Abstract
Currently, the effect of exposure to indoor air contaminants and the presence of dampness at home on respiratory/atopic health is of particular concern to physicians. The measurement of volatile organic compounds (VOCs) in exhaled breath is a useful approach for monitoring environmental exposures. A great advantage of this strategy is that it allows the study of the impact of pollutants on the metabolism through a non-invasive method. In this paper, the levels of nine VOCs (acetone, isoprene, toluene, p/m-xylene, o-xylene, styrene, benzaldehyde, naphthalene, and 2-ethyl-1-hexanol) in the exhaled breath of subjects exposed and not exposed to home dampness were assessed. Exhaled breath samples were collected from 337 mother–child pairs of a birth cohort and analysed by gas-chromatography–mass-spectrometry. It was observed that the levels of 2-ethyl-1-hexanol in the exhaled breath of the mothers were significantly influenced by exposure to household humidity. In the case of the infants, differences in some of the VOC levels related to home dampness exposure; however, they did not reach statistical significance. In addition, it was also found that the eosinophil counts of the mothers exposed to home dampness were significantly elevated compared to those of the non-exposed mothers. To our knowledge, these findings show, for the first time, that exposure to home dampness may influence VOC patterns in exhaled breath.
1. Introduction
In recent years, a considerable increase in the number of people worldwide suffering with asthma or atopic diseases, such as rhinitis and dermatitis, has been reported [1]. The problem is critical in pediatric populations, where asthma is currently the most prevalent chronic disease [2]. The appearance of atopic eczema and recurrent wheezing during the first years of life is linked to asthma development in the future [3,4]. Atopic disorders are complex diseases, and their development can be affected by certain environmental factors through the mediation of epigenetic mechanisms [5,6]. Over the last decades, the presence of dampness at home and the exposure to both indoor and outdoor environmental pollutants have been identified as possible risk factors for atopic disease development [7,8,9]. Currently, people spend long hours indoors (at home, workplaces, schools, etc.). Therefore, the study of the impact of indoor pollutants and the presence of home dampness on the development of asthma and other atopic diseases has been a priority objective of the scientific community in the last few years [10,11].
Indoor contaminants can arise from environmental tobacco smoke (ETS) and household products or construction materials, among others. Several volatile organic compounds (VOCs) can be emitted from these sources and enter human airways [12]. Moreover, it has been observed that VOCs emission is most noticeable in newly constructed buildings [13,14]. Exposure to pollutant VOCs is often measured by indoor-air analysis. In addition, personal exposure can be assessed by OVM (organic vapor monitor) samplers. These devices are worn by subjects and allow the measurement of the VOCs to which they are exposed [15,16]. Volatile organic compounds can be determined with multiple types of analytical platform, but technologies based on mass spectrometry (e.g., gas-chromatography–mass-spectrometry (GC/MS)) are the most widespread [17]. Furthermore, VOCs can also be detected in human matrices, such as urine and exhaled breath [18,19,20]. In fact, VOC analysis in exhaled breath provides relevant information about personal environmental pollutant exposure (exposome). On the other hand, the endogenous VOCs derived from human metabolism and intestinal flora can be also detected in exhaled breath [21]. Thus, the analysis of VOCs is a useful strategy for monitoring inflammation or changes in human metabolism due to diseases or environmental contaminant exposure [13,22]. Indeed, the VOCs involved in oxidative stress can be detected in exhaled breath [23]. In this regard, it has been reported that the metabolite profiles detected in exhaled breath or exhaled breath condensate (EBC) can discriminate between asthmatics and healthy controls, and between controlled asthmatics and asthmatics with exacerbations [24,25,26,27].
The effect of dampness exposure on the development of respiratory symptoms has been widely studied. In addition, it has been suggested that prenatal and postnatal exposure to home dampness may be involved in the occurrence of wheezing, rhinitis, and asthma in children [28,29,30]. Furthermore, indoor dampness and indoor levels of VOCs such as toluene, ethylbenzene, or 2-ethyl-1-hexanol are also associated with the sick-building syndrome (SBS), which is marked by respiratory problems, as well as other symptoms related to the built environment, and can lead to asthma [31,32,33,34]. In this sense, it has been reported that mould microorganisms and VOCs released from high-humidity locations could be the main causes of the role of exposure to indoor dampness in changes in health status [35,36]. The analysis of the influence of exposure to indoor dampness on health outcomes would be helpful in establishing guidelines for reducing the risk of disease development [37]. To date, the inflammation induced by exposure to residential dampness and its impact on human metabolism has been measured by fractional exhaled nitric oxide (FeNO) analysis and eosinophil counts in the nasal lavage and blood, respectively [38,39,40,41].
For all these reasons, this paper presents a study set in a country with a Mediterranean climate whose goal was to assess the effects of dampness exposure at home on VOC patterns in exhaled breath and on the appearance of atopic disorders. This is the first study, to our knowledge, in which the levels of nine VOCs (acetone, isoprene, toluene, p/m-xylene, o-xylene, styrene, benzaldehyde, naphthalene, and 2-ethyl-1-hexanol) in exhaled breath have been analysed in women and their infants for this purpose. Acetone and isoprene would originate in human metabolism (they are two of the most frequent VOCs in exhaled breath), whereas the other seven VOCs are derived from air pollutants. Among them, aromatic compounds (toluene, styrene, etc.) could derive from several sources, such as fuel combustion, smoking, household renovations, etc. [18,33,42].
2. Results
2.1. Characteristics of the Study Population
The characteristics of the study population are shown in Table 1 and Table 2 and Supplementary Figure S1. More than 25% of the homes showed damp in the walls, 20% of the homes had one parent with asthma, 50% of the homes had one parent with allergic rhinitis, and 17% of the households had one parent with atopic dermatitis. During their first three months of life, 12% of the children evidenced atopic eczema and 3% suffered some episode of respiratory infection. No significant association between the existence of dampness at home and the demographic characteristics of the study population, such as age, weight and height, social class, or level of education was shown (Table 1 and Table 2). A total of 674 exhaled breath samples from mothers and 3-month-old children were collected and analysed. In addition, 337 ambient air samples (one per each mother-child pair) were collected from the room in which the sampling was carried out, in order to confirm that the differences observed in the human exhaled breath were caused by the exposure to humidity in the home rather than by interference from the ambient air in the breath-sampling room. The average minimum time of home-dampness exposure of the subjects was 7 months (4 months of exposure during pregnancy and 3 months after childbirth).

Table 1.
Demographic characteristics of mothers included in this study.

Table 2.
Demographic characteristics of children included in this study.
2.2. Home Dampness and Atopic Disorders
Moisture stains or mould were present on the walls of the usual residence of 18% of asthmatic parents, 19% of parents with allergic rhinitis, 29% of parents with atopic dermatitis, 24% of parents with allergic conjunctivitis, and 13% of children who developed an atopic or respiratory disease during their first three months of life (Supplementary Figure S2). Figure 1 shows the ORs and their 95% confidence intervals that associate the risk of suffering from an atopic disease with the presence of dampness in the home. As can be seen, indoor dampness significantly increased the risk of one parent suffering from allergic rhinitis. On the other hand, although the development of atopic or respiratory disease in the first months of infants’ lives was not significantly associated with the exposure to home dampness, the risk was close to significant (p = 0.095). In fact, the observed frequency of respiratory and atopic symptoms was higher than the expected frequency in the children whose homes had damp stains.
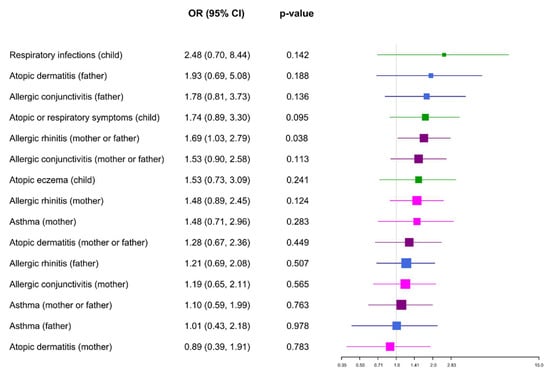
Figure 1.
Dampness and atopic disorders. Odds ratios (ORs) and their 95% confidence intervals (CI) that show the risk of suffering an atopic disease related to indoor dampness.
2.3. Home Dampness and Analysis of White-Blood-Cell (WBC) Counts
Table 3 shows the white-blood-cell counts of the mothers who lived in houses with damp stains and mothers with no damp in walls. Statistically significant differences in the eosinophil blood counts were observed. The eosinophil blood counts were higher in the mothers exposed to dampness than in those with no dampness in their usual residence (Supplementary Figure S3).

Table 3.
Relationship between white-blood-cell count and indoor-dampness exposure.
2.4. Home Dampness and Exhaled Breath of Mothers
Figure 2 shows the impact of home dampness on the exhaled breath of the mothers. The results indicate that the levels of 2-ethyl-1-hexanol and toluene were significantly higher in the air exhaled by the women whose homes had damp in their walls. However, the toluene levels were also significantly high in the ambient-air samples from the rooms in which the human exhaled breath samples were collected (Figure 3). Therefore, only the exhaled-breath levels of 2-ethyl-1-hexanol were significantly associated with the presence of dampness in the houses.
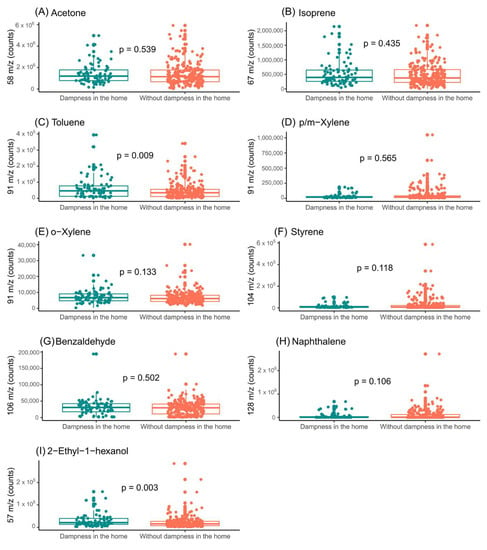
Figure 2.
Assessment of indoor-dampness exposure influence on the levels of the features selected in exhaled breath of mothers. (A) Impact of indoor-dampness exposure on levels of acetone (m/z = 58); (B) impact of indoor-dampness exposure on levels of isoprene (m/z = 67); (C) impact of indoor-dampness exposure on levels of toluene (m/z = 91); (D) impact of indoor-dampness exposure on levels of p/m-xylene (m/z = 91); (E) impact of indoor-dampness exposure on levels of o-xylene (m/z = 91); (F) impact of indoor-dampness exposure on levels of styrene (m/z = 104); (G) impact of indoor-dampness exposure on levels of benzaldehyde (m/z = 106); (H) impact of indoor-dampness exposure on levels of naphthalene (m/z = 128); and (I) impact of indoor-dampness exposure on levels of 2-ethyl-1-hexanol (m/z = 57) in exhaled breath of mothers.
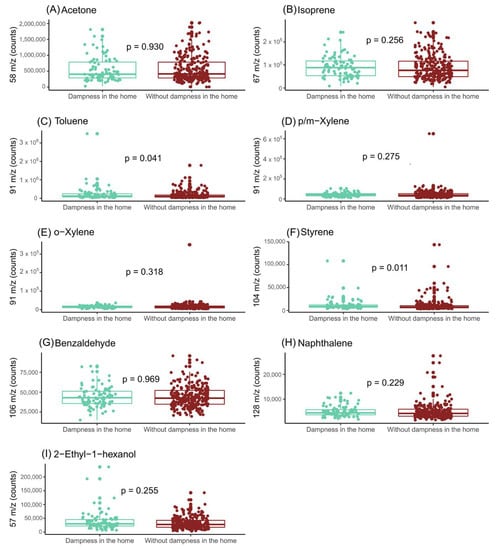
Figure 3.
Assessment of influence of indoor-dampness exposure on the levels of the features selected in ambient air from room collection. (A) Impact of indoor-dampness exposure on levels of acetone (m/z = 58); (B) impact of indoor-dampness exposure on levels of isoprene (m/z = 67); (C) impact of indoor-dampness exposure on levels of toluene (m/z = 91); (D) impact of indoor-dampness exposure on levels of p/m-xylene (m/z = 91); (E) impact of indoor-dampness exposure on levels of o-xylene (m/z = 91); (F) impact of indoor-dampness exposure on levels of styrene (m/z = 104); (G) impact of indoor-dampness exposure on levels of benzaldehyde (m/z = 106); (H) impact of indoor-dampness exposure on levels of naphthalene (m/z = 128); and (I) Impact of indoor-dampness exposure on levels of 2-ethyl-1-hexanol (m/z = 57) in room air-content samples.
2.5. Home Dampness and Exhaled Breath of Infants
Figure 4 shows the influence of home humidity on the exhaled breath of infants. Although some differences were observed, none of the selected VOCs showed a statistically significant association with the presence of dampness in the households of the subjects. The association of the VOC levels with the presence of dampness in the home was also stratified by the children’s sex (Figure 5 and Figure 6).
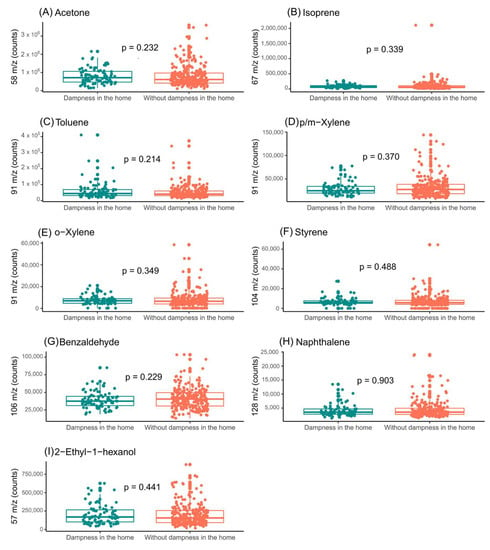
Figure 4.
Assessment of influence of indoor-dampness exposure on the levels of the features selected in exhaled breath from children. (A) Impact of indoor-dampness exposure on levels of acetone (m/z = 58); (B) Impact of indoor-dampness exposure on levels of isoprene (m/z = 67); (C) impact of indoor-dampness exposure on levels of toluene (m/z = 91); (D) impact of indoor-dampness exposure on levels of p/m-xylene (m/z = 91); (E) impact of indoor-dampness exposure on levels of o-xylene (m/z = 91); (F) impact of indoor-dampness exposure on levels of styrene (m/z = 104); (G) impact of indoor-dampness exposure on levels of benzaldehyde (m/z = 106); (H) impact of indoor-dampness exposure on levels of naphthalene (m/z = 128); and (I) impact of indoor-dampness exposure on levels of 2-ethyl-1-hexanol (m/z = 57) in exhaled breath of children.
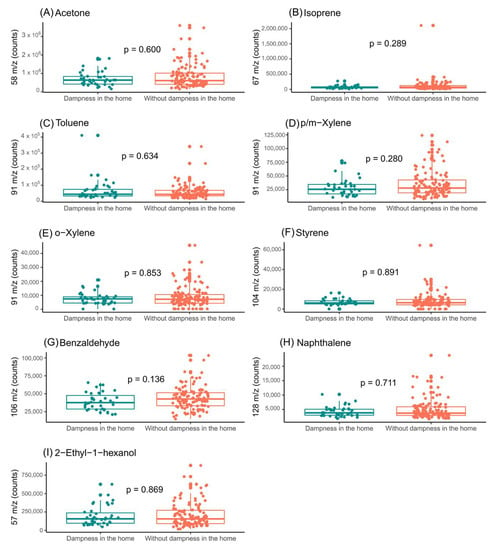
Figure 5.
Assessment of influence of indoor-dampness exposure on the levels of the features selected in exhaled breath from boys. (A) Impact of indoor-dampness exposure on levels of acetone (m/z = 58); (B) impact of indoor-dampness exposure on levels of isoprene (m/z = 67); (C) impact of indoor-dampness exposure on levels of toluene (m/z = 91); (D) impact of indoor-dampness exposure on levels of p/m-xylene (m/z = 91); (E) impact of indoor-dampness exposure on levels of o-xylene (m/z = 91); (F) impact of indoor-dampness exposure on levels of styrene (m/z = 104); (G) impact of indoor-dampness exposure on levels of benzaldehyde (m/z = 106); (H) impact of indoor-dampness exposure on levels of naphthalene (m/z = 128); and (I) impact of indoor-dampness exposure on levels of 2-ethyl-1-hexanol (m/z = 57) in exhaled breath of boys.
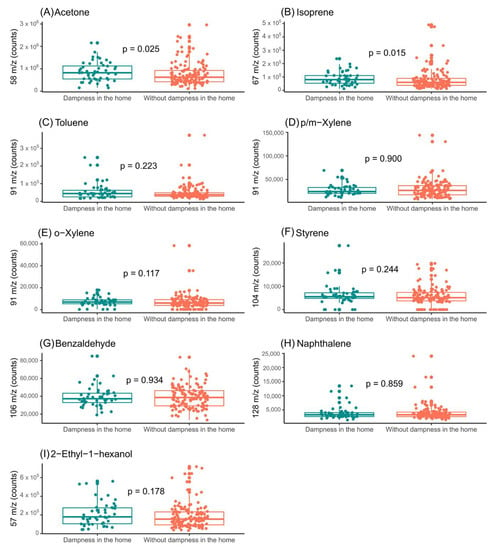
Figure 6.
Assessment of influence of indoor-dampness exposure on the levels of the features selected in exhaled breath from girls. (A) Impact of indoor-dampness exposure on levels of acetone (m/z = 58); (B) impact of indoor-dampness exposure on levels of isoprene (m/z = 67); (C) impact of indoor-dampness exposure on levels of toluene (m/z = 91); (D) impact of indoor-dampness exposure on levels of p/m-xylene (m/z = 91); (E) impact of indoor-dampness exposure on levels of o-xylene (m/z = 91); (F) impact of indoor-dampness exposure on levels of styrene (m/z = 104); (G) impact of indoor-dampness exposure on levels of benzaldehyde (m/z = 106); (H) impact of indoor-dampness exposure on levels of naphthalene (m/z = 128); and (I) impact of indoor-dampness exposure on levels of 2-ethyl-1-hexanol (m/z = 57) in exhaled breath of girls.
In the exhaled breath of the boys, there were no significant differences in the VOC levels (Figure 5). However, in the case of the exhaled breath of the girls, significant differences were observed in the levels of acetone and isoprene (Figure 6). In addition, the levels of the selected compounds in the ambient air of the sample-collection room were assessed (Supplementary Figures S4 and S5).
3. Discussion
This is the first study, to our knowledge, that aims to identify changes in human exhaled breath due to exposure to indoor dampness. The analysis of VOCs in exhaled breath is an emerging strategy for monitoring human metabolism [43,44]. For this reason, it has been proposed as a promising approach to assess the effects caused by environmental contaminant exposure [21]. Our results clearly demonstrate that the exhaled breath from mothers from the NELA cohort [45] was affected by the presence of damp stains in their homes. Thus, the levels of 2-ethyl-1-hexanol in the exhaled breath were influenced by the residential dampness. In this regard, it was previously reported that this compound increases its concentration owing to the rise in relative humidity [46]. 2-Ethyl-1-hexanol is an indoor-air contaminant derived from di-(2-ethylhexyl) phthalate (DEHP), which is the most common plasticizer of poly(vinyl chloride) (PVC) [33] (Figure 7). Plasticizers provide PVC with new properties, such as flexibility. However, plasticizer migration is frequent when plastic products suffer from deterioration, since plasticizers are not strongly joined to polymer chains [47]. 2-Ethyl-1-hexanol is emitted after the hydrolysis (KEGG reaction number: R04202) of DEHP, and this reaction is increased in the presence of humidity [33]. In this sense, it has been shown that several microorganisms, such as bacteria, fungi and yeasts degrade the plasticizers, DEHP or di-2-ethylhexyl adipate (DEHA), producing 2-ethyl-1-hexanol from the hydrolysis of their ester bonds [48]. Further, previous studies indicated that the concentration of 2-ethyl-1-hexanol in indoor air was higher in the presence of damp stains [14,40,49,50]. In this sense, our results show, for the first time, that levels of 2-ethyl-1-hexanol in human exhaled breath also fluctuate with the exposure to moisture in the home. Furthermore, exposure to this compound has been determined as the main cause of SBS. In addition, 2-Ethyl-1-hexanol is an endocrine disruptor associated with the development of several respiratory diseases, such as asthma [46,49,50,51,52,53]. Indeed, high levels of 2-ethyl-1-hexanol in exhaled breath have recently been linked to asthma with coexisting atopic diseases in women [27]. By contrast, in the present study, a significant relationship was not observed between dampness exposure and human-exhaled-breath levels of aromatic compounds, which are traditionally reported to be linked with SBS development [32].
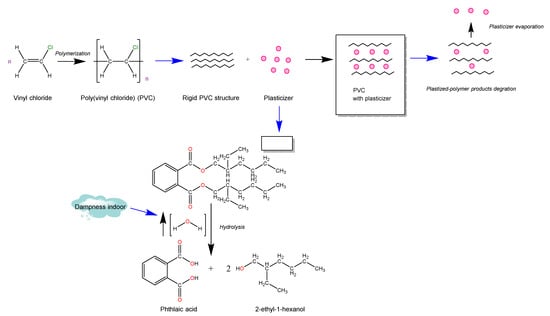
Figure 7.
Production of 2-ethyl-1-hexanol detected in human exhaled breath in presence of residential humidity.
Additionally, the eosinophil blood counts of the mothers in this study who lived in homes with humidity were higher than in those not exposed to -moisture. This result is in line with previous research outcomes [40,41]. Indeed, eosinophil count in both blood and nasal lavage is a usual strategy for inflammation detection and has been suggested as a tool for the diagnosis of asthma and other respiratory diseases [22,54,55,56]. Hence, the high eosinophil blood counts in mothers exposed to indoor dampness are evidence that prolonged exposure could cause inflammation. In this sense, although no significant association was found between humidity exposure and asthma in the adults, there was a significant association between allergic rhinitis and the presence of home humidity. In fact, a significant relationship between rhinitis in adults and the presence of humidity in houses was also reported by Zhang et al. [57]. In addition, a relationship between home dampness exposure and allergic rhinitis was also previously observed in a pediatric population [58].
Despite the fact that the association between the presence of humidity stains in houses and the appearance of respiratory or atopic diseases in children was not significant, the children who were exposed to them suffered more respiratory or atopic symptoms than the others. Nevertheless, this relationship may not have been significant, because the children were only a few months old. If risk assessments were to be conducted as the children grew up, in future phases of our study, the association would likely be significant. This is an ongoing project for our group. In this regard, the relevance of the exposure to indoor dampness in the development of asthma in children was highlighted in a recent systematic review [59]. With respect to the levels of compounds in exhaled breath, the differences in the children due to exposure to residential humidity were less noticeable than in their mothers. However, some compounds, such as 2-ethyl-1-hexanol, were found to be higher in the children exposed to moisture than in the unexposed infants. Thus, the trends observed could become as significant as those observed in the mothers if the exhaled breath of the children were analysed within a few months or years (i.e., longer exposure period). In addition, it is worth mentioning the fact that the differences in exposure to indoor humidity in exhaled air for the girls were more noticeable than for the boys. Thus, the levels of acetone and isoprene (two of the most typical endogenous compounds in human exhaled breath [60]) were significantly elevated in the girls exposed to indoor dampness. This study demonstrates the need to also perform statistical analyses separating data by sex, since differences could be masked.
Therefore, in view of the close relationship between residential humidity and the development of respiratory/atopic diseases, such as asthma or rhinitis, it is crucial to implement strategies to keep homes free of dampness and ensure the best possible indoor-air quality. In fact, some home-remediation projects have had encouraging respiratory health outcomes [61,62].
Current Limitations
The main limitation of this study is that the home damp stains affecting the mother–child pairs were not checked by a specialist. The qualitative indicator of dampness exposure was the collection of responses from the mothers to the structured questionnaires about the presence of visible dampness in the walls and the exposure time. Furthermore, the lack of significant differences in VOC levels in the exhaled breath due to indoor-dampness exposure could have been conditioned by the short lives of the children, who only experienced a few months of exposure. However, several follow-up visits are planned as part of the NELA birth-cohort study.
4. Materials and Methods
4.1. Study Design and Participants
This study included mother–child pairs from the NELA (Nutrition in Early Childhood Asthma) study, a population-based prospective birth cohort. The main objective of NELA is to unravel the developmental origins and mechanisms of asthma and allergies. The study protocol, sample size, recruiting methods, and data collection processes have been described elsewhere [45]. The study was approved by Clinical Research Ethics Committee (CEIC) of the University Hospital Virgen de la Arrixaca of Murcia (Spain) and written informed consent was obtained from all the subjects. Mother–child pairs who completed the three-month follow-up visit between May 2017 and October 2018 were included in the present study [27].
4.2. Information on Indoor-Dampness Exposure, Health Outcomes and Other Variables
During the recruitment (20–24 weeks of gestation), trained interviewers conducted a structured questionnaire. Subjects were divided into two groups (“dampness in the home” and “without dampness in the home”) based on whether they answered yes or no to the following statement: “presence of damp stains or mould on the walls of the usual residence”. In addition, the following information about baseline data and parental health status were obtained: age of mother; age of father; prepregnancy body-mass index (BMI) of mother; social class of mother (defined as occupation during pregnancy based on the highest social class by using a widely used Spanish adaptation of the international ISCO88 coding system: I–II, managers/technicians; III, skilled; IV–V, semiskilled/unskilled; and unemployed) [63]; social class of father; educational level of mother (incomplete secondary or less, complete secondary, and university); educational level of father; maternal smoking during pregnancy (yes/no); asthma in the mother (yes/no); asthma in the father (yes/no); allergic rhinitis of mother (yes/no); allergic rhinitis of father (yes/no); atopic dermatitis in mother (yes/no); atopic dermatitis in father (yes/no); allergic conjunctivitis in mother (yes/no); allergic conjunctivitis in father (yes/no). Subjects were considered to have an atopic disease if they answered on the structured questionnaire that they had been previously diagnosed by a physician. Moreover, white-blood-cell counts (WBC) were obtained from blood samples of the pregnant women by a Sysmex® XN9000 (Sysmex Corporation, Kobe, Japan) hematology analyser [27]. After childbirth, the following information on the children was also collected: sex, birth weight and birth height. During the three-month follow-up visit of the children, another structured questionnaire was administered to the mothers, which included questions related to the atopic and respiratory health of the children: atopic eczema in infants (yes/no); respiratory infections in infants (yes/no). In addition, the minimum time of exposure to humidity in the home was defined as the period between the date of the follow-up visit at 20–24 weeks of pregnancy and the date of the three-month follow-up visit.
4.3. Collection and Analysis of Exhaled Breath
The protocols used for collection and analysis of exhaled breath are described above [64]. The mixed-expiratory-breath portion of 3-month-old infants and their mothers was collected in 1-litre Tedlar® gas sampling bags. However, a previous step was included in breath sampling of infants, since they are passive subjects and Tedlar® bags showed too much resistance to be filled by them. Therefore, the exhaled breath of infants was previously collected by a facemask connected to Quintron® 400-millilitre gas sampling bags. Next, air content of Quintron® bags was transferred to a Tedlar® bag. In addition, ambient samples were actively pumped onto sorbent tube using an Easy-VOC syringe (Markes International, Bridgend, UK) to check the influence of the air in the room where the breath samples were collected. In this study, analysis of exhaled breath was conducted by a thermal desorption system coupled with gas-chromatography–single-quadrupole-mass-spectrometry (TD-GC/q-MS). For this purpose, both exhaled breath and room-air-content samples were stored in thermal desorption tubes (Tenax TA/carbograph 5td, Markes International) prior to analysis. In addition, two chemical standards (C7-C30 Saturated Alkanes Standard and VOC Calibration Standard, Sigma-Aldrich, Taufkirchen, Germany) were analysed for computation of VOC retention indexes.
4.4. Data Preprocessing
A workflow based on open sources was implemented for data preprocessing [64]. Firstly, it was necessary to transform raw data into an easy-to-use format, such as mzXML, by means of conversion from Proteowizard [65,66]. This workflow integrates the functions of three R packages (xcms, cliqueMS, and eRah) [67,68,69] and brings together the two most important strategies for GC/MS data preprocessing (ion-fragment/feature determination and compound determination). Through this workflow, a matrix with intensities of features or ion peaks detected in exhaled-air samples was generated and compounds to which they corresponded were determined and identified. The compound identification was carried out by matching with the NIST (National Institute of Standard and Technology) spectral library based on mass spectra and retention indexes. For the present study, nine compounds were selected and theses levels were assessed using their most characteristic features: acetone (m/z = 58), isoprene (m/z = 67), toluene (m/z = 91), p/m-xylene (m/z = 91), o-xylene (m/z = 91), styrene (m/z = 104), benzaldehyde (m/z = 106), naphthalene (m/z = 128), and 2-ethyl-1-hexanol (m/z = 57).
4.5. Statistical Analysis
The statistical analysis was carried out by version R 4.0.5. Chi-square test or Fisher’s exact test were performed to check the relationship between dampness indoor exposure and categorical variables. Associations between presence of dampness in usual residence of mother–child pairs and continuous variables, levels of selected features from exhaled breath of mothers and children, or results of white-blood-cell count (WBC) analysis of mothers during pregnancy, were assessed by Student’s t-tests or Mann–Whitney U tests (normal distribution was evaluated by Lilliefors tests using nortest package). Differences between both groups (“dampness in the home” and “without dampness in the home”) were considered significant when p-value < 0.05. In addition, the levels of selected features in both groups were also checked in the ambient air of the collection room to differentiate between fluctuations in human exhaled breath influenced by exposure to dampness in the home and those due to exhaled breath samples’ contamination by room air. In addition, logistic regression models were constructed to estimate odds ratio (OR) values between atopic disorders in parents and children with damp in the walls of houses.
5. Conclusions
The results of this study show, for the first time, to our knowledge, that exposure to dampness in the home may influence the VOC levels in the exhaled breath of mothers and infants. A significant association was found between home-indoor-dampness exposure and allergic rhinitis in adults. However, a follow-up of infants from the NELA birth cohort is required to confirm that indoor-dampness exposure causes atopic disease development in children. Moreover, it is essential to determine the effects that this exposure may have on human metabolism and health. Furthermore, in order to design home remediation strategies, a search for the sources of volatile organic compounds with elevated levels associated with residential dampness should be mandatory.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/app12146864/s1. Figure S1: Distribution of atopic disorders in study population. Figure S2: Distribution of indoor-dampness -exposure atopic disorders in study population. Figure S3: Indoor-dampness exposure influence on eosinophil blood count in mothers in NELA cohort. Figure S4: Assessment of indoor-dampness-exposure influence on the levels of the features selected in ambient air from room collection (only samples collected when infants were boys). Figure S5: Assessment of indoor-dampness-exposure influence on the levels of the features selected in ambient air from room collection (samples only collected when infants were girls).
Author Contributions
Conceptualization, R.A.S.-M. and T.d.D.P.; methodology, R.A.S.-M., G.L.T., J.G.-J., M.C.D. and T.d.D.P.; software, R.A.S.-M.; validation, R.A.S.-M.; formal analysis, R.A.S.-M., G.L.T. and J.G.-J.; investigation, R.A.S.-M. and J.A.N.-V.; resources, L.G.-M. and E.M.; data curation, R.A.S.-M. and E.M.; writing—original draft preparation, R.A.S.-M.; writing—review and editing, R.A.S.-M., G.L.T., J.G.-J., E.M., M.C.D. and T.d.D.P.; visualization, R.A.S.-M. and T.d.D.P.; supervision, T.d.D.P.; project administration, E.M., L.G.-M., M.C.D. and T.d.D.P.; funding acquisition, E.M., L.G.-M., M.C.D. and T.d.D.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the Instituto de Salud Carlos III, Spanish Ministry of Science, Innovation, and Universities and Fondos FEDER (CP14/00046, PIE15/00051, PI16/00422, and ARADyAL network RD160006), the Ministry of Science, Innovation, and Universities (MCIU), the State Research Agency (AEI) and the European Regional Development Fund (FEDER), RTI2018-094393-B-C21-MCIU/AEI/FEDER, UE, through “ERDF: A way of making Europe”, through the European Union, and through the “European Union NextGenerationEU/PRTR” and the Seneca Foundation, CARM, 20786/PI/18. Rosa A. Sola-Martínez is the recipient of a FPU-PhD fellowship from the Ministry of Science, Innovation, and Universities (FPU18/00545), and Gema Lozano Terol is a recipient of a PhD fellowship from the Seneca Foundation (20715/FPI/18). Eva Morales was funded by Miguel Servet Fellowships (MS14/00046 and CPII19/00019), awarded by the Instituto de Salud Carlos III (ISCIII), Spanish Ministry of Science, Innovation, and Universities, and Fondos FEDER.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by Clinical Research Ethics Committee (CEIC) of the University Hospital Virgen de la Arrixaca of Murcia (report 9/14; 29 September 2014).
Informed Consent Statement
Written informed consent was obtained from all subjects involved in the study.
Acknowledgments
The members of the NELA study group are as follows: ME Candel-Torralba1, L Garcia-Marcos (PI)1,2,4,18, MJ Gimenez-Bañon1, A Martinez-Torres1,2,18, E Morales (PI)1,3, V Perez-Fernandez1,4,18, M Sanchez-Solis1,2,4,18, A Nieto1,5, MT Prieto-Sanchez1,5, M Sanchez-Ferrer1,5, L Fernandez-Palacios1,6, VP Gomez-Gomez1,6, C Martinez-Gracia1,6, P Peso-Echarri1,6, G Ros-Berruezo1,6, M Santaella-Pacual1,6, A Gazquez1,7, E Larque1,7, MT Pastor-Fajardo1,7, M Sanchez-Campillo,1,7, A Serrano-Munuera1,7, M Zornoza-Moreno1,7, P Jimenez-Guerrero8, E Adomnei1,9, JJ Arense-Gonzalo1,9, J Mendiola1,9, F Navarro-Lafuente1,9, AM Torres-Cantero1,9, C Salvador-Garcia10, M Segovia-Hernández1,11, G Yagüe-Guirao1,11, PL Valero-Guillén1,12, FV Aviles-Plaza1,13, J Cabezas-Herrera1,13, A Martinez-Lopez1,13, M Martinez-Villanueva1,13, JA Noguera-Velasco1,13, E Cantero-Cano1, A Franco-Garcia1,14, AM Garcia-Serna,1,14, T Hernandez-Caselles1,14,18, E Martin-Orozco1,14,18, M Norte-Muñoz1,14, M Canovas1,14, T de Diego1,14, JM Pastor1,14, RA Sola-Martínez1,14, A Esteban-Gil1,17, JT Fernández-Breis1,15, MV Alcántara16, S Hernández16, C López-Soler16. 1 Biomedical Research Institute of Murcia, IMIB-Arrixaca, Murcia, Spain; 2 Paediatric Respiratory Unit, “Virgen de la Arrixaca” Children’s University Clinical Hospital, University of Murcia, Spain; 3 Department of Public Health Sciences, University of Murcia, Spain; 4 Department of Paediatrics, University of Murcia, Spain; 5 Obstetrics and Gynaecology Service, “Virgen de la Arrixaca” University Clinical Hospital, University of Murcia, Spain; 6 Food Science and Technology Department, Veterinary Faculty of Veterinary, University of Murcia, Spain; 7 Department of Physiology, Faculty of Biology, Campus Mare Nostrum, University of Murcia, Spain; 8 Regional Atmospheric Modelling Group, Department of Physics, University of Murcia, Spain; 9 Department of Public Health Sciences, University of Murcia, Spain; 10 Microbiology Service, General University Hospital Consortium, University of Valencia, Spain; 11 Microbiology Service, University Clinical Hospital “Virgen de la Arrixaca”, University of Murcia, Spain; 12 Microbiology and Genetics Department, University of Murcia, Spain; 13 Molecular Therapy and Biomarkers Research Group, Clinical Analysis Service, University Clinical Hospital “Virgen de la Arrixaca”, University of Murcia, Spain; 14 Department of Biochemistry and Molecular Biology B and Immunology, University of Murcia, Spain; 15 Department of Informatics and Systems, University of Murcia, Spain; 16 Paediatric and Adolescent Clinical Psychology University Research Group (GUIIA-PC), University of Murcia, Spain; 17 Foundation for Healthcare Training and Research of the Region of Murcia (FFIS); 18 Network of Asthma and Adverse and Allergic Reactions (ARADyAL).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Beckhaus, A.A.; Garcia-Marcos, L.; Forno, E.; Pacheco-Gonzalez, R.M.; Celedõn, J.C.; Castro-Rodriguez, J.A. Maternal nutrition during pregnancy and risk of asthma, wheeze, and atopic diseases during childhood: A systematic review and meta-analysis. Allergy 2015, 70, 1588–1604. [Google Scholar] [CrossRef]
- Kansen, H.M.; Le, T.M.; Uiterwaal, C.S.P.M.; van Ewijk, B.E.; Balemans, W.A.F.; Gorissen, D.M.W.; de Vries, E.; van Velzen, M.F.; Slabbers, G.H.P.R.; Meijer, Y.; et al. Prevalence and predictors of uncontrolled asthma in children referred for asthma and other atopic diseases. J. Asthma Allergy 2020, 13, 67–75. [Google Scholar] [CrossRef] [Green Version]
- Amat, F.; Soria, A.; Tallon, P.; Bourgoin-Heck, M.; Lambert, N.; Deschildre, A.; Just, J. New insights into the phenotypes of atopic dermatitis linked with allergies and asthma in children: An overview. Clin. Exp. Allergy 2018, 48, 919–934. [Google Scholar] [CrossRef]
- van de Kant, K.D.G.; van Berkel, J.J.B.N.; Jobsis, Q.; Lima Passos, V.; Klaassen, E.M.M.; van der Sande, L.; van Schayck, O.C.P.; de Jongste, J.C.; van Schooten, F.J.; Derks, E.; et al. Exhaled breath profiling in diagnosing wheezy preschool children. Eur. Respir. J. 2013, 41, 183–188. [Google Scholar] [CrossRef]
- Potaczek, D.P.; Harb, H.; Michel, S.; Alhamwe, B.A.; Renz, H.; Tost, J. Epigenetics and allergy: From basic mechanisms to clinical applications. Epigenomics 2017, 9, 539–571. [Google Scholar] [CrossRef]
- Garn, H.; Potaczek, D.P.; Pfefferle, P.I. The Hygiene Hypothesis and New Perspectives-Current Challenges Meeting an Old Postulate. Front. Immunol. 2021, 12, 637087. [Google Scholar] [CrossRef]
- Adgate, J.L.; Church, T.R.; Ryan, A.D.; Ramachandran, G.; Fredrickson, A.L.; Stock, T.H.; Morandi, M.T.; Sexton, K. Outdoor, indoor, and personal exposure to VOCs in children. Environ. Health Perspect. 2004, 112, 1386–1392. [Google Scholar] [CrossRef] [Green Version]
- Sharpe, R.A.; Bearman, N.; Thornton, C.R.; Husk, K.; Osborne, N.J. Indoor fungal diversity and asthma: A meta-analysis and systematic review of risk factors. J. Allergy Clin. Immunol. 2015, 135, 110–122. [Google Scholar] [CrossRef]
- Testa, D.; Di Bari, M.; Nunziata, M.; De Cristofaro, G.; Massaro, G.; Marcuccio, G.; Motta, G. Allergic rhinitis and asthma assessment of risk factors in pediatric patients: A systematic review. Int. J. Pediatr. Otorhinolaryngol. 2020, 129, 109759. [Google Scholar] [CrossRef]
- Mendell, M.J.; Kumagai, K. Observation-based metrics for residential dampness and mold with dose–response relationships to health: A review. Indoor Air 2017, 27, 506–517. [Google Scholar] [CrossRef]
- Madureira, J.; Paciência, I.; Pereira, C.; Teixeira, J.P.; de O. Fernandes, E. Indoor air quality in Portuguese schools: Levels and sources of pollutants. Indoor Air 2016, 26, 526–537. [Google Scholar] [CrossRef] [Green Version]
- Billionnet, C.; Gay, E.; Kirchner, S.; Leynaert, B.; Annesi-Maesano, I. Quantitative assessments of indoor air pollution and respiratory health in a population-based sample of French dwellings. Environ. Res. 2011, 111, 425–434. [Google Scholar] [CrossRef]
- Kwon, J.-W.; Park, H.-W.; Kim, W.J.; Kim, M.-G.; Lee, S.-J. Exposure to volatile organic compounds and airway inflammation. Environ. Health 2018, 17, 65. [Google Scholar] [CrossRef]
- Nielsen, G.D.; Larsen, S.T.; Olsen, O.; Løvik, M.; Poulsen, L.K.; Glue, C.; Wolkoff, P. Do indoor chemicals promote development of airway allergy? Indoor Air 2007, 17, 236–255. [Google Scholar] [CrossRef]
- Son, B.; Breysse, P.; Yang, W. Volatile organic compounds concentrations in residential indoor and outdoor and its personal exposure in Korea. Environ. Int. 2003, 29, 79–85. [Google Scholar] [CrossRef]
- Arif, A.A.; Shah, S.M. Association between personal exposure to volatile organic compounds and asthma among US adult population. Int. Arch. Occup. Environ. Health 2007, 80, 711–719. [Google Scholar] [CrossRef]
- Rattray, N.J.W.; Hamrang, Z.; Trivedi, D.K.; Goodacre, R.; Fowler, S.J. Taking your breath away: Metabolomics breathes life in to personalized medicine. Trends Biotechnol. 2014, 32, 538–548. [Google Scholar] [CrossRef]
- de Lacy Costello, B.; Amann, A.; Al-Kateb, H.; Flynn, C.; Filipiak, W.; Khalid, T.; Osborne, D.; Ratcliffe, N.M. A review of the volatiles from the healthy human body. J. Breath Res. 2014, 8, 014001. [Google Scholar] [CrossRef]
- Caron-Beaudoin, É.; Valter, N.; Chevrier, J.; Ayotte, P.; Frohlich, K.; Verner, M.A. Gestational exposure to volatile organic compounds (VOCs) in Northeastern British Columbia, Canada: A pilot study. Environ. Int. 2018, 110, 131–138. [Google Scholar] [CrossRef]
- Storer, M.; Curry, K.; Squire, M.; Kingham, S.; Epton, M. Breath testing and personal exposure--SIFT-MS detection of breath acetonitrile for exposure monitoring. J. Breath Res. 2015, 9, 036006. [Google Scholar] [CrossRef]
- Amann, A.; de Lacy Costello, B.; Miekisch, W.; Schubert, J.; Buszewski, B.; Pleil, J.; Ratcliffe, N.; Risby, T. The human volatilome: Volatile organic compounds (VOCs) in exhaled breath, skin emanations, urine, feces and saliva. J. Breath Res. 2014, 8, 034001. [Google Scholar] [CrossRef]
- van de Kant, K.D.G.; van der Sande, L.J.T.M.; Jöbsis, Q.; van Schayck, O.C.P.; Dompeling, E. Clinical use of exhaled volatile organic compounds in pulmonary diseases: A systematic review. Respir. Res. 2012, 13, 117. [Google Scholar] [CrossRef] [Green Version]
- Jalali, M.; Zare Sakhvidi, M.J.; Bahrami, A.; Berijani, N.; Mahjub, H. Oxidative stress biomarkers in exhaled breath of workers exposed to crystalline silica dust by SPME-GC-MS. J. Res. Health Sci. 2016, 16, 153–161. [Google Scholar]
- Ferraro, V.A.; Carraro, S.; Pirillo, P.; Gucciardi, A.; Poloniato, G.; Stocchero, M.; Giordano, G.; Zanconato, S.; Baraldi, E. Breathomics in Asthmatic Children Treated with Inhaled Corticosteroids. Metabolites 2020, 10, 390. [Google Scholar] [CrossRef]
- Di Palmo, E.; Cantarelli, E.; Catelli, A.; Ricci, G.; Gallucci, M.; Miniaci, A.; Pession, A. The predictive role of biomarkers and genetics in childhood asthma exacerbations. Int. J. Mol. Sci. 2021, 22, 4651. [Google Scholar] [CrossRef]
- Ibrahim, W.; Carr, L.; Cordell, R.; Wilde, M.J.; Salman, D.; Monks, P.S.; Thomas, P.; Brightling, C.E.; Siddiqui, S.; Greening, N.J. Breathomics for the Clinician: The use of volatile organic compounds in respiratory diseases. Thorax 2021, 76, 514–521. [Google Scholar] [CrossRef]
- Sola-Martínez, R.A.; Lozano-Terol, G.; Gallego-Jara, J.; Morales, E.; Cantero-Cano, E.; Sanchez-Solis, M.; García-Marcos, L.; Jiménez-Guerrero, P.; Noguera-Velasco, J.; Cánovas Díaz, M.; et al. Exhaled volatilome analysis as a useful tool to discriminate asthma with other coexisting atopic diseases in women of childbearing age. Sci. Rep. 2021, 11, 13823. [Google Scholar] [CrossRef]
- Norbäck, D.; Lu, C.; Zhang, Y.; Li, B.; Zhao, Z.; Huang, C.; Zhang, X.; Qian, H.; Sun, Y.; Sundell, J.; et al. Onset and remission of childhood wheeze and rhinitis across China—Associations with early life indoor and outdoor air pollution. Environ. Int. 2019, 123, 61–69. [Google Scholar] [CrossRef]
- Deng, Q.; Lu, C.; Ou, C.; Chen, L.; Yuan, H. Preconceptional, prenatal and postnatal exposure to outdoor and indoor environmental factors on allergic diseases/symptoms in preschool children. Chemosphere 2016, 152, 459–467. [Google Scholar] [CrossRef]
- Lu, C.; Norbäck, D.; Zhang, Y.; Li, B.; Zhao, Z.; Huang, C.; Zhang, X.; Qian, H.; Sun, Y.; Wang, J.; et al. Furry pet-related wheeze and rhinitis in pre-school children across China: Associations with early life dampness and mould, furry pet keeping, outdoor temperature, PM10 and PM2.5. Environ. Int. 2020, 144, 106033. [Google Scholar] [CrossRef]
- Valtonen, V. Clinical diagnosis of the dampness and mold hypersensitivity syndrome: Review of the literature and suggested diagnostic criteria. Front. Immunol. 2017, 8, 951. [Google Scholar] [CrossRef] [Green Version]
- Saijo, Y.; Kishi, R.; Sata, F.; Katakura, Y.; Urashima, Y.; Hatakeyama, A.; Kobayashi, S.; Jin, K.; Kurahashi, N.; Kondo, T.; et al. Symptoms in relation to chemicals and dampness in newly built dwellings. Int. Arch. Occup. Environ. Health 2004, 77, 461–470. [Google Scholar] [CrossRef]
- Wakayama, T.; Ito, Y.; Sakai, K.; Miyake, M.; Shibata, E.; Ohno, H.; Kamijima, M. Comprehensive review of 2-ethyl-1-hexanol as an indoor air pollutant. J. Occup. Health 2019, 61, 19–35. [Google Scholar] [CrossRef] [Green Version]
- Fu, X.; Norbäck, D.; Yuan, Q.; Li, Y.; Zhu, X.; Hashim, J.H.; Hashim, Z.; Ali, F.; Hu, Q.; Deng, Y.; et al. Association between indoor microbiome exposure and sick building syndrome (SBS) in junior high schools of Johor Bahru, Malaysia. Sci. Total Environ. 2021, 753, 141904. [Google Scholar] [CrossRef]
- Fisk, W.J.; Lei-Gomez, Q.; Mendell, M.J. Meta-analyses of the associations of respiratory health effects with dampness and mold in homes. Indoor Air 2007, 17, 284–296. [Google Scholar] [CrossRef]
- Hope, A.P.; Simon, R.A. Excess dampness and mold growth in homes: An evidence-based review of the aeroirritant effect and its potential causes. Allergy Asthma Proc. 2007, 28, 262–270. [Google Scholar] [CrossRef]
- Quansah, R.; Jaakkola, M.S.; Hugg, T.T.; Heikkinen, S.A.M.; Jaakkola, J.J.K. Residential Dampness and Molds and the Risk of Developing Asthma: A Systematic Review and Meta-Analysis. PLoS ONE 2012, 7, 47526. [Google Scholar] [CrossRef]
- Norbäck, D.; Hashim, J.H.; Hashim, Z.; Cai, G.H.; Sooria, V.; Ismail, S.A.; Wieslander, G. Respiratory symptoms and fractional exhaled nitric oxide (FeNO) among students in Penang, Malaysia in relation to signs of dampness at school and fungal DNA in school dust. Sci. Total Environ. 2017, 577, 148–154. [Google Scholar] [CrossRef] [Green Version]
- Rufo, J.C.; Madureira, J.; Paciência, I.; Aguiar, L.; Teixeira, J.P.; Moreira, A.; de Oliveira Fernandes, E. Indoor air quality and atopic sensitization in primary schools: A follow-up study. Porto Biomed. J. 2016, 1, 142–146. [Google Scholar] [CrossRef] [Green Version]
- Wålinder, R.; Wieslander, G.; Norbäck, D.; Wessen, B.; Venge, P. Nasal lavage biomarkers: Effects of water damage and microbial growth in an office building. Arch. Environ. Health 2001, 56, 30–36. [Google Scholar] [CrossRef]
- Zhang, X.; Sahlberg, B.; Wieslander, G.; Janson, C.; Gislason, T.; Norback, D. Dampness and moulds in workplace buildings: Associations with incidence and remission of sick building syndrome (SBS) and biomarkers of inflammation in a 10year follow-up study. Sci. Total Environ. 2012, 430, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Vardoulakis, S.; Giagloglou, E.; Steinle, S.; Davis, A.; Sleeuwenhoek, A.; Galea, K.S.; Dixon, K.; Crawford, J.O. Indoor Exposure to Selected Air Pollutants in the Home Environment: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 8972. [Google Scholar] [CrossRef] [PubMed]
- Boots, A.W.; van Berkel, J.J.B.N.; Dallinga, J.W.; Smolinska, A.; Wouters, E.F.; van Schooten, F.J. The versatile use of exhaled volatile organic compounds in human health and disease. J. Breath Res. 2012, 6, 027108. [Google Scholar] [CrossRef] [PubMed]
- Sola-Martínez, R.A.; Pastor Hernández, J.M.; Yanes Torrado, Ó.; Cánovas Díaz, M.; de Diego Puente, T.; Vinaixa Crevillent, M. Exhaled volatile organic compounds analysis in clinical pediatrics: A systematic review. Pediatr. Res. 2021, 89, 1352–1363. [Google Scholar] [CrossRef] [PubMed]
- Morales, E.; Alcantara-Lopez, M.V.; Cabezas-Herrera, J.; de Diego, T.; Hernandez-Caselles, T.; Jimenez-Guerrero, P.; Larque, E.; Lopez-Soler, C.; Martinez-Gracia, C.; Martinez-Torres, A.; et al. The Nutrition in Early Life and Asthma (NELA) birth cohort study: Rationale, design, and methods. Paediatr. Perinat. Epidemiol. 2022, 36, 310–324. [Google Scholar] [CrossRef]
- Markowicz, P.; Larsson, L. Influence of relative humidity on VOC concentrations in indoor air. Environ. Sci. Pollut. Res. 2015, 22, 5772–5779. [Google Scholar] [CrossRef]
- Wei, X.-F.; Linde, E.; Hedenqvist, M.S. Plasticiser loss from plastic or rubber products through diffusion and evaporation. NPJ Mater. Degrad. 2019, 3, 18. [Google Scholar] [CrossRef] [Green Version]
- Nalli, S.; Horn, O.J.; Grochowalski, A.R.; Cooper, D.G.; Nicell, J.A. Origin of 2-ethylhexanol as a VOC. Environ. Pollut. 2006, 140, 181–185. [Google Scholar] [CrossRef]
- Wieslander, G.; Norbäck, D.; Nordström, K.; Wålinder, R.; Venge, P. Nasal and ocular symptoms, tear film stability and biomarkers in nasal lavage, in relation to building-dampness and building design in hospitals. Int. Arch. Occup. Environ. Health 1999, 72, 451–461. [Google Scholar] [CrossRef]
- Wieslander, G.; Kumlin, A.; Norbäck, D. Dampness and 2-ethyl-1-hexanol in floor construction of rehabilitation center: Health effects in staff. Arch. Environ. Occup. Health 2010, 65, 3–11. [Google Scholar] [CrossRef]
- Paciência, I.; Cavaleiro Rufo, J.; Silva, D.; Martins, C.; Mendes, F.; Farraia, M.; Delgado, L.; de Oliveira Fernandes, E.; Padrão, P.; Moreira, P.; et al. Exposure to indoor endocrine-disrupting chemicals and childhood asthma and obesity. Allergy 2019, 74, 1277–1291. [Google Scholar] [CrossRef] [PubMed]
- Sur, U.; Erkekoglu, P.; Bulus, A.D.; Andiran, N.; Kocer-Gumusel, B. Oxidative stress markers, trace elements, and endocrine disrupting chemicals in children with Hashimoto’s thyroiditis. Toxicol. Mech. Methods 2019, 29, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Norback, D.; Wieslander, G.; Nordstrom, K.; Walinder, R. Asthma symptoms in relation to measured building dampness in upper concrete floor construction, and 2-ethyl-1-hexanol in indoor air. Int. J. Tuberc. Lung Dis. 2000, 4, 1016–1025. [Google Scholar] [PubMed]
- Gibson, P.G. Variability of blood eosinophils as a biomarker in asthma and COPD. Respirology 2018, 23, 12–13. [Google Scholar] [CrossRef] [Green Version]
- Pignatti, P.; Visca, D.; Cherubino, F.; Zampogna, E.; Lucini, E.; Saderi, L.; Sotgiu, G.; Spanevello, A. Do blood eosinophils strictly reflect airway inflammation in COPD? Comparison with asthmatic patients. Respir. Res. 2019, 20, 145. [Google Scholar] [CrossRef] [Green Version]
- Soma, T.; Iemura, H.; Naito, E.; Miyauchi, S.; Uchida, Y.; Nakagome, K.; Nagata, M. Implication of fraction of exhaled nitric oxide and blood eosinophil count in severe asthma. Allergol. Int. 2018, 67, S3–S11. [Google Scholar] [CrossRef]
- Zhang, X.; Norbäck, D.; Fan, Q.; Bai, X.; Li, T.; Zhang, Y.; Li, B.; Zhao, Z.; Huang, C.; Deng, Q.; et al. Dampness and mold in homes across China: Associations with rhinitis, ocular, throat and dermal symptoms, headache and fatigue among adults. Indoor Air 2019, 29, 30–42. [Google Scholar] [CrossRef]
- Jaakkola, J.J.K.; Hwang, B.F.; Jaakkola, M.S. Home dampness and molds as determinants of allergic rhinitis in childhood: A 6-year, population-based cohort study. Am. J. Epidemiol. 2010, 172, 451–459. [Google Scholar] [CrossRef] [Green Version]
- Caillaud, D.; Leynaert, B.; Keirsbulck, M.; Nadif, R.; Roussel, S.; Ashan-Leygonie, C.; Bex, V.; Bretagne, S.; Caillaud, D.; Colleville, A.C.; et al. Indoor mould exposure, asthma and rhinitis: Findings from systematic reviews and recent longitudinal studies. Eur. Respir. Rev. 2018, 27, 170137. [Google Scholar] [CrossRef] [Green Version]
- Miekisch, W.; Schubert, J.K.; Noeldge-Schomburg, G.F. Diagnostic potential of breath analysis—Focus on volatile organic compounds. Clin. Chim. Acta 2004, 347, 25–39. [Google Scholar] [CrossRef]
- Kercsmar, C.M.; Dearborn, D.G.; Schluchter, M.; Xue, L.; Kirchner, H.L.; Sobolewski, J.; Greenberg, S.J.; Vesper, S.J.; Allan, T. Reduction in asthma morbidity in children as a result of home remediation aimed at moisture sources. Environ. Health Perspect. 2006, 114, 1574–1580. [Google Scholar] [CrossRef] [PubMed]
- Barnes, C.S.; Horner, W.E.; Kennedy, K.; Grimes, C.; Miller, J.D.; Baxi, S.; Larenas-Linnemann, D.; Levetin, E.; Phipatanakul, W.; Williams, P.B.; et al. Home Assessment and Remediation. J. Allergy Clin. Immunol. Pract. 2016, 4, 423–431.e15. [Google Scholar] [CrossRef]
- Domingo-Salvany, A.; Regidor, E.; Alonso, J.; Alvarez-Dardet, C. Proposal for a social class measure. Working Group of the Spanish Society of Epidemiology and the Spanish Society of Family and Community Medicine. Aten. Primaria 2000, 25, 350–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sola-Martínez, R.A.; Pastor Hernández, J.M.; Lozano Terol, G.; Gallego-Jara, J.; García-Marcos, L.; Cánovas Díaz, M.; De Diego Puente, T. Data preprocessing workflow for exhaled breath analysis by GC/MS using open sources. Sci. Rep. 2020, 10, 22008. [Google Scholar] [CrossRef] [PubMed]
- Martens, L.; Chambers, M.; Sturm, M.; Kessner, D.; Levander, F.; Shofstahl, J.; Tang, W.H.; Römpp, A.; Neumann, S.; Pizarro, A.D.; et al. mzML—A community standard for mass spectrometry data. Mol. Cell. Proteomics 2011, 10, R110.000133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adusumilli, R.; Mallick, P. Data conversion with proteoWizard msConvert. In Proteomics: Methods and Protocols; Comai, U., Katz, J.E., Mallick, P., Eds.; Humana: New York, NY, USA, 2017; Volume 1550, pp. 339–368. [Google Scholar]
- Smith, C.A.; Want, E.J.; O’Maille, G.; Abagyan, R.; Siuzdak, G. XCMS: Processing Mass Spectrometry Data for Metabolite Profiling Using Nonlinear Peak Alignment, Matching, and Identification. Anal. Chem. 2006, 78, 779–787. [Google Scholar] [CrossRef]
- Senan, O.; Aguilar-Mogas, A.; Navarro, M.; Capellades, J.; Noon, L.; Burks, D.; Yanes, O.; Guimerà, R.; Sales-Pardo, M. CliqueMS: A computational tool for annotating in-source metabolite ions from LC-MS untargeted metabolomics data based on a coelution similarity network. Bioinformatics 2019, 35, 4089–4097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domingo-Almenara, X.; Brezmes, J.; Vinaixa, M.; Samino, S.; Ramirez, N.; Ramon-Krauel, M.; Lerin, C.; Díaz, M.; Ibáñez, L.; Correig, X.; et al. eRah: A Computational Tool Integrating Spectral Deconvolution and Alignment with Quantification and Identification of Metabolites in GC/MS-Based Metabolomics. Anal. Chem. 2016, 88, 9821–9829. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).