Abstract
In hamburger manufacturing, meat is subjected to four main processing steps (pre-grinding, mixing, grinding, and forming), whereby muscle fibers are disintegrated. In this study, the influence of these process steps was characterized by structural (amount of non-intact cells (ANIC), CLS-Microscopy), functional (drip loss) and qualitative (soluble protein content, lactate dehydrogenase (LDH) activity, myoglobin content (Mb)) parameters of the meat. Therefore, meat samples were analyzed after each process step. Histological analyses revealed an increased ANIC with progressive processing. Thereby, the first and second grinding steps caused the strongest increases (factors 2.43 and 2.69). Comparable results were found in the relative LDH activity (factor 2.20 and 1.62) and the Mb concentration (factor 2.24 and 1.33) of the extracted meat solution. The findings suggest that the disintegration of the meat structure increases with progressive processing, causing more vulnerable structures which result in increased leakage of intramuscular substances. Further, the type of stress acting on the meat determines the extent of the changes. The presented findings enable manufacturers to precisely adjust their process towards more gentle production parameters and thus, to meet the legal regulations.
1. Introduction
The disintegration of muscle fibers during beef hamburger manufacturing affects their physicochemical properties. The term hamburger is protected by the guidelines of the German Food Code and must be prepared from 100% beef [1], thereby not exceeding the legal limit of 20% Vol. disintegrated muscle fibers [2]. Production of beef hamburgers usually involves four processing steps, namely pre-cutting during the first grinding, mixing, main grinding in the second grinding, and forming of the hamburgers [3].
Grinding is a mechanical way of comminution [4] and is widely used for size reduction of meat [5]. During grinding, the meat is continuously conveyed towards the cutting set [6], extruded through the hole plates, and cut by the rotating knife [5], whereby the pressures of 6–8 bars act on lean meat. Due to increased grinder efficiency and material homogeneity and better ground meat quality, the size reduction is done gradually [4,7]. In the pre-cutting (first grinding), meat is ground to 13–19 mm particle size [5], which is important to guarantee optimal conveying and comminution [4]. Mixing is performed to obtain an even distribution of meat and fat particles, often using paddle mixers with low energy input to ensure gentle handling [5]. In the main grinding step (second grinding), the meat is ground to the final particle size of approximately 3 mm [5]. Following, hamburgers are formed by filling the ground meat in a defined mold and applying pressure [8].
Meat is considered a plastic–elastic material that is able to store force acting on it to a certain degree [9,10]. During hamburger manufacturing, meat is exposed to three main mechanical forces. First, wall friction forces are acting on the meat during grinding and mixing [6]. Second, pressure acts on the meat during forming and as it is forced through the hole blades. This pressure is higher, the smaller the hole plate diameter is [4], whereby the structure of the meat is disintegrated. It was found that the pressure within the hole plate is higher in beef meat than in pork meat and is higher when the fat content of the meat is higher [4]. Third, the meat is exposed to shear force due to conveying and due to cutting of the meat strands by the rotating knife at the end of the hole plate [6]. In this stage, the structural and mechanical properties of the meat are altered due to size reduction [4], and muscle fiber structures are degraded [11].
A mechanical rupture of the muscle fiber opens its internal structure, thus making proteins and intramuscular substances available for extraction [12]. Thereby, the degree of muscle fiber disintegration depends on the applied force and stress, the type of raw material, and the used technology. Hence, mechanical treatments, such as mixing or forming under pressure, are key parameters in muscle fiber disintegration [2] and significantly change the meat structure. During grinding, the ordered fibrillar structures and connective tissue are disrupted by mechanical force [13], thus leading to an increased amount of non-intact muscle fibers (ANIC). The ANIC is evaluated by histological techniques [2], influences quality, functionality, and sensorial perception of beef hamburgers and is, thus, legally limited to 20 Vol.% in Germany [2,11]. Histological approaches are used to quantify muscle fiber disintegration in muscle tissue to identify, characterize, and evaluate changes in the muscle fiber structure and enable the assessment of the meat in terms of legal rules [2].
As muscle fiber structures are disintegrated during grinding, resulting in leakage of meat juice, the meats drip loss will increase and meat quality is reduced [6]. The ability of meat to retain water is described as water-holding capacity [14], which is closely linked to the meat quality and the sensorial perceptions of the consumer, as it depends on muscle fibrillar changes during processing [14]. Thus, the amount of released water depends on the extent of muscle fiber disintegration during processing.
Enzymatic methods are also based on the release of intramuscular fibrillar enzymes, which increases lactate dehydrogenase (LDH) activity [15]. Additionally, the determination of soluble protein and myoglobin content is based on the fragmentation of muscle fibers during grinding, thereby creating more open ends [13] where intramuscular fibrillar substances, such as proteins, leak out.
By now, it has not been determined which processing step in beef hamburger manufacturing influences the characteristics and properties of the meat the strongest. The aim of this study was, therefore, to identify the influence of the single process steps on structural, functional, and qualitative parameters of the meat. With the gained knowledge, hamburger manufacturers can identify the most influencing parameter and are, therefore, able to carefully design their processing steps and adjust the parameters to ensure a gentler production. It was hypothesized, that the process steps, including size reduction (such as grinding), might alter the properties of the meat the strongest as they accompany a stronger mechanical load [10].
2. Materials and Methods
2.1. Material and Beef Hamburger Manufacturing
Hamburgers were produced, and samples were taken according to the production scheme (Figure 1); details on all used equipment are summarized in Table 1.
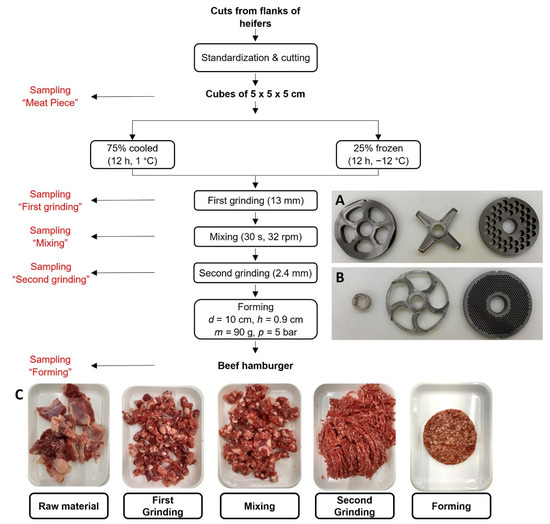
Figure 1.
Flow chart of the hamburger manufacturing with different points of sampling, cutting set composition of (A) first grinding step (3-part cutting system: pre-cutter, 4-bladed knife, 13 mm inclined perforated disc) and (B) second grinding step (3-part cutting set: spacer, 5-bladed sickle knife, 2.4 mm inclined perforated disc) and photographs of the samples (C), n = 3.

Table 1.
Details on equipment used for hamburger manufacturing.
Cuts from flank of heifers, purchased at a local meat trading company (MEGA—Das Fachzentrum für die Metzgerei und Gastronomie eG, Stuttgart, Germany), were visually standardized four days after slaughtering and cut into cubes of 5 × 5 × 5 cm. Thereby, visible tendons were removed, and the fat content was adjusted to approximately 20%. Approximately 25% of the meat was stored overnight to a core temperature of T = −12 °C and 75% of the meat to a core temperature of T = 1 °C. The meat was mixed in a horizontal mixer for 30 s in a clockwise direction and 30 s in a counterclockwise direction at 32 rpm. The meat cubes were ground with a meat grinder equipped with a 3-part cutting system (precutting device, 4-wing knife, 13 mm inclined end-hole plate). The feeding screw rotated with a speed of 20 rpm and the grinder screw at 187 rpm. The meat was mixed again at 32 rpm for 15 s in a clockwise direction and 15 s in a counterclockwise direction and ground to a final particle size of 2.4 mm using a 3-part grinding system (plastic spacer, 5-bladed sickle knife, 2.4 mm inclined perforated end-hole plate) with a speed of 20 rpm at the feeding screw and 187 rpm at the grinder screw. The ground meat was then formed into hamburgers of m = 90 g weight, h = 0.9 cm height, and d = 10 cm diameter by applying p = 5 bar forming pressure with a modified hamburger press. Samples were taken after each processing step, namely, raw material, first grinding step, mixing, second grinding step, and forming (Figure 1), and were then stored airtight at 1 °C until further analysis.
2.2. Methods
2.2.1. Chemical Composition of Beef Hamburgers
First, one hamburger was homogenized in a blender (Blixer® 2, M 02, robot-coupe®, Montceau-en-Bourgogne, France) for 30 s and analyzed for each repetition in triplicate.
The sea–sand method was used to determine the water content (§64 LFGB L 06.00-3 [16]). The fat content was determined by Soxhlet extraction (Büchi 810, Büchi Laboratoriums-Technik AG, Flawil, Switzerland), utilizing the leftovers of the water determination (§64 LFGB L 06.00-6). Dumas’ combustion method (Dumatherm N Pro, C. Gerhardt GmbH & Co. KG, Königswinter, Germany) was used to assess the protein content (§64 LFGB method L 06.00-20) by applying a nitrogen to protein conversion factor of 6.25 [17].
2.2.2. Histochemical Analyses
To assess the amount of non-intact muscle fibers (ANIC) in the meat samples, histochemical analyses were performed, according to §64 LFGB method L 06.00-13 [16]. Cryo-cuts (h = 5 µm) were prepared, dyed with picro-indigo carmine (CALLEJA) staining, and transferred to high-resolution images of 27,881 dpi (Labor Kneissler, Burglengenfeld, Germany). The number of non-intact muscle fibers in the cryo-cut scans were detected by point-counting at 20-fold magnification (NDP.view 2.7.52, Hamamatsu Photonics K.K., Shizuoka, Japan), and the ANIC was calculated according to Equation (1) [16]:
with = lower limit, = upper limit, , , n = number of non-intact muscle fibers counted, x = number of total muscle fibers counted. Per sample at least six cross-sections were analyzed, whereby two cross-sections each were averaged. The mean and the standard deviation of the three repetitions were calculated.
2.2.3. Drip Loss (DL)
The drip loss of the meat samples was determined in triplicate by the centrifugation method [14]. In short, 10 g of meat were weighed in tubes (Nalgene 50 mL PP tubes, Nalgene Nunc International Corporation, Rochester, NY, USA) and centrifuged (20 min, 5 °C, 16,000 rpm) (Z32HK, Hermle Labortechnik GmbH, Wehingen, Germany). The excess meat juice was removed by placing the pellet on a paper tissue for 1 min. The percentage weight loss (drip loss) of the meat sample was calculated, as shown in Equation (2):
with = weight of meat sample before centrifugation (g) and = weight of meat sample after centrifugation (g).
2.2.4. Confocal Laser Scanning Microscopy (CLSM)
A Nikon CLSM (Nikon D Eclipse C1, Nikon GmbH, Düsseldorf, Germany) equipped with a “Cobolt 06-MLD” laser was used to study the microstructure of the raw meat samples in the style of Irmscher [18]. A total of 20 µL of Calcofluor White solution (Sigma-Aldrich Chemie GmbH, Munich, Germany) for protein staining was applied onto a CLSM tray equipped with a cover glass. Round-shaped samples, taken from the raw samples with a special circular cutter, were placed in the tray and excited at 638 nm. Images of the representative areas were taken at 10-fold magnification (Plan-Apochromat Plan Fluor 4/0.13, Plan Fluor 10/0.30; Nikon GmbH, Düsseldorf, Germany) with the help of E-CZ1 software (NIS-Elements Confocal, Version 4.50, Nikon GmbH, Düsseldorf, Germany).
2.2.5. Preparation of Extracted Meat Solution (EMS)
As necessary for further analyses, the extracted meat solutions (EMSs) of the samples were prepared by diluting meat in the ratio of 1:10 with 10 mM potassium phosphate buffer pH 7 at 2 °C. The mixture was incubated (20 min, 7 °C, 85 rpm) (innova® 42R, New Brunswick Scientific/Eppendorf AG, Hamburg, Germany) and stored for 1 h at 7 °C for further extraction. The meat was separated by using folded filters (Rotilabo®-folded filters, type 113P, Carl Roth GmbH & Co. KG, Karlsruhe, Germany). Until further analyses, the EMSs were stored at 7 °C in brown glass bottles.
2.2.6. Soluble Protein Content (SPC)
Rapid nitrogen analysis, according to Dumas’ combustion method (Dumatherm N Pro, C. Gerhardt GmbH & Co. KG, Königswinter, Germany), with a nitrogen to protein conversion factor of 6.25 [17] was applied to quantify the amount of soluble protein in the EMS.
2.2.7. Lactate Dehydrogenase Activity (LDH)
The LDH activity of the EMS was determined by an enzyme detection kit (lactate dehydrogenase activity assay kit MAK066, Sigma-Aldrich Chemie GmbH, Munich, Germany) based on an NADH-dependent indicator reaction. A colored compound is formed that is photometrically quantifiable at 450 nm [19]. The EMS was first diluted 1:400 using 10 mM of a potassium–phosphate buffer adjusted to pH 7 and then 1:40,000 using the LDH sample buffer (part of the enzyme kit). Triplicates of each sample were carried out. The enzyme activity was calculated according to the manufacturer’s instructions, the relative LDH activity was then calculated by dividing the respective enzyme activities by the activity of the meat piece.
2.2.8. Myoglobin Content (Mb)
The myoglobin content of the EMS was photometrically detected following the method of Trout [20]. In short, 100 µL of the previously prepared EMSs were transferred into a 96-well transparent plate (Nunclon Delta Surface, Thermo Fisher Scientific, Roskilde, Denmark) in triplicate for each sample. The absorption spectra of the EMSs were recorded from 300 nm–800 nm in 1 nm steps at 25 °C (Biotek Synergy HT, Agilent Technologies, Inc., Santa Clara, CA, USA). The myoglobin content was then calculated according to Trout [20].
2.2.9. Statistical Analyses
Three independent experiments (biological replicates) with at least two to three analytical replicates (technical replicates) were performed. All results are shown as mean ± standard deviation or mean ± standard error (pressure profiles), calculated by MS Excel (Microsoft, Redmond, WA, USA) and plotted by OriginPro 2020 (OriginLab Corporation, North Hampton, MA, USA). Statistical analyses were done with SPSS (IBM SPSS Statistics 25, IBM Deutschland GmbH, Ehningen, Germany). The Shapiro–Wilk and Levene tests were used to test the normal distribution of data and variance homogeneity, respectively. All data were normally distributed. A significance analysis by univariant ANOVA (analysis of variance) was performed if the data showed variance homogeneity, applying the Tukey post hoc test (confidence interval of 95% (α = 0.05)). The Welch-ANOVA (analysis of variance) was conducted for data without variance homogeneity using the Games-Howell post hoc (confidence interval of 95% (α = 0.05)). Small letters attached to the mean values of the samples indicate statistical significance.
3. Results and Discussion
3.1. Chemical Composition
The hamburgers of this study were produced following the German guidelines for meat and meat products [1], where hamburgers typically contain beef meat only. Incorporation of salt and spices into the hamburger patties are uncommon and not of industrial relevance. Usage of salt in the hamburger formulation would facilitate the solubilization of myofibrillar proteins and thus, influence product parameters, such as drip loss. The raw material used for the experiments was composed of 62.6 ± 0.4% water, 19.0 ± 0.6% fat, 18.765 ± 1.1719% protein, and 0.87 ± 0.05% ash, resulting in a sum parameter [21] of 101.25%. The slightly increased sum parameter might be caused by the protein determination by Dumas’ method, which is based on the determination of the total nitrogen content of the sample. According to Berry and Abraham [22], a fat content of ca. 20% is usually used for hamburger production. Keeton and Eddy [23] report that the protein and ash content of skeletal muscle tissue decreases with increased fat content, typically having approximately 18% protein and 64% water in meat with 20% fat. Thus, the determined values are within the expected range.
3.2. Structural and Functional Properties
To check the influence of each process step on the ANIC, the meat samples were histologically analyzed for their degree of non-intact muscle fibers. During the first grinding step, the ANIC was approximately doubled (factor 2.4) from 3.79 ± 2.61 Vol.% in raw material to 9.21 ± 4.81 Vol.% after the first grinding, thereby reducing the volume of the particle by a factor of ca. 100 (Figure 2A).
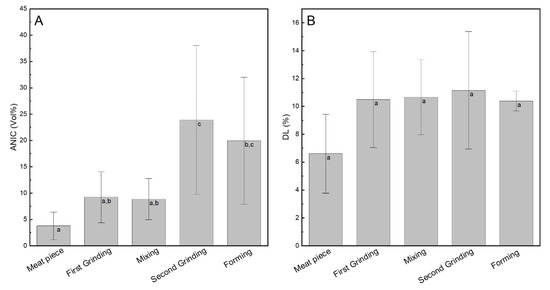
Figure 2.
Characterization of the structural and functional parameters: (A) amount of non-intact muscle fibers (ANIC) and (B) drip loss (DL) of the meat samples at different processing stages. Data points with different letters are significantly different (p < 0.05), n = 3.
During the second grinding step, the volume of the particles was further reduced by a factor ca. 81, and the ANIC significantly increased about threefold (factor 2.7, Figure 2A) from 8.87 ± 3.91 Vol.% after mixing to 23.86 ± 14.16 Vol.% after the second grinding. Both the mixing and the forming steps did not significantly change the ANIC (p > 0.05). Hence, the grinding steps have the biggest influence on muscle fiber disintegration, whereby the increase is higher in the second grinding compared to the first grinding. The higher standard deviations in Figure 2A might be caused by variations in the raw material meat, as the displayed mean value is composed out of three biological replicates made from different meat cut at different days. It is known that parameters, such as physiological parameters of the animal [23], sex, age, or slaughtering conditions [24] influence the meat quality, wherefore changes between different batches are reasonable. Additionally, the histological determination of ANIC is based on a subjective, visual evaluation of the histological cryo-cut, thus making the determination suspectable to greater variations. The histological images (Figure 3) depict the previously described changes in the meat structure, which are underlined by the CLSM images (Figure 3). Naturally organized muscle fibers are present in the raw material after the first grinding and mixing, whereas the ordered structure is reduced after the second grinding and forming.
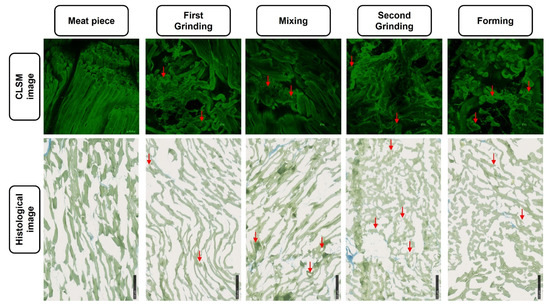
Figure 3.
Confocal laser scanning microscopy (CLSM) and histological images of the meat samples at different processing stages. Red arrows exemplarily indicate non-intact cells; images from the same processing step do not represent the identical samples section.
This outcome was expected, as grinding decreases the particle size through cutting and shearing the meat through end-hole plates, which disintegrates muscle structures [2]. The findings of Beneke [2] that mechanical treatment, such as mixing or forming, under pressure are key parameters in muscle fiber disintegration could not be verified in this study. This might be explained by quite gentle mixing and forming conditions in the present study. Increasing mixing time, speed, or higher formation pressure might cause stronger disintegration of meat muscle fibers [25,26].
To check the influence of each process step on the water-holding capacity, the samples were analyzed for their drip loss (DL). As muscle fiber disintegration is associated with increased DL [6], it is considered as a quality determining product parameter in this study. The centrifugation method was chosen over the sedimentation method, i.e., without applying an external force other than gravity (e.g., 24 h, 1 g) [14], as preliminary experiments revealed no DL on hamburgers over several days (data not shown). Hamm [27] described that all methods determining the free-water content can be used to determine the water-holding capacity of meat. Both traditional sedimentation, as well as accelerated methods applying centrifugal forces, are applicable. It is assumed that the sedimentation method might be more suitable for meat samples releasing free water easily, e.g., due to meat defects [21]. The DL increased slightly but not significantly from 6.61 ± 2.83% in the meat piece to 10.50 ± 3.45% after the first grinding (Figure 2B). For all other process steps, the DL remains nearly constant at 10.38 to 11.15%. As the volume reduction of the meat particles in the first grinding is stronger compared to the reduction in the second grinding, more muscle fibers are cut, and more intramuscular fibrillar substances might leak out. Thus, it is reasonable that the DL slightly increased during the first grinding step. In contrast to the present study, Tyszkiewicz et al. [12] found a more pronounced positive correlation between muscle fiber disintegration caused by rupture of myofibrils and water release from mechanically stressed pork meat. Additionally, they found that meat treated with a meat grinder lost more water compared to meat treated gentler with a meat activator or tenderizer. This underlines the hypothesis of a higher DL with increasing ANIC [12] but could not be fully confirmed in this study. Following the idea of Hughes et al. [28], that loss of moisture reduces the muscles’ rigidity and structure, it is reasonable that the histologically determined ANIC increases (Figure 2A) due to the grinding steps.
3.3. Changes in Chemical and Quality Properties
To check changes in the chemical and quality properties, EMS were analyzed for their relative LDH activity, their SPC, and Mb. As the aim of the study was to detect changes in the structural, functional, and chemical properties of samples with different mechanical treatment, the EMS were produced by a gentle extraction process without homogenization. A homogenization would mechanically disrupt the muscle fiber structure completely; this structural damage would superpose the process related changes and would, thus, rule out the differentiation between the samples.
LDH doubled (factor 2.20) during the first grinding step and further increased during the second grinding (Figure 4A). Only slight changes were caused by mixing, whereas forming caused a comparable increase to the second grinding (factor 1.70, Figure 4B). This might be attributed to a stronger LDH release from the muscle fibers due to the applied pressure. Considering the statics, the statistically significant increases in LDH were caused by the first and second grinding steps and forming. This result is partly comparable to the histologically determined ANIC (Figure 2A). Moreover, this shows that LDH might serve as a chemical indicator for muscle fiber disintegration when the raw material is known and analyzed as a reference. An LDH increase was expected, as a disruption of muscle fibers by grinding and a subsequent release of muscle fiber content leading to an increase in LDH activity was reported earlier [12,15]. Similarly, LDH activity determination is a common measure to distinguish between chilled and frozen meat caused by increased enzyme release upon freezing [15].
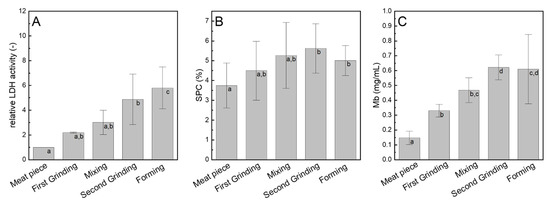
Figure 4.
Characterization of the chemical and quality properties: (A) relative lactate dehydrogenase activity (LDH), (B) myoglobin content (Mb), and (C) soluble protein content (SPC) of the extracted meat solutions at different processing stages. Data points with different letters are significantly different (p < 0.05), n = 3.
A slight increase in the amount of SPC during processing could be found (Figure 4B). However, statistically significant differences were only analyzed between the second grinding step and forming and the meat piece. Moreover, SPC did not change in the same manner as the histologically determined ANIC. An increase in the soluble protein content was expected, as Tyszkiewicz et al. [12] stated, that a mechanical rupture of the meat muscle fibers opens the internal structure of meat tissue, thus making the (myofibrillar) proteins available for extraction. They found a significantly higher proportion of extracted water-soluble (sarcoplasmic) protein in the ground sample compared with the sample treated with the tenderizer or activator. However, this trend was only slightly pronounced in the study. This might be traced back to the use of different analytical methods. Tyszkiewicz et al. [12] used the Helander procedure [29] which is used to detect the protein availability, whereas in this study the total amount of nitrogen compounds was determined. It is possible that other soluble nitrogen compounds, such as non-protein nitrogen compounds, dipeptides, or amino acids remain in the extract upon filtration and overlay the effect of the processing steps on the SPC.
During the first grinding step, Mb approximately doubled from 0.15 ± 0.05 mg/mL in raw material to 0.33 ± 0.04 mg/mL after the first grinding (Figure 4C). The mixing and second grinding step further increased Mb (0.62 ± 0.08 mg/mL after second grinding). The subsequent forming step slightly reduced Mb. It can be concluded that the first and second grinding steps increase Mb the strongest. The Mb analysis is based on specific absorbance spectra of myoglobin derivates [30]. As myoglobin is present in cardiac and skeletal muscles and is released upon muscle fiber damage during acute myocardial infarctions, it serves as a biochemical indicator [31,32]. Meat grinding also disintegrates muscle structures, thus an increase of Mb with increasing ANIC is assumed. The strongest increase in the myoglobin content in the first and second grinding steps is in accordance with the findings of the histologically determined ANIC (Figure 2A) and SPC (Figure 4B). This proves the hypothesis that a higher ANIC leads to an enhanced release of intramuscular fibrillar compounds, such as myoglobin, and that the myoglobin content can serve as a chemical indicator for estimation of muscle fiber disintegration in this study.
The outcomes suggest that the grinding steps are key points for muscle fiber disintegration. When it comes to optimization toward a gentler beef hamburger manufacturing process, those should be taken under close investigation. Based on the present findings, it is assumed that adjustments in the grinding steps might lead to optimizations of the beef hamburger manufacturing process, thus optimizing the burger’s quality.
3.4. Mechanical Considerations
Figure 5 illustrates changes in the meat structure as well as the mechanical stress acting on the meat during the different processing steps.
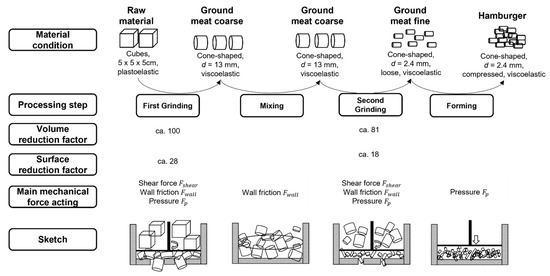
Figure 5.
Changes in material conditions and main mechanical forces in meat upon hamburger manufacturing.
As previously discussed, the first and second grinding steps account for the strongest structural, functional, and chemical changes. During grinding, particle sizes are reduced by cutting and shearing the meat through end-hole plates. Muscle fibers are fragmented into smaller pieces, muscle structures are disintegrated, and more open ends are created [2,10,13]. This can be underlined by the mechanical forces acting on the meat in comparison to the mixing and forming steps where mainly wall friction and pressure applied to the meat; shear force, wall friction, and pressure stresses the meat during grinding, therefore, applying a higher mechanical load. This leads to stronger disintegration of the material [10].
In the first grinding step, meat is cut from a cube into cylindrical-cone-shaped particles of 13 mm diameter, thereby reducing the surface area and volume per particle but increasing the total number of particles and the total surface area. This leads to an increased number of cut muscle fibers. During the second grinding, the particles are ground to a final particle size of 2.4 mm diameter, which further decreases the surface area (factor 18, Figure 5) and volume per particle and the increasing total number of particles and total surface area. A stronger increase during grinding is in accordance with the findings of Beneke [2] who reported a cumulative increase in muscle fiber disintegration if several mechanical methods are combined. Another reason for the stronger increase in ANIC after the second grinding is the higher grinding screw pressure of 3.63 ± 0.68 bar in the second grinding step (Figure 6) compared to a strongly variating and lower pressure in the first grinding step of 0.72 ± 0.29 bar (Figure 6).
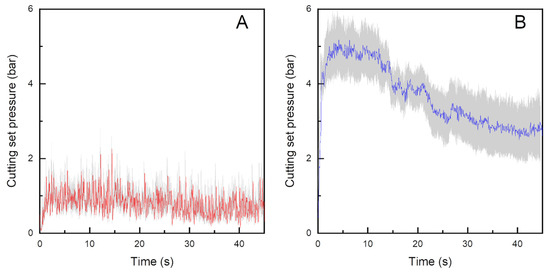
Figure 6.
Mean (red and blue lines) and standard errors (grey lines) of cutting set pressures during the first (red line) (A) and second (blue line) (B) grinding steps in beef patty manufacturing, n = 12.
This might be attributed to the higher cutting resistance in already ground meat, which has more viscoelastic properties compared to the intact meat part in which elastoplastic properties dominate [10]. Higher pressure is, according to Wild et al. [33], associated with increased friction and destruction of the meat. Fluctuations in cutting set pressure might be caused by fluctuating raw material quality, as the processability of the meat strongly depends on the meat’s properties and thus, determine the cutting set pressures.
4. Conclusions
It was shown in this study, that meat muscle fiber structure changes upon production of beef hamburgers, thus altering those attributes. Each process step contributes to a different extent to the material changes, as different forces act on the meat. The first and the second grinding were found to have the strongest impact as they caused a pronounced increase in the analyzed attributes, ANIC, LDH, Mb, and SPC. Those findings allow for an improvement of hamburger production by adjusting the grinding parameters, now known as the most influential processing step, and thus, to meet product quality and legal requirements.
Author Contributions
Conceptualization, L.M.B. and M.G.; data curation, L.M.B.; formal analysis, L.M.B. and M.G.; funding acquisition, F.W., N.T., J.W. and M.G.; investigation, L.M.B.; methodology, L.M.B.; project administration, L.M.B., F.W., N.T., J.W. and M.G.; resources, L.M.B.; software, L.M.B.; supervision, J.W. and M.G.; validation, L.M.B. and M.G.; visualization, L.M.B.; writing—original draft, L.M.B.; writing—review and editing, L.M.B., J.W. and M.G. All authors have read and agreed to the published version of the manuscript.
Funding
This IGF Project of the FEI was supported via AiF within the program for promoting the Industrial Collective Research (IGF) of the German Ministry of Economics and Climate Action (BMWK) based on a resolution of the German Parliament. Project AiF 20384 N.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Most data are included in the article.
Acknowledgments
We would like to thank our head butcher Kurt Herrmann for his help during manufacturing as well as Mona Esch for her support during the analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bundesanstalt für Landwirtschaft und Ernährung; Leitsätze für Fleisch & Fleischerzeugnisse: Bonn, Germany, 2015.
- Beneke, B. Technologie verändert die Muskelstruktur: Histologische Identifikation und Beurteilung bei Fleisch und Fleischerzeugnissen. Fleischwirtschaft 2018, 2018, 62–68. [Google Scholar]
- Tornberg, E. Effects of heat on meat proteins—Implications on structure and quality of meat products. Meat Sci. 2005, 70, 493–508. [Google Scholar] [CrossRef] [PubMed]
- Haack, E.; Schnäckel, W.; Haack, O. Optimal Fördern und Zerkleinern: Grundlagen und Vorgänge bei der Fleischbearbeitung mit Maschinen der Wolftechnologie. Fleischwirtschaft 2003, 2003, 41–47. [Google Scholar]
- Knipe, C.L.; Rust, R.E. Processing equipment—Mixing and cutting equipment. In Encyclopedia of Meat Sciences; Dikeman, M., Devine, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 2, pp. 126–130. [Google Scholar]
- Haack, E.; Schnäckel, W.; Haack, O. Probleme, Ursachen und Lösungen: Grundlagen und Vorgänge bei der Fleischbearbeitung mit Maschinen der Wolftechnologie. Fleischwirtschaft 2003, 2003, 52–56. [Google Scholar]
- Schnäckel, W.; Krickmeier, J.; Schnäckel, D.; Micklisch, I.; Haack, O. Untersuchungen zur Optimierung des Wolfprozesses: Teil 4: Anwendung des Wolfprozesses auf die Feinbrätherstellung. Fleischwirtschaft 2012, 92, 91–96. [Google Scholar]
- Jurgens, A.; Mooij, J.D.; Logtenberg, H.; Verkleij, T.J. Physico-chemical characteristics of ground meat relevant for patty forming and end product quality. In Proceedings of the XVIIth European Symposium on the Quality of Poultry Meat, Doorwerth, The Netherlands, 23–26 May 2005. [Google Scholar]
- Haack, E.; Sielaff, H. Der Rohstoff Fleisch hat seine Tücken. Fleischwirtschaft 2005, 2005, 59–64. [Google Scholar]
- Krickmeier, J. Modellierung der Bedingungen Beim Schneiden Insbesondere in Einer Wolfmaschine mit dem Ziel der Optimierung des Zerkleinerungsprozesses Sowie der Erhöhung der Produktqualität. Ph.D. Thesis, Martin-Luther-Universität Halle-Wittenberg, Halle, Germany, 2015. [Google Scholar]
- Raudsepp, P.; Brüggemann, D.A.; Henckel, P.; Vyberg, M.; Groves, K.; Oksbjerg, N.; Therkildsen, M. Performance of conventional histochemical methods relative to a novel immunolabeling technique in assessing degree of degradation in comminuted chicken meat. Food Control 2017, 73, 133–139. [Google Scholar] [CrossRef]
- Tyszkiewicz, I.; Kłossowska, B.M.; Wieczorek, U.; Jakubiec-Puka, A. Mechanical tenderisation of pork meat: Protein and water release due to tissue damage. J. Sci. Food Agric. 1997, 73, 179–185. [Google Scholar] [CrossRef]
- Honikel, K.O. Minced meats. In Encyclopedia of Meat Sciences; Dikeman, M., Devine, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 2, pp. 422–424. [Google Scholar]
- Honikel, K.O.; Hamm, R. Measurement of water-holding capacity and juiciness. In Quality Attributes and Their Measurement in Meat, Poultry and Fish Products; Pearson, A.M., Dutson, T.R., Eds.; Springer: Boston, MA, USA, 1994; Volume 8, pp. 125–161. [Google Scholar]
- Ballin, N.Z.; Lametsch, R. Analytical methods for authentication of fresh vs. thawed meat—A review. Meat Sci. 2008, 80, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Lebensmittelsicherheit BfVu. Amtliche Sammlung von Untersuchungsverfahren nach § 64 LFGB, § 38 TabakerzG, § 28b GenTG: Verfahren zur Probenahme und Untersuchung von Lebensmitteln; Beuth: Berlin, Germany, 2006. [Google Scholar]
- Mariotti, F.; Tomé, D.; Mirand, P.P. Converting nitrogen into protein—beyond 6.25 and Jones’ factors. Crit. Rev. Food Sci. Nutr. 2008, 48, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Irmscher, S.B. Continuous Structure Formation in Meat Products Using the Vane Pump Grinder Technology. Ph.D. Thesis, Universität Hohenheim, Stuttgart, Germany, 2015. [Google Scholar]
- GmbH, S.-A.C. Laktatdehydrogenase-Aktivitätsassay-Kit: Product Information. Available online: https://www.sigmaaldrich.com/DE/de/product/sigma/mak066 (accessed on 19 November 2021).
- Trout, G.R. Variation in myoglobin denaturation and color of cooked beef, pork, and turkey meat as influenced by pH, sodium chloride, sodium tripolyphosphate, and cooking temperature. J. Food Sci. 1989, 54, 536–540. [Google Scholar] [CrossRef]
- Belitz, H.-D.; Grosch, W.; Schieberle, P. Lehrbuch der Lebensmittelchemie, 6th ed.; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Berry, B.W.; Abraham, H.C. Sensory, shear force and cooking properties of commercially processed ground beef patties. Food Qual. Prefer. 1996, 7, 55–59. [Google Scholar] [CrossRef]
- Keeton, J.T.; Eddy, S. Chemical and physical characteristics of meat: Chemical Composition. In Encyclopedia of Meat Sciences; Jensen, W.K., Devine, C., Dikemann, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2004; pp. 210–218. [Google Scholar]
- Xing, T.; Gao, F.; Tume, R.K.; Zhou, G.; Xu, X. Stress effects on meat quality: A mechanistic perspective. Compr. Rev. Food Sci. Food Saf. 2019, 18, 380–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walsh, H.M.; Kerry, J.P. Meat packaging. In Meat Processing; Kerry, J., Kerry, J., Ledward, D., Eds.; Woodhead Publishing in Food Science and Technology; CRC Press: Boca Raton, FL, USA; Cambridge, UK, 2002. [Google Scholar]
- Pearson, A.M.; Tauber, F.W. Processed Meats, 2nd ed.; Springer: Dordrecht, The Netherlands, 1984; p. 438. [Google Scholar]
- Hamm, R. Kolloidchemie des Fleisches: Das Wasserbindungsvermögen des Muskeleiweisses in Theorie und Praxis; Parey: Berlin, Germany, 1972; p. 275. [Google Scholar]
- Hughes, J.M.; Oiseth, S.K.; Purslow, P.P.; Warner, R.D. A structural approach to understanding the interactions between colour, water-holding capacity and tenderness. Meat Sci. 2014, 98, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Helander, E. On quantitative muscle protein determination; sarcoplasm and myofibril protein content of normal and atrophic skeletal muscles. Acta Physiol. Scand. Suppl. 1957, 41, 1–99. [Google Scholar] [PubMed]
- Millar, S.J.; Moss, B.W.; Stevenson, M.H. Some observations on the absorption spectra of various myoglobin derivatives found in meat. Meat Sci. 1996, 42, 277–288. [Google Scholar] [CrossRef]
- Woo, J.; Lacbawan, F.L.; Sunheimer, R.; LeFever, D.; McCabe, J.B. Is myoglobin useful in the diagnosis of acute myocardial infarction in the emergency department setting? Am. J. Clin. Pathol. 1995, 103, 725–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plebani, M.; Zaninotto, M. Diagnostic strategies using myoglobin measurement in myocardial infarction. Clin. Chim. Acta 1998, 272, 69–77. [Google Scholar] [CrossRef]
- Wild, J.L.; Sebranek, J.G.; Olson, D.G. Grinding time and pressure developed in beef and pork: Effects of temperature and fat. J. Food Sci. 1991, 56, 1171–1175. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).