Abstract
Tissue engineering and regenerative medicine (TERM) may be defined as a translational discipline focused on the development of novel techniques, devices, and materials to replace or repair injured or diseased tissue and organs. The main approaches typically use cells, scaffolds, and signaling molecules, either alone or in combination, to promote repair and regeneration. Although cells are required to create new functional tissue, the source of cells, either from an exogenous allogeneic or autologous source or through the recruitment of endogenous (autologous) cells, is technically challenging and risks the host rejection of new tissue. Regardless of the cell source, these approaches also require appropriate instruction for proliferation, differentiation, and in vivo spatial organization to create new functional tissue. Such instruction is supplied through the microenvironment where cells reside, environments which largely consist of the extracellular matrix (ECM). The specific components of the ECM, and broadly the extracellular space, responsible for promoting tissue regeneration and repair, are not fully understood, however extracellular vesicles (EVs) found in body fluids and solid phases of ECM have emerged as key mediators of tissue regeneration and repair. Additionally, these EVs might serve as potential cell-free tools in TERM to promote tissue repair and regeneration with minimal risk for host rejection and adverse sequelae. The past two decades have shown a substantial interest in understanding the therapeutic role of EVs and their applications in the context of TERM. Therefore, the purpose of this review is to highlight the fundamental characteristics of EVs, the current pre-clinical and clinical applications of EVs in TERM, and the future of EV-based strategies in TERM.
1. Introduction
Tissue engineering and regenerative medicine (TERM) is a translational discipline in biomedical science focused on developing novel strategies to regenerate and replace damaged tissues with the ultimate goal of restoring native tissue function and physiology [1,2]. Such strategies in TERM implement the use of cells, scaffolds, and signaling molecules—either alone or in combination—that, when implanted into the host, will promote constructive and functional tissue remodeling, integration, and regeneration. Ultimately, cells are required to create new functional tissue, and such cells can be supplied from an exogenous allogeneic or autologous source and injected into the host, or through the implantation of bioactive materials that recruit endogenous stem cells to the site of injury [3,4]. Regardless of the cell source, the relevant cells require appropriate instruction for proliferation, differentiation, and in vivo spatial organization to create new functional tissue. This instruction is supplied through the microenvironment in which cells reside. In mammalian tissues, this microenvironment is largely represented by the extracellular matrix (ECM) [5,6]. The ECM is a complex, three-dimensional network of proteins, proteoglycans, and glycosaminoglycans that provides cells and tissues with both structural and physiologic support [5]. In addition, the ECM hosts an abundant landscape of sequestered growth factors, morphogens, cryptic peptides, and extracellular vesicles (EVs), all of which function to regulate normal cell physiology and promote tissue regeneration and repair [6].
The specific components of the ECM, and broadly the extracellular space, responsible for promoting tissue regeneration and repair are not fully understood, but EVs found in body fluids and solid phases of ECM have emerged as key mediators of tissue regeneration and repair [7]. Given their potential as therapeutic mediators of tissue repair in a wide variety of pathophysiological conditions [8], EVs are being explored as a cell-free approach to supporting tissue regeneration and repair through influencing host cell phenotype and function entirely independent of a requirement for cell source or scaffold material. This approach could potentially mitigate the issues of cell and scaffold rejection by the host, a major concern for the translational application of TERM therapies [9]. Over the past two decades, there has been substantial interest in understanding the therapeutic role of EVs and their applications in the context of TERM. Therefore, the purpose of this review is to highlight the fundamental characteristics of EVs, the current pre-clinical and clinical applications of EVs in TERM and other disciplines, and the future of EV-based strategies in TERM.
2. Extracellular Vesicles (EVs)
EVs are lipid-bound vesicles produced by cells and secreted into the extracellular space [10]. EVs have been shown to transfer functional cargo (including lipids, proteins, and nucleic acids) to recipient cells whereby they influence cell function and phenotype [11]. EVs have been shown to be involved in development, tissue homeostasis, and wound healing, and have also been shown to be dysregulated during disease progression [12,13]. There are five major subclasses of EVs currently identified: exosomes, microvesicles, apoptotic bodies, biomineralization vesicles, and matrix-bound nanovesicles (Figure 1). The cargo of EVs includes intraluminal proteins and nucleic acids as well as the lipids contained within the membrane of these vesicles. While all five subclasses share overlapping similarities in the types of cargo they contain, the biogenesis, size, specific cargo, and biological function of these subclasses are distinct from each other. The characteristics and functions of these five subclasses are reviewed below.
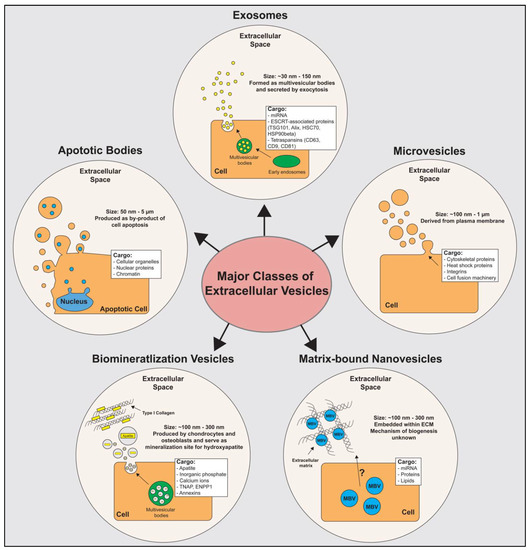
Figure 1.
Major subclasses of extracellular vesicles. The five major subclasses of extracellular vesicles used in tissue engineering and regenerative medicine include exosomes, microvesicles, matrix-bound nanovesicles (MBVs), biomineralization vesicles, and apoptotic bodies. Size, cargo, and biogenesis of each vesicle subclass are illustrated. Abbreviations; ESCRT: endosomal sorting complexes required for transport machinery; TSG101: tumor susceptibility gene 101; HSC70: heat shock cognate 71 kDa protein; HSP90beta: heat shock protein 90 kDa beta; CD: cluster of differentiation; TNAP: tissue-nonspecific alkaline phosphatase; ENPP1: ectonucleotide pyrophosphatase/phosphodiesterase; MBV: Matrix-bound Nanovesicles.
2.1. Exosomes
Exosomes are a subclass of EVs secreted by all cell types; they have been identified in plasma, urine, saliva, semen, bronchial lavage fluid, cerebral spinal fluid, amniotic fluid, synovial fluid, tears, lymph, bile, breast milk, and gastric acid [14,15,16,17,18,19,20,21,22,23,24,25,26,27]. Exosomes participate in cell-to-cell communication can induce physiological changes in recipient cells by transferring their molecular cargo including proteins, lipids, and nucleic acids [28]. Exosomes, which range in size from 30–150 nm in diameter, have a biogenesis that occurs via the inward budding of intracellular endosomes [29,30,31,32,33]. Repeated inward budding of the endosomal membrane results in the formation of a multivesicular body. The multivesicular body then fuses with the cell membrane, and the exosomes within are released into the extracellular fluid [29,30,34,35,36]. Although the molecular cargo of exosomes can vary depending on the cell source, exosome-specific marker proteins have been identified. These include proteins involved in the formation of multivesicular bodies such as ESCRT proteins, ESCRT-accessory proteins (TSG101, Alix, HSC70, and HSP90beta), and select tetraspanins (CD63, CD9, and CD81) [34,37,38,39,40,41,42,43,44].
2.2. Microvesicles
Microvesicles are formed through the outward budding of the cell membrane [45]. Microvesicles range in size from 100 nm to 1 µm in diameter and are thought to be involved in cell-to-cell communication, both locally and systemically [29,31,32,33,35]. Unlike exosomes that have distinctive marker proteins, there are no specific marker proteins for microvesicles. The biogenesis of microvesicles occurs through the trafficking of molecular cargo to the plasma membrane where contractile machinery induces budding and ultimately the pinching off of these vesicles [46,47]. Since microvesicles are produced from the outward budding of the cell membrane, the proteins found on the surface of microvesicles are similar to the surface proteins of the parent cell. The intracellular cargo of microvesicles is diverse and contains proteins including, but not limited to, cytoskeletal proteins, heat shock proteins, integrins, and proteins involved in cell fusion machinery (SNAREs and tethering proteins) [35,48,49,50,51,52,53,54].
2.3. Apoptotic Bodies
Apoptotic bodies are produced as a by-product of cells undergoing programmed cell death [55]. These vesicles are the largest subclass of EVs, ranging from 50 nm to 5000 nm in diameter [30]. The aptly-named apoptotic bodies are formed during apoptosis from the release of cell membrane fragments as cells contract and hydrostatic forces separate pieces of the lipid membrane away from the cytoskeleton of cells [56]. These large EVs can contain much larger cargo than the other EVs reviewed. This cargo can include large, modified proteins, entire cellular organelles, and/or pieces of chromatin. Specifically these bodies are also known to contain components of the nucleus, mitochondria, endoplasmic reticulum, and Golgi apparatus [30,36,57,58].
2.4. Biomineralization Vesicles
Biomineralization vesicles (also known as matrix vesicles) are EVs produced by hypertrophic chondrocytes and mature osteoblasts [59,60,61,62]. These biomineralization vesicles range in size from 100–300 nm in diameter and assist in bone formation in both endochondral and membranous ossification [59,60,61,62]. While the specific mechanisms for biogenesis are not fully understood, current evidence supports a similar biogenesis mechanism to that of microvesicles which occurs through the outward budding of the plasma membrane [59,63]. These vesicles are thought to bind ECM molecules including collagen and serve as nucleating foci to initiate hydroxyapatite crystallization necessary for bone formation [59,60,61]. Biomineralization vesicles initiate apatite formation through a combination of luminal and surface proteins. Within these vesicles, orphan phosphatase (PHOSPHO1) and sphingomyelin phosphodiesterase 3 (SMPD3) enzymes are located and function to increase the luminal concentration of inorganic pyrophosphate (Pi) [64]. On the surface of these vesicles, tissue-nonspecific alkaline phosphatase (TNAP) and ectonucleotide pyrophosphatase/phosphodiesterase 1 (ENPP1) function to increase luminal Pi concentrations [65,66,67]. Extracellular calcium (Ca2+) is thought to be transported into the vesicles through annexins A5 and A6 [68]. The combination of elevated Pi and Ca2+ within the vesicles causes intraluminal apatite to crystalize, and these apatite crystals are necessary for bone mineralization and the formation of hydroxyapatite in bone [59].
2.5. Matrix-Bound Nanovesicles (MBVs)
MBVs are a subtype of EVs embedded within the extracellular matrix (ECM). Ranging in size from 100–300 nm in diameter, MBVs are composed of a lipid membrane that stores and transports a diverse portfolio of signaling molecules. These molecules include proteins, miRNAs, and bioactive lipids [69,70,71,72].
MBVs are secreted by tissue-resident cells and are integrated into the solid-phase, fibrillar structure of the ECM [70]. This solid-phase localization of MBVs to the ECM, rather than to the surrounding extracellular fluid, suggests a unique mechanism for MBV synthesis, cellular export, and matrix association that, when taken cumulatively, differs from the mechanisms of synthesis and the release of other EVs [70]. Although MBVs have been shown to be produced by multiple cell types, the mechanisms for the biogenesis of the other EV subtypes are better understood; the mechanisms for the release and matrix association of MBVs remain unknown [73]. MBVs can be isolated from a wide variety of ECM source materials through enzymatic digestion [69,70,71,74]. While MBVs are similar in size and morphology to exosomes secreted into body fluids or cell culture supernatant, recent evidence show that MBVs contain a distinctive protein, miRNA, and lipid signature compared to liquid-phase exosomes produced from the same cell type [70]. Furthermore, in contrast to liquid-phase EVs, MBVs possess a marked reduction in CD63, CD81, and CD9, with all three of these protein markers being used to identify and characterize liquid-phase EVs (the fourth marker being Hsp70, which has not shown any differences between EVs and MBVs) [69,71,75].
3. Pre-Clinical Applications of EVs in Tissue Engineering and Regenerative Medicine
Given the broad diversity and biologic functions of EVs, there has been a growing interest in the implementation of both naturally derived and bioengineered EV therapies in TERM. Examples of potential regenerative medicine applications of EV-based therapy ranging across a variety of organ systems are reviewed below.
3.1. Cardiovascular System
The cardiovascular system, specifically the heart, represents a challenge in the field of TERM, as the inherent regenerative capacity of the heart cannot compensate for extensive cardiomyocyte damage and death [76,77]. Therefore, there is great interest in developing novel treatment strategies to promote cardiac tissue regeneration and repair. One specific approach is the implementation of EV-based therapies for cardiac regeneration.
There has been notable success in implementing EV-based therapies in animal models of cardiac injury, including myocardial infarction (MI), ischemia-reperfusion injury, and heart transplantation. Exosomes derived from mesenchymal stem cells (MSCs) have been shown to reduce disease progression in models of myocardial infarction and ischemia-reperfusion injury, with an end result of decreased cardiac fibrosis and increased blood flow to the site of injury [8,78,79,80,81,82]. In a separate study, placental MSC-derived exosomes were shown to promote angiogenesis in a model of an ischemic cerebral vascular accident [83]. In addition to unaltered, MSC-derived exosomes, exosomes released from CD34+ human cells engineered to contain Sonic Hedgehog (Shh), a potent mediator of angiogenesis, have shown great promise in reducing infarct size, increasing capillary density, and improving cardiac function in a model of MI [84].
In addition to applications related to MI and ischemia-reperfusion injury, EV-based therapies for promoting tissue transplantation have also been investigated. Specifically, local delivery of MBVs containing IL-33 in a rodent model of cardiac transplantation have been shown as a promising chronic graft rejection prophylaxis via the immunomodulation of pro-inflammatory macrophages towards an anti-inflammatory phenotype [85].
3.2. Respiratory System
Major challenges for TERM in the context of the respiratory system involve inhibiting the pro-fibrotic and pro-pulmonary hypertension pathways central to the healing of damaged lung tissue. MSC- and lung-derived EVs have been considered as potential therapies for the mitigation of these adverse healing pathways. Specifically, MSC-derived exosomes have been shown to inhibit pulmonary hypertension and suppress hyperproliferative and fibrotic pathways [86]. In addition, lung-derived microvesicles have been shown to be particularly useful in TERM as there is evidence that these EVs can reprogram marrow cells into a pulmonary epithelial phenotype which could help provide a stable cell source for lung engineering [87,88].
3.3. Renal System
While EV research regarding the renal system has primarily focused on EV-based diagnostics, there have been a few recent examples of EV-based therapies in TERM. Specifically, there is evidence supporting the use of microvesicles from both endothelial progenitor cells and MSCs to protect kidney epithelial cells in models of acute kidney injury as well as an ischemia-reperfusion injury of the kidney [89,90]. These microvesicles have great promise in aiding tissue regeneration and cellular survival of bio-engineered kidney devices.
3.4. Nervous System
A major focus in the field of TERM has been on the regeneration and repair of injuries to the central and peripheral nervous system. Like the cardiovascular system, the innate regenerative capacity of the nervous system is limited; consequently, there is a growing interest to implement novel strategies such as EV-based therapies to promote neural regeneration.
There have been several studies showing the promise of EV-based therapy for neural regeneration in both central and peripheral nerve injury. In the central nervous system (CNS), exosomes derived from bone marrow MSC and stromal MSC containing miR-133b have been shown to promote neurite outgrowth and the re-myelination of CNS nerve fibers after injury [91,92]. Exosomes derived from MSC are also understood to inhibit pericyte migration after spinal cord injury and, as a result, these exosomes improve motor function [8,93]. Intravitreal administration of MBVs derived from porcine urinary bladder ECM protect against ischemia-induced retinal ganglion cell axon degeneration and death, as well as preserved visual function in an in vivo rodent model of retinal damage [94]. In the peripheral nervous system (PNS), Schwann cell-derived exosomes have been shown to promote axon growth and regeneration in a peripheral nerve injury model [95]. Broadly, there is a growing body of literature supporting the use of EVs in peripheral nerve repair and showing pre-clinical promise [96].
3.5. Liver
Unlike previously reviewed organ systems, the liver possesses a far greater innate ability for regeneration and repair. However, this regenerative capacity is not without limitations as liver disease and failure represent a significant burden on worldwide health. EVs represent a complementary strategy to the extensive approaches previously implemented in liver TERM. Applications of EVs in liver TERM have focused primarily on the use of hepatocyte and liver stem-cell derived EVs. Hepatocyte-derived EVs have been shown to increase hepatocyte proliferation, viability, and regeneration through the activation of stellate cells in the liver [97,98,99]. In addition, microvesicles derived from human liver stem cells have been shown to accelerate liver regeneration in hepatectomized rats through the means of EV-mediated RNA transfer to target hepatocytes [100].
3.6. Skin and Wound Healing
The application of TERM in skin and topical wound healing has been extensively studied; the result is that there is an extensive number of products available and in development for this purpose. EVs are a logical supplement to these TERM approaches to skin and wound healing and have shown initial promise in pre-clinical studies. Across various applications of EVs in skin and wound healing, results have consistently shown that EVs derived from a variety of cell sources promote skin and topical wound healing through promoting re-epithelialization, angiogenesis, ECM remodeling, and the migration of keratinocytes and fibroblasts to the wound region [101,102,103,104,105,106,107,108,109,110]. Specific applications of note include adipose MSC-derived exosomes and gingival MSC-derived exosomes embedded in a hydrogel formulation [109,110]. In addition, there has been extensive interest in using EVs derived from adipose-derived stem cells for application in skin regeneration and hair follicle regeneration [111].
3.7. Bone and Cartilage Regeneration
The regeneration of bone and cartilage are active research interests in TERM and the utility of EVs in enhancing bone and cartilage regeneration has been shown as promising in pre-clinical results. EV-based therapies derived from MSC sources have been shown to promote cartilage regeneration in vitro and in vivo through increasing type II collagen production and inhibiting cartilage degradation [112,113,114,115]. Furthermore, MSC-derived exosomes have been shown to promote cartilage regeneration in vivo when printed into hydrogel scaffolds [116]. In bone regeneration, osteoblast-produced EVs and periodontal-ligament stem cell-derived EVs, and specifically exosomes, have been shown to promote bone regeneration and facilitate osteoclast activation [117,118,119,120,121]. Biomineralization vesicles (matrix vesicles) have also shown promise in the engineering of bone tissue through promoting bone formation and the calcification of bone matrix [60,62,122,123].
Moreover, there has been extensive research focused on the therapeutic efficacy of EVs in the axial skeleton, with particular emphasis on treating intervertebral disc degeneration. Specifically, exosomes derived from MSC sources, such as bone marrow [124,125,126,127,128,129], human urine [130,131] adipose [132], human placenta [133], and human umbilical cord [134], have all shown promise in reducing various aspects of inflammation and damage in intervertebral disc degeneration. In addition to MSC-derived exosomes, exosomes isolated from nucleus pulposus cells [135,136,137], endplate chondrocytes [138,139,140], annulus fibrosus cells [141], platelet-rich plasma [142], and notochordal cells [143] have shown similar early promise in the treatment of intervertebral disc degeneration.
In addition to regeneration of bone and cartilage, EVs have also been explored as a therapy for pathophysiological conditions that affect the joint such as rheumatoid arthritis (RA); for example, bone marrow-derived dendritic cells can be genetically modified to produce exosomes enriched with anti-inflammatory molecules, such as IL-10 [144,145] and IL-4, [146] or pro-apoptotic molecules, such as FasL [147], or immune checkpoint inhibitors, such as CTLA4-Ig. [148] EV have also been isolatedfrom the plasma of antigen-immunized mice [149]. All these approaches have shown pre-clinical efficacy in RA through the immunomodulation of innate and adaptive immune cell populations [144,145,146,147,148,149]. In addition, MBVs have been shown in vivo to mitigate RA through the immunomodulation of macrophage phenotypes locally and systemically in a rat model [150]. MBVs, a distinct population of extracellular vesicles, represent an alternative to these fluid-phase exosome therapies, with minimal processing and manipulation in comparison [70,74].
4. Clinical Development of EVs
4.1. Companies Developing EV Products
Despite the relatively early stage of EV therapy and diagnostics, EV technology has generated considerable traction in the pharmaceutic industry over the past decade. For example, more than 2000 patents related to EVs (Google Patents, Query in June 2022) have been filed since 2010. The companies involved in the development of EV products represent small- to medium-sized enterprises. These companies are generally spin-offs from larger companies and the majority are currently in the early process of raising funds, developing technology, and working toward becoming leaders in the growing market (Table 1). In addition, several multinational pharmaceutical companies such as Pfizer, AstraZeneca, Roche, and Merk are also expanding their research and development portfolios to focus on EV therapies. Although no leaders have established market dominance in this growing industry, several companies have been making considerable progress.

Table 1.
Companies developing EV-based products.
Codiak Bioscience (Cambridge, MA, USA) is a company dedicated to EV-based diagnostics and therapeutics. Specifically, the company is interested in using cell culture-derived EVs for immune activation in the treatment of cancer. Codiak Biosciences filed 11 patents for exosome manufacturing and purification in 2021 and has experienced an exponential growth in revenue over the last few years, reaching a total of $22.9 million in 2021 (Pitchbook, June 2022). Evox Therapeutics (Oxford, UK) has developed modified EVs for drug delivery with a special interest in siRNA and mRNA delivery for protein replacement or gene therapy. This company has accrued $155 million in venture capital support. ExoCoBio (Seoul, South Korea) has developed EV-based therapies for cosmetic and skin regeneration applications and has submitted five patents related to this technology in 2021. Other companies like Aegle Therapeutics (Woburn, MA, USA), Panacea Pharmaceuticals (New Delhi, India), and Exopharm (Melbourne, Australia) are in early funding stages and are following similar strategies to the companies mentioned above through an approach to protecting intellectual property and evaluating various potential applications for their own EV technology [151].
4.2. Clinical Trials Testing EV Therapies
Currently, there are 20 active clinical trials investigating EV therapies and six clinical trials that have been concluded. Given the early stage of EV technology, most of these trials or studies are Phase I/II clinical trials focused on evaluating the safety and early efficacy of EV therapies for a wide range of clinical indications, directly and indirectly related to the traditional scope of TERM. Recently, the SARS-nCOVID-19 pandemic has accelerated the clinical translation of EV therapies in general, and these trials have been primarily directed toward testing EV-based therapies in the context of inflammatory conditions with a particular focus on EV-based therapies for SARS-nCOVID-19 [152,153,154]. Targeted cancer therapies are also common application of EVs in clinical trials, with these trials using modified EVs to deliver drugs to specific cellular targets as well as to mitigate the effects of aggressive therapies such as chemotherapy and radiation. Table 2 summarizes completed or active clinical trials for EV therapies. While not all of the trials reviewed herein were developed specifically to address questions in TERM, these studies are worth highlighting as the success of EV-based therapies across a wide variety of clinical indications will only help to facilitate the growth and translation of EV-based approaches in TERM.

Table 2.
Clinical trials investigating EV-based therapies.
4.3. Future Challenges
The translation of EV-based therapies has grown rapidly over the past two decades and has shown early potential for applications in TERM. However, there remain important challenges that must be addressed to facilitate the translation of these technologies into the clinic. These challenges primarily concern issues of large-scale production, understanding the biodistribution of EVs, and the absence of a thorough characterization of EVs to ensure safe and efficacious clinical translation.
While the production of EVs shares similarities with the manufacturing of traditional biologic products (i.e., industrial production of antibodies and recombinant proteins), the optimal cell source and culture conditions for the large-scale production of EVs have not yet been established. MBVs are a potential solution to this limitation since they are isolated from readily available tissue. However, the preparation of MBVs also requires additional tissue decellularization and ECM solubilization steps that may affect their properties [74]. With regard to the collection, isolation, and purification of EV-based therapies, several studies have established a consensus that standardized protocols and outcome measurements are necessary for large-scale translation [155,156,157,158]. To address concerns in EV isolation and purity, the manufacturing trends are shifting from techniques including ultracentrifugation (which is time consuming and can produce contaminants) towards isolation techniques potentially capable of large-scale manufacturing such as tangential flow filtration (TFF) or size-exclusion chromatography (SEC) [74,157,158,159]. However, these techniques still have their limitations and further studies are necessary to determine the optimal technique for the isolation and purification of EVs at the large-scale, commercial level. In addition to concerns regarding the manufacturing of EV therapeutics, the long-term storage and stability of EV therapies pose a significant hurdle to clinical translation. The effects of various preservation techniques on EV stability and bioactivity are not well understood [157,160]. Initial studies suggested that cryopreservation at −80 °C was superior to storage at 4 °C and would preserve EV structure and composition. However, storage at −80 °C comes with increased costs and technical challenges. The lyophilization of EVs might address this concern for long-term storage as long as the optimal concentration of cryoprotectants is used to protect the biologic activity of the EVs through this process [156,160]. Altogether, the optimal conditions for the preservation of EVs depend on multiple factors including, but not limited to, the EV source, isolation and purification technique, and the route of administration.
The functional and structural characterization of EVs represent an additional and unavoidable hurdle for the translation of EV-based therapies, and fundamental understanding of the heterogeneity and specificity of each kind of EV is critical. Relevant properties, such as size, shape, molecular cargo (i.e., sRNA, miRNA, cytokines, proteins, or DNA), membrane composition, or surface markers, vary not only between different EV kinds or cell sources but also within EVs derived from the same source [70,155,159,161,162]. Technical limitations for separating EV subpopulations and the lack of specific markers for EV subtypes obstruct the understanding of how these subpopulations affect bioactivity and safety [156,157,159]. Further research and technical innovations are necessary to overcome this hurdle.
The safety of EV-based therapies remains a major barrier to clinical translation. Many pre-clinical and early clinical studies have shown that EV therapies are generally biocompatible and have a low risk of immunogenicity in humans [156,163,164,165]. However, these clinical studies seldom discuss the safety of repeated administrations as well as the safety of a wide variety of administration routes [156]. Several pre-clinical studies have assessed the biodistribution of EVs in rodents and have shown that EVs are rapidly cleared from the circulatory system [166,167,168]. However, the accumulation of EVs in the liver or kidneys, and the EV-mediated effects on these organs, are unknown and therefore must be investigated in future studies. Moreover, the relatively rapid clearance of EVs from the systemic circulation could affect the specificity of EV therapies and increase the frequency of off-target, non-specific effects. To address these non-specific, off-target effects, EV membrane decorating techniques have been developed and have shown early potential for more focused, targeted therapy as seen in specific models of cancer [156,168]. In addition to the off-target effects, there remain safety concerns including the possibility of viral vector contaminants in EV preparation. These viral contaminants cannot be overlooked as they present similar characteristics to EVs and are often capable of surpassing filtration systems during the harvest of EVs [155,156,157].
5. Conclusions
During the past two decades of growing research and interest in EVs, it is clear that EVs represent a promising therapy applicable across a wide variety of TERM applications. It is evident from extensive preclinical and clinical research as well as the rapid growth of companies developing EV-based therapies that there exists a reproducible proof-of-concept model for using EVs in TERM. However, future studies will be necessary to support the development and clinical translation of these therapies. Major hurdles remain in the full biologic and functional characterization of EV subtypes, as well as in establishing cost-effective, safe, and reproducible preparation techniques at the commercial scale. Finally, future regulatory concerns exist regarding how to define the efficacy, consistency, and safety of EVs, as these EV-based therapies are inherently multimodal in their mechanisms of action due to compositional heterogeneity. Nevertheless, the field of EV-based therapies in TERM is promising and the potentially wide-reaching applications of these therapies continue to inspire the important work of researchers, innovators, and investors alike.
Author Contributions
Writing—original draft preparation, R.J.C. and H.C.-M.; writing—review and editing, R.J.C., H.C.-M., S.F.B. and G.S.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yahya, E.B.; Amirul, A.; HPS, A.K.; Olaiya, N.G.; Iqbal, M.O.; Jummaat, F.; AK, A.S.; Adnan, A. Insights into the Role of Biopolymer Aerogel Scaffolds in Tissue Engineering and Regenerative Medicine. Polymers 2021, 13, 1612. [Google Scholar] [CrossRef] [PubMed]
- Frey, B.M.; Zeisberger, S.M.; Hoerstrup, S.P. Tissue Engineering and Regenerative Medicine—New Initiatives for Individual Treatment Offers. Transfus. Med. Hemother. 2016, 43, 318–319. [Google Scholar] [CrossRef] [PubMed]
- Pacelli, S.; Basu, S.; Whitlow, J.; Chakravarti, A.; Acosta, F.; Varshney, A.; Modaresi, S.; Berkland, C.; Paul, A. Strategies to develop endogenous stem cell-recruiting bioactive materials for tissue repair and regeneration. Adv. Drug Deliv. Rev. 2017, 120, 50–70. [Google Scholar] [CrossRef] [PubMed]
- Abraham, E.; Trainor, N.; Ostrout, N.; Hewitt, M.M. Cell therapy products are revolutionizing multiple therapeutic areas; to maintain this pace of innovation, manufacturing solutions must adapt. Curr. Opin. Biomed. Eng. 2021, 20, 100340. [Google Scholar] [CrossRef]
- Hussey, G.S.; Dziki, J.L.; Badylak, S.F. Extracellular matrix-based materials for regenerative medicine. Nat. Rev. Mater. 2018, 3, 159–173. [Google Scholar] [CrossRef]
- Bissell, M.J.; Hall, H.G.; Parry, G. How does the extracellular matrix direct gene expression? J. Theor. Biol. 1982, 99, 31–68. [Google Scholar] [CrossRef]
- Nagelkerke, A.; Ojansivu, M.; van der Koog, L.; Whittaker, T.E.; Cunnane, E.M.; Silva, A.M.; Dekker, N.; Stevens, M.M. Extracellular vesicles for tissue repair and regeneration: Evidence, challenges and opportunities. Adv. Drug Deliv. Rev. 2021, 175, 113775. [Google Scholar] [CrossRef]
- Tsiapalis, D.; O’Driscoll, L. Mesenchymal stem cell derived extracellular vesicles for tissue engineering and regenerative medicine applications. Cells 2020, 9, 991. [Google Scholar] [CrossRef]
- Bolton, E.M.; Bradley, J.A. Avoiding immunological rejection in regenerative medicine. Regen. Med. 2015, 10, 287–304. [Google Scholar] [CrossRef]
- Couch, Y.; Buzàs, E.I.; Di Vizio, D.; Gho, Y.S.; Harrison, P.; Hill, A.F.; Lötvall, J.; Raposo, G.; Stahl, P.D.; Théry, C.; et al. A brief history of nearly EV-erything—The rise and rise of extracellular vesicles. J. Extracell. Vesicles 2021, 10, e12144. [Google Scholar] [CrossRef]
- Doyle, L.M.; Wang, M.Z. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; van Niel, G.; Stahl, P.D. Extracellular vesicles and homeostasis-An emerging field in bioscience research. FASEB Bioadv. 2021, 3, 456–458. [Google Scholar] [CrossRef] [PubMed]
- Roefs, M.T.; Sluijter, J.P.G.; Vader, P. Extracellular Vesicle-Associated Proteins in Tissue Repair. Trends Cell Biol. 2020, 30, 990–1013. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H. Detection of DNA methylation of gastric juice-derived exosomes in gastric cancer. Integr. Mol. Med. 2014, 1. [Google Scholar] [CrossRef]
- Yoon, S.B.; Chang, J.H. Extracellular vesicles in bile: A game changer in the diagnosis of indeterminate biliary stenoses? Hepatobiliary Surg. Nutr. 2017, 6, 408–410. [Google Scholar] [CrossRef]
- Grigor’eva, A.E.; Tamkovich, S.N.; Eremina, A.V.; Tupikin, A.E.; Kabilov, M.R.; Chernykh, V.V.; Vlassov, V.V.; Laktionov, P.P.; Ryabchikova, E.I. Exosomes in tears of healthy individuals: Isolation, identification, and characterization. Biochem. Moscow Suppl. Ser. B 2016, 10, 165–172. [Google Scholar] [CrossRef]
- Dixon, C.L.; Sheller-Miller, S.; Saade, G.R.; Fortunato, S.J.; Lai, A.; Palma, C.; Guanzon, D.; Salomon, C.; Menon, R. Amniotic fluid exosome proteomic profile exhibits unique pathways of term and preterm labor. Endocrinology 2018, 159, 2229–2240. [Google Scholar] [CrossRef]
- Yuan, Z.; Bedi, B.; Sadikot, R.T. Bronchoalveolar Lavage Exosomes in Lipopolysaccharide-induced Septic Lung Injury. J. Vis. Exp. 2018, 135, e57737. [Google Scholar] [CrossRef]
- Zlotogorski-Hurvitz, A.; Dayan, D.; Chaushu, G.; Korvala, J.; Salo, T.; Sormunen, R.; Vered, M. Human saliva-derived exosomes: Comparing methods of isolation. J. Histochem. Cytochem. 2015, 63, 181–189. [Google Scholar] [CrossRef]
- Vojtech, L.; Woo, S.; Hughes, S.; Levy, C.; Ballweber, L.; Sauteraud, R.P.; Strobl, J.; Westerberg, K.; Gottardo, R.; Tewari, M.; et al. Exosomes in human semen carry a distinctive repertoire of small non-coding RNAs with potential regulatory functions. Nucleic Acids Res. 2014, 42, 7290–7304. [Google Scholar] [CrossRef]
- Caby, M.-P.; Lankar, D.; Vincendeau-Scherrer, C.; Raposo, G.; Bonnerot, C. Exosomal-like vesicles are present in human blood plasma. Int. Immunol. 2005, 17, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Pisitkun, T.; Shen, R.-F.; Knepper, M.A. Identification and proteomic profiling of exosomes in human urine. Proc. Natl. Acad. Sci. USA 2004, 101, 13368–13373. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Fujiya, M.; Konishi, H.; Sasajima, J.; Fujibayashi, S.; Hayashi, A.; Utsumi, T.; Sato, H.; Iwama, T.; Ijiri, M.; et al. An elevated expression of serum exosomal microRNA-191, - 21, -451a of pancreatic neoplasm is considered to be efficient diagnostic marker. BMC Cancer 2018, 18, 116. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Wang, P.-Y.; Li, X.-Y.; Chen, J.-X.; Li, Y.; Zhang, X.-Z.; Zhang, C.-G.; Jiang, T.; Li, W.-B.; Ding, W.; et al. Exosomal levels of miRNA-21 from cerebrospinal fluids associated with poor prognosis and tumor recurrence of glioma patients. Oncotarget 2015, 6, 26971–26981. [Google Scholar] [CrossRef]
- Akers, J.C.; Ramakrishnan, V.; Kim, R.; Skog, J.; Nakano, I.; Pingle, S.; Kalinina, J.; Hua, W.; Kesari, S.; Mao, Y.; et al. MiR-21 in the extracellular vesicles (EVs) of cerebrospinal fluid (CSF): A platform for glioblastoma biomarker development. PLoS ONE 2013, 8, e78115. [Google Scholar] [CrossRef]
- Hornick, N.I.; Huan, J.; Doron, B.; Goloviznina, N.A.; Lapidus, J.; Chang, B.H.; Kurre, P. Serum Exosome MicroRNA as a Minimally-Invasive Early Biomarker of AML. Sci. Rep. 2015, 5, 11295. [Google Scholar] [CrossRef]
- Harding, C.V.; Heuser, J.E.; Stahl, P.D. Exosomes: Looking back three decades and into the future. J. Cell Biol. 2013, 200, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bi, J.; Huang, J.; Tang, Y.; Du, S.; Li, P. Exosome: A review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int. J. Nanomed. 2020, 15, 6917–6934. [Google Scholar] [CrossRef] [PubMed]
- Zaborowski, M.P.; Balaj, L.; Breakefield, X.O.; Lai, C.P. Extracellular vesicles: Composition, biological relevance, and methods of study. Bioscience 2015, 65, 783–797. [Google Scholar] [CrossRef]
- Borges, F.T.; Reis, L.A.; Schor, N. Extracellular vesicles: Structure, function, and potential clinical uses in renal diseases. Braz. J. Med. Biol. Res. 2013, 46, 824–830. [Google Scholar] [CrossRef]
- Bebelman, M.P.; Smit, M.J.; Pegtel, D.M.; Baglio, S.R. Biogenesis and function of extracellular vesicles in cancer. Pharmacol. Ther. 2018, 188, 1–11. [Google Scholar] [PubMed]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed]
- Wollert, T.; Hurley, J.H. Molecular mechanism of multivesicular body biogenesis by ESCRT complexes. Nature 2010, 464, 864–869. [Google Scholar] [PubMed]
- Simons, M.; Raposo, G. Exosomes--vesicular carriers for intercellular communication. Curr. Opin. Cell Biol. 2009, 21, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Boussac, M.; Véron, P.; Ricciardi-Castagnoli, P.; Raposo, G.; Garin, J.; Amigorena, S. Proteomic analysis of dendritic cell-derived exosomes: A secreted subcellular compartment distinct from apoptotic vesicles. J. Immunol. 2001, 166, 7309–7318. [Google Scholar] [CrossRef] [PubMed]
- Witwer, K.W.; Buzás, E.I.; Bemis, L.T.; Bora, A.; Lässer, C.; Lötvall, J.; Nolte-’t Hoen, E.N.; Piper, M.G.; Sivaraman, S.; Skog, J.; et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J. Extracell. Vesicles 2013, 2, 20360. [Google Scholar]
- Géminard, C.; De Gassart, A.; Blanc, L.; Vidal, M. Degradation of AP2 during reticulocyte maturation enhances binding of hsc70 and Alix to a common site on TFR for sorting into exosomes. Traffic 2004, 5, 181–193. [Google Scholar] [PubMed]
- van Niel, G.; Porto-Carreiro, I.; Simoes, S.; Raposo, G. Exosomes: A common pathway for a specialized function. J. Biochem. 2006, 140, 13–21. [Google Scholar] [CrossRef]
- Morita, E.; Sandrin, V.; Chung, H.-Y.; Morham, S.G.; Gygi, S.P.; Rodesch, C.K.; Sundquist, W.I. Human ESCRT and ALIX proteins interact with proteins of the midbody and function in cytokinesis. EMBO J. 2007, 26, 4215–4227. [Google Scholar] [CrossRef] [PubMed]
- Babst, M.; Katzmann, D.J.; Estepa-Sabal, E.J.; Meerloo, T.; Emr, S.D. Escrt-III: An endosome-associated heterooligomeric protein complex required for mvb sorting. Dev. Cell 2002, 3, 271–282. [Google Scholar] [CrossRef]
- Möbius, W.; Ohno-Iwashita, Y.; van Donselaar, E.G.; Oorschot, V.M.J.; Shimada, Y.; Fujimoto, T.; Heijnen, H.F.G.; Geuze, H.J.; Slot, J.W. Immunoelectron microscopic localization of cholesterol using biotinylated and non-cytolytic perfringolysin O. J. Histochem. Cytochem. 2002, 50, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Mathivanan, S.; Simpson, R.J. ExoCarta: A compendium of exosomal proteins and RNA. Proteomics 2009, 9, 4997–5000. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Ignatchenko, V.; Ignatchenko, A.; Mejia-Guerrero, S.; Kislinger, T. In-depth proteomic analyses of ovarian cancer cell line exosomes reveals differential enrichment of functional categories compared to the NCI 60 proteome. Biochem. Biophys. Res. Commun. 2014, 445, 694–701. [Google Scholar] [CrossRef]
- Stahl, P.D.; Raposo, G. Extracellular vesicles: Exosomes and microvesicles, integrators of homeostasis. Physiology 2019, 34, 169–177. [Google Scholar] [CrossRef]
- D’Souza-Schorey, C.; Clancy, J.W. Tumor-derived microvesicles: Shedding light on novel microenvironment modulators and prospective cancer biomarkers. Genes Dev. 2012, 26, 1287–1299. [Google Scholar] [CrossRef]
- Tricarico, C.; Clancy, J.; D’Souza-Schorey, C. Biology and biogenesis of shed microvesicles. Small GTPases 2017, 8, 220–232. [Google Scholar] [CrossRef]
- Morello, M.; Minciacchi, V.R.; de Candia, P.; Yang, J.; Posadas, E.; Kim, H.; Griffiths, D.; Bhowmick, N.; Chung, L.W.K.; Gandellini, P.; et al. Large oncosomes mediate intercellular transfer of functional microRNA. Cell Cycle 2013, 12, 3526–3536. [Google Scholar] [CrossRef]
- Di Vizio, D.; Morello, M.; Dudley, A.C.; Schow, P.W.; Adam, R.M.; Morley, S.; Mulholland, D.; Rotinen, M.; Hager, M.H.; Insabato, L.; et al. Large oncosomes in human prostate cancer tissues and in the circulation of mice with metastatic disease. Am. J. Pathol. 2012, 181, 1573–1584. [Google Scholar] [CrossRef]
- Heijnen, H.F.; Schiel, A.E.; Fijnheer, R.; Geuze, H.J.; Sixma, J.J. Activated platelets release two types of membrane vesicles: Microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood 1999, 94, 3791–3799. [Google Scholar] [CrossRef] [PubMed]
- Østergaard, O.; Nielsen, C.T.; Iversen, L.V.; Jacobsen, S.; Tanassi, J.T.; Heegaard, N.H.H. Quantitative proteome profiling of normal human circulating microparticles. J. Proteome Res. 2012, 11, 2154–2163. [Google Scholar] [CrossRef] [PubMed]
- Zöller, M. Tetraspanins: Push and pull in suppressing and promoting metastasis. Nat. Rev. Cancer 2009, 9, 40–55. [Google Scholar] [CrossRef]
- Escola, J.M.; Kleijmeer, M.J.; Stoorvogel, W.; Griffith, J.M.; Yoshie, O.; Geuze, H.J. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J. Biol. Chem. 1998, 273, 20121–20127. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Reinisch, K.; Ferro-Novick, S. Coats, tethers, Rabs, and SNAREs work together to mediate the intracellular destination of a transport vesicle. Dev. Cell 2007, 12, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Battistelli, M.; Falcieri, E. Apoptotic bodies: Particular extracellular vesicles involved in intercellular communication. Biology 2020, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Wickman, G.; Julian, L.; Olson, M.F. How apoptotic cells aid in the removal of their own cold dead bodies. Cell Death Differ. 2012, 19, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Escrevente, C.; Keller, S.; Altevogt, P.; Costa, J. Interaction and uptake of exosomes by ovarian cancer cells. BMC Cancer 2011, 11, 108. [Google Scholar] [CrossRef]
- Kerr, J.F.; Wyllie, A.H.; Currie, A.R. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef]
- Bottini, M.; Mebarek, S.; Anderson, K.L.; Strzelecka-Kiliszek, A.; Bozycki, L.; Simão, A.M.S.; Bolean, M.; Ciancaglini, P.; Pikula, J.B.; Pikula, S.; et al. Matrix vesicles from chondrocytes and osteoblasts: Their biogenesis, properties, functions and biomimetic models. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 532–546. [Google Scholar] [CrossRef]
- Golub, E.E. Role of matrix vesicles in biomineralization. Biochim. Biophys. Acta 2009, 1790, 1592–1598. [Google Scholar] [CrossRef]
- Anderson, H.C. Matrix vesicles and calcification. Curr. Rheumatol. Rep. 2003, 5, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Houston, D.A.; Farquharson, C.; MacRae, V.E. Characterisation of matrix vesicles in skeletal and soft tissue mineralisation. Bone 2016, 87, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Anderson, H.C. Molecular biology of matrix vesicles. Clin. Orthop. Relat. Res. 1995, 314, 266–280. [Google Scholar] [CrossRef]
- Roberts, S.; Narisawa, S.; Harmey, D.; Millán, J.L.; Farquharson, C. Functional involvement of PHOSPHO1 in matrix vesicle-mediated skeletal mineralization. J. Bone Miner. Res. 2007, 22, 617–627. [Google Scholar] [CrossRef]
- Yadav, M.C.; Bottini, M.; Cory, E.; Bhattacharya, K.; Kuss, P.; Narisawa, S.; Sah, R.L.; Beck, L.; Fadeel, B.; Farquharson, C.; et al. Skeletal Mineralization Deficits and Impaired Biogenesis and Function of Chondrocyte-Derived Matrix Vesicles in Phospho1(-/-) and Phospho1/Pi t1 Double-Knockout Mice. J. Bone Miner. Res. 2016, 31, 1275–1286. [Google Scholar] [CrossRef] [PubMed]
- Millán, J.L. The role of phosphatases in the initiation of skeletal mineralization. Calcif. Tissue Int. 2013, 93, 299–306. [Google Scholar] [CrossRef]
- Bossi, M.; Hoylaerts, M.F.; Millán, J.L. Modifications in a flexible surface loop modulate the isozyme-specific properties of mammalian alkaline phosphatases. J. Biol. Chem. 1993, 268, 25409–25416. [Google Scholar] [CrossRef]
- Wang, W.; Xu, J.; Kirsch, T. Annexin-mediated Ca2+ influx regulates growth plate chondrocyte maturation and apoptosis. J. Biol. Chem. 2003, 278, 3762–3769. [Google Scholar] [CrossRef]
- Huleihel, L.; Bartolacci, J.G.; Dziki, J.L.; Vorobyov, T.; Arnold, B.; Scarritt, M.E.; Pineda Molina, C.; LoPresti, S.T.; Brown, B.N.; Naranjo, J.D.; et al. Matrix-Bound Nanovesicles Recapitulate Extracellular Matrix Effects on Macrophage Phenotype. Tissue Eng. Part A 2017, 23, 1283–1294. [Google Scholar] [CrossRef]
- Hussey, G.S.; Pineda Molina, C.; Cramer, M.C.; Tyurina, Y.Y.; Tyurin, V.A.; Lee, Y.C.; El-Mossier, S.O.; Murdock, M.H.; Timashev, P.S.; Kagan, V.E.; et al. Lipidomics and RNA sequencing reveal a novel subpopulation of nanovesicle within extracellular matrix biomaterials. Sci. Adv. 2020, 6, eaay4361. [Google Scholar] [CrossRef]
- Huleihel, L.; Hussey, G.S.; Naranjo, J.D.; Zhang, L.; Dziki, J.L.; Turner, N.J.; Stolz, D.B.; Badylak, S.F. Matrix-bound nanovesicles within ECM bioscaffolds. Sci. Adv. 2016, 2, e1600502. [Google Scholar] [CrossRef]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Petrovčíková, E.; Vičíková, K.; Leksa, V. Extracellular vesicles—Biogenesis, composition, function, uptake and therapeutic applications. Biologia 2018, 73, 437–448. [Google Scholar] [CrossRef]
- Quijano, L.M.; Naranjo, J.D.; El-Mossier, S.O.; Turner, N.J.; Pineda Molina, C.; Bartolacci, J.; Zhang, L.; White, L.; Li, H.; Badylak, S.F. Matrix-Bound Nanovesicles: The Effects of Isolation Method upon Yield, Purity, and Function. Tissue Eng. Part C Methods 2020, 26, 528–540. [Google Scholar] [CrossRef] [PubMed]
- Lötvall, J.; Hill, A.F.; Hochberg, F.; Buzás, E.I.; Di Vizio, D.; Gardiner, C.; Gho, Y.S.; Kurochkin, I.V.; Mathivanan, S.; Quesenberry, P.; et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: A position statement from the International Society for Extracellular Vesicles. J. Extracell. Vesicles 2014, 3, 26913. [Google Scholar] [CrossRef] [PubMed]
- Senyo, S.E.; Steinhauser, M.L.; Pizzimenti, C.L.; Yang, V.K.; Cai, L.; Wang, M.; Wu, T.-D.; Guerquin-Kern, J.-L.; Lechene, C.P.; Lee, R.T. Mammalian heart renewal by pre-existing cardiomyocytes. Nature 2013, 493, 433–436. [Google Scholar] [CrossRef]
- Bergmann, O.; Bhardwaj, R.D.; Bernard, S.; Zdunek, S.; Barnabé-Heider, F.; Walsh, S.; Zupicich, J.; Alkass, K.; Buchholz, B.A.; Druid, H.; et al. Evidence for cardiomyocyte renewal in humans. Science 2009, 324, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Bian, S.; Zhang, L.; Duan, L.; Wang, X.; Min, Y.; Yu, H. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J. Mol. Med. 2014, 92, 387–397. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, M.; Deng, S.; Lu, J.; Huang, H.; Zhang, Y.; Gong, P.; Shen, X.; Ruan, H.; Jin, M.; et al. miR-93-5p-Containing Exosomes Treatment Attenuates Acute Myocardial Infarction-Induced Myocardial Damage. Mol. Ther. Nucleic Acids 2018, 11, 103–115. [Google Scholar] [CrossRef]
- Sahoo, S.; Losordo, D.W. Exosomes and cardiac repair after myocardial infarction. Circ. Res. 2014, 114, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.C.; Arslan, F.; Lee, M.M.; Sze, N.S.K.; Choo, A.; Chen, T.S.; Salto-Tellez, M.; Timmers, L.; Lee, C.N.; El Oakley, R.M.; et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010, 4, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Vrijsen, K.R.; Sluijter, J.P.G.; Schuchardt, M.W.L.; van Balkom, B.W.M.; Noort, W.A.; Chamuleau, S.A.J.; Doevendans, P.A.F.M. Cardiomyocyte progenitor cell-derived exosomes stimulate migration of endothelial cells. J. Cell Mol. Med. 2010, 14, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Salomon, C.; Ryan, J.; Sobrevia, L.; Kobayashi, M.; Ashman, K.; Mitchell, M.; Rice, G.E. Exosomal signaling during hypoxia mediates microvascular endothelial cell migration and vasculogenesis. PLoS ONE 2013, 8, e68451. [Google Scholar] [CrossRef]
- Mackie, A.R.; Klyachko, E.; Thorne, T.; Schultz, K.M.; Millay, M.; Ito, A.; Kamide, C.E.; Liu, T.; Gupta, R.; Sahoo, S.; et al. Sonic hedgehog-modified human CD34+ cells preserve cardiac function after acute myocardial infarction. Circ. Res. 2012, 111, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhang, Z.; Bartolacci, J.G.; Dwyer, G.K.; Liu, Q.; Mathews, L.R.; Velayutham, M.; Roessing, A.S.; Lee, Y.C.; Dai, H.; et al. Graft IL-33 regulates infiltrating macrophages to protect against chronic rejection. J. Clin. Investig. 2020, 130, 5397–5412. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Mitsialis, S.A.; Aslam, M.; Vitali, S.H.; Vergadi, E.; Konstantinou, G.; Sdrimas, K.; Fernandez-Gonzalez, A.; Kourembanas, S. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation 2012, 126, 2601–2611. [Google Scholar] [CrossRef]
- Aliotta, J.M.; Pereira, M.; Li, M.; Amaral, A.; Sorokina, A.; Dooner, M.S.; Sears, E.H.; Brilliant, K.; Ramratnam, B.; Hixson, D.C.; et al. Stable cell fate changes in marrow cells induced by lung-derived microvesicles. J. Extracell. Vesicles 2012, 1, 18163. [Google Scholar] [CrossRef]
- Aliotta, J.M.; Sanchez-Guijo, F.M.; Dooner, G.J.; Johnson, K.W.; Dooner, M.S.; Greer, K.A.; Greer, D.; Pimentel, J.; Kolankiewicz, L.M.; Puente, N.; et al. Alteration of marrow cell gene expression, protein production, and engraftment into lung by lung-derived microvesicles: A novel mechanism for phenotype modulation. Stem Cells 2007, 25, 2245–2256. [Google Scholar] [CrossRef] [PubMed]
- Anderson, H.C.; Mulhall, D.; Garimella, R. Role of extracellular membrane vesicles in the pathogenesis of various diseases, including cancer, renal diseases, atherosclerosis, and arthritis. Lab. Investig. 2010, 90, 1549–1557. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, J.; Hu, J.; Fu, B.; Mao, Z.; Zhang, H.; Cai, G.; Chen, X.; Sun, X. Extracellular vesicles for acute kidney injury in preclinical rodent models: A meta-analysis. Stem Cell Res. Ther. 2020, 11, 11. [Google Scholar] [CrossRef]
- Xin, H.; Li, Y.; Buller, B.; Katakowski, M.; Zhang, Y.; Wang, X.; Shang, X.; Zhang, Z.G.; Chopp, M. Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells 2012, 30, 1556–1564. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Li, Y.; Liu, Z.; Wang, X.; Shang, X.; Cui, Y.; Zhang, Z.G.; Chopp, M. MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells 2013, 31, 2737–2746. [Google Scholar] [CrossRef]
- Lu, Y.; Zhou, Y.; Zhang, R.; Wen, L.; Wu, K.; Li, Y.; Yao, Y.; Duan, R.; Jia, Y. Bone Mesenchymal Stem Cell-Derived Extracellular Vesicles Promote Recovery Following Spinal Cord Injury via Improvement of the Integrity of the Blood-Spinal Cord Barrier. Front. Neurosci. 2019, 13, 209. [Google Scholar] [CrossRef] [PubMed]
- van der Merwe, Y.; Faust, A.E.; Sakalli, E.T.; Westrick, C.C.; Hussey, G.; Chan, K.C.; Conner, I.P.; Fu, V.L.N.; Badylak, S.F.; Steketee, M.B. Matrix-bound nanovesicles prevent ischemia-induced retinal ganglion cell axon degeneration and death and preserve visual function. Sci. Rep. 2019, 9, 3482. [Google Scholar] [CrossRef]
- Lopez-Verrilli, M.A.; Picou, F.; Court, F.A. Schwann cell-derived exosomes enhance axonal regeneration in the peripheral nervous system. Glia 2013, 61, 1795–1806. [Google Scholar] [CrossRef]
- Hercher, D.; Nguyen, M.Q.; Dworak, H. Extracellular vesicles and their role in peripheral nerve regeneration. Exp. Neurol. 2022, 350, 113968. [Google Scholar] [CrossRef]
- Royo, F.; Schlangen, K.; Palomo, L.; Gonzalez, E.; Conde-Vancells, J.; Berisa, A.; Aransay, A.M.; Falcon-Perez, J.M. Transcriptome of extracellular vesicles released by hepatocytes. PLoS ONE 2013, 8, e68693. [Google Scholar] [CrossRef]
- Mabuchi, A.; Mullaney, I.; Sheard, P.; Hessian, P.; Zimmermann, A.; Senoo, H.; Wheatley, A.M. Role of hepatic stellate cells in the early phase of liver regeneration in rat: Formation of tight adhesion to parenchymal cells. Comp. Hepatol. 2004, 3 (Suppl. S1), S29. [Google Scholar] [CrossRef]
- Rodríguez-Suárez, E.; Gonzalez, E.; Hughes, C.; Conde-Vancells, J.; Rudella, A.; Royo, F.; Palomo, L.; Elortza, F.; Lu, S.C.; Mato, J.M.; et al. Quantitative proteomic analysis of hepatocyte-secreted extracellular vesicles reveals candidate markers for liver toxicity. J. Proteom. 2014, 103, 227–240. [Google Scholar] [CrossRef]
- Herrera, M.B.; Fonsato, V.; Gatti, S.; Deregibus, M.C.; Sordi, A.; Cantarella, D.; Calogero, R.; Bussolati, B.; Tetta, C.; Camussi, G. Human liver stem cell-derived microvesicles accelerate hepatic regeneration in hepatectomized rats. J. Cell Mol. Med. 2010, 14, 1605–1618. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, Z.; Li, Y.; Wang, Y.; Li, Q.; Han, D. GelMA combined with sustained release of HUVECs derived exosomes for promoting cutaneous wound healing and facilitating skin regeneration. J. Mol. Histol. 2020, 51, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Xiao, C.; Miao, Y.; Wang, J.; Chen, R.; Fan, Z.; Hu, Z. Human acellular amniotic membrane incorporating exosomes from adipose-derived mesenchymal stem cells promotes diabetic wound healing. Stem Cell Res. Ther. 2021, 12, 255. [Google Scholar] [CrossRef]
- Narauskaitė, D.; Vydmantaitė, G.; Rusteikaitė, J.; Sampath, R.; Rudaitytė, A.; Stašytė, G.; Aparicio Calvente, M.I.; Jekabsone, A. Extracellular vesicles in skin wound healing. Pharmaceuticals 2021, 14, 811. [Google Scholar] [CrossRef]
- Nooshabadi, V.T.; Khanmohamadi, M.; Valipour, E.; Mahdipour, S.; Salati, A.; Malekshahi, Z.V.; Shafei, S.; Amini, E.; Farzamfar, S.; Ai, J. Impact of exosome-loaded chitosan hydrogel in wound repair and layered dermal reconstitution in mice animal model. J. Biomed. Mater. Res. A 2020, 108, 2138–2149. [Google Scholar] [CrossRef]
- Qian, Z.; Bai, Y.; Zhou, J.; Li, L.; Na, J.; Fan, Y.; Guo, X.; Liu, H. A moisturizing chitosan-silk fibroin dressing with silver nanoparticles-adsorbed exosomes for repairing infected wounds. J. Mater. Chem. B Mater. Biol. Med. 2020, 8, 7197–7212. [Google Scholar] [CrossRef]
- Shiekh, P.A.; Singh, A.; Kumar, A. Data supporting exosome laden oxygen releasing antioxidant and antibacterial cryogel wound dressing OxOBand alleviate diabetic and infectious wound healing. Data Brief 2020, 31, 105671. [Google Scholar] [CrossRef]
- Wang, C.; Liang, C.; Wang, R.; Yao, X.; Guo, P.; Yuan, W.; Liu, Y.; Song, Y.; Li, Z.; Xie, X. The fabrication of a highly efficient self-healing hydrogel from natural biopolymers loaded with exosomes for the synergistic promotion of severe wound healing. Biomater Sci. 2019, 8, 313–324. [Google Scholar] [CrossRef]
- da Fonseca Ferreira, A.; da Silva Cunha, P.; Carregal, V.M.; de Cássia da Silva, P.; de Miranda, M.C.; Kunrath-Lima, M.; de Melo, M.I.A.; Faraco, C.C.F.; Barbosa, J.L.; Frezard, F.; et al. Extracellular Vesicles from Adipose-Derived Mesenchymal Stem/Stromal Cells Accelerate Migration and Activate AKT Pathway in Human Keratinocytes and Fibroblasts Independently of miR-205 Activity. Stem Cells Int. 2017, 2017, 9841035. [Google Scholar]
- Pelizzo, G.; Avanzini, M.A.; Icaro Cornaglia, A.; De Silvestri, A.; Mantelli, M.; Travaglino, P.; Croce, S.; Romano, P.; Avolio, L.; Iacob, G.; et al. Extracellular vesicles derived from mesenchymal cells: Perspective treatment for cutaneous wound healing in pediatrics. Regen. Med. 2018, 13, 385–394. [Google Scholar] [CrossRef]
- Shi, Q.; Qian, Z.; Liu, D.; Sun, J.; Wang, X.; Liu, H.; Xu, J.; Guo, X. GMSC-Derived Exosomes Combined with a Chitosan/Silk Hydrogel Sponge Accelerates Wound Healing in a Diabetic Rat Skin Defect Model. Front. Physiol. 2017, 8, 904. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, L.; Zhao, H.; Li, Z.; Chen, J.; Cen, Y.; Zhang, Z. The Therapeutic Role of ADSC-EVs in Skin Regeneration. Front. Med. 2022, 9, 858824. [Google Scholar] [CrossRef]
- Vonk, L.A.; van Dooremalen, S.F.J.; Liv, N.; Klumperman, J.; Coffer, P.J.; Saris, D.B.F.; Lorenowicz, M.J. Mesenchymal Stromal/stem Cell-derived Extracellular Vesicles Promote Human Cartilage Regeneration In Vitro. Theranostics 2018, 8, 906–920. [Google Scholar] [CrossRef] [PubMed]
- Sondergaard, B.C.; Henriksen, K.; Wulf, H.; Oestergaard, S.; Schurigt, U.; Bräuer, R.; Danielsen, I.; Christiansen, C.; Qvist, P.; Karsdal, M.A. Relative contribution of matrix metalloprotease and cysteine protease activities to cytokine-stimulated articular cartilage degradation. Osteoarthr. Cartil. 2006, 14, 738–748. [Google Scholar] [CrossRef][Green Version]
- Tseng, W.J.; Huang, S.W.; Fang, C.H.; Hsu, L.T.; Chen, C.Y.; Shen, H.H.; Chang, J.Z.; Sun, J.S.; Lin, F.H. Treatment of osteoarthritis with collagen-based scaffold: A porcine animal model with xenograft mesenchymal stem cells. Histol Histopathol. 2018, 33, 1271–1286. [Google Scholar] [CrossRef]
- Mao, G.; Zhang, Z.; Hu, S.; Zhang, Z.; Chang, Z.; Huang, Z.; Liao, W.; Kang, Y. Exosomes derived from miR-92a-3p-overexpressing human mesenchymal stem cells enhance chondrogenesis and suppress cartilage degradation via targeting WNT5A. Stem Cell Res. Ther. 2018, 9, 247. [Google Scholar] [CrossRef]
- Chen, P.; Zheng, L.; Wang, Y.; Tao, M.; Xie, Z.; Xia, C.; Gu, C.; Chen, J.; Qiu, P.; Mei, S.; et al. Desktop-stereolithography 3D printing of a radially oriented extracellular matrix/mesenchymal stem cell exosome bioink for osteochondral defect regeneration. Theranostics 2019, 9, 2439–2459. [Google Scholar] [CrossRef]
- Man, K.; Brunet, M.Y.; Federici, A.S.; Hoey, D.A.; Cox, S.C. An ECM-Mimetic Hydrogel to Promote the Therapeutic Efficacy of Osteoblast-Derived Extracellular Vesicles for Bone Regeneration. Front. Bioeng. Biotechnol. 2022, 10, 829969. [Google Scholar]
- Behera, J.; Tyagi, N. Exosomes: Mediators of bone diseases, protection, and therapeutics potential. Oncoscience 2018, 5, 181–195. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, Y.; Zhang, L.; Ge, W.; Tang, P. The roles of bone-derived exosomes and exosomal microRNAs in regulating bone remodelling. J. Cell Mol. Med. 2017, 21, 1033–1041. [Google Scholar] [CrossRef]
- Gholami, L.; Nooshabadi, V.T.; Shahabi, S.; Jazayeri, M.; Tarzemany, R.; Afsartala, Z.; Khorsandi, K. Extracellular vesicles in bone and periodontal regeneration: Current and potential therapeutic applications. Cell Biosci. 2021, 11, 16. [Google Scholar] [CrossRef]
- Wang, M.; Li, J.; Ye, Y.; He, S.; Song, J. SHED-derived conditioned exosomes enhance the osteogenic differentiation of PDLSCs via Wnt and BMP signaling in vitro. Differentiation 2020, 111, 1–11. [Google Scholar] [CrossRef]
- Ansari, S.; de Wildt, B.W.M.; Vis, M.A.M.; de Korte, C.E.; Ito, K.; Hofmann, S.; Yuana, Y. Matrix vesicles: Role in bone mineralization and potential use as therapeutics. Pharmaceuticals 2021, 14, 289. [Google Scholar] [CrossRef] [PubMed]
- Yi, G.; Zhang, S.; Ma, Y.; Yang, X.; Huo, F.; Chen, Y.; Yang, B.; Tian, W. Matrix vesicles from dental follicle cells improve alveolar bone regeneration via activation of the PLC/PKC/MAPK pathway. Stem Cell Res. Ther. 2022, 13, 41. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Li, H.-Y.; Yang, K.; Wu, J.-L.; Cai, X.-W.; Zhou, Y.; Li, C.-Q. Exosomes as potential alternatives to stem cell therapy for intervertebral disc degeneration: In-vitro study on exosomes in interaction of nucleus pulposus cells and bone marrow mesenchymal stem cells. Stem Cell Res. Ther. 2017, 8, 108. [Google Scholar] [CrossRef]
- Zhu, L.; Shi, Y.; Liu, L.; Wang, H.; Shen, P.; Yang, H. Mesenchymal stem cells-derived exosomes ameliorate nucleus pulposus cells apoptosis via delivering miR-142-3p: Therapeutic potential for intervertebral disc degenerative diseases. Cell Cycle 2020, 19, 1727–1739. [Google Scholar] [CrossRef]
- Li, Z.-Q.; Kong, L.; Liu, C.; Xu, H.-G. Human Bone Marrow Mesenchymal Stem Cell-derived Exosomes Attenuate IL-1β-induced Annulus Fibrosus Cell Damage. Am. J. Med. Sci. 2020, 360, 693–700. [Google Scholar] [CrossRef]
- Zhu, G.; Yang, X.; Peng, C.; Yu, L.; Hao, Y. Exosomal miR-532-5p from bone marrow mesenchymal stem cells reduce intervertebral disc degeneration by targeting RASSF. Exp. Cell Res. 2020, 393, 112109. [Google Scholar] [CrossRef]
- Xia, C.; Zeng, Z.; Fang, B.; Tao, M.; Gu, C.; Zheng, L.; Wang, Y.; Shi, Y.; Fang, C.; Mei, S.; et al. Mesenchymal stem cell-derived exosomes ameliorate intervertebral disc degeneration via anti-oxidant and anti-inflammatory effects. Free Radic. Biol. Med. 2019, 143, 1–15. [Google Scholar] [CrossRef]
- Liao, Z.; Luo, R.; Li, G.; Song, Y.; Zhan, S.; Zhao, K.; Hua, W.; Zhang, Y.; Wu, X.; Yang, C. Exosomes from mesenchymal stem cells modulate endoplasmic reticulum stress to protect against nucleus pulposus cell death and ameliorate intervertebral disc degeneration in vivo. Theranostics 2019, 9, 4084–4100. [Google Scholar] [CrossRef]
- Guo, Z.; Su, W.; Zhou, R.; Zhang, G.; Yang, S.; Wu, X.; Qiu, C.; Cong, W.; Shen, N.; Guo, J.; et al. Exosomal MATN3 of Urine-Derived Stem Cells Ameliorates Intervertebral Disc Degeneration by Antisenescence Effects and Promotes NPC Proliferation and ECM Synthesis by Activating TGF-β. Oxid. Med. Cell. Longev. 2021, 2021, 5542241. [Google Scholar] [CrossRef]
- Xiang, H.; Su, W.; Wu, X.; Chen, W.; Cong, W.; Yang, S.; Liu, C.; Qiu, C.; Yang, S.-Y.; Wang, Y.; et al. Exosomes Derived from Human Urine-Derived Stem Cells Inhibit Intervertebral Disc Degeneration by Ameliorating Endoplasmic Reticulum Stress. Oxid. Med. Cell. Longev. 2020, 2020, 6697577. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Zhang, Z.; Mao, Q.; Wang, C.; Zhou, Y.; Zhou, X.; Ying, L.; Xu, H.; Hu, S.; Zhang, N. Injectable exosome-functionalized extracellular matrix hydrogel for metabolism balance and pyroptosis regulation in intervertebral disc degeneration. J. Nanobiotechnol. 2021, 19, 264. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Wang, X.; Liu, L.; Cai, Y.; Zhao, X.; Ma, H.; Zhang, Y. Exosomes Derived from Human Placental Mesenchymal Stromal Cells Carrying AntagomiR-4450 Alleviate Intervertebral Disc Degeneration Through Upregulation of ZNF 121. Stem Cells Dev. 2020, 29, 1038–1058. [Google Scholar] [CrossRef]
- Yuan, X.; Li, T.; Shi, L.; Miao, J.; Guo, Y.; Chen, Y. Human umbilical cord mesenchymal stem cells deliver exogenous miR-26a-5p via exosomes to inhibit nucleus pulposus cell pyroptosis through METTL14/NLRP3. Mol. Med. 2021, 27, 91. [Google Scholar] [CrossRef] [PubMed]
- Hingert, D.; Ekström, K.; Aldridge, J.; Crescitelli, R.; Brisby, H. Extracellular vesicles from human mesenchymal stem cells expedite chondrogenesis in 3D human degenerative disc cell cultures. Stem Cell Res. Ther. 2020, 11, 323. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.-R.; Pan, S.; Li, H.-Y.; Sun, C.; Chang, X.; Lu, K.; Jiang, C.-Q.; Zuo, R.; Zhou, Y.; Li, C.-Q. Inhibition of the Notch1 Pathway Promotes the Effects of Nucleus Pulposus Cell-Derived Exosomes on the Differentiation of Mesenchymal Stem Cells into Nucleus Pulposus-Like Cells in Rats. Stem Cells Int. 2019, 2019, 8404168. [Google Scholar] [CrossRef]
- Zhang, Q.-C.; Hu, S.-Q.; Hu, A.-N.; Zhang, T.-W.; Jiang, L.-B.; Li, X.-L. Autophagy-activated nucleus pulposus cells deliver exosomal miR-27a to prevent extracellular matrix degradation by targeting MMP-13. J. Orthop. Res. 2020, 1, 1921–1932. [Google Scholar] [CrossRef]
- Luo, L.; Gong, J.; Zhang, H.; Qin, J.; Li, C.; Zhang, J.; Tang, Y.; Zhang, Y.; Chen, J.; Zhou, Y.; et al. Cartilage endplate stem cells transdifferentiate into nucleus pulposus cells via autocrine exosomes. Front. Cell Dev. Biol. 2021, 9, 648201. [Google Scholar] [CrossRef]
- Luo, L.; Jian, X.; Sun, H.; Qin, J.; Wang, Y.; Zhang, J.; Shen, Z.; Yang, D.; Li, C.; Zhao, P.; et al. Cartilage endplate stem cells inhibit intervertebral disc degeneration by releasing exosomes to nucleus pulposus cells to activate Akt/autophagy. Stem Cells 2021, 39, 467. [Google Scholar] [CrossRef]
- Xie, L.; Chen, Z.; Liu, M.; Huang, W.; Zou, F.; Ma, X.; Tao, J.; Guo, J.; Xia, X.; Lyu, F.; et al. MSC-Derived Exosomes Protect Vertebral Endplate Chondrocytes against Apoptosis and Calcification via the miR-31-5p/ATF6 Axis. Mol. Ther. Nucleic Acids 2020, 22, 601–614. [Google Scholar] [CrossRef]
- Sun, Z.; Zhao, H.; Liu, B.; Gao, Y.; Tang, W.-H.; Liu, Z.-H.; Luo, Z.-J. AF cell derived exosomes regulate endothelial cell migration and inflammation: Implications for vascularization in intervertebral disc degeneration. Life Sci. 2021, 265, 118778. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xie, G.; Yang, W.; Wang, W.; Zuo, Z.; Wang, W. Platelet-rich plasma attenuates intervertebral disc degeneration via delivering miR-141-3p-containing exosomes. Cell Cycle 2021, 20, 1487–1499. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Liu, B.; Liu, Z.-H.; Song, W.; Wang, D.; Chen, B.-Y.; Fan, J.; Xu, Z.; Geng, D.; Luo, Z.-J. Notochordal-Cell-Derived Exosomes Induced by Compressive Load Inhibit Angiogenesis via the miR-140-5p/Wnt/β-Catenin Axis. Mol. Ther. Nucleic Acids 2020, 22, 1092–1106. [Google Scholar] [CrossRef]
- Kim, S.-H.; Lechman, E.R.; Bianco, N.; Menon, R.; Keravala, A.; Nash, J.; Mi, Z.; Watkins, S.C.; Gambotto, A.; Robbins, P.D. Exosomes derived from IL-10-treated dendritic cells can suppress inflammation and collagen-induced arthritis. J. Immunol. 2005, 174, 6440–6448. [Google Scholar] [CrossRef]
- Ruffner, M.A.; Kim, S.H.; Bianco, N.R.; Francisco, L.M.; Sharpe, A.H.; Robbins, P.D. B7-1/2, but not PD-L1/2 molecules, are required on IL-10-treated tolerogenic DC and DC-derived exosomes for in vivo function. Eur. J. Immunol. 2009, 39, 3084–3090. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Bianco, N.R.; Shufesky, W.J.; Morelli, A.E.; Robbins, P.D. Effective treatment of inflammatory disease models with exosomes derived from dendritic cells genetically modified to express IL-4. J. Immunol. 2007, 179, 2242–2249. [Google Scholar] [CrossRef]
- Kim, S.H.; Bianco, N.; Menon, R.; Lechman, E.R.; Shufesky, W.J.; Morelli, A.E.; Robbins, P.D. Exosomes derived from genetically modified DC expressing FasL are anti-inflammatory and immunosuppressive. Mol. Ther. 2006, 13, 289–300. [Google Scholar] [CrossRef]
- Bianco, N.R.; Kim, S.H.; Ruffner, M.A.; Robbins, P.D. Therapeutic effect of exosomes from indoleamine 2,3-dioxygenase-positive dendritic cells in collagen-induced arthritis and delayed-type hypersensitivity disease models. Arthritis Rheum. 2009, 60, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Bianco, N.R.; Shufesky, W.J.; Morelli, A.E.; Robbins, P.D. MHC class II+ exosomes in plasma suppress inflammation in an antigen-specific and Fas ligand/Fas-dependent manner. J. Immunol. 2007, 179, 2235–2241. [Google Scholar] [CrossRef]
- Crum, R.J.; Hall, K.; Molina, C.P.; Hussey, G.S.; Graham, E.; Li, H.; Badylak, S.F. Immunomodulatory matrix-bound nanovesicles mitigate acute and chronic pristane-induced rheumatoid arthritis. NPJ Regen. Med. 2022, 7, 13. [Google Scholar] [CrossRef]
- Exosome Diagnostics Market Size & Growth Analysis Report. Available online: https://www.bccresearch.com/market-research/biotechnology/exosome-diagnostics-and-therapeutics-global-markets-report.html (accessed on 7 June 2022).
- Sengupta, V.; Sengupta, S.; Lazo, A.; Woods, P.; Nolan, A.; Bremer, N. Exosomes Derived from Bone Marrow Mesenchymal Stem Cells as Treatment for Severe COVID-19. Stem Cells Dev. 2020, 29, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-G.; Shi, M.-M.; Monsel, A.; Dai, C.-X.; Dong, X.; Shen, H.; Li, S.-K.; Chang, J.; Xu, C.-L.; Li, P.; et al. Nebulized exosomes derived from allogenic adipose tissue mesenchymal stromal cells in patients with severe COVID-19: A pilot study. Stem Cell Res. Ther. 2022, 13, 220. [Google Scholar] [CrossRef]
- Shi, M.-M.; Yang, Q.-Y.; Monsel, A.; Yan, J.-Y.; Dai, C.-X.; Zhao, J.-Y.; Shi, G.-C.; Zhou, M.; Zhu, X.-M.; Li, S.-K.; et al. Preclinical efficacy and clinical safety of clinical-grade nebulized allogenic adipose mesenchymal stromal cells-derived extracellular vesicles. J. Extracell. Vesicles 2021, 10, e12134. [Google Scholar] [CrossRef]
- Sahoo, S.; Adamiak, M.; Mathiyalagan, P.; Kenneweg, F.; Kafert-Kasting, S.; Thum, T. Therapeutic and diagnostic translation of extracellular vesicles in cardiovascular diseases: Roadmap to the clinic. Circulation 2021, 143, 1426–1449. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xu, J.; Qian, J.; Gao, X. Engineering extracellular vesicles for cancer therapy: Recent advances and challenges in clinical translation. Biomater. Sci. 2020, 8, 6978–6991. [Google Scholar] [CrossRef]
- Burnouf, T.; Agrahari, V.; Agrahari, V. Extracellular vesicles as nanomedicine: Hopes and hurdles in clinical translation. Int. J. Nanomed. 2019, 14, 8847–8859. [Google Scholar] [CrossRef] [PubMed]
- Stam, J.; Bartel, S.; Bischoff, R.; Wolters, J.C. Isolation of extracellular vesicles with combined enrichment methods. J. Chromatogr. B, Analyt. Technol. Biomed. Life Sci. 2021, 1169, 122604. [Google Scholar] [CrossRef]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun. Signal. 2021, 19, 47. [Google Scholar] [CrossRef]
- Bahr, M.M.; Amer, M.S.; Abo-El-Sooud, K.; Abdallah, A.N.; El-Tookhy, O.S. Preservation techniques of stem cells extracellular vesicles: A gate for manufacturing of clinical grade therapeutic extracellular vesicles and long-term clinical trials. Int. J. Vet. Sci. Med. 2020, 8, 1–8. [Google Scholar] [CrossRef]
- Crescitelli, R.; Lässer, C.; Szabó, T.G.; Kittel, A.; Eldh, M.; Dianzani, I.; Buzás, E.I.; Lötvall, J. Distinct RNA profiles in subpopulations of extracellular vesicles: Apoptotic bodies, microvesicles and exosomes. J. Extracell. Vesicles 2013, 2, 20677. [Google Scholar] [CrossRef]
- Willms, E.; Cabañas, C.; Mäger, I.; Wood, M.J.A.; Vader, P. Extracellular vesicle heterogeneity: Subpopulations, isolation techniques, and diverse functions in cancer progression. Front. Immunol. 2018, 9, 738. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Huang, X.; Xiu, H.; Sun, Y.; Chen, J.; Cheng, G.; Song, Z.; Peng, Y.; Shen, Y.; Wang, J.; et al. Extracellular vesicles: Natural liver-accumulating drug delivery vehicles for the treatment of liver diseases. J. Extracell. Vesicles 2020, 10, e12030. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, A.; Moirangthem, A.; Angom, R.S.; Ishiguro, K.; Driscoll, J.; Yan, I.K.; Mukhopadhyay, D.; Patel, T. Safety of bovine milk derived extracellular vesicles used for delivery of RNA therapeutics in zebrafish and mice. J. Appl. Toxicol. 2020, 40, 706–718. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.F.; Lázaro-Ibáñez, E.; Forsgard, M.A.-M.; Shatnyeva, O.; Osteikoetxea, X.; Karlsson, F.; Heath, N.; Ingelsten, M.; Rose, J.; Harris, J.; et al. Extracellular vesicles induce minimal hepatotoxicity and immunogenicity. Nanoscale 2019, 11, 6990–7001. [Google Scholar] [CrossRef] [PubMed]
- Wiklander, O.P.B.; Nordin, J.Z.; O’Loughlin, A.; Gustafsson, Y.; Corso, G.; Mäger, I.; Vader, P.; Lee, Y.; Sork, H.; Seow, Y.; et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J. Extracell. Vesicles 2015, 4, 26316. [Google Scholar] [CrossRef] [PubMed]
- Smyth, T.; Kullberg, M.; Malik, N.; Smith-Jones, P.; Graner, M.W.; Anchordoquy, T.J. Biodistribution and delivery efficiency of unmodified tumor-derived exosomes. J. Control. Release 2015, 199, 145–155. [Google Scholar] [CrossRef]
- Salunkhe, S.; Dheeraj; Basak, M.; Chitkara, D.; Mittal, A. Surface functionalization of exosomes for target-specific delivery and in vivo imaging & tracking: Strategies and significance. J. Control. Release 2020, 326, 599–614. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).