Effects of Caffeine and 5-Caffeoylquinic Acid on Blood Cell In Vitro Cytokine Production in Response to Lipopolysaccharide Stimulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Laboratory Visit
2.3. Plasma Caffeine Measurement
2.4. Blood Culture, In Vitro Blood LPS Stimulation and Effects of Caffeine and 5-CQA
2.5. Cytokine Measurements in Cell Culture Supernatants
2.6. Adenosine-3′,5′-Cyclic Monophosphate (cAMP) Measurement
2.7. Malondialdehyde (MDA) Measurement
2.8. Statistical Analysis
3. Results
3.1. Characteristics of Participants in the Study
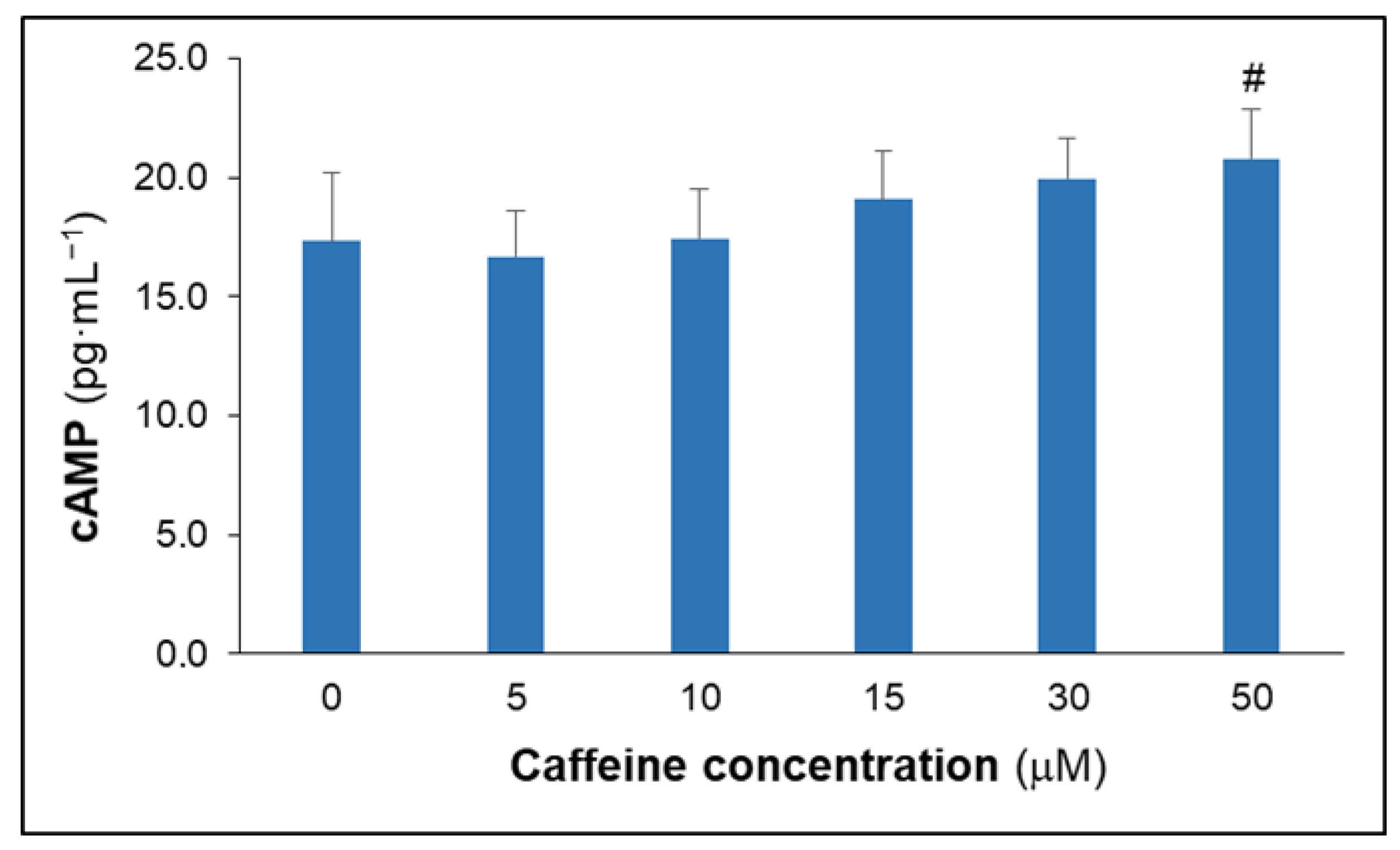
3.2. Effects of Caffeine on Culture Cytokine, MDA and cAMP Concentrations
3.3. Effects of 5-CQA on Culture Cytokine and MDA Concentrations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reyes, C.M.; Cornelis, M.C. Caffeine in the diet: Country-level consumption and guidelines. Nutrients 2018, 10, 1772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faudone, G.; Arifi, S.; Merk, D. The medicinal chemistry of caffeine. J. Med. Chem. 2021, 64, 7156–7178. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Kitts, D.D. Role of Chlorogenic Acids in Controlling Oxidative and Inflammatory Stress Conditions. Nutrients 2015, 8, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neveu, V.; Perez-Jiménez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database 2010, 2010, bap024. [Google Scholar] [CrossRef]
- Farah, A.; de Paula Lima, J. Consumption of chlorogenic acids through coffee and health implications. Beverages 2019, 5, 11. [Google Scholar] [CrossRef] [Green Version]
- Akash, M.S.H.; Rehman, K.; Chen, S. Effects of coffee on type 2 diabetes mellitus. Nutrition 2014, 30, 755–763. [Google Scholar] [CrossRef]
- Ding, M.; Bhupathiraju, S.N.; Chen, M.; van Dam, R.M.; Hu, F.B. Caffeinated and decaffeinated coffee consumption and risk of type 2 diabetes: A systematic review and a dose-response meta-analysis. Diabetes Care 2014, 37, 569–586. [Google Scholar] [CrossRef] [Green Version]
- Shang, F.; Li, X.; Jiang, X. Coffee consumption and risk of the metabolic syndrome: A meta-analysis. Diabetes Metab. 2016, 42, 80–87. [Google Scholar] [CrossRef]
- Ding, M.; Satija, A.; Bhupathiraju, S.N.; Hu, Y.; Sun, Q.; Han, J.; Lopez-Garcia, E.; Willett, W.; van Dam, R.M.; Hu, F.B. Association of coffee consumption with total and cause-specific mortality in 3 large prospective cohorts. Circulation 2015, 132, 2305–2315. [Google Scholar] [CrossRef] [Green Version]
- Grosso, G.; Godos, J.; Galvano, F.; Giovannucci, E.L. Coffee, Caffeine, and Health Outcomes: An Umbrella Review. Annu. Rev. Nutr. 2017, 37, 131–156. [Google Scholar] [CrossRef] [Green Version]
- Torres-Collado, L.; Compañ-Gabucio, L.M.; González-Palacios, S.; Leyre, N.-B.; Oncina-Cánovas, A.; Vioque, J.; García-de la Hera, M. Coffee Consumption and All-Cause, Cardiovascular, and Cancer Mortality in an Adult Mediterranean Population. Nutrients 2021, 13, 1241. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.J.; Fargnoli, J.L.; Hwang, J.J.; van Dam, R.M.; Blackburn, G.L.; Hu, F.B.; Mantzoros, C.S. Coffee Consumption Is Associated with Higher Plasma Adiponectin Concentrations in Women with or Without Type 2 Diabetes A prospective cohort study. Diabetes Care 2008, 31, 504–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotani, K.; Tsuzaki, K.; Sano, Y.; Maekawa, M.; Fujiwara, S.; Hamada, T.; Sakane, N. The relationship between usual coffee consumption and serum C-reactive protein level in a Japanese female population. Clin. Chem. Lab. Med. 2008, 46, 1434–1437. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Garcia, E.; van Dam, R.M.; Qi, L.; Hu, F.B. Coffee consumption and markers of inflammation and endothelial dysfunction in healthy and diabetic women. Am. J. Clin. Nutr. 2006, 84, 888–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maki, T.; Pham, N.M.; Yoshida, D.; Yin, G.; Ohnaka, K.; Takayanagi, R.; Kono, S. The relationship of coffee and green tea consumption with high-sensitivity C-reactive protein in Japanese men and women. Clin. Chem. Lab. Med. 2010, 48, 849–854. [Google Scholar] [CrossRef]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: A comprehensive review of the literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef]
- Hwang, S.J.; Kim, Y.-W.W.; Park, Y.; Lee, H.-J.J.; Kim, K.-W.W. Anti-inflammatory effects of chlorogenic acid in lipopolysaccharide-stimulated RAW 264.7 cells. Inflamm. Res. 2013, 63, 81–90. [Google Scholar] [CrossRef]
- Shan, J.; Fu, J.; Zhao, Z.; Kong, X.; Huang, H.; Luo, L.; Yin, Z. Chlorogenic acid inhibits lipopolysaccharide-induced cyclooxygenase-2 expression in RAW264.7 cells through suppressing NF-KB and JNK/AP-1 activation. Int. Immunopharmacol. 2009, 9, 1042–1048. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Satti, N.K.; Sharma, P.; Sharma, V.K.; Suri, K.A.; Bani, S. Differential effects of chlorogenic acid on various immunological parameters relevant to rheumatoid arthritis. Phytother. Res. 2012, 26, 1156–1165. [Google Scholar] [CrossRef]
- Yun, N.; Kang, J.-W.; Lee, S.-M. Protective effects of chlorogenic acid against ischemia/reperfusion injury in rat liver: Molecular evidence of its antioxidant and anti-inflammatory properties. J. Nutr. Biochem. 2012, 23, 1249–1255. [Google Scholar] [CrossRef]
- Shin, H.S.; Satsu, H.; Bae, M.J.; Zhao, Z.; Ogiwara, H.; Totsuka, M.; Shimizu, M. Anti-inflammatory effect of chlorogenic acid on the IL-8 production in Caco-2 cells and the dextran sulphate sodium-induced colitis symptoms in C57BL/6 mice. Food Chem. 2015, 168, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, M.; Farah, A.; Perrone, D.; Trugo, L.C.; Donangelo, C. Chlorogenic acid compounds from coffee are differentially absorbed and metabolized in humans. J. Nutr. 2007, 137, 2196–2201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horrigan, L.A.; Kelly, J.P.; Connor, T.J. Immunomodulatory effects of caffeine: Friend or foe? Pharmacol. Ther. 2006, 111, 877–892. [Google Scholar] [CrossRef] [PubMed]
- Van Furth, A.M.; Seijmonsbergen, E.M.; Langermans, J.A.; van der Meide, P.H.; van Furth, R. Effect of xanthine derivates and dexamethasone on Streptococcus pneumoniae-stimulated production of tumor necrosis factor alpha, interleukin-1 beta (IL-1 beta), and IL-10 by human leukocytes. Clin. Diagn. Lab. Immunol. 1995, 2, 689–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horrigan, L.A.; Kelly, J.P.; Connor, T.J. Caffeine suppresses TNF-alpha production via activation of the cyclic AMP/protein kinase A pathway. Int. Immunopharmacol. 2004, 4, 1409–1417. [Google Scholar] [CrossRef]
- Tauler, P.; Martinez, S.; Moreno, C.; Monjo, M.; Martinez, P.; Aguilo, A. Effects of caffeine on the inflammatory response induced by a 15-km run competition. Med. Sci. Sport. Exerc. 2013, 45, 1269–1276. [Google Scholar] [CrossRef]
- Segre, E.; Fullerton, J.N. Stimulated whole blood cytokine release as a biomarker of immunosuppression in the critically ill: The need for a standardized methodology. Shock 2016, 45, 490–494. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, R.; Chase, S.D. Rapid, fluorimetric-liquid chromatographic determination of malondialdehyde in biological samples. J. Chromatogr. B. Anal. Technol. Biomed. Life Sci. 2002, 775, 121–126. [Google Scholar] [CrossRef]
- Damsgaard, C.T.; Lauritzen, L.; Calder, P.C.; Kjær, T.M.R.; Frøkiær, H. Whole-blood culture is a valid low-cost method to measure monocytic cytokines—A comparison of cytokine production in cultures of human whole-blood, mononuclear cells and monocytes. J. Immunol. Methods 2009, 340, 95–101. [Google Scholar] [CrossRef]
- Tauler, P.; Martinez, S.; Martinez, P.; Lozano, L.; Moreno, C.; Aguiló, A. Effects of Caffeine Supplementation on Plasma and Blood Mononuclear Cell IL-10 Levels After Exercise. Int. J. Sport Nutr. Exerc. Metab. 2016, 26, 8–16. [Google Scholar] [CrossRef]
- De Leon, J.; Diaz, F.J.; Rogers, T.; Browne, D.; Dinsmore, L.; Ghosheh, O.H.; Dwoskin, L.P.; Crooks, P.A. A pilot study of plasma caffeine concentrations in a US sample of smoker and nonsmoker volunteers. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2003, 27, 165–171. [Google Scholar] [CrossRef]
- Horrigan, L.A.; Diamond, M.; Connor, T.J.; Kelly, J.P. Caffeine inhibits monocyte and neutrophil chemotaxis at concentrations relevant to normal human consumption. In Proceedings of the International Cytokine Society Annual Meeting, Dublin, Ireland, 20–24 September 2003; pp. 49–54. [Google Scholar]
- Dray, C.; Daviaud, D.; Guigné, C.; Valet, P.; Castan-Laurell, I. Caffeine reduces TNFα up-regulation in human adipose tissue primary culture. J. Physiol. Biochem. 2007, 63, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Eigler, A.; Siegmund, B.; Emmerich, U.; Baumann, K.H.; Hartmann, G.; Endres, S. Anti-inflammatory activities of cAMP-elevating agents: Enhancement of IL-10 synthesis and concurrent suppression of TNF production. J. Leukoc. Biol. 1998, 63, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Montoya, G.A.; Bakuradze, T.; Eirich, M.; Erk, T.; Baum, M.; Habermeyer, M.; Eisenbrand, G.; Richling, E. Modulation of 3′,5′-cyclic AMP homeostasis in human platelets by coffee and individual coffee constituents. Br. J. Nutr. 2014, 112, 1427–1437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcinkiewicz, J.; Grabowska, A.; Lauterbach, R.; Bobek, M. Differential effects of pentoxifylline, a non-specific phosphodiesterase inhibitor, on the production of IL-10, IL-12 p40 and p35 subunits by murine peritoneal macrophages. Immunopharmacology 2000, 49, 335–343. [Google Scholar] [CrossRef]
- Haskó, G.; Szabó, C.; Németh, Z.H.; Kvetan, V.; Pastores, S.M.; Vizi, E.S. Adenosine receptor agonists differentially regulate IL-10, TNF-alpha, and nitric oxide production in RAW 264.7 macrophages and in endotoxemic mice. J. Immunol. 1996, 157, 4634–4640. [Google Scholar]
- Socała, K.; Szopa, A.; Serefko, A.; Poleszak, E.; Wlaź, P. Neuroprotective effects of coffee bioactive compounds: A review. Int. L. Mol. Sci. 2020, 22, 107. [Google Scholar] [CrossRef]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [Green Version]
- Barcelos, R.P.; Lima, F.D.; Carvalho, N.R.; Bresciani, G.; Royes, L.F. Caffeine effects on systemic metabolism, oxidative-inflammatory pathways, and exercise performance. Nutr. Res. 2020, 80, 1–17. [Google Scholar] [CrossRef]
- Gómez-Ruiz, J.Á.; Leake, D.S.; Ames, J.M. In vitro antioxidant activity of coffee compounds and their metabolites. J. Agric. Food Chem. 2007, 55, 6962–6969. [Google Scholar] [CrossRef]
- Zatorski, H.; Sałaga, M.; Zielińska, M.; Piechota-Polańczyk, A.; Owczarek, K.; Kordek, R.; Lewandowska, U.; Chen, C.; Fichna, J. Experimental colitis in mice is attenuated by topical administration of chlorogenic acid. Naunyn-Schmiedebergs Arch. Pharmacol. 2015, 388, 643–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paiva, C.L.R.S.; Beserra, B.T.S.; Reis, C.E.G.; Dorea, J.G.; Da Costa, T.H.M.; Amato, A.A. Consumption of coffee or caffeine and serum concentration of inflammatory markers: A systematic review. Crit. Rev. Food Sci. Nutr. 2019, 59, 652–663. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Goodman, C.L.; Capps, C.R.; Shue, Z.L.; Arnot, R. Influence of 2-weeks ingestion of high chlorogenic acid coffee on mood state, performance, and postexercise inflammation and oxidative stress: A randomized, placebo-controlled trial. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 55–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Parameter | All (n = 10) | Men (n = 5) | Women (n = 5) | p Value |
|---|---|---|---|---|
| Age (years) | 36.5 ± 6.5 | 35.8 ± 8.1 | 37.2 ± 5.3 | 0.754 |
| Body mass (kg) | 68.5 ± 11.6 | 70.6 ± 14.6 | 66.4 ± 9.0 | 0.599 |
| Stature (cm) | 169.7 ± 7.0 | 173.6 ± 5.7 | 165.8 ± 6.3 | 0.073 |
| BMI (kg·m−2) | 23.8 ± 3.4 | 23.3 ± 3.9 | 24.2 ± 3.3 | 0.722 |
| Leukocytes (103·μL−1) | 5.28 ± 1.07 | 5.34 ± 1.40 | 5.22 ± 0.78 | 0.871 |
| Lymphocytes (103·μL−1) | 1.53 ± 0.37 | 1.65 ± 0.39 | 1.41 ± 0.35 | 0.334 |
| Monocytes (103·μL−1) | 0.35 ± 0.13 | 0.38 ± 0.17 | 0.32 ± 0.07 | 0.495 |
| Caffeine Concentrations (μM) | ANOVA | ||||||
|---|---|---|---|---|---|---|---|
| Control | 5 | 10 | 15 | 30 | 50 | p (η2) | |
| IL-10 (pg·10−3 cells) | 21.9 ± 3.4 | 22.1 ± 6.5 | 20.6 ± 3.9 | 22.7 ± 4.5 | 24.0 ± 4.9 | 24.6 ± 3.9 | 0.208 (0.137) |
| IL-6 (pg·10−3 cells) | 385.2 ± 88.6 | 448.2 ± 96.3 | 451.5 ± 99.8 | 412.4 ± 82.5 | 440.6 ± 107 | 396.1 ± 93.6 | 0.543 (0.070) |
| TNF-α (pg·10−3 cells) | 58.6 ± 10.4 | 57.5 ± 9.8 | 55.2 ± 9.4 | 49.2 ± 9.1 | 47.9 ± 8.3 | 45.1 ± 7.7 # | 0.014 * (0.250) |
| IL-12 (pg·10−3 cells) | 4.13 ± 0.84 | 4.50 ± 0.78 | 5.01 ± 0.11 | 4.91 ± 0.66 | 4.36 ± 0.8 | 4.50 ± 0.95 | 0.700 (0.053) |
| IL-1β (pg·10−3 cells) | 17.2 ± 5.0 | 19.3 ± 6.0 | 18.2 ± 5.3 | 19.4 ± 4.8 | 16.8 ± 6.3 | 20.1 ± 5.1 | 0.501 (0.073) |
| MDA (nM) | 0.62 ± 0.10 | 0.63 ± 0.12 | 0.72 ± 0.13 | 0.72 ± 0.12 | 0.62 ± 0.11 | 0.58 ± 0.11 | 0.227 (0.147) |
| 5-CQA Concentrations | ANOVA | ||||||
|---|---|---|---|---|---|---|---|
| Control | 5 nM | 20 nM | 200 nM | 2 μM | 20 μM | p (η2) | |
| IL-10 (pg·10−3 cells) | 21.2 ± 4.5 | 20.8 ± 6.6 | 20.2 ± 5.7 | 19.2 ± 6.8 | 17.4 ± 6.2 | 17.8 ± 6.5 | 0.438 (0.083) |
| IL-6 (pg·10−3 cells) | 385.2 ± 92.4 | 351.2 ± 83.1 | 326.5 ± 93.9 | 291.9 ± 86.1 | 252.0 ± 85.8 # | 202.2 ± 82.5 # | 0.002 * (0.286) |
| TNF-α (pg·10−3 cells) | 58.2 ± 9.8 | 55.1 ± 10.1 | 55.4 ± 8.0 | 49.2 ± 9.9 | 46.9 ± 7.3 | 44.7 ± 8.5 | 0.068 (0.169) |
| IL-12 (pg·10−3 cells) | 4.48 ± 0.10 | 4.22 ± 0.97 | 4.53 ± 0.12 | 4.24 ± 0.14 | 4.05 ± 0.98 | 3.75 ± 0.10 | 0.650 (0.058) |
| IL-1β (pg·10−3 cells) | 16.2 ± 4.0 | 18.5 ± 5.0 | 17.8 ± 5.3 | 18.4 ± 5.8 | 15.8 ± 7.7 | 14.8 ± 5.7 | 0.571 (0.067) |
| MDA (nM) | 0.61 ± 0.11 | 0.65 ± 0.14 | 0.70 ± 0.14 | 0.68 ± 0.14 | 0.70 ± 0.11 | 0.65 ± 0.10 | 0.259 (0.111) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodas, L.; Martínez, S.; Riera-Sampol, A.; Moir, H.J.; Tauler, P. Effects of Caffeine and 5-Caffeoylquinic Acid on Blood Cell In Vitro Cytokine Production in Response to Lipopolysaccharide Stimulation. Appl. Sci. 2022, 12, 7322. https://doi.org/10.3390/app12147322
Rodas L, Martínez S, Riera-Sampol A, Moir HJ, Tauler P. Effects of Caffeine and 5-Caffeoylquinic Acid on Blood Cell In Vitro Cytokine Production in Response to Lipopolysaccharide Stimulation. Applied Sciences. 2022; 12(14):7322. https://doi.org/10.3390/app12147322
Chicago/Turabian StyleRodas, Lluis, Sonia Martínez, Aina Riera-Sampol, Hannah J. Moir, and Pedro Tauler. 2022. "Effects of Caffeine and 5-Caffeoylquinic Acid on Blood Cell In Vitro Cytokine Production in Response to Lipopolysaccharide Stimulation" Applied Sciences 12, no. 14: 7322. https://doi.org/10.3390/app12147322
APA StyleRodas, L., Martínez, S., Riera-Sampol, A., Moir, H. J., & Tauler, P. (2022). Effects of Caffeine and 5-Caffeoylquinic Acid on Blood Cell In Vitro Cytokine Production in Response to Lipopolysaccharide Stimulation. Applied Sciences, 12(14), 7322. https://doi.org/10.3390/app12147322








