Abstract
Roxarsone (ROX) is used extensively in the broiler chicken industry, and most is excreted in poultry litter. ROX degradation produces inorganic arsenic, which causes arsenic contamination of soil and aquatic environment. Furthermore, elevated arsenic concentrations are found in livers of chickens fed ROX. Microorganisms, light, and ions are the main factors that promote ROX degradation in the environment. The adsorption of ROX on different substances and its influencing factors have also been studied extensively. Additionally, the remediation method, combining adsorption and degradation, can effectively restore ROX contamination. Based on this, the review reports the ecological hazards, discussed the transformation and adsorption of ROX in environmental systems, documents the biological response to ROX, and summarizes the remediation methods of ROX contamination. Most previous studies of ROX have been focused on identifying the mechanisms involved under theoretical conditions, but more attention should be paid to the behavior of ROX under real environmental conditions, including the fate and transport of ROX in the real environment. ROX remediation methods at real contaminated sites should also be assessed and verified. The summary of previous studies on the environmental behavior and remediation methods of ROX is helpful for further research in the future.
1. Introduction
Roxarsone (3-nitro-4-hydroxyphenylarsone acid; ROX) is a low-toxic form of an organoarsenical, that has been widely used as an additive in livestock feed to control coccidiosis, improve feed efficiency, and promote weight gain [1]. Their disease prevention and growth accelerative effects were first observed in the 1940s and 1950s. It was later approved for use in poultry and pig production [2,3]. However, almost all of the ROX consumed by poultry is excreted in feces and urine chemically unchanged because ROX is not metabolized by animals [4]. Around the world, thousands of tons of poultry waste are used on land every year. This treatment is economical because poultry waste is rich in nutrients and is an effective fertilizer. In any case, repeated use of poultry waste on land introduces organoarsenicals into the environment and increases arsenic levels in the soil [5,6]. Figure 1 illustrates the environmental behavior of ROX. ROX that enters the environment can quickly and completely be transformed on soil surfaces through biological and abiotic processes [7]. The catabolites of ROX include 3-amino-4-hydroxyphenylarsine acid (HAPA) [8,9,10], monomethylarsine acid (MMA), dimethylarsine acid (DMA), As(III), As(V), and other arsenic-containing compounds [11]. Inorganic arsenic species (As(III) and As(V)) more readily migrate and are more toxic than organoarsenic compounds, and inorganic arsenic species can be adsorbed to and accumulated in soil. This can lead to high arsenic concentrations in soil, surface water, and groundwater [12,13] that can pose risks to human and environmental health. Concern about arsenic pollution in environmental media and arsenic residues in chickens has led to arylarsenic feed additives, including ROX, to be banned in China, the European Union, and the USA [14,15]. However, ROX has already accumulated in the environment after decades of use. And ROX is still used in many countries, including Australia, Brazil, and India [16,17].
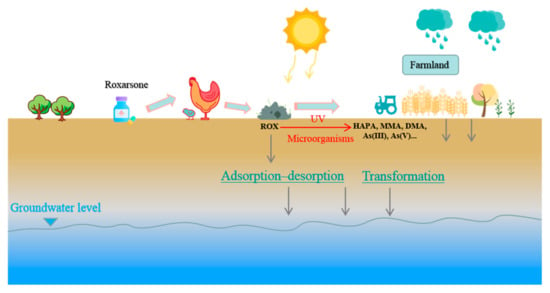
Figure 1.
Environmental behavior of ROX used as feed additives.
ROX degradation, conversion, migration, and the effects of ROX on organisms are currently receiving attention around the world. In order to comprehensively master the research progress of ROX on environmental behavior and remediation, the existing literature were fully reviewed. In the Web of Science Core Collection, we searched for “roxarsone*environment” and “roxarsone*remediation” and 235 and 38 records were obtained, respectively. After removing the literature with weak correlation, the keywords of all downloaded articles were analyzed, and the correlation diagram between ROX and the keywords was drawn (Figure 2). Keywords “degradation” and “adsorption” appeared most frequently, 9 times and 11 times, respectively, and other keywords appeared 2~3 times. Keywords that appeared only once were not plotted. These keywords have been completely covered in this review. The aim of this review is to compile and analyze currently available information about the environmental behavior and remediation methods of ROX, including ecological hazards, transformation, adsorption, biological response, and remediation methods.
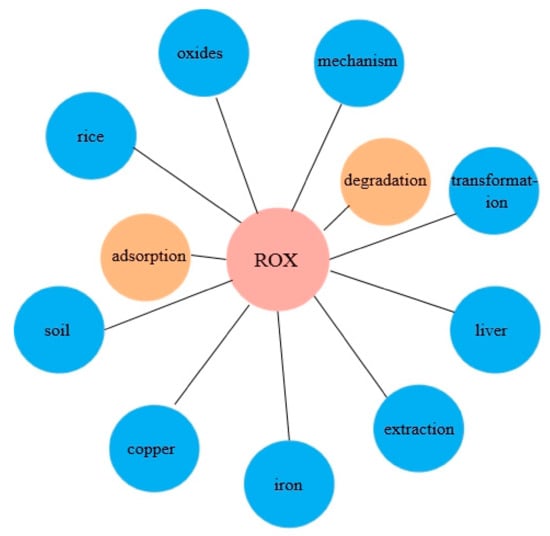
Figure 2.
The correlation diagram between ROX and keywords. The yellow circles indicate frequent occurrences, and the blue circles indicate rare occurrences.
2. Ecological Hazards of ROX
2.1. Arsenic Residues Caused by ROX Use
The U.S. Food and Drug Administration (USFDA) requires animals fed with arsenic to have a 5-day rest period before slaughter to prevent residues in poultry. The World Health Organization recommends that the arsenic content of food should be ≤0.1 mg/kg, and the Chinese national standard for “pollution-free food” requires the arsenic content of animal-based food (including aquatic animal products) to be ≤0.5 mg/kg [11]. However, small amounts of ROX in food can be absorbed by chickens, meaning chicken flesh can contain arsenic. In 2011, the USFDA found elevated arsenic concentrations in livers of chickens fed ROX [18]. In 2011–2012, Nachman et al. [19] found ROX residues in half of the samples they analyzed and found inorganic arsenic contents twice as high in samples of chickens fed ROX than samples of chickens not fed ROX. The allowed total arsenic content of feed in China is 2 mg/kg, but the total arsenic content of feed is higher than this in some areas [20]. If the arsenic dose is high, the arsenic will not be completely excreted during the arsenic-free feeding period before slaughter, and the meat will contain arsenic. Drug withdrawal periods before slaughter are not complied with for some livestock in China, so it is likely that some meat will contain arsenic, which can seriously harm human health.
2.2. Arsenic Contamination Caused by ROX
When broiler chickens consumed food containing ROX at a content of 45.4 g/t, about 150 mg of ROX was excreted by each broiler chicken during the 42 d growing period [4]. In an area in which 100 × 106 chickens were reared and with 70% of the chickens consuming feed containing ROX at a content of 45.4 g/t, 4300 kg of arsenic would therefore be present in the manure produced by the chickens each year [21]. Poultry manure is usually applied to farmland to minimize disposal costs. This would cause large amounts of arsenic to enter the environment. The US Geological Survey found arsenic concentrations in rivers and silt in livestock farming areas that were higher than the average arsenic concentrations in rivers and silt in the USA, and they found arsenic levels much higher than the relevant limit in groundwater in livestock farming areas [22]. Gupta et al. [23] found high arsenic content in the vadose zones of farmland and in river sediment near poultry farms, the manure from which was applied to farmland. Dust near stored poultry waste containing ROX in Arkansas had a high arsenic content, the total arsenic contents being 10.7–130 mg/kg [24]. The main arsenic species was As(V), but small amounts of As(III) and ROX were found. The arsenic species found in house dust were similar to the arsenic species found in poultry waste and suspended particles in the atmosphere. It was found that arsenic in poultry waste in intensive poultry-rearing areas can contaminate the environment through being transported in the air and can pose risks to human health [24]. The toxicity of ROX leachate increased after passing through the soil column. Therefore, applying manure containing ROX to fields may lead to ROX being transferred to nearby soil, surface water, and groundwater through leaching and transfer in run-off of rainwater and irrigation water. Pig farm waste has also been found to have a high ROX content and to contain other forms of arsenic [25,26].
2.3. Enrichment of Arsenic Supplied by ROX
Introducing ROX to the environment in livestock manure has been found to affect the soil microbial community structure and diversity and to lead to arsenic becoming enriched in plants. When livestock are fed ROX and manure containing ROX is applied to farmland, the arsenic contamination sequence will be ROX in feed→animal→animal feces→soil→crops. ROX or p-arsanilic acid can markedly decrease the heights rice plants grow to, the effective number of tillers, the mass of straw produced, and the yield of rice grains and cause the rice grains to contain arsenic at contents that can pose risks to human health [27]. Roots were found to be more sensitive than above-ground plant tissues to stress from ROX because the ROX content of the plant roots were higher than the ROX content of the above-ground plant tissues [28]. The median effective concentration for inhibition of wheat root elongation by ROX is about 20 mg/L [29]. Different forms of arsenic can accumulate to different degrees in plants and different plant organs. ROX metabolites (DMA, As(III), and As(V)) have been found to accumulate in plants (turnips and water spinach), but ROX and MMA were not found to be absorbed by the plants. Only As(III) was detected in garland chrysanthemum shoots, but both As(III) and As(V) were detected in the roots [30,31,32,33]. Applying phosphorus or nitrogen fertilizer to farmland can increase arsenic absorption by crops, but adding Fenton reagents to soil can decrease arsenic absorption and enrichment in rice [34]. Soil containing abundant free iron oxides and/or with a high pH causes straight-head disorder in rice to be more likely when ROX is present [35].
3. Transformation of ROX
When ROX is introduced to the environment in livestock manure, reactions, such as methylation, oxidation, and photolysis, can occur through biological and abiotic processes, and various degradation and transformation products are generated. Microorganisms, light, and ions are the main factors that promote ROX degradation and transformation.
3.1. Microbial Degradation
It has been found in many studies that microbes mainly control ROX degradation in livestock waste and environmental media. Sterilizing soil markedly decreases the degree to which ROX is degraded, and ROX degradation processes are strongly affected by environmental factors, such as the temperature, humidity, and organic matter content [36]. ROX is stable in fresh and dried animal feces, but composting manure with a moisture content of 50% at 40 °C causes ROX to be rapidly converted into inorganic arsenic within 30 d through mainly biological processes [4]. ROX can be degraded by both anaerobic and aerobic microorganisms, as shown in Table 1. ROX is more readily and more efficiently degraded in an anaerobic environment than an aerobic environment [37]. ROX-degrading bacteria have been isolated from animal manure, soil, and sediment. Organic-rich manure and soil provide the necessary conditions for microorganisms to transform ROX into more toxic inorganic arsenic.
The metabolic pathway involved in microbial degradation of ROX begins with nitro-reduction, which produces HAPA. Glucose, hydrogen, and lactate can be used as electron donors to accelerate the reduction of nitrogen substituents. As(III) and As(V) are produced through the cleavage of As–C bonds and aromatic rings in HAPA [38]. HAPA can also be acetylated to give N-acetyl-4-hydroxy-m-arsanilic acid [6]. A novel sulfur-containing arsenic species (AsC9H13N2O6S) has also been detected during the biotransformation of ROX by Enterobacter sp. CZ-1 [39]. Various proteins and genes are involved in ROX degradation, including 816 proteins involved in ROX degradation by Alkaliphilus oremlandii OhILAs [40]. Two nhoA genes (nhoA1 and nhoA2), encoding N-hydroxyarylamine O-acetyltransferases, were found to be responsible for HAPA acetylation by Enterobacter sp. strain CZ-1 [41]. Exoelectrogenic bacteria can effectively use ROX as an electron acceptor for anaerobic respiration. MtrC and UndA are the key cytochromes involved in extracellular reduction in ROX by Shewanella putrefaciens CN32, but extracellular and intracellular reduction occur simultaneously and As(III) is the main inorganic arsenic species that is produced [42]. ROX degradation is also associated with proteins that detoxify arsenic (ars operon) and proteins that allow respiration using arsenic (arr operon). The arsI gene encodes ArsI C-As lyase [43]. The arsM gene abundances in samples of anaerobic granular sludge were found to positively correlate with the arsenic volatilization rates for ROX-loaded digesters [44]. arsEFG genes cloned from Shewanella putrefaciens 200 were found to be responsible for the production and efflux of HAPA(III) and to contribute to HAPA mobilization and toxicity [45]. ArsK is a novel efflux protein for As(III), trivalent ROX (ROX(III)), and methylarsenite (MAs(III)) [46].

Table 1.
Microorganisms that degrade ROX.
Table 1.
Microorganisms that degrade ROX.
| Type | Phylum | Species | Isolation Environment | Degradation Efficiency (Additive Concentration, Time, Degradation Rate) | Catabolites | Reference |
|---|---|---|---|---|---|---|
| Anaerobic microorganisms | Firmicutes | Clostridium sp. strain OhILAs | Manure | 84 h, 100% | HAPA and inorganic arsenic | [47] |
| Firmicutes | Alkaliphilus oremlandii sp. nov. strain OhILAs | Sediments | -- | HAPA and As(V) | [48] | |
| Proteobacteria | Shewanella oneidensis MR-1 | -- | 1 mmol/L,96 h, 100% | HAPA | [49] | |
| Shewanella putrefaciens CN32 | -- | 0.2 mM, 84 h, 100% | HAPA, As(III), As(V) | [42] | ||
| Shewanella decolorationis S12 | -- | 0.2 mM, 24 h, 100% | HAPA, As(III), As(V) | |||
| Aeromonas hydrophila ATCC 7966 | -- | 0.2 mM,4 d, 50% | HAPA, As(III) | |||
| Geobacter sulfurreducens PCA | Sediments | 0.2 mM,4 d, 100% | HAPA, As(III) | |||
| Aerobic microorganisms | Alphaproteobacteria and Firmicutes | Uncultured Rhizobiales bacterium, Rhizobium sp., Agrobacterium sp., Sphingomonas sp., Aurantimonas sp., Bacillus sp. | Soil fertilized with poultry litter containing ROX | 7 d, 81.04% | inorganic arsenic, mainly As(V) | [50] |
| Proteobacteria | Enterobacter sp. CZ-1 | As-contaminated paddy soil | 72 h, 60% | As(V), As(III), N-AHPAA, HAPA, AsC9H13N2O6S | [39] |
3.2. Abiotic Degradation
3.2.1. Photolysis
Light can cause ROX to be transformed through photodegradation or photocatalytic oxidation. At pH 4–8, ROX can be converted into As(III) through photolysis, and the degradation rate increases as the pH increases. As(III) can then be photochemically transformed into As(V), which is less mobile and toxic than As(III). With the strengthening of light intensity, the degradation efficiency of ROX gradually increased [51]. Nitrate and organic matter increase the photolysis rate, so storage of livestock waste and composting can affect ROX degradation through photolysis [52].
Advanced oxidation processes involving ultraviolet light (UV), such as UV/chlorine treatment [53], UV/ZnO treatment [54], UV/TiO2 treatment [55], UV/H2O2 treatment [56], and UV/photosensitizer treatment [57], can effectively degrade ROX. The UV/H2O2 treatment process requires high chemical doses and large amounts of energy because radicals are not efficiently produced because of poor UV absorbance [58]. ROX can be degraded to As(III) and then to As(V) using a derivative of riboflavin as a photosensitizer [57].
3.2.2. Chemical Degradation
Chemical reactions, particularly oxidation of metal ions, such as copper and iron ions, can transform ROX. Andra et al. [59] added ROX to give a concentration of 500 μg/L to aliquots of wastewater containing different concentrations of copper. After 16 d, ROX degradation products were detected in the wastewater with high copper concentrations, but no ROX degradation products were detected in the wastewater with low copper concentrations, indicating that organic copper reagents promote ROX degradation. Iron ions also promote ROX transformations. Microorganisms can reduce Fe(III) to Fe(II), which is a very effective reducing agent [49]. ROX can be transformed in the presence of Fe(II) and tetrapolyphosphate, the tetrapolyphosphate efficiently promoting activation of oxygen by Fe(II) to give ·OH radicals [60]. Ferrate ions can attack As–C bonds and cause cleavage of –AsO(OH)2 groups and oxidation to give As(V) [61].
4. Adsorption of ROX
Adsorption of ROX to soil occurs to a similar degree to adsorption of As(V) because of the presence of arsenate. ROX strongly adsorbs to metal oxides and clay minerals in soil, and the physical and chemical properties of soil strongly affect ROX adsorption [62]. Environmental factors that affect ROX adsorption include the mineral type, metal ions, ion strength, pH, and dissolved organic matter (DOM) content.
Soil minerals are mostly responsible for providing ROX adsorption sites. Adsorption of ROX depends strongly on the characteristics of the soil minerals, including the particle size, specific surface area, hydrophobic partitioning, surface coordination, hydrogen bonding sites, and main adsorption sites, which all play important roles in the adsorption process. Goethite is an iron oxide mineral that is widely present in soil. Goethite has stable chemical properties, a high specific surface area, and high surface charge, so is an effective adsorbent for ROX. Aluminum oxides and hydroxides are widely present in acidic soil and aquatic environments. Fewer studies of arsenic adsorption by aluminum oxides than by goethite have been performed. Silicate minerals are widely present in the aquatic and terrestrial environment. Many water-bearing silicate minerals contain aluminum and magnesium, and the main minerals are kaolinite, illite, and montmorillonite. These minerals and aging of these minerals significantly affects arsenic adsorption, desorption, and oxidation.
Ions affect ROX adsorption to soil. Metal ions, particularly Cu2+, Fe3+, and Zn2+, can form complex precipitates with ROX and enhance ROX adsorption to goethite [63]. Conventional ions in natural water also affect ROX adsorption. Na+, K+, and other monovalent cations affect ROX adsorption through competitive adsorption. Divalent cations, such as Mg2+ and Ca2+, decrease the adsorption capacity for organoarsenical by competing for adsorption sites. Divalent cations can also form complexes with organoarsenical or form bridges between organoarsenical and the adsorption substrate to increase the adsorption capacity for organoarsenical. Sulfate and nitrate affect ROX adsorption little, but phosphate strongly affects ROX adsorption because As and P have relatively similar properties. It has been found in many studies that phosphate can strongly compete with ROX for adsorption sites [63,64].
The pH can affect ROX adsorption by changing the charge state of the adsorbent and the hydrolysis characteristics and stability of ROX. Wang et al. found that the pH affects ROX adsorption by changing the degree of ionization of montmorillonite modified with Fe/La, the morphologies of the ionizable chemicals, and the surface charge of the adsorbent [65]. At a low pH, ROX is poorly dissociated and strongly hydrophobic, which will be conducive for linear distributive adsorption of ROX [4].
The morphology, mobility, and bioavailability of arsenic can be affected by DOM. DOM can occupy adsorption sites on typical soil minerals (nano alumina, goethite, and kaolin) through electrostatic interactions, decreasing the amount of ROX that becomes adsorbed. ROX can form DOM–ROX complexes through interactions with amino, carboxyl, hydroxyl, and nitro groups in DOM [62,66]. Binding to DOM markedly changes the chemical and biological reactivities and bioavailability of arsenic, and so affects ROX bioavailability, mobility, and morphology.
5. Biological Responses to ROX
Various biological, chemical, and physical processes can transform ROX that has been released into the environment in livestock manure. ROX also causes various biological responses. ROX does not markedly affect soil respiration, but can suppress ammoniation, nitrification, and denitrification. ROX can stimulate alkaline phosphatase and urease activities to different degrees. At a high concentration, ROX can inhibit catalase and protease, which can have wide implications for the efficiency of nitrogen use and nitrogen cycling in agroecosystems [67]. ROX metabolites can decrease soil nitrate and nitrite contents [68].
Microorganisms are physiologically and biochemically affected by ROX, and ROX can affect the microbial community structure and diversity. Microbial populations have been found to increase more in the presence of ROX than the absence of ROX and to increase more as the ROX concentration increases. ROX can affect microbial community diversity, and the microbial community structure changes to different degrees at different ROX concentrations [69,70]. ROX can affect microbial activity but not always in a concentration-dependent manner. Microbial activity decreased as the ROX concentration increased in the range 0–100 mg/L but increased as the ROX concentration increased above 100 mg/L [71]. ROX is not toxic to soil microorganisms, but inorganic arsenic produced from ROX gradually inhibited the hydrolytic activity of fluorescein diacetate, indicating that the toxicity increased [36].
The presence of ROX strongly affects sewage treatment processes, so when treating wastewater containing ROX it is necessary to adjust the treatment scheme according to the ROX concentration. ROX can markedly inhibit the reduction in COD and the release and sorption of phosphorus during wastewater treatment [72]. In anaerobic environments, such as sediment, anoxic groundwater, and anaerobic wastewater treatment systems, methanogenesis is the last step of microbial degradation of organic matter. ROX, p-arsanilic acid, and HAPA inhibit methanogens, and particularly acetoclastic methanogenic activity, and the degree of inhibition increases rapidly with time [73,74,75]. Nevertheless, the addition of zero-valent iron (ZVI) has great potential to alleviate the methanogenesis inhibition induced by ROX, which can enhance anaerobic digestion and effectively treatment of organoarsenic-contaminated wastewater [76].
6. Remediation Methods of ROX Contamination
The livestock breeding industry has used ROX as a feed additive for a long time. However, ROX in the environment can be biologically and abiotically degraded to give inorganic arsenic, which is harmful to the environment. Avoiding arsenic pollution caused by livestock breeding and rearing therefore requires solid waste containing ROX to be remediated. The main methods for treating wastewater containing ROX involve adsorption [77], biodegradation [78], and photodegradation [52]. Adsorption is considered to be the most economical and effective method for removing ROX from contaminated water [79].
6.1. Adsorption
Adsorption is cheap and simple and is an effective method for removing benzene arsenic acid compounds from aqueous solutions. Commonly used adsorption materials include metal oxides, nanomaterials, and metal–organic frameworks (MOFs).
Materials based on iron and aluminum are often used to adsorb ROX. Aromatic organoarsenic compounds adsorb to adsorbent materials through the As–C bond on the benzene ring. The acidity of organoarsenical means arsenic-containing organic compounds strongly sorb to iron and alumina. The adsorption of ROX was carried out through the AsO43− functional group [4]. ROX adsorbs similarly strongly to alumina and goethite, but there are fewer adsorption sites on alumina surfaces than goethite surfaces, meaning that the adsorption efficiency is lower for alumina than goethite [64]. Research into nanomaterials for adsorbing ROX has mainly focused on the synthesis of efficient organoarsenical–adsorbent, including multiwalled carbon nanotubes, iron, and aluminum nanomaterials. The O–H bond plays a major role in the adsorption process of ROX on multiwalled carbon nanotubes, and the increase in pH value and ionic strength would reduce the adsorption capacity of ROX on multiwalled carbon nanotubes [80].
Researchers in China and abroad have recently been trying to develop new adsorbents or improve existing adsorbents to improve the removal efficiencies for ROX from water to allow the adsorbents to be used to prevent and remediate ROX pollution of the environment. The specific surface area and pore volume of montmorillonite are increased by modifying the montmorillonite with Fe/La, and the adsorbent was found to have a good affinity for ROX and an adsorption capacity of 32.82 mg/g [65]. More ROX was found to adsorb to goethite modified with humic acid than to unmodified goethite, and the maximum adsorption capacity of goethite modified with humic acid for ROX was 80.71 mg/g [81]. The adsorption process included diffusion through the outer liquid film, surface adsorption, and internal particle diffusion, but chemical adsorption was the key factor affecting the adsorption rate [81]. Fe3S4 nanosheets have been used to adsorb ROX, and ROX was first transformed into HAPA through structural sulfide in the Fe3S4 nanosheets and then the HAPA adsorbed to the Fe3S4 surface [82]. This occurred more strongly at neutral to alkaline pH values than at lower pH values [82]. MOFs, such as MIL-100-Fe, can be used to remove organoarsenic compounds from contaminated water because of its high adsorption capacity, and rapid adsorption [83]. The maximum adsorption capacities of modified sorghum straw biochar for ROX, As(III), and As(V) were 12.4, 5.3, and 23.0 mg/g, respectively, and the adsorption behavior was described well by the Langmuir model and a quasi-second-order rate model [84]. The results described above indicate that continual improvements are giving adsorbents with increasing adsorption capacities for ROX that offer great promise for economically and efficiently remediating water contaminated with ROX.
6.2. Combined Degradation and Adsorption
Removing ROX from a contaminated medium by adsorption often requires several hours for adsorption equilibrium to be reached. Organoarsenical is generally more difficult than inorganic arsenic to remove by adsorption [61]. Adsorbents, such as aluminum and iron, more strongly adsorb inorganic arsenic than organoarsenic [85], so first oxidizing organoarsenical to As(V) could improve the total arsenic removal efficiency achieved by adsorption. ROX is, therefore, usually removed by combining adsorption with degradation or oxidation. First, inorganic arsenic is produced through degradation or oxidation using light, then the degradation products are adsorbed to an adsorbent to effectively remove the arsenic completely. Combinations, such as photochemical oxidation + adsorption, chemical oxidation + adsorption, and heat treatment + adsorption, have been used, as shown in Table 2. Iron ore, Fe(II), and Fe(III) have been used in most combinations.
Combinations of photodegradation and adsorption have been used to remove ROX. Examples include UV/Fe(III) [86,87], Fe(II) + UV/chlorine [53], hematite + UV/oxalate [88], and UV/α-FeOOH@GCA activated persulfate [89]. Oxalate can chelate to surface Fe(III) in hematite to give plenty of •OH radicals through an oxygen activation process mediated by a surface Fe(III)/Fe(II) cycle under simulated solar light, and this can transform ROX into inorganic arsenic species that will adsorb to the hematite surfaces [88]. Almost 100% of ROX was transformed by α-FeOOH@GCA activated persulfate in an UV irradiation system using light at a wavelength of 365 nm, and the As(V) released immediately adsorbed to the α-FeOOH@GCA surfaces [89].
Bifunctional Co3O4–Y2O3 with excellent catalytic and adsorption performances has been used to activate peroxymonosulfate to eliminate ROX and simultaneously adsorb secondary inorganic arsenic, with Co3O4 acting as the primary catalyst and Y2O3 as the main adsorbent [90]. ROX can be effectively oxidized by ferrate, and the arsenic can then be removed by the ferric nanoparticles that form through the oxidation reaction [61,91,92].
A thermal treatment combined with a chemical stabilization technique can degrade ROX efficiently and completely and also stabilize the products and effectively and safely remediate soil contaminated with ROX [93].

Table 2.
ROX main pollution remediation methods.
Table 2.
ROX main pollution remediation methods.
| Remediation Type | Remediation Method | ROX Initial Concentration and Removal Rate | References |
|---|---|---|---|
| Adsorption method | Cellulose-goethite composites | 0.04 mM, 24 h, 100% | [94] |
| HA-a-FeOOH | 20 mg/L, 12 h, >60% | [81] | |
| Modified sorghum straw biochar(MSSB) | 1.0 mg/L, 4 h, 67.09% | [84] | |
| magnetic greigite (Fe3S4) | 1–10 mg/L, 4 h, 100% | [82] | |
| Goethite modified biochar(GMB) | 10 mg/L, 24 h, 98% | [95] | |
| Zerovalent Iron Nanocomposite | 30 mg/L, 40 min, 86.74% | [96] | |
| Bifunctional cationic cyclodextrin material (GD-DTAC) | 50 mg/L, 51% | [97] | |
| Zr-based metal–organic frameworks | 1 mg/L, >99.1% | [98] | |
| Photochemical oxidation + adsorption | UV/Fe(III) | 10 mg/L, 99% | [87] |
| UV/Fe(III) | 10 μmol/L, 90 min, 97.8% | [86] | |
| UV/chlorine + Fe(II) | 5 μmol/L, >98% | [53] | |
| UV/oxalate + hematite | 20 mg/L, 6 h, 85.1% | [88] | |
| UV/alpha-FeOOH@GCA/persulfate | 20 mg/L(in As), 2 h, 100% | [89] | |
| UV/hematite/sulfite | 19 μmol/L, 50 min, 92.55 ± 0.05% | [99] | |
| UV/TiO2/FeOOH hybrid | 10 mg/L, 12 h, 96% | [100] | |
| UV/a bifunctional membrane modified by BiOCl0.875Br0.125 and polydopamine | 17.5 mg/L, 5 h, 100% | [101] | |
| UV/permanganate | 14.29 μmol/L, 20 min, 78% | [102] | |
| FeS2 decorated resorcinol-formaldehyde resins (FeS2-RFR) photocatalyst | 20 mg/L, 2 h, >97% | [103] | |
| Chemical oxidation + adsorption | Co3O4-Y2O3+peroxymonosulfate | 50 μmol/L, 15 min, 100% | [90] |
| Ferrate | 5 μmol/L, 10 min, >95% | [61] | |
| KMnO4-Fe(III) | 0.13 mmol/L, 240 min, >99% | [92] | |
| Heterogeneous Fenton | 10 mg/L, 3 h, >80% | [104] | |
| 0.1-ball milling multi-walled carbon nanotubes/electrolytic manganese residue/peroxydisulfate | 40 mg/L, 60 min, 90.96% | [105] | |
| Chlorination + Fe(II) | 5 μmol/L, 120 min, 100% | [106] | |
| Heat treatment + adsorption | Heat treatment +FeSO4.7H2O | 500 mg/kg (in As), 300 °C, 60 min, 98% | [93] |
7. Conclusions
The environmental behavior and remediation methods of ROX, including ecological hazards, transformation, adsorption, biological response, and remediation methods, were reviewed. Several treatment techniques (e.g., adsorption and degradation + adsorption) have been used to remove ROX from environmental media, and a list of methods that have been used was compiled.
Through the summary and analysis of existing studies, the following two gaps are identified to the future research needs. Firstly, the fate and transport of ROX in the real environment need to be characterized. Organic matter in manure can markedly increase the organic matter content of a shallow layer in the vadose zone of soil. ROX will migrate and be transformed to different degrees in a vadose zone with a high organic matter content and a natural vadose zone, and such differences will affect the ROX removal efficiency and arsenic compound speciation and concentrations into the groundwater. It is therefore necessary to study ROX migration and transformation mechanisms in a vadose zone with a high organic matter content at an open manure dump.
Secondly, there have been many attempts to develop materials to remove ROX from environmental media, and the removal mechanisms have also been studied. However, most of the current research on remediation methods is carried out through liquid phase experiments in laboratory, which have not been used in practice. The remediation effect will be affected by actual environmental conditions. Remediation methods should therefore be applied to real contaminated sites and the remediation effects assessed.
ROX, as a feed additive for livestock, has been increasingly proved to be a great threat to human and the environment. Therefore, it is necessary to ban the use of ROX all over the world, especially in areas where scientific research and regulation are weak, and to raise people’s awareness of the drug.
Author Contributions
Conceptualization, Y.L. (Yasong Li); methodology, M.W.; validation, Y.L. (Yaci Liu) and L.W.; formal analysis, S.C.; investigation, Y.L. (Yasong Li); resources, S.C.; data curation, M.W.; writing—original draft preparation, Y.L. (Yaci Liu); writing—review and editing, Y.L. (Yasong Li); visualization, L.W.; supervision, S.C.; project administration, Y.L. (Yaci Liu); funding acquisition, Y.L. (Yaci Liu). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China (No. 41907175), and China Geological Survey project (No. DD20221773).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Gareth Thomas from Liwen Bianji (Edanz), for editing the language of a draft of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shelver, W.L. Generation of antibody and development of an enzyme-linked immunosorbant assay for the feed additive roxarsone. Food Agric. Immunol. 2011, 22, 171–184. [Google Scholar] [CrossRef]
- Bridges, J.H.; Hale, F.; Kunkel, H.O.; Lyman, C.M. The Effects of Bacitracin, Penicillin and Arsanilic Acid on Growth Rate and Feed Efficiency in Swine. J. Anim. Sci. 1954, 13, 912–917. [Google Scholar] [CrossRef]
- Morehouse, N.F.; Mayfield, O.J. The Effect of Some Aryl Arsonic Acids on Experimental Coccidiosis Infection in Chickens. J. Parasitol. 1946, 32, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Garbarino, J.R.; Bednar, A.J.; Rutherford, D.W.; Beyer, R.S.; Wershaw, R.L. Environmental Fate of Roxarsone in Poultry Litter. I. Degradation of Roxarsone during Composting. Environ. Sci. Technol. 2003, 37, 1509–1514. [Google Scholar] [CrossRef] [PubMed]
- Nachman, K.E.; Graham, J.; Price, L.B.; Silbergeld, E.K. Arsenic: A Roadblock to Potential Animal Waste Management Solutions. Environ. Health Perspect. 2005, 113, 1123–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.M.; Chen, Z.L.; Sun, Y.X.; Gao, Y.L.; Yu, J.X. Investigation on the pollution of organoarsenical additives to animal feed in the surroundings and farmland near hog farms. Acta Ecol. Sin. 2006, 26, 154–162. (In Chinese) [Google Scholar]
- Liu, Y.C.; Zhang, Z.J.; Zhao, X.Y.; Wen, M.T.; Cao, S.W.; Li, Y.S. Arsenic contamination caused by roxarsone transformation with spatiotemporal variation of microbial community structure in a column experiment. J. Groundw. Sci. Eng. 2021, 9, 13. [Google Scholar] [CrossRef]
- Cullen, W.R.; Reimer, K.J. Arsenic Speciation in the Environment. Chem. Rev. 1989, 89, 713–764. [Google Scholar] [CrossRef] [Green Version]
- Moody, J.; Williams, R. The metabolism of 4-hydroxy-3-nitrophenylarsonic acid in hens. Food Cosmet. Toxicol. 1964, 2, 707–715. [Google Scholar] [CrossRef]
- Jackson, B.P.; Bertsch, P.M.; Cabrera, M.L.; Camberato, J.J.; Seaman, J.C.; Wood, C.W. Trace Element Speciation in Poultry Litter. J. Environ. Qual. 2003, 32, 535–540. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, H.; Lu, X.; Liu, Q.; Huang, R.; Hu, B.; Kachanoski, G.; Zuidhof, M.J.; Le, X.C. Arsenic Metabolites, Including N-Acetyl-4-hydroxy-m-arsanilic Acid, in Chicken Litter from a Roxarsone-Feeding Study Involving 1600 Chickens. Environ. Sci. Technol. 2016, 50, 6737–6743. [Google Scholar] [CrossRef] [Green Version]
- Morrison, J.L. Distribution of arsenic from poultry litter in broiler chickens, soil, and crops. J. Agric. Food Chem. 1969, 17, 1288–1290. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, X.; Cao, S.; Li, Y.; Dong, H.; Li, Y. Pollution characteristics and health risk assessment of arsenic transformed from feed additive organoarsenicals around chicken farms on the North China Plain. Chemosphere 2021, 278, 130438. [Google Scholar] [CrossRef]
- Fisher, D.J.; Yonkos, L.T.; Staver, K.W. Environmental Concerns of Roxarsone in Broiler Poultry Feed and Litter in Maryland, USA. Environ. Sci. Technol. 2015, 49, 1999–2012. [Google Scholar] [CrossRef]
- Hu, Y.; Cheng, H.; Tao, S.; Schnoor, J.L. China’s Ban on Phenylarsonic Feed Additives, A Major Step toward Reducing the Human and Ecosystem Health Risk from Arsenic. Environ. Sci. Technol. 2019, 53, 12177–12187. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, W.; Cheng, H.; Tao, S. Public Health Risk of Arsenic Species in Chicken Tissues from Live Poultry Markets of Guangdong Province, China. Environ. Sci. Technol. 2017, 51, 3508–3517. [Google Scholar] [CrossRef]
- Silbergeld, E.K.; Nachman, K. The Environmental and Public Health Risks Associated with Arsenical Use in Animal Feeds. Ann. N. Y. Acad. Sci. 2008, 1140, 346–357. [Google Scholar] [CrossRef] [Green Version]
- Kawalek, J.C.; Carson, M.; Conklin, S.; Lancaster, V.; Howard, K.; Ward, J.; Farrell, D.; Myers, M.; Swain, H.; Jeanettes, P.; et al. Provide Data on Various Arsenic Species Present in Broilers Treated with Roxarsone: Comparison with Untreated Birds; Final Report on Study 275.30; US Food and Drug Administration: Laurel, MD, USA, 2011. [Google Scholar]
- Nachman, K.E.; Baron, P.A.; Raber, G.; Francesconi, K.A.; Navas-Acien, A.; Love, D.C. Roxarsone, Inorganic Arsenic, and Other Arsenic Species in Chicken: A U.S.-Based Market Basket Sample. Environ. Health Perspect. 2013, 121, 818–824. [Google Scholar] [CrossRef] [Green Version]
- Yuan, H.; Chen, J.F.; Xiang, J.Z.; Peng, T.; Li, J.F. Arsenic pollution in livestock and poultry feed and its analysis report. Hunan Feed 2000, 3, 2–3. (In Chinese) [Google Scholar]
- Mangalgiri, K.P.; Adak, A.; Blaney, L. Organoarsenicals in poultry litter: Detection, fate, and toxicity. Environ. Int. 2015, 75, 68–80. [Google Scholar] [CrossRef]
- Miller, C.V.; Foster, G.D.; Huff, T.B.; Garbarino, J.R. Organic Compounds and Trace Elements in the Pocomoke River and Its Tributaries; U.S. Department of the Interior, U.S. Geological Survey: Baltimore, MD, USA, 1999. [Google Scholar] [CrossRef]
- Gupta, G.; Karuppiah, M. Heavy metals in sediments of two Chesapeake Bay tributaries—Wicomico and Pocomoke Rivers. J. Hazard. Mater. 1996, 50, 15–29. [Google Scholar] [CrossRef]
- O’Connor, R.; O’Connor, M.; Irgolic, K.; Sabrsula, J.; Gürleyük, H.; Brunette, R.; Howard, C.; Garcia, J.; Brien, J.; Brien, J.; et al. Transformations, Air Transport, and Human Impact of Arsenic from Poultry Litter. Environ. Forensics 2006, 6, 83–89. [Google Scholar] [CrossRef]
- Mafla, S.; Moraga, R.; León, C.G.; Guzmán-Fierro, V.G.; Yañez, J.; Smith, C.T.; Mondaca, M.A.; Campos, V.L. Biodegradation of roxarsone by a bacterial community of underground water and its toxic impact. World J. Microbiol. Biotechnol. 2015, 31, 1267–1277. [Google Scholar] [CrossRef]
- Makris, K.C.; Quazi, S.; Punamiya, P.; Sarkar, D.; Datta, R. Fate of Arsenic in Swine Waste from Concentrated Animal Feeding Operations. J. Environ. Qual. 2008, 37, 1626–1633. [Google Scholar] [CrossRef]
- Wang, F.M.; Chen, Z.L.; Zhang, L.; Gao, Y.L.; Sun, Y.X. Arsenic Uptake and Accumulation in Rice (Oryza sativa L.) at Different Growth Stages following Soil Incorporation of Roxarsone and Arsanilic Acid. Plant Soil 2006, 285, 359–367. [Google Scholar] [CrossRef]
- Fu, Q.-L.; Blaney, L.; Zhou, D.-M. Identifying Plant Stress Responses to Roxarsone in Soybean Root Exudates: New Insights from Two-Dimensional Correlation Spectroscopy. J. Agric. Food Chem. 2018, 66, 53–62. [Google Scholar] [CrossRef]
- Fu, Q.-L.; Blaney, L.; Zhou, D.-M. Phytotoxicity and uptake of roxarsone by wheat (Triticum aestivum L.) seedlings. Environ. Pollut. 2016, 219, 210–218. [Google Scholar] [CrossRef]
- Yao, L.; Li, G.; Dang, Z.; He, Z.; Zhou, C.; Yang, B. Arsenic speciation in turnip as affected by application of chicken manure bearing roxarsone and its metabolites. Plant Soil 2009, 316, 117–124. [Google Scholar] [CrossRef]
- Yao, L.; Li, G.; Dang, Z.; Yang, B.; He, Z.; Zhou, C. Uptake and transport of roxarsone and its metabolites in water spinach as affected by phosphate supply. Environ. Toxicol. Chem. 2010, 29, 947–951. [Google Scholar] [CrossRef]
- Yao, L.; Huang, L.; He, Z.; Zhou, C.; Li, G.; Yang, B.; Deng, X. External inorganic N source enhances the uptake of As species in garland chrysanthemum (C. coronarium) amended with chicken manure bearing roxarsone and its metabolites. J. Hazard. Mater. 2013, 254–255, 270–276. [Google Scholar] [CrossRef]
- Yao, L.; Huang, L.; He, Z.; Zhou, C.; Li, G.; Deng, X. Phosphate enhances uptake of As species in garland chrysanthemum (C. coronarium) applied with chicken manure bearing roxarsone and its metabolites. Environ. Sci. Pollut. Res. 2015, 22, 4654–4659. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, H.; Lin, C. Fenton process-affected transformation of roxarsone in paddy rice soils: Effects on plant growth and arsenic accumulation in rice grain. Ecotoxicol. Environ. Saf. 2016, 130, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Carey, M.P.; Zhong, J.; Bai, C.; Zhou, C.; Meharg, A.A. Soil attribute regulates assimilation of roxarsone metabolites by rice (Oryza sativa L.). Ecotoxicol. Environ. Saf. 2019, 184, 109660. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Ke, Z.; Chen, Q.; Liu, L.; Chen, G. Degradation of roxarsone in a silt loam soil and its toxicity assessment. Chemosphere 2014, 112, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Z.; Li, Y.; Fei, Y. Response of microbial communities to roxarsone under different culture conditions. Can. J. Microbiol. 2017, 63, 661–670. [Google Scholar] [CrossRef] [Green Version]
- Cortinas, I.; Field, J.A.; Kopplin, M.; Garbarino, J.R.; Gandolfi, A.J.; Sierra-Alvarez, R. Anaerobic Biotransformation of Roxarsone and Related N-Substituted Phenylarsonic Acids. Environ. Sci. Technol. 2006, 40, 2951–2957. [Google Scholar] [CrossRef]
- Huang, K.; Peng, H.; Gao, F.; Liu, Q.; Lu, X.; Shen, Q.; Le, X.C.; Zhao, F.-J. Biotransformation of arsenic-containing roxarsone by an aerobic soil bacterium Enterobacter sp. CZ-1. Environ. Pollut. 2019, 247, 482–487. [Google Scholar] [CrossRef]
- Thomas, J.A.; Chovanec, P.; Stolz, J.F.; Basu, P. Mapping the protein profile involved in the biotransformation of organoarsenicals using an arsenic metabolizing bacterium. Metallomics 2014, 6, 1958–1969. [Google Scholar] [CrossRef]
- Huang, K.; Gao, F.; Le, X.C.; Zhao, F.J. N-Hydroxyarylamine O-Acetyltransferases Catalyze Acetylation of 3-Amino-4-Hydroxyphenylarsonic Acid in the 4-Hydroxy-3-Nitrobenzenearsonic Acid Transformation Pathway of Enterobacter sp. Strain CZ-1. Appl. Environ. Microbiol. 2020, 86, e02050-19. [Google Scholar] [CrossRef]
- Han, J.C.; Zhang, F.; Cheng, L.; Mu, Y.; Liu, D.F.; Li, W.W.; Yu, H.Q. Rapid Release of Arsenite from Roxarsone Bioreduction by Exoelectrogenic Bacteria. Environ. Sci. Technol. Lett. 2017, 4, 350–355. [Google Scholar] [CrossRef]
- Nadar, V.S.; Kandavelu, P.; Sankaran, B.; Rosen, B.P.; Yoshinaga, M. The ArsI C-As lyase: Elucidating the catalytic mechanism of degradation of organoarsenicals. J. Inorg. Biochem. 2022, 232, 111836. [Google Scholar] [CrossRef]
- Tang, R.; Yuan, S.; Wang, Y.; Wang, W.; Wu, G.; Zhan, X.; Hu, Z. Arsenic volatilization in roxarsone-loaded digester: Insight into the main factors and arsM genes. Sci. Total Environ. 2020, 711, 135123. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, J.; Rosen, B.P. Role of ArsEFG in Roxarsone and Nitarsone Detoxification and Resistance. Environ. Sci. Technol. 2019, 53, 6182–6191. [Google Scholar] [CrossRef]
- Shi, K.; Li, C.; Rensing, C.; Dai, X.; Fan, X.; Wang, G. Efflux Transporter ArsK is Responsible for Bacterial Resistance to Arsenite, Antimonite, Trivalent Roxarsone, and Methylarsenite. Appl. Environ. Microbiol. 2018, 84, e01842-18. [Google Scholar] [CrossRef] [Green Version]
- Stolz, J.F.; Perera, E.; Kilonzo, B.; Kail, B.; Crable, B.; Fisher, E.; Ranganathan, M.; Wormer, L.; Basu, P. Biotransformation of 3-Nitro-4-hydroxybenzene Arsonic Acid (Roxarsone) and Release of Inorganic Arsenic by Clostridium Species. Environ. Sci. Technol. 2007, 41, 818–823. [Google Scholar] [CrossRef]
- Fisher, E.; Dawson, A.M.; Polshyna, G.; Lisak, J.; Crable, B.; Perera, E.; Ranganathan, M.; Thangavelu, M.; Basu, P.; Stolz, J.F. Transformation of Inorganic and Organic Arsenic by Alkaliphilus oremlandii sp. nov. Strain OhILAs. Ann. N. Y. Acad. Sci. 2008, 1125, 230–241. [Google Scholar] [CrossRef]
- Chen, G.; Ke, Z.; Liang, T.; Liu, L.; Wang, G. Shewanella oneidensis MR-1-Induced Fe(III) Reduction Facilitates Roxarsone Transformation. PLoS ONE 2016, 11, e0154017. [Google Scholar] [CrossRef]
- Guzmán-Fierro, V.G.; Moraga, R.; León, C.G.; Campos, V.L.; Smith, C.T.; Mondaca, M.A. Isolation and characterization of an aerobic bacterial consortium able to degrade roxarsone. Int. J. Environ. Sci. Technol. 2015, 12, 1353–1362. [Google Scholar] [CrossRef] [Green Version]
- Meng, J.; Arong; Yuan, S.; Wang, W.; Jin, J.; Zhan, X.; Xiao, L.; Hu, Z. Photodegradation of roxarsone in the aquatic environment: Influencing factors, mechanisms, and artificial neural network modeling. Environ. Sci. Pollut. Res. 2022, 29, 7844–7852. [Google Scholar] [CrossRef]
- Bednar, A.; Garbarino, J.; Ferrer, I.; Rutherford, D.; Wershaw, R.; Ranville, J.; Wildeman, T. Photodegradation of roxarsone in poultry litter leachates. Sci. Total Environ. 2003, 302, 237–245. [Google Scholar] [CrossRef]
- Yang, T.; Wu, S.; Liu, C.; Liu, Y.; Zhang, H.; Cheng, H.; Wang, L.; Guo, L.; Li, Y.; Liu, M.; et al. Efficient Degradation of Organoarsenic by UV/Chlorine Treatment: Kinetics, Mechanism, Enhanced Arsenic Removal, and Cytotoxicity. Environ. Sci. Technol. 2021, 55, 2037–2047. [Google Scholar] [CrossRef]
- Acuña, K.; Yáñez, J.; Ranganathan, S.; Ramírez, E.; Cuevas, J.P.; Mansilla, H.D.; Santander, P. Photocatalytic degradation of roxarsone by using synthesized ZnO nanoplates. Sol. Energy 2017, 157, 335–341. [Google Scholar] [CrossRef]
- Lu, D.; Ji, F.; Wang, W.; Yuan, S.; Hu, Z.-H.; Chen, T. Adsorption and photocatalytic decomposition of roxarsone by TiO2 and its mechanism. Environ. Sci. Pollut. Res. 2014, 21, 8025–8035. [Google Scholar] [CrossRef]
- Adak, A.; Mangalgiri, K.; Lee, J.; Blaney, L. UV irradiation and UV-H2O2 advanced oxidation of the roxarsone and nitarsone organoarsenicals. Water Res. 2015, 70, 74–85. [Google Scholar] [CrossRef]
- Meng, J.; Xu, F.; Yuan, S.; Mu, Y.; Wang, W.; Hu, Z.-H. Photocatalytic oxidation of roxarsone using riboflavin-derivative as a photosensitizer. Chem. Eng. J. 2019, 355, 130–136. [Google Scholar] [CrossRef]
- Wols, B.; Hofman-Caris, C.; Harmsen, D.; Beerendonk, E. Degradation of 40 selected pharmaceuticals by UV/H2O2. Water Res. 2013, 47, 5876–5888. [Google Scholar] [CrossRef]
- Andra, S.S.; Makris, K.C.; Quazi, S.; Sarkar, D.; Datta, R.; Bach, S.B.H. Organocopper complexes during roxarsone degradation in wastewater lagoons. Environ. Sci. Pollut. Res. 2010, 17, 1167–1173. [Google Scholar] [CrossRef]
- Chen, N.; Wan, Y.; Ai, Z.; Jia, F.; Zhang, L. Fast transformation of roxarsone into toxic arsenic species with ferrous iron and tetrapolyphosphate. Environ. Chem. Lett. 2019, 17, 1077–1084. [Google Scholar] [CrossRef]
- Yang, T.; Liu, Y.; Wang, L.; Jiang, J.; Huang, Z.; Pang, S.-Y.; Cheng, H.; Gao, D.; Ma, J. Highly effective oxidation of roxarsone by ferrate and simultaneous arsenic removal with in situ formed ferric nanoparticles. Water Res. 2018, 147, 321–330. [Google Scholar] [CrossRef]
- Fu, Q.-L.; He, J.-Z.; Blaney, L.; Zhou, D.-M. Sorption of roxarsone onto soils with different physicochemical properties. Chemosphere 2016, 159, 103–112. [Google Scholar] [CrossRef]
- Wang, L.-Y.; Wang, S.-W.; Chen, W.-R. Roxarsone desorption from the surface of goethite by competitive anions, phosphate and hydroxide ions: Significance of the presence of metal ions. Chemosphere 2016, 152, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-R.; Huang, C.-H. Surface adsorption of organoarsenic roxarsone and arsanilic acid on iron and aluminum oxides. J. Hazard. Mater. 2012, 227–228, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-J.; Ji, F.; Wang, W.; Yuan, S.-J.; Hu, Z.-H. Removal of roxarsone from aqueous solution by Fe/La-modified montmorillonite. Desalin. Water Treat. 2016, 57, 20520–20533. [Google Scholar] [CrossRef]
- Fu, Q.-L.; He, J.; Blaney, L.; Zhou, D.-M. Roxarsone binding to soil-derived dissolved organic matter: Insights from multi-spectroscopic techniques. Chemosphere 2016, 155, 225–233. [Google Scholar] [CrossRef]
- Chen, G.; Liu, H.; Zhang, W.; Li, B.; Liu, L.; Wang, G. Roxarsone exposure jeopardizes nitrogen removal and regulates bacterial community in biological sequential batch reactors. Ecotoxicol. Environ. Saf. 2018, 159, 232–239. [Google Scholar] [CrossRef]
- Yao, L.; Huang, L.; Bai, C.; Zhou, C.; He, Z. Effect of roxarsone metabolites in chicken manure on soil biological property. Ecotoxicol. Environ. Saf. 2019, 171, 493–501. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Zhang, Z.; Fei, Y.; Tian, X.; Cao, S. Identification of an anaerobic bacterial consortium that degrades roxarsone. MicrobiologyOpen 2020, 9, e1003. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.; Li, Y.; Wen, Y.; Fei, Y. Response of soil microbial communities to roxarsone pollution along a concentration gradient. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2017, 52, 819–827. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, P.; Wang, Y.; Li, B.; Wang, Y. Effects of roxarsone on the functional diversity of soil microbial community. Int. Biodeterior. Biodegrad. 2013, 76, 32–35. [Google Scholar] [CrossRef]
- Guo, Q.; Liu, L.; Hu, Z.; Chen, G. Biological phosphorus removal inhibition by roxarsone in batch culture systems. Chemosphere 2013, 92, 138–142. [Google Scholar] [CrossRef]
- Tang, R.; Yuan, S.; Chen, F.; Zhan, X.; Wang, W.; Hu, Z. Effects of roxarsone and sulfadiazine on biogas production and their degradation during anaerobic digestion. Int. Biodeterior. Biodegrad. 2019, 140, 113–118. [Google Scholar] [CrossRef]
- Zhang, F.F.; Wang, W.; Yuan, S.J.; Hu, Z.H. Biodegradation and speciation of roxarsone in an anaerobic granular sludge system and its impacts. J. Hazard. Mater. 2014, 279, 562–568. [Google Scholar] [CrossRef]
- Tang, R.; Luo, H.; Prommer, H.; Yue, Z.; Wang, W.; Su, K.; Hu, Z.-H. Response of anaerobic granular sludge to long-term loading of roxarsone: From macro- to micro-scale perspective. Water Res. 2021, 204, 117599. [Google Scholar] [CrossRef]
- Yao, G.; Tang, R.; Luo, H.; Yuan, S.; Wang, W.; Xiao, L.; Chu, X.; Hu, Z.H. Zero-valent iron mediated alleviation of methanogenesis inhibition induced by organoarsenic roxarsone. Sci. Total Environ. 2022, 819, 152080. [Google Scholar] [CrossRef]
- Li, B.; Zhu, X.; Hu, K.; Li, Y.; Feng, J.; Shi, J.; Gu, J. Defect creation in metal-organic frameworks for rapid and controllable decontamination of roxarsone from aqueous solution. J. Hazard. Mater. 2016, 302, 57–64. [Google Scholar] [CrossRef]
- Fei, J.; Wang, T.; Zhou, Y.; Wang, Z.; Min, X.; Ke, Y.; Hu, W.; Chai, L. Aromatic organoarsenic compounds (AOCs) occurrence and remediation methods. Chemosphere 2018, 207, 665–675. [Google Scholar] [CrossRef]
- Simeonidis, K.; Gkinis, T.; Tresintsi, S.; Boubeta, C.M.; Vourlias, G.; Tsiaoussis, I.; Stavropoulos, G.; Mitrakas, M.; Angelakeris, M. Magnetic separation of hematite-coated Fe3O4 particles used as arsenic adsorbents. Chem. Eng. J. 2011, 168, 1008–1015. [Google Scholar] [CrossRef]
- Hu, J.; Tong, Z.; Hu, Z.; Chen, G.; Chen, T. Adsorption of roxarsone from aqueous solution by multi-walled carbon nanotubes. J. Colloid Interface Sci. 2012, 377, 355–361. [Google Scholar] [CrossRef]
- Song, J.; Yu, J.; Wang, W.; Mi, N.; Wei, W.; Li, S.; Zhang, Y. Enhanced adsorption of roxarsone onto humic acid modified goethite from aqueous solution. J. Dispers. Sci. Technol. 2019, 40, 25–32. [Google Scholar] [CrossRef]
- Liu, W.; Ai, Z.; Dahlgren, R.A.; Zhang, L.; Wang, X. Adsorption and reduction of roxarsone on magnetic greigite (Fe3S4): Indispensable role of structural sulfide. Chem. Eng. J. 2017, 330, 1232–1239. [Google Scholar] [CrossRef]
- Jun, J.W.; Tong, M.; Jung, B.K.; Hasan, Z.; Zhong, C.; Jhung, S.H. Effect of Central Metal Ions of Analogous Metal-Organic Frameworks on Adsorption of Organoarsenic Compounds from Water: Plausible Mechanism of Adsorption and Water Purification. Chem—Eur. J. 2014, 21, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Zang, S.; Zuo, Y.; Wang, J.; Liu, X.; Gomez, M.A.; Wei, L. Adsorption removal of roxarsone, arsenite(III), and arsenate(V) using iron-modified sorghum straw biochar and its kinetics. Acta Geochim. 2021, 40, 409–418. [Google Scholar] [CrossRef]
- Depalma, S.; Cowen, S.; Hoang, T.; Al-Abadleh, H.A. Adsorption Thermodynamics of p-Arsanilic Acid on Iron (Oxyhydr)Oxides: In-Situ ATR-FTIR Studies. Environ. Sci. Technol. 2008, 42, 1922–1927. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lin, C.; Zhou, Y.; Long, L.; Li, L.; Tang, M.; Liu, Z.; Pozdnyakov, I.P.; Huang, L.-Z. Transformation of roxarsone during UV disinfection in the presence of ferric ions. Chemosphere 2019, 233, 431–439. [Google Scholar] [CrossRef]
- Chen, N.; Wang, X.; Wan, Y.; Luo, Y.; Huang, Y.; Zhang, L. Simulated solar light driven Fe(III)/Fe(II) redox cycle for roxarsone degradation and simultaneous arsenate immobilization. J. Hazard. Mater. 2020, 394, 121635. [Google Scholar] [CrossRef]
- Chen, N.; Wan, Y.; Zhan, G.; Wang, X.; Li, M.; Zhang, L. Simulated solar light driven roxarsone degradation and arsenic immobilization with hematite and oxalate. Chem. Eng. J. 2019, 384, 123254. [Google Scholar] [CrossRef]
- Su, S.; Cao, C.; Zhao, Y.; Dionysiou, D.D. Efficient transformation and elimination of roxarsone and its metabolites by a new α-FeOOH@GCA activating persulfate system under UV irradiation with subsequent As(V) recovery. Appl. Catal. B Environ. 2019, 245, 207–219. [Google Scholar] [CrossRef]
- Chen, C.; Liu, L.; Li, Y.; Zhou, L.; Lan, Y. Efficient degradation of roxarsone and simultaneous in-situ adsorption of secondary inorganic arsenic by a combination of Co3O4-Y2O3 and peroxymonosulfate. J. Hazard. Mater. 2021, 407, 124559. [Google Scholar] [CrossRef]
- Xie, X.; Cheng, H. A simple treatment method for phenylarsenic compounds: Oxidation by ferrate (VI) and simultaneous removal of the arsenate released with in situ formed Fe(III) oxide-hydroxide. Environ. Int. 2019, 127, 730–741. [Google Scholar] [CrossRef]
- Xie, X.; Zhao, W.; Hu, Y.; Xu, X.; Cheng, H. Permanganate oxidation and ferric ion precipitation (KMnO4-Fe(III)) process for treating phenylarsenic compounds. Chem. Eng. J. 2019, 357, 600–610. [Google Scholar] [CrossRef]
- Zhan, L.; Xia, Z.; Xu, Z.; Xie, B. Study on the remediation of tetracycline antibiotics and roxarsone contaminated soil. Environ. Pollut. 2020, 271, 116312. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Wilson, L.D. Synthesis and characterization of cellulose-goethite composites and their adsorption properties with roxarsone. Carbohydr. Polym. 2017, 169, 282–294. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zhao, J.; Zhao, N.; Yang, X.; Chen, C.; Shang, J. Goethite modified biochar as a multifunctional amendment for cationic Cd(II), anionic As(III), roxarsone, and phosphorus in soil and water. J. Clean. Prod. 2019, 247, 119579. [Google Scholar] [CrossRef]
- Li, B.; Wei, D.; Li, Z.; Zhou, Y.; Li, Y.; Huang, C.; Long, J.; Huang, H.; Tie, B.; Lei, M. Mechanistic insights into the enhanced removal of roxsarsone and its metabolites by a sludge-based, biochar supported zerovalent iron nanocomposite: Adsorption and redox transformation. J. Hazard. Mater. 2020, 389, 122091. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, J.; Duan, C.; Wang, T.; Zhou, Y. A novel cationic graphene modified cyclodextrin adsorbent with enhanced removal performance of organic micropollutants and high antibacterial activity. J. Hazard. Mater. 2021, 426, 128074. [Google Scholar] [CrossRef]
- Xu, Y.; Lv, J.; Song, Y.; Zhou, X.; Tian, C.; Hong, X.; Cai, Y.; Zhao, C.; Lin, Z. Efficient removal of low-concentration organoarsenic by Zr-based metal–organic frameworks: Cooperation of defects and hydrogen bonds. Environ. Sci. Nano 2019, 6, 3590–3600. [Google Scholar] [CrossRef]
- Li, G.; Wang, C.; Yan, Y.; Yan, X.; Li, W.; Feng, X.; Li, J.; Xiang, Q.; Tan, W.; Liu, F.; et al. Highly enhanced degradation of organic pollutants in hematite/sulfite/photo system. Chem. Eng. J. 2020, 386, 124007. [Google Scholar] [CrossRef]
- Fu, W.; Lu, D.-L.; Yao, H.; Yuan, S.; Wang, W.; Gong, M.; Hu, Z.-H. Simultaneous roxarsone photocatalytic degradation and arsenic adsorption removal by TiO2/FeOOH hybrid. Environ. Sci. Pollut. Res. 2020, 27, 18434–18442. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, Z.; Wang, Y.; Ding, Z.; Xu, X.; Peng, W.; Fan, J.; Zhou, X.; Liu, J. BiOCl0.875Br0.125/polydopamine functionalized PVDF membrane for highly efficient visible-light-driven photocatalytic degradation of roxarsone and simultaneous arsenic immobilization. Chem. Eng. J. 2020, 402, 126048. [Google Scholar] [CrossRef]
- Wei, W.; Guo, K.; Kang, X.; Zhang, J.; Li, C.; Fang, J. Complete Removal of Organoarsenic by the UV/Permanganate Process via HO• Oxidation and in Situ-Formed Manganese Dioxide Adsorption. ACS EST Eng. 2021, 1, 794–803. [Google Scholar] [CrossRef]
- Xia, L.A.; Jie, H.A.; Jian, L.; Yi, Z.; Yza, B. In-situ production and activation of H2O2 for enhanced degradation of roxarsone by FeS2 decorated resorcinol-formaldehyde resins. J. Hazard. Mater. 2022, 424 Pt D, 127650. [Google Scholar]
- Zhang, A.Y.; Huang, N.H.; Zhang, C.; Zhao, P.C.; Lin, T.; He, Y.Y.; Feng, J.W. Heterogeneous Fenton decontamination of organoarsenicals and simultaneous adsorption of released arsenic with reduced secondary pollution. Chem. Eng. J. 2018, 344, 1–11. [Google Scholar] [CrossRef]
- Li, M.; He, Z.; Zhong, H.; Hu, L.; Sun, W. Multi-walled carbon nanotubes facilitated Roxarsone elimination in SR-AOPs by accelerating electron transfer in modified electrolytic manganese residue and forming surface activated-complexes. Water Res. 2021, 200, 117266. [Google Scholar] [CrossRef]
- Wu, S.; Yang, T.; Mai, J.; Tang, L.; Liang, P.; Zhu, M.; Huang, C.; Li, Q.; Cheng, X.; Liu, M.; et al. Enhanced removal of organoarsenic by chlorination: Kinetics, effect of humic acid, and adsorbable chlorinated organoarsenic. J. Hazard. Mater. 2022, 422, 126820. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).