Sasa borealis Ethanol Extract Protects PC12 Neuronal Cells against Oxidative Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Antioxidant Activity Assay
2.2.1. ABTS+ Radical Scavenging Assay
2.2.2. DPPH Radical Scavenging Assay
2.3. Cell Culture
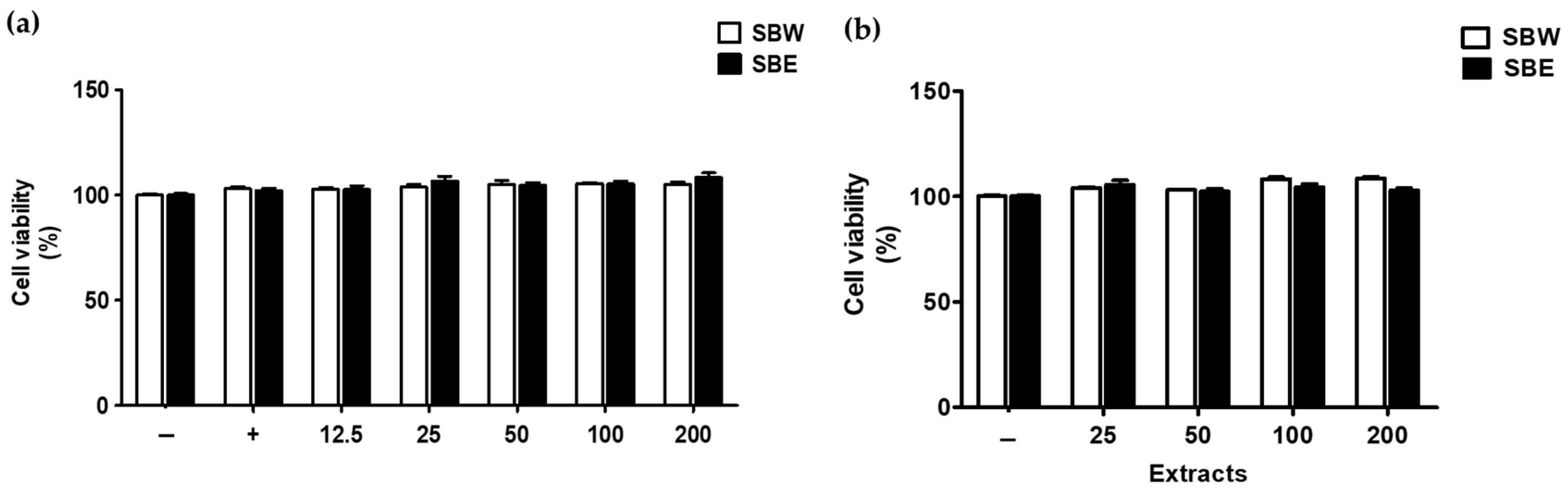
2.4. Cell Viability Assay
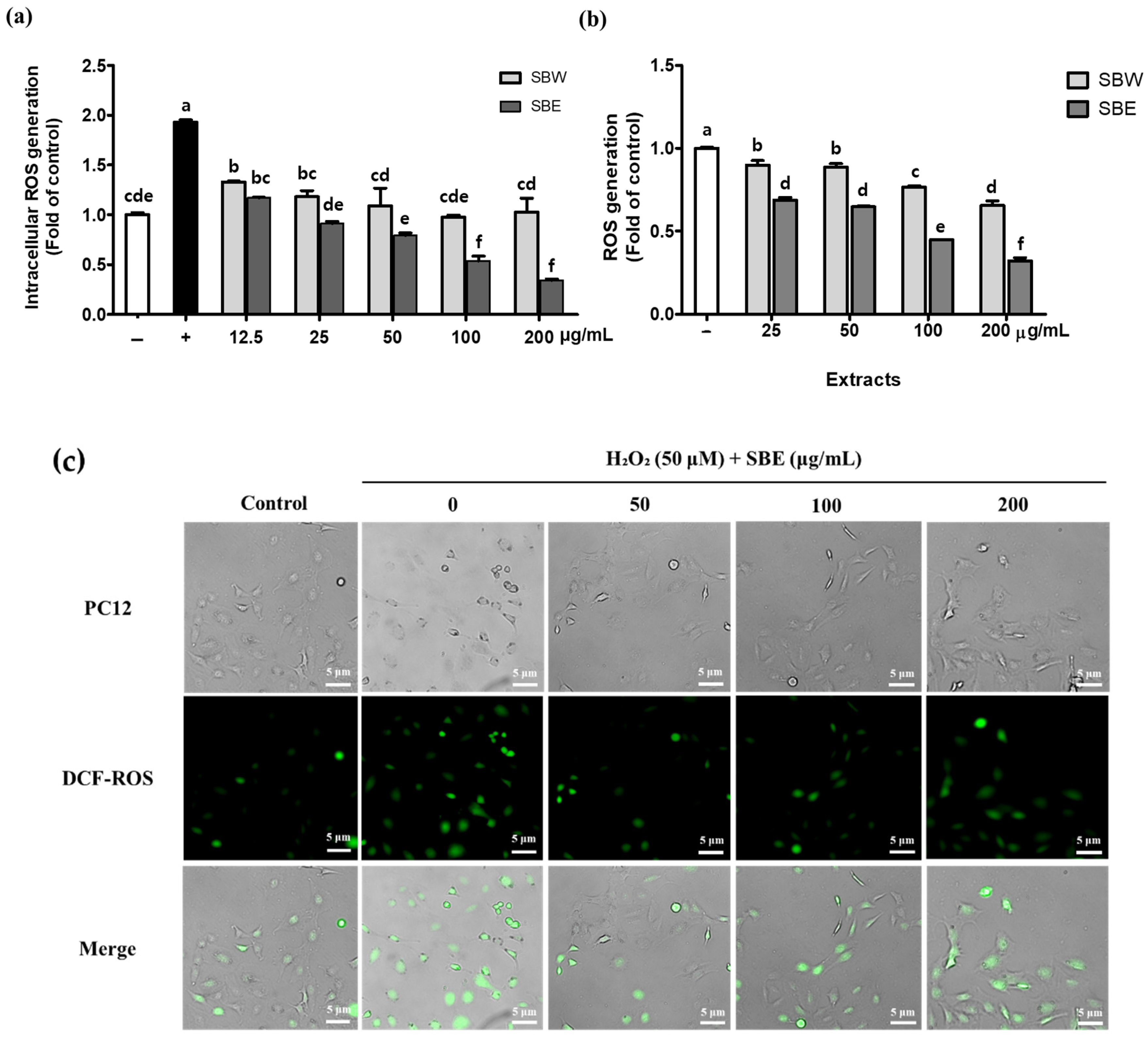
2.5. Measurement of Intracellular ROS
2.6. High-Performance Liquid Chromatography Analysis of Phenolic Acids and Flavonoids
2.7. Western Blotting
2.8. Statistics
3. Results
3.1. SB Extracts Show an Antioxidant Effect by Scavenging Free Radicals
3.2. SB Extracts Decrease H2O2-Induced ROS Production in PC12 Neuronal Cells
3.3. Isoorientin and Caffeic Acid of SB Extracts Were Identified by HPLC Analysis
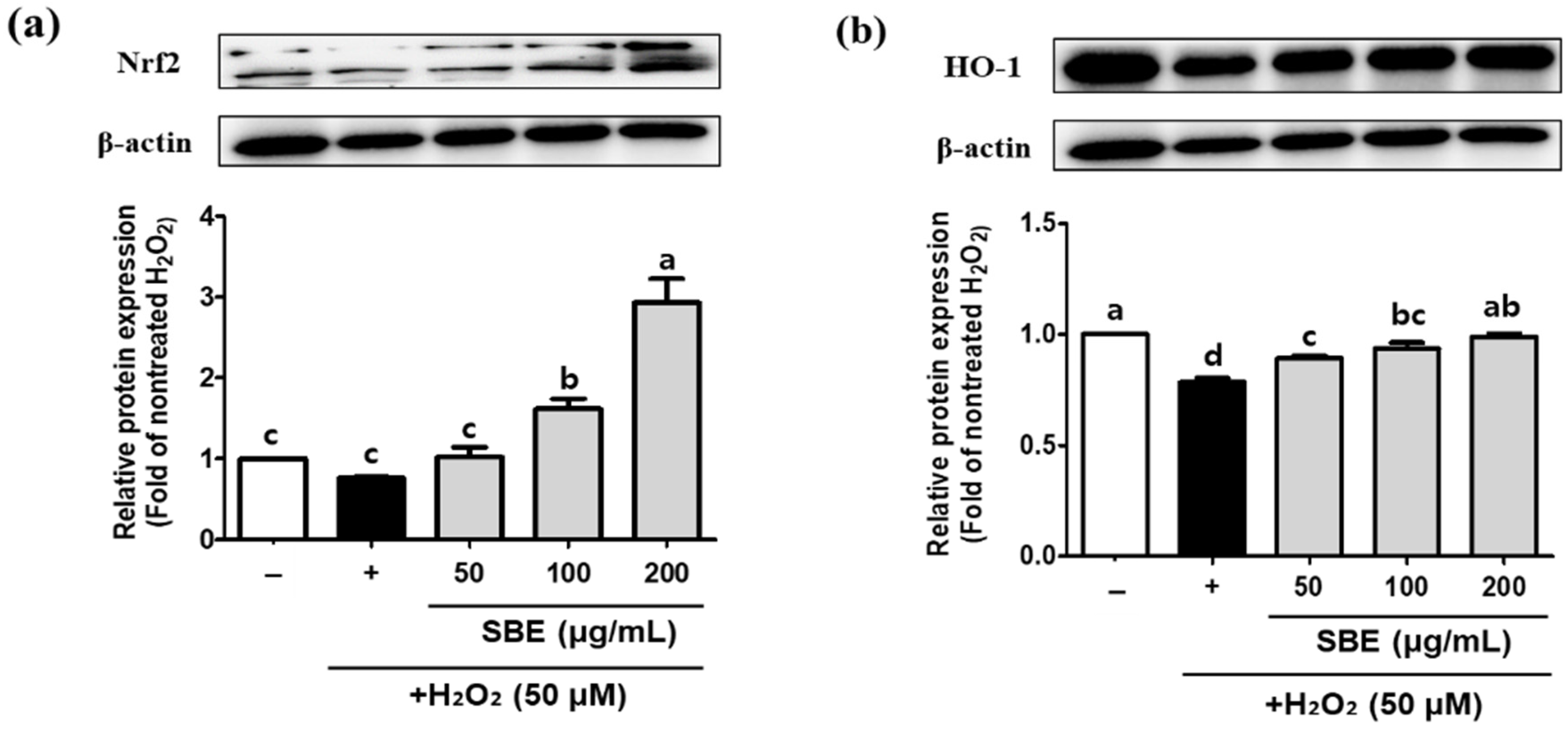
3.4. SBE Increases the Protein Levels of Nrf2, HO-1, SOD2, CAT, and GPx
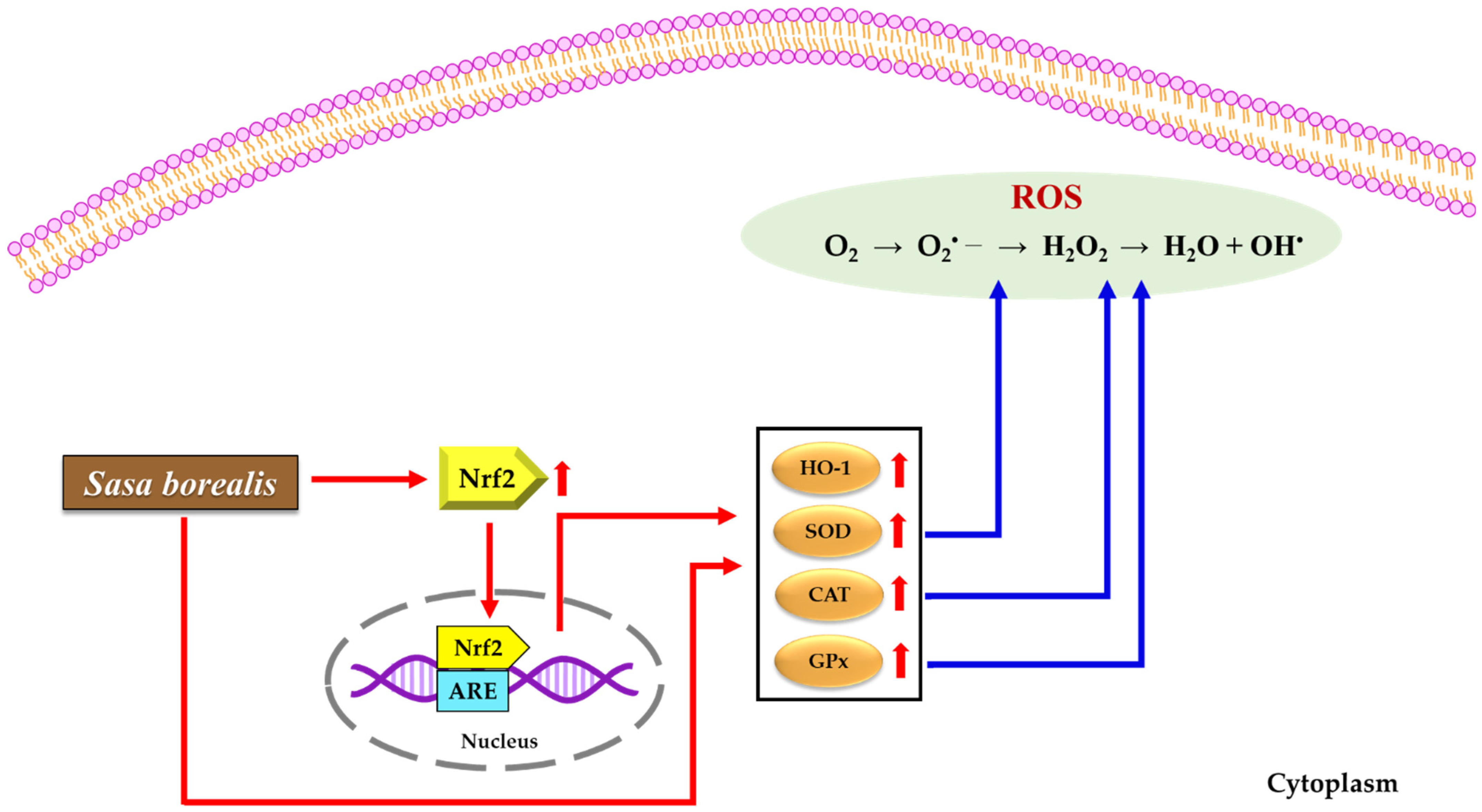
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Limón-Pacheco, J.; Gonsebatt, M.E. The role of antioxidants and antioxidant-related enzymes in protective responses to environmentally induced oxidative stress. Mutat. Res. Genet. Toxicol. Environ. Mutagenes. 2009, 674, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, V.; Sharma, S. Role of mitochondrial dysfunction, oxidative stress and autophagy in progression of Alzheimer’s disease. J. Neurol. Sci. 2021, 421, 117253. [Google Scholar] [CrossRef]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Wu, X.; Shi, J.; Dong, Y.; Zhang, Y. Toxicology and safety of antioxidant of bamboo leaves. Part 2: Developmental toxicity test in rats with antioxidant of bamboo leaves. Food Chem. Toxicol. 2006, 44, 1739–1743. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Wu, X.; Tie, X.; Zhang, Y.; Zhang, Y. Toxicology and safety of anti-oxidant of bamboo leaves. Part 1: Acute and subchronic toxicity studies on anti-oxidant of bamboo leaves. Food Chem. Toxicol. 2005, 43, 783–792. [Google Scholar] [CrossRef]
- Ko, B.-S.; Jun, D.-W.; Jang, J.-S.; Kim, J.-H.; Park, S.-M. Effect of Sasa borealis and white lotus roots and leaves on insulin action and secretion in vitro. Korean J. Food Sci. Technol. 2006, 38, 114–120. [Google Scholar]
- Choi, Y.-J.; Lim, H.-S.; Choi, J.-S.; Shin, S.-Y.; Bae, J.-Y.; Kang, S.-W.; Kang, I.-J.; Kang, Y.-H. Blockade of chronic high glucose–induced endothelial apoptosis by Sasa borealis bamboo extract. Exp. Biol. Med. 2008, 233, 580–591. [Google Scholar] [CrossRef]
- Kim, E.-Y.; Jung, E.-Y.; Lim, H.-S.; Heo, Y.-R. The effects of the Sasa borealis leaves extract on plasma adiponectin, resistin, C-reactive protein and homocysteine levels in high fat diet-induced obese C57/BL6J mice. J. Nutr. Health 2007, 40, 303–311. [Google Scholar]
- Park, H.-S.; Lim, J.H.; Kim, H.J.; Choi, H.J.; Lee, I.-S. Antioxidant flavone glycosides from the leaves of Sasa borealis. Arch. Pharmacal. Res. 2007, 30, 161–166. [Google Scholar] [CrossRef]
- Sangeetha, R.; Diea, Y.; Chaitra, C.; Malvi, P.; Shinomol, G. The amazing bamboo: A review on its medicinal and pharmacological potential. Indian J. Nutr. 2015, 2, 1–7. [Google Scholar]
- Sun, A.; Ren, G.; Deng, C.; Zhang, J.; Luo, X.; Wu, X.; Mani, S.; Dou, W.; Wang, Z. C-glycosyl flavonoid orientin improves chemically induced inflammatory bowel disease in mice. J. Funct. Foods 2016, 21, 418–430. [Google Scholar] [CrossRef]
- Park, E.-J.; Jhon, D.-Y. Effects of bamboo shoot consumption on lipid profiles and bowel function in healthy young women. Nutrition 2009, 25, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Molina-Cortes, A.; Sanchez-Motta, T.; Tobar-Tosse, F.; Quimbaya, M. Spectrophotometric estimation of total phenolic content and antioxidant capacity of molasses and vinasses generated from the sugarcane industry. Waste Biomass Valorization 2020, 11, 3453–3463. [Google Scholar] [CrossRef] [Green Version]
- Bello, O.M.; Ogbesejana, A.B.; Adetunji, C.O.; Oguntoye, S.O. Flavonoids isolated from Vitex grandifolia, an underutilized vegetable, exert monoamine A & B inhibitory and anti-inflammatory effects and their structure-activity relationship. Turk. J. Pharm. Sci. 2019, 16, 437. [Google Scholar]
- Fan, X.; Lv, H.; Wang, L.; Deng, X.; Ci, X. Isoorientin ameliorates APAP-induced hepatotoxicity via activation Nrf2 antioxidative pathway: The involvement of AMPK/Akt/GSK3β. Front. Pharmacol. 2018, 9, 1334. [Google Scholar] [CrossRef]
- Wu, Q.-Y.; Wong, Z.C.-F.; Wang, C.; Fung, A.H.-Y.; Wong, E.O.-Y.; Chan, G.K.-L.; Dong, T.T.-X.; Chen, Y.; Tsim, K.W.-K. Isoorientin derived from Gentiana veitchiorum Hemsl. flowers inhibits melanogenesis by down-regulating MITF-induced tyrosinase expression. Phytomedicine 2019, 57, 129–136. [Google Scholar] [CrossRef]
- Yuan, L.; Wang, J.; Xiao, H.; Xiao, C.; Wang, Y.; Liu, X. Isoorientin induces apoptosis through mitochondrial dysfunction and inhibition of PI3K/Akt signaling pathway in HepG2 cancer cells. Toxicol. Appl. Pharmacol. 2012, 265, 83–92. [Google Scholar] [CrossRef]
- Lee, W.; Ku, S.-K.; Bae, J.-S. Vascular barrier protective effects of orientin and isoorientin in LPS-induced inflammation in vitro and in vivo. Vasc. Pharmacol. 2014, 62, 3–14. [Google Scholar] [CrossRef]
- Magesh, S.; Chen, Y.; Hu, L. Small molecule modulators of K eap1-N rf2-ARE pathway as potential preventive and therapeutic agents. Med. Res. Rev. 2012, 32, 687–726. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.; Lee, K.Y.; Park, B. Isoorientin Inhibits Amyloid β25–35-Induced Neuronal Inflammation in BV2 Cells by Blocking the NF-κB Signaling Pathway. Molecules 2021, 26, 7056. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liu, N.; Zhu, C.; Ma, L.; Yang, J.; Du, J.; Zhang, W.; Sun, T.; Niu, J.; Yu, J. Antinociceptive effect of isoorientin against neuropathic pain induced by the chronic constriction injury of the sciatic nerve in mice. Int. Immunopharmacol. 2019, 75, 105753. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velichkova, M.; Hasson, T. Keap1 regulates the oxidation-sensitive shuttling of Nrf2 into and out of the nucleus via a Crm1-dependent nuclear export mechanism. Mol. Cell. Biol. 2005, 25, 4501–4513. [Google Scholar] [CrossRef] [Green Version]
- Cai, S.-Q.; Zhang, K.-F.; Cai, X.-H. Recent Advances in Dimethyl Sulfoxide (DMSO) Used as a Multipurpose Reactant. Curr. Org. Chem. 2022, 26, 91–121. [Google Scholar] [CrossRef]
- Truong, D.-H.; Nguyen, D.H.; Ta, N.T.A.; Bui, A.V.; Do, T.H.; Nguyen, H.C. Evaluation of the Use of Different Solvents for Phytochemical Constituents, Antioxidants, and In Vitro Anti-Inflammatory Activities of Severinia buxifolia. J. Food Qual. 2019, 2019, 8178294. [Google Scholar] [CrossRef] [Green Version]
- Van den Berg, R.; Haenen, G.R.; van den Berg, H.; Bast, A. Applicability of an improved Trolox equivalent antioxidant capacity (TEAC) assay for evaluation of antioxidant capacity measurements of mixtures. Food Chem. 1999, 66, 511–517. [Google Scholar] [CrossRef]
- Bitalebi, S.; Nikoo, M.; Rahmanifarah, K.; Noori, F.; Ahmadi Gavlighi, H. Effect of apple peel extract as natural antioxidant on lipid and protein oxidation of rainbow trout (Oncorhynchus mykiss) mince. Int. Aquat. Res. 2019, 11, 135–146. [Google Scholar] [CrossRef] [Green Version]
- Kelly, E.; Vyas, P.; Weber, J.T. Biochemical properties and neuroprotective effects of compounds in various species of berries. Molecules 2017, 23, 26. [Google Scholar] [CrossRef] [Green Version]
- Tsiepe, T.J.; Kabanda, M.M.; Serobatse, K. Antioxidant properties of kanakugiol revealed through the hydrogen atom transfer, electron transfer and M2+ (M2+ = Cu (II) or Co (II) ion) coordination ability mechanisms. A DFT study in vacuo and in solution. Food Biophys. 2015, 10, 342–359. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, G.; Zang, X.; Ye, F. Optimization, antioxidant activity and bile salts adsorption capacity of the aqueous enzymatic extract from rice bran. Czech J. Food Sci. 2018, 36, 338–348. [Google Scholar]
- Park, Y.-O.; Lim, H.-S. Antioxidant activities of bamboo (Sasa borealis) leaf extract according to extraction solvent. J. Korean Soc. Food Sci. Nutr. 2009, 38, 1640–1648. [Google Scholar] [CrossRef]
- Furger, C. Live Cell Assays for the Assessment of Antioxidant Activities of Plant Extracts. Antioxidants 2021, 10, 944. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zhang, B.; Liu, J.; Qiao, C.; Liu, Y.; Li, S.; Lv, H. Isoorientin exerts a protective effect against 6-OHDA-induced neurotoxicity by activating the AMPK/AKT/Nrf2 signalling pathway. Food Funct. 2020, 11, 10774–10785. [Google Scholar] [CrossRef]
- Yuan, L.; Ren, X.; Wu, Y.; Wang, J.; Xiao, H.; Liu, X. Isoorientin protects BRL-3A rat liver cell against hydrogen peroxide-induced apoptosis by inhibiting mitochondrial dysfunction, inactivating MAPKs, activating Akt and scavenging ROS and NO. Biomed. Aging Pathol. 2013, 3, 153–159. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, M.; Luo, H.; Li, H. Isoorientin alleviates UVB-induced skin injury by regulating mitochondrial ROS and cellular autophagy. Biochem. Biophys. Res. Commun. 2019, 514, 1133–1139. [Google Scholar] [CrossRef]
- Anilkumar, K.; Reddy, G.V.; Azad, R.; Yarla, N.S.; Dharmapuri, G.; Srivastava, A.; Kamal, M.A.; Pallu, R. Evaluation of anti-inflammatory properties of isoorientin isolated from tubers of Pueraria tuberosa. Oxidative Med. Cell. Longev. 2017, 2017, 5498054. [Google Scholar] [CrossRef] [Green Version]
- Mthembu, S.X.; Muller, C.J.; Dludla, P.V.; Madoroba, E.; Kappo, A.P.; Mazibuko-Mbeje, S.E. Rooibos Flavonoids, Aspalathin, Isoorientin, and Orientin Ameliorate Antimycin A-Induced Mitochondrial Dysfunction by Improving Mitochondrial Bioenergetics in Cultured Skeletal Muscle Cells. Molecules 2021, 26, 6289. [Google Scholar] [CrossRef]
- Luo, T. Protective effect of isoorientin on oleic acid-induced oxidative damage and steatosis in rat liver cells. Front. Pharmacol. 2022, 13, 818159. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Talalay, P. Direct and indirect antioxidant properties of inducers of cytoprotective proteins. Mol. Nutr. Food Res. 2008, 52, S128–S138. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Alam, M.B.; Quan, K.T.; Kwon, K.-R.; Ju, M.-K.; Choi, H.-J.; Lee, J.S.; Yoon, J.-I.; Majumder, R.; Rather, I.A. Antioxidant efficacy and the upregulation of Nrf2-mediated HO-1 expression by (+)-lariciresinol, a lignan isolated from Rubia philippinensis, through the activation of p38. Sci. Rep. 2017, 7, 46035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lakhan, S.E.; Kirchgessner, A.; Hofer, M. Inflammatory mechanisms in ischemic stroke: Therapeutic approaches. J. Transl. Med. 2009, 7, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jun, Y.-J.; Lee, M.; Shin, T.; Yoon, N.; Kim, J.-H.; Kim, H.-R. Eckol enhances heme oxygenase-1 expression through activation of Nrf2/JNK pathway in HepG2 cells. Molecules 2014, 19, 15638–15652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ighodaro, O.; Akinloye, O. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.Q.; Kosten, T.R.; Zhang, X.Y. Free radicals, antioxidant defense systems, and schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 46, 200–206. [Google Scholar] [CrossRef]
- Battin, E.E.; Brumaghim, J.L. Antioxidant activity of sulfur and selenium: A review of reactive oxygen species scavenging, glutathione peroxidase, and metal-binding antioxidant mechanisms. Cell Biochem. Biophys. 2009, 55, 1–23. [Google Scholar] [CrossRef]


| Extract | DPPH 1 (mg AAE 5/g, d.b.) | ABTS+ 2 (mg AAE 5/g, d.b.) | Yields (%) |
|---|---|---|---|
| SBW 3 | 60.91 ± 0.13 a | 165.14 ± 5.08 a | 14.2 |
| SBE 4 | 49.63 ± 1.02 b | 144.84 ± 2.82 b | 16.9 |
| No. | Phenolic Acids | Content (mg/g Extract, Dried Basis) | |
|---|---|---|---|
| SBW 1 | SBE 2 | ||
| 6 | Caffeic acid | 0.72 ± 0.00 b | 0.73 ± 0.01 a |
| 10 | Isoorientin | 2.70 ± 0.03 b | 6.75 ± 0.17 a |
| Factors | DPPH 1 | ABTS+ 1 | Isoorientin | Caffeic Acid | ROS 2 |
|---|---|---|---|---|---|
| DPPH 1 | 1.000 | ||||
| ABTS+ 1 | 0.933 ** | 1.000 | |||
| Isoorientin | 0.995 *** | 0.959 ** | 1.000 | ||
| Caffeic acid | 0.889 * | 0.902 * | 0.893 * | 1.000 | |
| ROS 2 | −0.932 ** | −0.887 * | −0.925 ** | −0.768 | 1.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, M.H.; Ji, Y.-J.; Han, Y.M.; Jang, G.Y.; Kim, D.H.; Lee, J.H.; Kim, G.-S.; Choi, S.J.; Kim, H.D. Sasa borealis Ethanol Extract Protects PC12 Neuronal Cells against Oxidative Stress. Appl. Sci. 2022, 12, 6155. https://doi.org/10.3390/app12126155
Kang MH, Ji Y-J, Han YM, Jang GY, Kim DH, Lee JH, Kim G-S, Choi SJ, Kim HD. Sasa borealis Ethanol Extract Protects PC12 Neuronal Cells against Oxidative Stress. Applied Sciences. 2022; 12(12):6155. https://doi.org/10.3390/app12126155
Chicago/Turabian StyleKang, Min Hye, Yun-Jeong Ji, Yu Mi Han, Gwi Yeong Jang, Dong Hwi Kim, Jeong Hoon Lee, Geum-Soog Kim, Su Ji Choi, and Hyung Don Kim. 2022. "Sasa borealis Ethanol Extract Protects PC12 Neuronal Cells against Oxidative Stress" Applied Sciences 12, no. 12: 6155. https://doi.org/10.3390/app12126155
APA StyleKang, M. H., Ji, Y.-J., Han, Y. M., Jang, G. Y., Kim, D. H., Lee, J. H., Kim, G.-S., Choi, S. J., & Kim, H. D. (2022). Sasa borealis Ethanol Extract Protects PC12 Neuronal Cells against Oxidative Stress. Applied Sciences, 12(12), 6155. https://doi.org/10.3390/app12126155






