klimesch, W. Verification of a central pacemaker in brain stem by phase-coupling analysis between HR interval- and BOLD-oscillations in the 0.10–0.15 Hz frequency Band. Front. Neurosci. 2020, 14, 922. [Google Scholar] [CrossRef]
- Kamshilin, A.A.; Miridonov, S.; Teplov, V.; Saarenheimo, R.; Nippolainen, E. Photoplethysmographic imaging of high spatial resolution. Biomed. Opt. Express 2011, 2, 996. [Google Scholar] [CrossRef] [Green Version]
- Borik, S.; Procka, P.; Kubicek, J.; Antink, C.H. Skin tissue perfusion mapping triggered by an audio-(de) modulated reference signal. Biomed. Opt. Express 2022, 13, 4058–4070. [Google Scholar] [CrossRef]
- Low, P.A.; Neumann, C.; Dyck, P.J.; Fealey, R.D.; Tuck, R.R. Evaluation of skin vasomotor reflexes by using laser doppler velocimetry. Mayo Clin. Proc. 1983, 58, 583–592. [Google Scholar]
- Wallin, B.G.; Båtelsson, K.; Kienbaum, P.; Karlsson, T.; Gazelius, B.; Elam, M. Two neural mechanisms for respiration-induced cutaneous vasodilatation in humans? J. Physiol. 1998, 513, 559–569. [Google Scholar] [CrossRef]
- Rauh, R.; Posfay, A.; Mück-Weymann, M. Quantification of inspiratory-induced vasoconstrictive episodes: A comparison of laser doppler fluxmetry and photoplethysmography. Clin. Physiol. Funct. Imaging 2003, 23, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, L.; Radaelli, A.; Solda’, P.L.; Coats, A.J.S.; Reeder, M.; Calciati, A.; Garrard, C.S.; Sleight, P. Autonomic control of skin microvessels: Assessment by power spectrum of photoplethysmographic waves. Clin. Sci. 1996, 90, 345–355. [Google Scholar] [CrossRef]
- Nilsson, L.; Johansson, A.; Kalman, S. Macrocirculation is not the sole determinant of respiratory induced variations in the reflection mode photoplethysmographic signal. Physiol. Meas. 2003, 24, 925. [Google Scholar] [CrossRef]
- Allen, J.; Frame, J.R.; Murray, A. Microvascular blood flow and skin temperature changes in the fingers following a deep inspiratory gasp. Physiol. Meas. 2002, 23, 365. [Google Scholar] [CrossRef] [PubMed]
- Nitzan, M.; Faib, I.; Friedman, H. Respiration-induced changes in tissue blood volume distal to occluded artery, Measured by photoplethysmography. J. Biomed. Opt. 2006, 11, 40506. [Google Scholar] [CrossRef] [PubMed]
- Borik, S.; Lyra, S.; Perlitz, V.; Keller, M.; Leonhardt, S.; Blazek, V. On the spatial phase distribution of cutaneous low-frequency perfusion oscillations. Sci. Rep. 2022, 12, 5997. [Google Scholar] [CrossRef]
- van Netten, J.J.; van Baal, J.G.; Liu, C.; van Der Heijden, F.; Bus, S.A. Infrared thermal imaging for automated detection of diabetic foot complications. J. Diabetes Sci. Technol. 2013, 7, 1122–1129. [Google Scholar] [CrossRef] [Green Version]
- Urbancic-Rovan, V.; Stefanovska, A.; Bernjak, A.; Ažman-Juvan, K.; Kocijančič, A. Skin blood flow in the upper and lower extremities of diabetic patients with and without autonomic neuropathy. J. Vasc. Res. 2004, 41, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, S.A.; Stansberry, K.B.; Hill, M.A.; Meyer, M.D.; McNitt, P.M.; Bhatt, B.A.; Vinik, A.I. Normal blood flow response and vasomotion in the diabetic charcot foot. J. Diabetes Complicat. 1998, 12, 147–153. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Procka, P.; Celovska, D.; Smondrk, M.; Borik, S. Correlation Mapping of Perfusion Patterns in Cutaneous Tissue. Appl. Sci. 2022, 12, 7658. https://doi.org/10.3390/app12157658
Procka P, Celovska D, Smondrk M, Borik S. Correlation Mapping of Perfusion Patterns in Cutaneous Tissue. Applied Sciences. 2022; 12(15):7658. https://doi.org/10.3390/app12157658
Chicago/Turabian StyleProcka, Patrik, Denisa Celovska, Maros Smondrk, and Stefan Borik. 2022. "Correlation Mapping of Perfusion Patterns in Cutaneous Tissue" Applied Sciences 12, no. 15: 7658. https://doi.org/10.3390/app12157658
APA StyleProcka, P., Celovska, D., Smondrk, M., & Borik, S. (2022). Correlation Mapping of Perfusion Patterns in Cutaneous Tissue. Applied Sciences, 12(15), 7658. https://doi.org/10.3390/app12157658





