Abstract
The functional beverages market is one of the fastest-growing sectors of functional food production. An innovative recipe for powdered fruit and vegetable drinks fortified with lentil proteins (AGF) and stabilized with flax seed gums (FSG) was developed. The study focused on the analysis of potentially bioaccessible fractions from the produced beverages in terms of their antioxidant, antiproliferative activities and physicochemical properties. The contents of bioactive components were tailored by the incorporation of lyophilized fruits and vegetables, the FSG and the AGF. Digestion in vitro effectively released phenolics from all matrices. The highest contents of potentially bioavailable polyphenols were recorded for the AGF based beverages enriched with 5% of FSG and green-leafy vegetables (58 mg/100 mL) and those with lyophilized fruit (54 mg/100 mL). The reducing power of the beverages was mainly affected by the presence of the AGF, while the FSG and lyophilized fruit improved the chelating power. The digests applied in the concentrations mimicking physiological concentrations showed antiproliferative properties against gastric and colon adenocarcinoma—they seemed to be tailored by bioactive peptides and phenolics, respectively. The addition of the FSG improved the stability of the beverages increasing the time required for a reduction of 20% of the initial optical density by 16- and 28-times in the beverages without additives or enriched with vegetables. Both, the AGF and FSG stabilize the beverages after rehydration and are sources of bioaccessible antioxidant and anticancer components, which create their functionality.
1. Introduction
The staggering amount of food waste, mainly related to the rapid deterioration of vegetables and fruit, prompts researchers to find novel methods for using fresh fruits and vegetables as well as extending their shelf-life and suitability for consumption. The use of whole fruits or vegetables for the production of functional food, including powdered drinks, in contrast to the traditional production of juices, does not deprive the finished product of the beneficial dietary fiber and many active compounds associated with cell walls, such as phenolic acids. Secondly, it is pro-ecological as it does not generate waste, which is a significant problem due to its low microbiological stability and high disposal costs [1]. A lot of emphasis is placed on the possibility of drying and powdering vegetables and fruits. After all such products might be used as commercial and value-added products, which can find applications in preparing smoothie-like beverages, or as additives rich in vitamins and antioxidants dedicated to, e.g., ice creams, noodles, cakes, etc. Such a premix of high protein vegetables and fruits in dehydrated form also gains a ready-to-drink character after dissolving in water [2]. The functional beverages production sector is the fastest-growing of the functional food market and, has become increasingly popular among consumers conscientious about the impact of the diet on their health and wellbeing [3]. The powdered drinks have long-term constancy and the nutrients might be better preserved compared to liquid form. As their retarded deterioration rate is related to the little amount of water in the powder and the prevention of air ingress, powdered drinks are ideal products dedicated to fortification with nutrients and bioactive compounds [2].
Nonthermal techniques, such as lyophilization, despite its relatively high price, could be a good solution for the fruit-vegetable functional beverages food market, due to the possibility of long-term storage without significant loss of their biological activity, and ease of preparation and possibility of fortification with components with high nutritional value [3,4,5]. Besides the instant properties, such as wettability, dispersibility, and solubility, other factors, such as the dissolution time, hygroscopicity, compressibility, or flowability characters determine the quality and preference of powdered products [6]. In powdered beverages, maltodextrin (or other polysaccharides) is usually used for their stabilization after rehydration [5,7,8]. Unfortunately, it has a high glycemic index, which is undesirable in the case of the products dedicated to the elderly, especially diabetics [9]. So far, alternative carriers were used, such as modified starch, inulin, alginate, gum Arabic, or combinations of them [10]. The carrier agents may additionally protect sensitive food components, such as probiotics, and carotenoids; preserve flavors, and reduce volatility and reactivity [11,12]. Additionally, in our studies, an alternative, natural stabilizer was used—flaxseed gum (FSG) exhibiting strong prebiotic potential and pro-health properties [13].
The use of biologically active substances in nutrition and disease prevention generates great interest in the food industry [14]. The expanding range of products classified as functional foods creates an opportunity to consciously shape the diet in the aspect of broadly understood preventive healthcare. Powdered drinks seem to be one of the meal alternatives. They are easy to prepare and can be carriers of desirable components dedicated to different consumer groups, e.g., the elderly who often struggle to bite, swallow and/or have deficiencies in micro- and macronutrients [15]. The composition, and thus bioactivity, of powdered beverages, can be easily tailored to specific needs. Beverages can be fortified with antioxidants [15], minerals, vitamins [16], dietary fibre [17], amino acids, peptides and proteins [18], pro- and prebiotics [19]. On the other hand, such beverages can be also prepared for the special needs of consumers; for example, non-dairy instant beverage powder is an alternative for lactose-intolerant people [4], and beverages with special rheological properties enable easier swallowing for dysphagia patients [20]. Following this trend, new powdered beverages produced from lyophilized fruit and vegetables [21,22] were tailored by adding efficient nutrients and functional ingredients—lentil proteins (AGF) and flaxseed gum (FSG) [23].
One of the key factors influencing food’s nutritional value and pro-health properties is bioaccessibility [24]. It is defined as an amount of ingested nutrient/active compound that is available for absorption in the gut after digestion, whereas bioavailability refers to the fraction of an ingested nutrient that reaches the systemic circulation and the specific sites where it can exert its biological action [25]. There are many studies assessing the in vitro bioaccessibility of selected bioactive compounds; however, the research on some complicated heterogeneous matrices, such as beverages [26], bread [27]), and pasta [28] are rare. In such matrices, the nutritional and pro-health qualities are also tailored by interactions between selected components of the system (e.g., nutrients, polyphenols, carotenoids, vitamins, digestive enzymes, and other components of digestive fluids) occurring during processing, dispersing, and/or dissolving powders and finally during the digestion process [29,30].
Thus, the aim of this study was to investigate the effect of lentil protein (AGS) and flaxseed gum (FSG) on freeze-dried beverages’ functionality and stability. To our knowledge, such a recipe was developed for the first time. Particularly, using simulated digestion, we focused on potentially bioaccessible components with antioxidant and anticancer properties.
2. Materials and Methods
2.1. Chemicals
ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid), α-amylase (52.7 U/mg), pancreatin (4× UPS), pepsin (541 U/mg), bile extract, and Folin–Ciocalteau phenol reagent and media for cell lines were purchased from Sigma–Aldrich company (Poznan, Poland). All solvents and basic chemicals in the analytical grade were purchased from Sigma–Aldrich Company (Poznan, Poland).
2.2. Plant Material
All plant materials (carrot, pumpkin, lentil sprouts, lentil seeds, raspberry, strawberry, broccoli sprouts, parsley leaves, and defatted linseeds powder) were lyophilized and prepared as described previously [23]. Flaxseed gum (FSG) was prepared as described previously by Nikbakht Nasrabadi, Goli, Sedaghat Doost, and Van der Meeren [31]. The defatted powder was mixed with Milli-Q water with a ratio of 1:10 (w/v) and mixed at 200 rpm (80 °C, 2 h). Then, the mixture was passed through a screen (0.45 mm). Flaxseed gum (FSG) was precipitated by mixing with 96% ethanol (1:3, w/v), freeze-dried, and milled using the laboratory grinder (MRC GRINDING MACHINE, SM-450, Holon, Israel), and stored at −20 °C.
Lentil albumin/globulins-rich fraction (AGF) was isolated based on the solubility criterion according to Ribeiro, Teixeira, and Ferreira [32] with slight modifications. For the extraction of albumin/globulin-rich fraction (AGF), the flour (100 g) was extracted with 1 L of 10 mmol L−1 CaCl2, 10 mmol L−1 MgCl2, and 100 g kg−1 (w/v) NaCl in deionized water for 1 h at room temperature. The samples were centrifuged (15 min, 3860× g) and supernatants were collected. The solubilized proteins were precipitated at 4 °C overnight by decreasing the pH value to 4.5 with 0.1 N HCl. After that, centrifugation at 3860× g for 10 min at 4 °C followed. The received AGF was resuspended in water, freeze-dried (LABCONCO, Kansas City, MO, USA), and stored at −20 °C.
2.3. Powdered Beverages
The beverages were prepared based on the previously developed recipes [23]. The output drinks contained 30% carrot, 30% pumpkin, and 40% lentil sprouts (C); 30% carrot, 30% pumpkin, 30% lentil sprouts, and 10% of broccoli sprouts and parsley leaves (CV); 30% carrot, 30% pumpkin, 30% lentil sprouts and 10% raspberry, strawberry (CF). Powdered beverages (0.5 g) were rehydrated in 10 mL Milli-Q water and shaken (three intervals, 30 s) at room temperature using a multi-rotator (RS-60, Biosan, Riga, Latvia) (300 rpm) and used for analysis.
2.4. In Vitro Digestion
In vitro digestion was performed as described previously [33] with slight modifications. For simulated mastication and gastrointestinal digestion, 1 mL beverage samples was mixed with 1 mL of simulated salivary fluid [15.1 mmol/L KCl, 3.7 mmol/L KH2PO4, 13.6 mmol/L NaHCO3, 0.15 mmol/L MgCl2 (H2O)6, 0.06 mmol/L (NH4)2CO3, 1.5 mmol/L CaCl2, α-amylase (75 U/mL)] and shaken for 10 min at 37 °C. Next, the samples were adjusted to pH 3 with 6 mol/L HCl, suspended in 2 mL of simulated gastric fluid [6.9 mmol/L KCl, 0.9 mmol/L KH2PO4, 25 mmol/L NaHCO3, 47.2 mmol/L NaCl, 0.1 mmol/L MgCl2 (H2O)6, 0.5 mol/L (NH4)2CO3 0.15 mmol/L CaCl2, pepsin (2000 U/mL)] and shaken for 120 min. at 37 °C. After simulated gastric digestion, the samples were adjusted to pH 7 with 1 M NaOH and suspended in 4 mL simulated intestinal fluid [6.8 mmol/L KCl, 0.8 mmol/L KH2PO4, 85 mmol/L NaHCO3, 38.4 mmol/L NaCl, 0.33 mmol/L MgCl2 (H2O)6, 0.15 mmol/L CaCl2, 10 mol/L bile extract, pancreatin (2000 U/mL)]. The prepared samples underwent in vitro intestinal digestion for 120 min. After digestion, the samples were centrifuged (15 min, 6900× g) and the supernatants were mixed with an equal volume of methanol to stop enzyme activity.
2.5. Low-Molecular-Weight Antioxidants
The tests were performed directly for the hydrated beverages (raw) as well as for potentially bioaccessible fractions (the beverages subjected to in vitro digestion).
2.5.1. Total Phenolics Analysis
The amounts of total phenolics were determined using Folin–Ciocalteau reagent [34] and expressed as gallic acid equivalents (GAE) in mg per 100 mL of beverage. Briefly, a 10 μL sample of the extract was mixed with 10 μL of H2O, 40 μL ml of Folin reagent (1:5 H2O), and after 3 min with 250 μL of 10% Na2CO3. After 30 min, the absorbance of samples was measured at a wavelength of 725 nm.
2.5.2. Carotenoids and Chlorophylls Content
The beverages or digests were mixed with acetone (a final concentration of acetone of 80%) and extracted until the powders became colorless. The samples were centrifuged (15 min, 6000× g) and the absorbance was measured at 663, 647, and 470 nm. The carotenoid and chlorophyll contents were calculated using equations proposed by Sumanta, Haque, Nishika, and Suprakash [35].
Cha = 12.25A663 − 279A647
Chb = 21.5A647 − 5.1A663
Carotenoids = (1000A470 − 1.82Cha − 85.02Chb)/198
Chlorophylls and carotenoids were expressed in mg or g per 100 mL of beverages, respectively.
2.6. Antioxidant Activities
2.6.1. Reducing Power (RP)
Reducing power was determined by the method of Pulido, Bravo, and Saura-Calixto [36]. Briefly, the analyzed sample (0.05 mL) was mixed with phosphate buffer (0.05 mL, 200 mM, pH 6.6) and potassium ferricyanide K3(Fe[CN6]) (0.05 mL, 1%). The mixture was incubated at 50 °C for 20 min. Reactions were stopped with 0.05 mL 10% TCA and centrifuged for 10 min at 6500× g. The upper layer of the solution (0.1 mL) was mixed with distilled water (0.1 mL) and 0.04 mL of 0.1% FeCl3 and the absorbance was measured at 700 nm. Reducing power was expressed as Trolox equivalents (TE) in mg per 100 mL of beverages.
2.6.2. Ability to Quench ABTS Radicals (ABTS)
The experiments were carried out using the ABTS decolorization assay [37]. The ABTS solution was diluted to an absorbance of 0.7 ± 0.05 at 734 nm. The extract samples (10 μL) were added to 0.25 mL of ABTS solution, shaken vigorously and left to stand at room temperature for 2 h. The affinity of the test material to quench the ABTS free radical was evaluated according to the following equation:
where: AC—absorbance of the control, AA—absorbance of the sample.
scavenging % = ([AC − AA]/AC)) × 100,
The radical scavenging ability was expressed as Trolox equivalents in mg per 100 mL of beverages.
2.6.3. Ability to Quench Hydroxyl Radicals (OH)
The OH• scavenging ability was determined according to Su, Wang, and Liu [38]. The reaction mixture contained 20 μL of the sample, 70 μL H2O2, 24 μL 1.0 mM FeSO4, and 40 μL of 2 mM H2O2. Adding 10 μL of sodium salicylate started the reaction. After incubation at 37 °C for 30 min., the absorbance of the mixture at 560 nm was measured with a spectrophotometer. The affinity of the test material to quench OH• free radical was evaluated according to the following equation:
where AC—absorbance of the control (without salicylate), AA—absorbance of the sample.
scavenging % = ([AC − AA]/AC))× 100,
The activity was expressed as Trolox equivalents in mg per 100 mL of beverages.
2.6.4. Chelating Power (CHP)
Chelating power was determined by the method of Decker and Welch [39]. The extract samples (0.2 mL) were added to 0.01 mL of 2 mM FeCl2 solution and left for 10 min. Next, 0.01 mL 5 mM ferrozine was added, shaken vigorously and left to stand at room temperature for 10 min. Then, the absorbance of the solution was measured spectrophotometrically at 562 nm. The percentage of inhibition of ferrozine–Fe2+ complex formation was calculated according to the following formula:
where: Ac—absorbance of the control, Ap—absorbance of the sample.
% inhibition = [1 − (Ap/Ac)] × 100,
Chelating power was expressed as EDTA equivalents in mg per 100 mL of beverages.
2.7. The Relative Bioaccessibility Index (REF)
The factors were determined for a better understanding of the relationships between biologically active compounds as well as antioxidant activities in light of their bioaccessibility [40]. It was calculated for all low-molecular-weight antioxidants and all the studied activities.
where, CD—concentration of the selected antioxidants (phenolics, chlorophylls, carotenoids) in the digest of beverages, CB—concentration of the selected antioxidants (phenolics, chlorophylls, carotenoids) in the raw beverages.
where, AD—selected antioxidant activity (RP, ABTS, OH, CHP) in the digest of beverages, AB—selected antioxidant activity (RP, ABTS, OH, CHP) in the raw beverages.
REF = CD/CB
REF = AD/AB
2.8. Anticancer Properties
The potential anticancer properties of the beverages were tested using two cancer cell lines: AGS—Human Caucasian gastric adenocarcinoma (ECACC No. 89090402) and HT 29—Human Caucasian colon adenocarcinoma (ATCC HTB-38). The cells (0.5 × 106 cells/mL were seeded in 96 well plates and incubated in an air atmosphere humidified with 5% CO2 for 24 h at 37 °C. The growth medium consisted of DMEM F12 (for AGS) or RPMI 1640 medium (for HT29), 10% FBS (heat-inactivated fetal bovine serum), 2 mM L-glutamine and 1% antibiotic–antimycotic solution (Sigma-Aldrich, Poznań, Poland). The day after cell seeding the medium was replaced by a fresh one containing the lyophilized digests or potentially bioaccessible phenolics (the digest purified using Sep-Pak® C18 Cartridges). Extracts were applied in two concentrations: the equivalents of 0.7% and 2.1% beverage in medium (the equivalent of the amounts potentially present in living cells after consumption of 200 mL of the beverage and its triple). Cells were incubated for up to 24 h and then WST-1 assay kit (BioVision, Inc., San Francisco, CA, USA) for cytotoxicity evaluation was used according to the manufacturing procedure.
2.9. Physio-Chemical Properties of Beverages
2.9.1. Swelling potential
An amount of 0.5 g of the powdered sample (M) was placed into a graduated cylinder, and the volume (V1) was reported. After that, 10 mL of distilled water (DW) was added and the samples were shaken (3 intervals, 30 s) at room temperature using a multi-rotator (RS-60, Biosan, Riga, Latvia) (300 rpm). The suspensions were allowed to expand for 2 h. then the volumes of the wet powders (V2) were reported [41]. The swelling capacity was calculated as follows:
Swelling capacity [mL/g] = (V2 − V1)/M
2.9.2. Beverages Stability
The beverages were hydrated according to the procedure described in Section 2.3 and dissolved (1:4) with distilled water. Then, they were placed in a quartz cuvette and absorbance of 660 nm was recorded for 2 h. (30 s intervals). Finally, the curves describing the stability of the beverage were drawn and used to estimate some parameters characterizing the stability of the beverage. They included: (1) initial absorbance at 660 nm, (2) change of absorbance in time (Δ660), and (3) time required for reducing 20% of initial absorbance (T0.8).
2.10. Statistical Analysis
The distribution of the data was estimated using Shapiro–Wilk’s tests. A statistical significance was estimated by Tukey’s test for the data obtained from three independent samples of each extract in three parallel experiments (n = 9). The experimental data were shown as means ± S.D. Unless stated otherwise, the statistical tests were carried out at a significance level of α = 0.05. The statistical tests were performed using Statistica 13.1 software (StatSoft, Inc., Tulsa, OK, USA).
3. Results and Discussion
There are some studies concerning the production of instant beverages (fruit-based, coffee, etc.) where technology covers the production of beverages and further drying, e.g., spray-drying or lyophilization [4]. Contrary to them, in the case of powdered beverages, parts of the components are insoluble and form a suspension that must be stabilized to maintain the desired organoleptic properties. It strongly affects bioaccessibility and bioactivity. According to our knowledge, this is the first attempt at studying the pro-health properties of the potentially bioaccessible fractions. The contents of total phenolics, carotenoids and chlorophylls in the raw beverages and their digests are presented in Table 1. The content of low-molecular-antioxidants was tailored by the incorporation of lyophilized fruits and vegetables, the addition of flaxseed gum (FSG), as well as the replacement of a sprouted lentil flour with lentil proteins fraction (AGF). In the raw beverages the higher content of phenolics was determined in the beverages enriched with the FSG and lyophilized fruit (P15F; 18.62 mg/100 mL)—1.3, 1.6 and 0.93-fold increase compared to the control containing, respectively, the sprouted lentil flour (C), the beverages based on AGF (P0) and the beverages based on AGF enriched with lyophilized fruit. The results indicated that both the FSG and lyophilized additives are responsible for an elevation of total phenolics. An addition of FSG to the beverages containing AGF caused a linear increase in the phenolic content. Generally, such behavior was recorded in all the studied beverages. In the case of chlorophylls, and carotenoids only slight changes were observed. The contents of chlorophyll a and carotenoids were the most significantly increased by the incorporation of green lyophilized vegetables (the samples P0V–P15V). These results correspond well with our previous studies describing the changes to low-molecular antioxidants in a chemically-extractable fraction of powdered beverages [23]. Estimation of the nutritional value of new foods is usually based on the concentration of nutrients and phytochemicals and/or the level of pro-health properties; however, the real bioactivity of food is created by many additional factors. It is widely accepted that not all constituents present in the food matrix may be completely bioaccessible; thus, in vitro methodologies have been developed for better evaluation of food quality [42]. Digestion in vitro effectively released phenolics from all matrices which corresponds with other studies describing the bioaccessibility of these compounds from plant-origin food [28,43]. Relative bioaccessibility factors ranged from 2.62 to 6.72 in the beverages based on the sprouted lentil flour and enriched with lyophilized vegetables and those with AGF without any additives. The highest contents of polyphenols in the potentially bioavailable fraction were recorded for the P5V (57.6 mg/100 mL) and the P0F (53.8 mg/100 mL). The results indicated that the lentil protein isolates used in the beverages are a rich source of phenolics which confirms our previous results obtained for chemically extracted phenolics [23]. This phenomenon was also reported by Pedrosa and co-workers in pea and bean protein isolates which contained 3.8 and 5.5 mg of phenolics per g, respectively [44]. Additionally, as expected, the addition of lyophilized fruit and vegetables increased the content of low-molecular-weight antioxidants. A final concentration of phenolics is the result of several factors—the introduction of functional components, such as FSG and lyophilized vegetables and fruit at the same time caused a reduction in the phenolics-rich AGF level. The influence of inulin with different degrees of polymerization as a stabilizer of soursop whey beverage on the bioaccessibility of bioactive compounds was studied by Guimarães and co-workers [12]. They proved that despite the different behavior during in vitro digestion, the bioaccessibility of the bioactive compounds of both digesta was similar. Similar to our studies, an increase in phenolic content (c.a. 20%) was determined; however, it was slightly lower (20% vs. 34%). Contrary, the addition of 10% of inulin to tomato sauce decreased the bioaccessibility of phenolics by c.a. 30% [45].

Table 1.
Content of total phenolics, carotenoids and chlorophylls in rehydrated and digested beverages.
Compared to the phenolics, the pigments were not so effectively released from the beverages (REF factors ranged from 1.11 to 2.03). The highest content of carotenoids was found in beverages enriched with green-leafy vegetables and fruits. The slight variation within chlorophylls and carotenoids, both in the raw beverages and their digests, may be mainly due to changes in the matrix composition (an addition of FSG reduces the content of other basic ingredients).
Many studies also confirm that bioactive components, such as phenolics and plant pigments may interact, which influences their extractability [46]. It was proved that phenolics improve carotenoids’ release from the matrix, improving carotenoids’ bioaccessibility and suggesting that some synergistic mechanisms are overlapped. It was reported that co-digestion of carotenoid-rich vegetables (carrot, or baby spinach) with phenolic-rich vegetables increased the intestinal cellular bioaccessibility of carotenoids [46]. Such behavior was also observed in our study in the case of beverages enriched with lyophilized vegetables and fruits (especially P0V and P0F samples). On the other hand, carotenoids (unstable in light, acids and during processing) are protected by phenolics against oxidation, which was observed during digestion in vitro of mandarin pulp [47] or free lycopene and β-carotene [48]. Our study confirmed that low-molecular antioxidants are well bioaccessible in vitro. What is important, the contents determined in the fraction obtained after digestion in vitro were the result of not only the sum of ingredients, but also confirmed the presence of interactions.
The reducing power of beverages was mainly affected by the presence of the AGF (Figure 1A). The highest reducing power was determined for the P15F (105 TE mg/100 mL—a 2.7- and a 2.1-fold increase compared to the C and P0). It was also observed that the addition of FSG positively affected the reducing properties of the raw beverages. This tendency was not noted after digestion, where the values of reducing power seemed to be tailored by both FSG and lyophilized vegetables/fruit. What is important, the replacement of sprouted lentil flour with AGF allowed for increasing the activity of the potentially bioaccessible fraction. The release of peptides from legume flour during digestion in vitro was reported previously [49]. Generally, the compounds exhibiting reducing properties were bioaccessible: the REF values ranged from 1.1 (CV) to 2.6 (P0); however, a negative effect of FSG was visible.
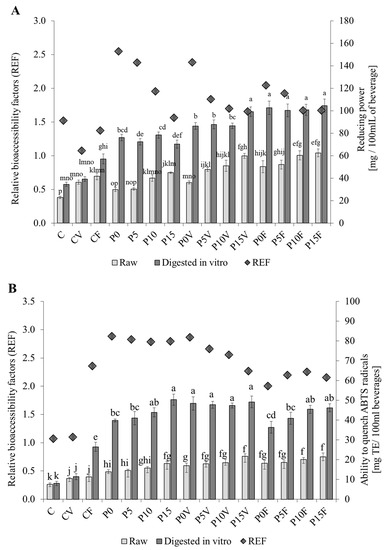
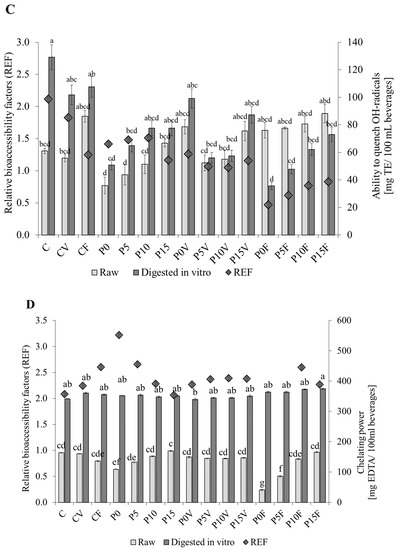
Figure 1.
Reducing properties of the powdered beverages. (A)—Reducing power; (B)—Ability to quench ABTS radicals; (C)—Ability to quench OH-radicals; (D)—Chelating power. C—beverages based on lentil sprouts; P—beverages based on lentil proteins; V—lyophilized parsley and broccoli sprouts; F—lyophilized strawberry and raspberry; 0–15—amounts of flaxseeds gum (%). REF—the relative bioaccessibility factor; TE—Trolox equivalents. Means (±SD) followed by different letters are significantly different (n = 9; p ≤ 0.05).
According to Figure 1B, the digests of AGF-based beverages represented a better ability to quench the ABTS radicals. The high bioavailability of antiradical compounds was evidenced by the REF coefficient, which ranged between 1.7 and 2.9. Generally, the best results were obtained for the beverages supplemented with green-leafy vegetables (activity ranged from 47 to 50 mg TE/100 mL). An increase was mainly associated with the introduction of AGF; however, a slight effect was also recorded in the samples with increasing content of FSG—the relatively high antiradical activity of flax polysaccharides is associated with a residual presence of phenolics [50]. It may be also supposed that lentil proteins were effectively hydrolyzed to peptides exhibiting antiradical properties. Such behavior was previously reported in Moreno et al. [51], where digestive enzymes (pepsin + pancreatin) effectively produced such peptides from lentil proteins (IC50 c.a. 0.9 Trolox equivalents (TE) mg/mL of hydrolysate). On the other hand, the digestive tract might effectively release phenolics from the matrix [40]. This was previously observed in studies of the potential bioaccessibility of phenolics from different foods, e.g., pasta fortified with powdered parsley leaves [52] or lentil sprouts enriched with probiotics [53]. Contrary, Seczyk et al. [54] proved that the potential accessibility of phenolics from bean pasta is limited by interactions occurring between phenolics and proteins, dietary fiber as well as starch.
The highest ability to quench hydroxyl radicals was found for the potentially bioaccessible fraction obtained from the control beverages produced with the sprouted lentil flour (129 mg TE/100 mL) (Figure 1C). The replacement of sprouted lentil flour with the AGF decreased this ability in the raw beverages; however, this undesirable trend was partially masked by the addition of FSG. A very interesting relationship was observed in the beverages supplemented with lyophilized fruit, where increasing amounts of anthocyanins-rich material were not reflected in a subsequent elevation of the activity in the potentially bioaccessible fraction. Although, anthocyanins effectively extracted into the water in the raw beverages exhibit a significant antiradical potential, in the case of digests their activity was significantly diminished. It may be due to their interactions with components of the digestive tract [55] and legume proteins [56]. Such an explanation supports also a slightly decreased digestibility of the proteins in those beverages [23].
A positive effect of FSG addition on the chelating power was recorded in the raw beverages based on lentil proteins and those enriched with lyophilized fruit (Figure 1D). Surprisingly, the incorporation of anthocyanins-rich material did not cause any expected increases. It is supposed that in the case of chelating powers, the activity is tailored by mechanisms similar to those observed in the case of the ability to quench hydroxyl radicals. Digestion in vitro effectively released the compounds able to chelate transition metal ions. There was no effect from beverages matrix, as well as functional supplements, which may suggest that a key role in this activity plays bioactive peptides driven by lentil proteins. It was previously shown in many studies, e.g., hydrolysate prepared from lupin protein using Protamex enzyme [57]. Such a mechanism is also supported by our previous study concerning protein digestibility, in particular, no effect of additives on this feature [23].
The bioactive compounds present in the beverages showed dose-independent cytotoxic activity against the AGS cancer lines (Figure 2A). Tests were performed for “pure” lyophilized digests as well as the purified phenolic fractions. The highest activity against the AGS cells was determined for the lyophilized digests of the beverages containing 15% of FSG and vegetables (proliferation reduced by 92%) applied in a higher studied concentration. In this case, also the purified phenolics exhibited high activity (proliferation reduced by 61%). The digests tested in lower concentrations (mimicking physiological contents) had no effect or only a slightly reduced proliferation of AGS cells. The high activity was also exhibited by the anthocyanins-rich fraction from the beverages based on the sprouted flour (proliferation reduced by 73%); this effect was also observed in the beverages containing the AGF and FSG. It was proved that anthocyanins are accumulated by AGS cells and induce apoptosis by the elevation of the Bax/Bcl-2 ratio and direct activation of caspase-3 [58]. The analysis of the samples from the beverages based on the AGS (without the addition of lyophilized supplements) showed that this matrix released the compounds effectively, limiting the growth of AGS. Additionally, this positive effect was enhanced by the incorporation of FSG. A comparison of the results for the digests and their phenolics fraction may suggest that in this case, other compounds than phenolics are responsible for the activity. It may be supposed that a key role is played by peptides realized from the lentil proteins. Previously, Luna-Vital and González de Mejía showed that peptides from legumes interact with matrix metalloproteases, cause the loss of mitochondrial membrane potential and induce DNA damage [59]. On the other hand, phenolics seemed to be responsible for the antiproliferative activity in the CF beverages (supplemented with lyophilized fruit). Surprisingly, this positive effect was significantly reduced in the beverages based on AGF; more enrichment with FSG caused a further reduction in the activity. A decrease in the antiproliferative activity of phenolics (caffeic acid, quercetin, catechin) studied in model systems (breast (MCF7), liver (HePG2) and colon (HCT116)) was previously reported and associated with their interactions with peptides and proteins (casein, whey protein isolates) [60]. Except for the beverages based on AGF and enriched with lyophilized fruit, the application of digests at lower concentrations did not cause a significant reduction in HT-29 proliferation. More, the beverages containing the sprouted lentil flour seemed to promote the growth of cells. It may be supposed that “nutrient-dense” digests provided glucose and free amino acids which offset an antiproliferative activity of phenolics. This explanation supports the results obtained for the purified phenolics fraction where a strong, dose-dependent effect was observed. Previously, a similar phenomenon was reported during the study of potentially bioaccessible fractions from adzuki and mung bean sprouts [61]. The highest antiproliferative activity was recorded for the purified phenolics applied in the higher studied concentrations; however, a key role was played by phenolics released from FSG whose activity was supported only by those released from green-leafy vegetables.
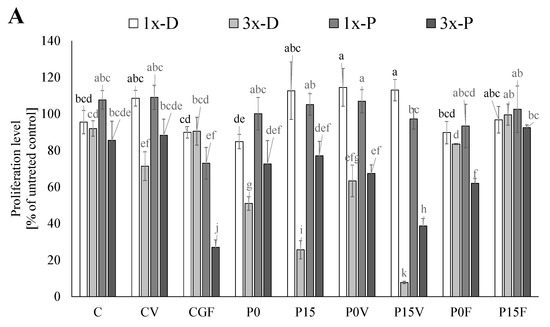
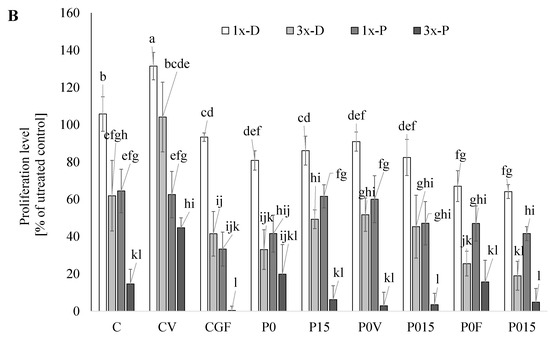
Figure 2.
Anticancer properties of the beverages. (A) AGS (Human Caucasian gastric adenocarcinoma); (B) HT 29 (Human Caucasian colon adenocarcinoma). 1x-D, 3x-D (lyophilized digests applied at concentrations being the equivalents of 0.7% and 2.1% beverage, respectively); 1x-P, 3x-P (potentially bioaccessible phenolics applied at concertation being the equivalents of 0.7% and 2.1% beverage, respectively). C—beverages based on lentil sprouts; P—beverages based on lentil proteins; V—lyophilized parsley and broccoli sprouts; F—lyophilized strawberry and raspberry; 0–15—amounts of flaxseeds gum (%). Means (±SD) followed by different letters are significantly different (n = 9; p ≤ 0.05).
An important factor affecting the consumer acceptance of powdered beverages is their stability after rehydration. In our study, a method describing the kinetics of sedimentation was employed to assay the stability of beverages (Figure 3). Additionally, based on the obtained results some parameters were proposed to better characterize the process (Table 2). It was proved that the replacement of sprouted lentil flour with the AGS significantly decreased stability. This undesirable effect was defuncted by the incorporation of FSG which is confirmed by the changes in optical density in time visible on the descent curves. Compared to the beverages based on the sprouted lentil flour those with the AGF were characterized by a significantly higher optical density after rehydration. A change in absorbance in time (Δ660) provides information about a range of sedimentation.
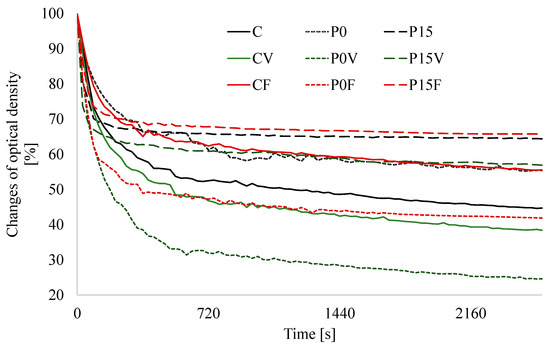
Figure 3.
Effect of the flaxseeds gum (FSG) and lentil proteins (AGS) on the stability of the beverage. C—beverages based on lentil sprouts; P—beverages based on lentil proteins; V—lyophilized parsley and broccoli sprouts; F—lyophilized strawberry and raspberry; 0–15—amounts of flaxseeds gum (%).

Table 2.
Parameters of the stability of the beverage.
Compared with the adequate controls (the beverages enriched with lyophilized vegetables, fruit and without additives, respectively), in the beverages based on AGF and containing 15% of FSG, an increase in stability of 56, 70 and 42% was recorded. What is important, the introduction of lyophilized green-leafy vegetables significantly deteriorated the stability both in the case of the beverages based on the sprouted lentil flour and AGF. The positive effect of the FSG was the highest during the analysis of the T0.8 parameter. The time required for a reduction of 20% of the initial optical density of the beverages without additives or enriched with lyophilized vegetables and fruit was extended 16-, 28- and 7-times when compared to the appropriate control, respectively. The swelling potential is mainly described as swelling capacity or swelling index and flours and powders with high swelling potential are good tools for combating hunger as they provide satiety [62]. The swelling potential of the beverages ranged from 25.6 (the beverages based on the AGS containing 5–10% of FSG) to c.a. 32.5 (the beverages based on AGS containing 15% of FSG and those based on the sprouted lentil flour enriched with lyophilized fruit CF). Porous particles, such as freeze-dried powders have a high swelling and water holding capacity which promote the formation of viscous solutions and play an important role in reconstitution properties [63]. Additionally, powdered fruits and vegetables are a good thickening agent and thus may play a vital role in the food industry. Lecumberri et al. studied cacao beverages enriched with apple and orange pectin and recorded a swelling capacity of 7.42 and 10.45, respectively [41]. The swelling values obtained in our study were slightly higher, which may be caused by AGS exhibiting high emulsifying properties [64]. More, Khalloufi et al. [65] proved that electrostatic interaction between FSG and proteins stabilizes the emulsion, thus, this polysaccharide may be used as a functional additive. More, the results obtained for FSG are comparable with those for maltodextrin used in the instant beverage powder from red beetroot, quince fruit, and cinnamon extracts [7].
4. Conclusions
The introduction of FSG allowed obtaining high-stable beverages which are confirmed by an increased initial density as well as sedimentation kinetics. The time required for a reduction of 20% of the initial optical density was increased by 16- and 28-times in the beverages without additives or those enriched with vegetables. It was also proved that the replacement of the sprouted lentil flour with its protein (AGF) did not deteriorate the stability and antioxidant potential. The digestion in vitro effectively released bioactive compounds with multidirectional pro-health properties. The results confirmed that different classes of antioxidants (phenolics, carotenoids, peptides) take a part in the creation of antioxidant properties. The highest contents of potentially bioavailable polyphenols were recorded for the AGF beverages containing FSG and green-leafy vegetables and those with lyophilized fruit. What is important, the digests applied in the concentrations mimicking physiological concentrations exhibit antiproliferative properties against gastric and colon adenocarcinoma, which seemed to be tailored by bioactive peptides and phenolics, respectively. The study provides knowledge about the FSG and AGF as valuable functional additives and being the improvers of the pro-health properties and structure of powdered drinks.
Author Contributions
Author Contributions: Conceptualization, J.B.-N. and M.Ś.; Methodology, J.B.-N., U.S. and M.Ś.; Formal analysis, J.B.-N.; Investigation, J.B.-N. and U.S. Data curation, J.B.-N.; writing—original draft preparation, J.B.-N.; writing—review and editing, M.Ś. and U.S.; supervision, M.Ś.; project administration, J.B.-N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All relevant data are included in the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mnisi, C.M.; Mhlongo, G.; Manyeula, F. Fruit Pomaces as Functional Ingredients in Poultry Nutrition: A Review. Front. Anim. Sci. 2022, 3, 40. [Google Scholar] [CrossRef]
- Çopur, Ö.U.; İncedayı, B.; Karabacak, A.Ö. Technology and Nutritional Value of Powdered Drinks; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128152607. [Google Scholar]
- Islam, J.; Kabir, Y. Effects and Mechanisms of Antioxidant-Rich Functional Beverages on Disease Prevention; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128163979. [Google Scholar]
- Chaturvedi, S.; Khartad, A.; Chakraborty, S. The Potential of Non-Dairy Synbiotic Instant Beverage Powder: Review on a New Generation of Healthy Ready-to-Reconstitute Drinks. Food Biosci. 2021, 42, 101195. [Google Scholar] [CrossRef]
- Caliskan, G.; Dirim, S.N. The Effect of Different Drying Processes and the Amounts of Maltodextrin Addition on the Powder Properties of Sumac Extract Powders. Powder Technol. 2016, 287, 308–314. [Google Scholar] [CrossRef]
- Kyaw Hla, P.; Hogekamp, S. Wetting Behaviour of Instantized Cocoa Beverage Powders. Int. J. Food Sci. Technol. 1999, 34, 335–342. [Google Scholar] [CrossRef]
- Hajiaghaei, M.; Sharifi, A. Physicochemical Properties of Red Beetroot and Quince Fruit Extracts Instant Beverage Powder: Effect of Drying Method and Maltodextrin Concentration. J. Food Qual. 2022, 2022, 7499994. [Google Scholar] [CrossRef]
- Michalska-Ciechanowska, A.; Majerska, J.; Brzezowska, J.; Wojdyło, A.; Figiel, A. The Influence of Maltodextrin and Inulin on the Physico-Chemical Properties of Cranberry Juice Powders. ChemEngineering 2020, 4, 12. [Google Scholar] [CrossRef] [Green Version]
- Gourineni, V.; Stewart, M.L.; Skorge, R.; Sekula, B.C. Slowly Digestible Carbohydrate for Balanced Energy: In Vitro and In Vivo Evidence. Nutrients 2017, 9, 1230. [Google Scholar] [CrossRef] [Green Version]
- Duar, R.M.; Ang, P.T.; Hoffman, M.; Wehling, R.; Hutkins, R.; Schlegel, V. Processing Effects on Four Prebiotic Carbohydrates Supplemented in an Extruded Cereal and a Low PH Drink. Cogent Food Agric. 2015, 1, 1013782. [Google Scholar] [CrossRef]
- Valero-Cases, E.; Frutos, M.J. Effect of Different Types of Encapsulation on the Survival of Lactobacillus Plantarum during Storage with Inulin and in Vitro Digestion. LWT-Food Sci. Technol. 2015, 64, 824–828. [Google Scholar] [CrossRef]
- Guimarães, J.T.; Silva, E.K.; Arruda, H.S.; Freitas, M.Q.; Pastore, G.M.; Meireles, M.A.A.; Cruz, A.G. How Does the Degree of Inulin Polymerization Affect the Bioaccessibility of Bioactive Compounds from Soursop Whey Beverage during in Vitro Gastrointestinal Digestion? Food Hydrocoll. 2020, 101, 105511. [Google Scholar] [CrossRef]
- Hu, Y.; Shim, Y.Y.; Reaney, M.J.T. Flaxseed Gum Solution Functional Properties. Foods 2020, 9, 681. [Google Scholar] [CrossRef]
- Kasapoğlu, K.N.; Daşkaya-Dikmen, C.; Yavuz-Düzgün, M.; Karaça, A.C.; Özçelik, B. Enrichment of Beverages with Health Beneficial Ingredients. In Value-Added Ingredients and Enrichments of Beverages; Academic Press: New York, NY, USA, 2019; Volume 14, pp. 63–99. ISBN 9780128166871. [Google Scholar]
- Sun-Waterhouse, D. The Development of Fruit-Based Functional Foods Targeting the Health and Wellness Market: A Review. Int. J. Food Sci. Technol. 2011, 46, 899–920. [Google Scholar] [CrossRef]
- Öztürk, B. Nanoemulsions for Food Fortification with Lipophilic Vitamins: Production Challenges, Stability, and Bioavailability. Eur. J. Lipid Sci. Technol. 2017, 119, 1500539. [Google Scholar] [CrossRef]
- Goff, H.D.; Repin, N.; Fabek, H.; El Khoury, D.; Gidley, M.J. Dietary Fibre for Glycaemia Control: Towards a Mechanistic Understanding. Bioact. Carbohydrates Diet. Fibre 2018, 14, 39–53. [Google Scholar] [CrossRef]
- Vidal-Lletjós, S.; Beaumont, M.; Tomé, D.; Benamouzig, R.; Blachier, F.; Lan, A. Dietary Protein and Amino Acid Supplementation in Inflammatory Bowel Disease Course: What Impact on the Colonic Mucosa? Nutrients 2017, 9, 310. [Google Scholar] [CrossRef] [Green Version]
- Nazhand, A.; Souto, E.B.; Lucarini, M.; Souto, S.B.; Durazzo, A.; Santini, A. Ready to Use Therapeutical Beverages: Focus on Functional Beverages Containing Probiotics, Prebiotics and Synbiotics. Beverages 2020, 6, 26. [Google Scholar] [CrossRef] [Green Version]
- Adeleye, B.; Rachal, C. Comparison of the Rheological Properties of Ready-to-Serve and Powdered Instant Food-Thickened Beverages at Different Temperatures for Dysphagic Patients. J. Am. Diet. Assoc. 2007, 107, 1176–1182. [Google Scholar] [CrossRef]
- Bochnak-Niedźwiecka, J.; Świeca, M. Quality of New Functional Powdered Beverages Enriched with Lyophilized Fruits-Potentially Bioaccessible Antioxidant Properties, Nutritional Value, and Consumer Analysis. Appl. Sci. 2020, 10, 3668. [Google Scholar] [CrossRef]
- Bochnak-Niedźwiecka, J.; Szymanowska, U.; Świeca, M. Studies on the Development of Vegetable-Based Powdered Beverages—Effect of the Composition and Dispersing Temperature on Potential Bioaccessibility of Main Low-Molecular Antioxidants and Antioxidant Properties. LWT 2020, 131, 109822. [Google Scholar] [CrossRef]
- Bochnak-Niedźwiecka, J.; Szymanowska, U.; Kapusta, I.; Świeca, M. Antioxidant Content and Antioxidant Capacity of the Protein-Rich Powdered Beverages Enriched with Flax Seeds Gum. Antioxidants 2022, 11, 582. [Google Scholar] [CrossRef]
- Carbonell-Capella, J.M.; Buniowska, M.; Barba, F.J.; Esteve, M.J.; Frígola, A. Analytical Methods for Determining Bioavailability and Bioaccessibility of Bioactive Compounds from Fruits and Vegetables: A Review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 155–171. [Google Scholar] [CrossRef]
- Wootton-Beard, P.C.; Moran, A.; Ryan, L. Stability of the Total Antioxidant Capacity and Total Polyphenol Content of 23 Commercially Available Vegetable Juices before and after in Vitro Digestion Measured by FRAP, DPPH, ABTS and Folin—Ciocalteu Methods. Food Res. Int. 2011, 44, 217–224. [Google Scholar] [CrossRef]
- Wootton-Beard, P.C.; Ryan, L. A Beetroot Juice Shot Is a Significant and Convenient Source of Bioaccessible Antioxidants. J. Funct. Foods 2011, 3, 329–334. [Google Scholar] [CrossRef]
- Lachowicz, S.; Świeca, M.; Pejcz, E. Biological Activity, Phytochemical Parameters, and Potential Bioaccessibility of Wheat Bread Enriched with Powder and Microcapsules Made from Saskatoon Berry. Food Chem. 2021, 338, 128026. [Google Scholar] [CrossRef]
- Melini, V.; Melini, F.; Acquistucci, R. Phenolic Compounds and Bioaccessibility Thereof in Functional Pasta. Antioxidants 2020, 9, 343. [Google Scholar] [CrossRef] [Green Version]
- Sęczyk, Ł.; Świeca, M.; Gawlik-Dziki, U.; Swieca, M.; Gawlik-Dziki, U. Nutritional and Health-Promoting Properties of Bean Paste Fortified with Onion Skin in the Light of Phenolic–Food Matrix Interactions. Food Funct. 2015, 6, 3560–3566. [Google Scholar] [CrossRef]
- Jakobek, L. Interactions of Polyphenols with Carbohydrates, Lipids and Proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef]
- Nikbakht Nasrabadi, M.; Goli, S.A.H.; Sedaghat Doost, A.; Van der Meeren, P. Characterization and Enhanced Functionality of Nanoparticles Based on Linseed Protein and Linseed Gum Biocomplexes. Int. J. Biol. Macromol. 2020, 151, 116–123. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, A.C.; Teixeira, A.R.; Ferreira, R.B. Characterization of Globulins from Common Vetch (Vicia sativa L.). J. Agric. Food Chem. 2004, 52, 4913–4920. [Google Scholar] [CrossRef] [PubMed]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static in Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Sumanta, N.; Haque, C.I.; Nishika, J.; Suprakash, R. Spectrophotometric Analysis of Chlorophylls and Carotenoids from Commonly Grown Fern Species by Using Various Extracting Solvents. Res. J. Chem. Sci. 2014, 4, 63–69. [Google Scholar]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant Activity of Dietary Polyphenols as Determined by a Modified Ferric Reducing/Antioxidant Power Assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef] [Green Version]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Su, X.Y.; Wang, Z.Y.; Liu, J.R. In Vitro and in Vivo Antioxidant Activity of Pinus Koraiensis Seed Extract Containing Phenolic Compounds. Food Chem. 2009, 117, 681–686. [Google Scholar] [CrossRef]
- Decker, E.A.; Welch, B. Role of Ferritin as a Lipid Oxidation Catalyst in Muscle Food. J. Agric. Food Chem. 1990, 38, 674–677. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U.; Dziki, D.; Świeca, M.; Sȩczyk, Ł.; Rózyło, R.; Szymanowska, U. Bread Enriched with Chenopodium Quinoa Leaves Powder—The Procedures for Assessing the Fortification Efficiency. LWT-Food Sci. Technol. 2015, 62, 1226–1234. [Google Scholar] [CrossRef]
- Lecumberri, E.; Mateos, R.; Izquierdo-Pulido, M.; Rupérez, P.; Goya, L.; Bravo, L. Dietary Fibre Composition, Antioxidant Capacity and Physico-Chemical Properties of a Fibre-Rich Product from Cocoa (Theobroma cacao L.). Food Chem. 2007, 104, 948–954. [Google Scholar] [CrossRef]
- Lucas-González, R.; Viuda-Martos, M.; Pérez-Alvarez, J.A.; Fernández-López, J. In Vitro Digestion Models Suitable for Foods: Opportunities for New Fields of Application and Challenges. Food Res. Int. 2018, 107, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Seczyk, L.; Swieca, M.; Kapusta, I.; Gawlik-Dziki, U. Protein–Phenolic Interactions as a Factor Affecting the Physicochemical Properties of White Bean Proteins. Molecules 2019, 24, 408. [Google Scholar] [CrossRef] [Green Version]
- Pedrosa, M.M.; Varela, A.; Domínguez-Timón, F.; Tovar, C.A.; Moreno, H.M.; Borderías, A.J.; Díaz, M.T. Comparison of Bioactive Compounds Content and Techno-Functional Properties of Pea and Bean Flours and Their Protein Isolates. Plant Foods Hum. Nutr. 2020, 75, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Tomas, M.; Beekwilder, J.; Hall, R.D.; Diez Simon, C.; Sagdic, O.; Capanoglu, E. Effect of Dietary Fiber (Inulin) Addition on Phenolics and in Vitro Bioaccessibility of Tomato Sauce. Food Res. Int. 2018, 106, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Marques, M.C.; Hacke, A.; Neto, C.A.C.; Mariutti, L.R. Impact of Phenolic Compounds in the Digestion and Absorption of Carotenoids. Curr. Opin. Food Sci. 2021, 39, 190–196. [Google Scholar] [CrossRef]
- Petry, F.C.; Mercadante, A.Z. Impact of: In Vitro Digestion Phases on the Stability and Bioaccessibility of Carotenoids and Their Esters in Mandarin Pulps. Food Funct. 2017, 8, 3951–3963. [Google Scholar] [CrossRef] [PubMed]
- Kopec, R.E.; Gleize, B.; Borel, P.; Desmarchelier, C.; Caris-Veyrat, C. Are Lutein, Lycopene, and β-Carotene Lost through the Digestive Process? Food Funct. 2017, 8, 1494–1503. [Google Scholar] [CrossRef]
- Matemu, A.; Nakamura, S.; Katayama, S. Health Benefits of Antioxidative Peptides Derived from Legume Proteins with a High Amino Acid Score. Antioxidants 2021, 10, 316. [Google Scholar] [CrossRef]
- Bouaziz, F.; Koubaa, M.; Barba, F.J.; Roohinejad, S.; Chaabouni, S.E. Antioxidant Properties of Water-Soluble Gum from Flaxseed Hulls. Antioxidants 2016, 5, 26. [Google Scholar] [CrossRef] [Green Version]
- Moreno, C.; Mojica, L.; de Mejía, E.G.; Camacho Ruiz, R.M.; Luna-Vital, D.A. Combinations of Legume Protein Hydrolysates Synergistically Inhibit Biological Markers Associated with Adipogenesis. Foods 2020, 9, 1678. [Google Scholar] [CrossRef]
- Sȩczyk, Ł.; Świeca, M.; Gawlik-Dziki, U.; Luty, M.; Czyz, J. Effect of Fortification with Parsley (Petroselinum crispum Mill.) Leaves on the Nutraceutical and Nutritional Quality of Wheat Pasta. Food Chem. 2016, 190, 419–428. [Google Scholar] [CrossRef]
- Swieca, M.; Kordowska-Wiater, M.; Pytka, M.; Gawlik-Dziki, U.; Seczyk, L.; Złotek, U.; Kapusta, I. Nutritional and Pro-Health Quality of Lentil and Adzuki Bean Sprouts Enriched with Probiotic Yeast Saccharomyces cerevisiae Var. Boulardii. LWT-Food Sci. Technol. 2019, 100, 220–226. [Google Scholar] [CrossRef]
- Sęczyk, Ł.; Gawlik-Dziki, U.; Świeca, M. Influence of Phenolic-Food Matrix Interactions on in Vitro Bioaccessibility of Selected Phenolic Compounds and Nutrients Digestibility in Fortified White Bean Paste. Antioxidants 2021, 10, 1825. [Google Scholar] [CrossRef] [PubMed]
- McDougall, G.J.; Stewart, D. The Inhibitory Effects of Berry Polyphenols on Digestive Enzymes. BioFactors 2005, 23, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xie, Y. Interaction of Protein Isolate with Anthocyanin Extracted from Black Soybean and Its Effect on the Anthocyanin Stability. J. Food Sci. 2019, 84, 3140–3146. [Google Scholar] [CrossRef] [PubMed]
- Fadimu, G.J.; Farahnaky, A.; Gill, H.; Truong, T. Influence of Ultrasonic Pretreatment on Structural Properties and Biological Activities of Lupin Protein Hydrolysate. Int. J. Food Sci. Technol. 2022, 57, 1729–1738. [Google Scholar] [CrossRef]
- Nikbakht Nasrabadi, M.; Goli, S.A.H.; Sedaghat Doost, A.; Van der Meeren, P.; Bochnak-Niedźwiecka, J.; Szymanowska, U.; Kapusta, I.; Świeca, M.; Tannous, S.; Haykal, T.; et al. Effects of Anthocyanidin on the Inhibition of Proliferation and Induction of Apoptosis in Human Gastric Adenocarcinoma Cells. Biomed. Pharmacother. 2020, 43, 116–123. [Google Scholar] [CrossRef]
- Luna-Vital, D.; González de Mejía, E. Peptides from Legumes with Antigastrointestinal Cancer Potential: Current Evidence for Their Molecular Mechanisms. Curr. Opin. Food Sci. 2018, 20, 13–18. [Google Scholar] [CrossRef]
- Mehanna, N.S.; Hassan, Z.M.R.; El-Din, H.M.F.; Ali, A.A.-E.; Amarowicz, R.; El-Messery, T.M. Effect of Interaction Phenolic Compounds with Milk Proteins on Cell Line. Food Nutr. Sci. 2014, 5, 2130–2146. [Google Scholar] [CrossRef] [Green Version]
- Świeca, M.; Herok, A.; Piwowarczyk, K.; Sikora, M.; Ostanek, P.; Gawlik-Dziki, U.; Kapusta, I.; Czyż, J. Potentially Bioaccessible Phenolics from Mung Bean and Adzuki Bean Sprouts Enriched with Probiotic—Antioxidant Properties and Effect on the Motility and Survival of AGS Human Gastric Carcinoma Cells. Molecules 2020, 25, 2963. [Google Scholar] [CrossRef]
- Mosca, A.C.; Torres, A.P.; Slob, E.; de Graaf, K.; McEwan, J.A.; Stieger, M. Small Food Texture Modifications Can Be Used to Change Oral Processing Behaviour and to Control Ad Libitum Food Intake. Appetite 2019, 142, 104375. [Google Scholar] [CrossRef]
- Marques, L.G.; Prado, M.M.; Freire, J.T. Rehydration Characteristics of Freeze-Dried Tropical Fruits. LWT-Food Sci. Technol. 2009, 42, 1232–1237. [Google Scholar] [CrossRef]
- Ladjal-Ettoumi, Y.; Boudries, H.; Chibane, M.; Romero, A. Pea, Chickpea and Lentil Protein Isolates: Physicochemical Characterization and Emulsifying Properties. Food Biophys. 2016, 11, 43–51. [Google Scholar] [CrossRef]
- Khalloufi, S.; Corredig, M.; Goff, H.D.; Alexander, M. Flaxseed Gums and Their Adsorption on Whey Protein-Stabilized Oil-in-Water Emulsions. Food Hydrocoll. 2009, 23, 611–618. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).