Prostaglandin D2 Attenuates Lipopolysaccharide-Induced Acute Lung Injury through the Modulation of Inflammation and Macrophage Polarization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. In Vitro Study
2.2.1. Cell Culture
2.2.2. Cell Viability Analysis
2.2.3. Nitric Oxide Measurement
2.2.4. Proinflammatory Cytokine Determination
2.3. In Vivo Study
2.3.1. Animal Model
2.3.2. Grouping of Animals According to the Treatment Plan
2.3.3. Harvesting the BALF
2.3.4. Determination of Lung Wet/Dry Weight Ratio
2.3.5. Determination of Inflammatory Markers
2.3.6. Histopathological Analysis
2.3.7. Immunohistochemical Study
2.3.8. Apoptosis Analysis by TUNEL Assay
2.3.9. Apoptosis Analysis by Flow Cytometry
2.3.10. Statistical Analysis
3. Results
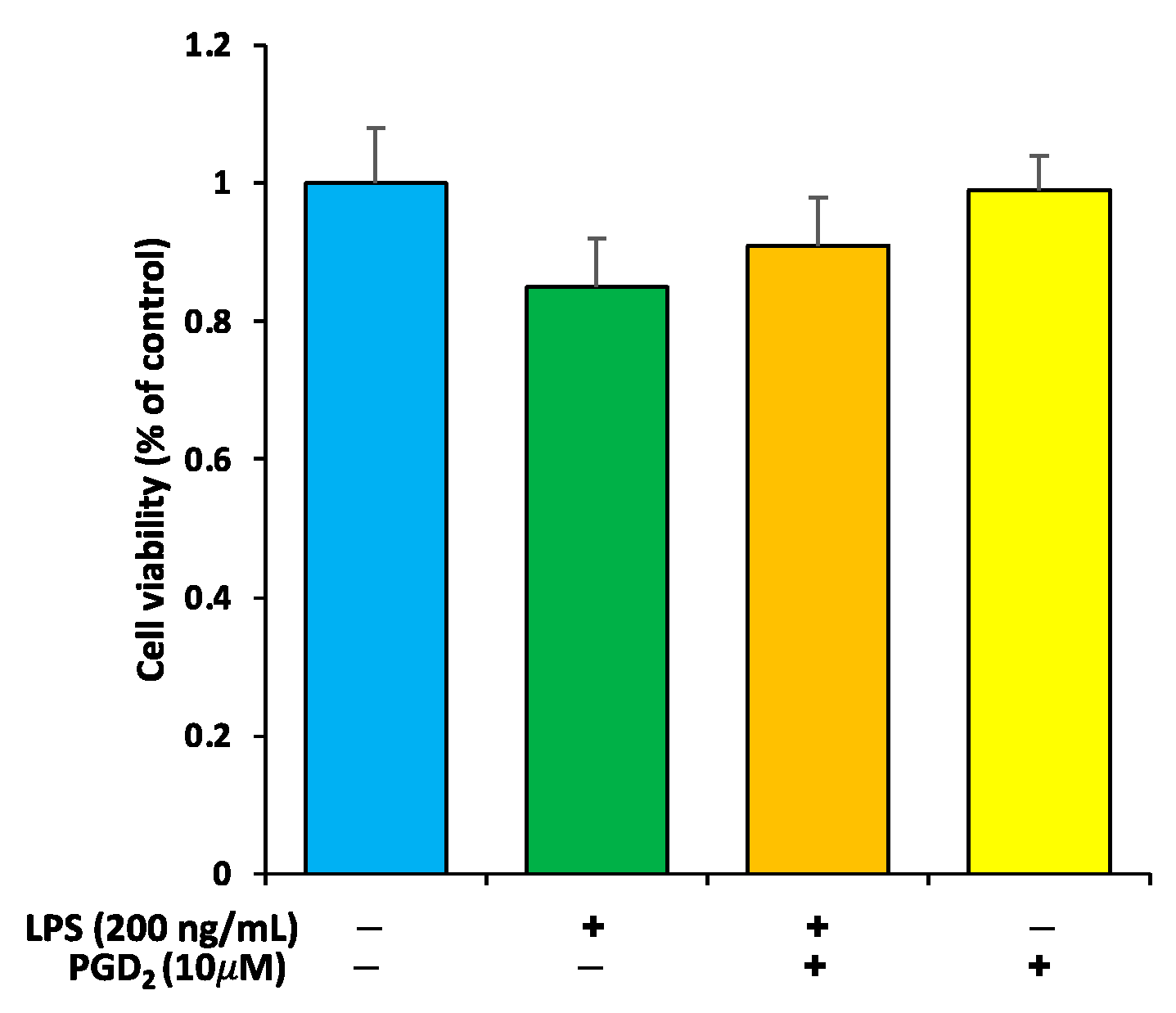
3.1. Viability Check of RAW264.7 Macrophages by PGD2 and LPS Treatment
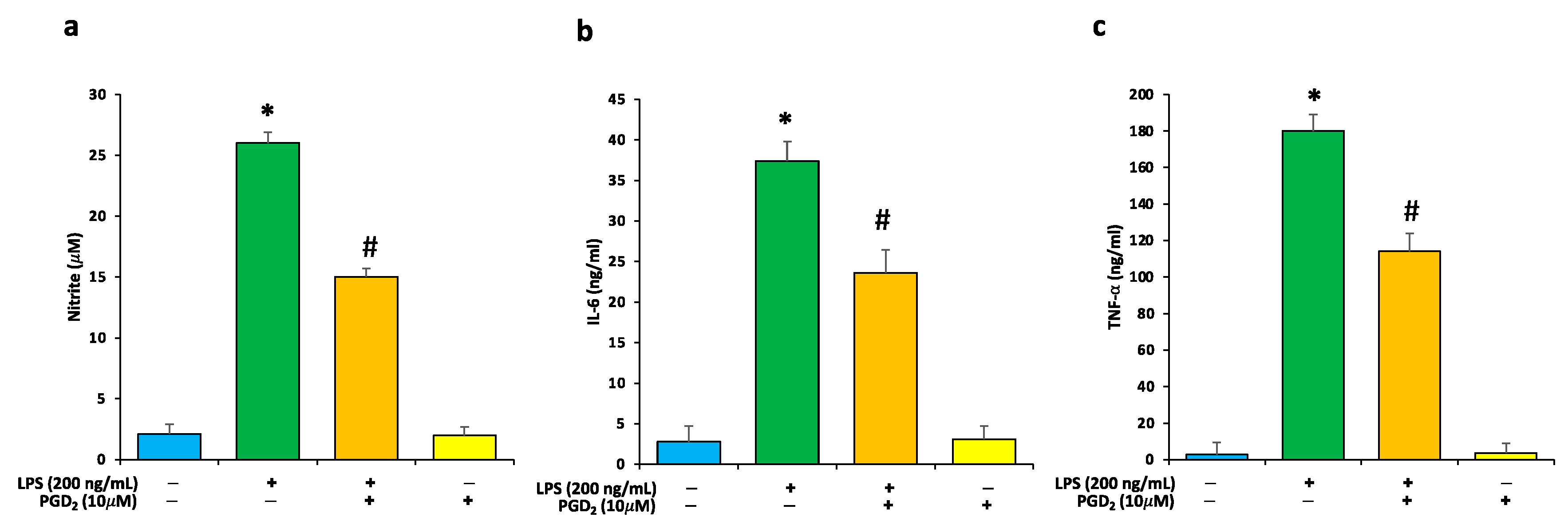
3.2. Effects of LPS and PGD2 Treatment on NO and Proinflammatory Cytokines
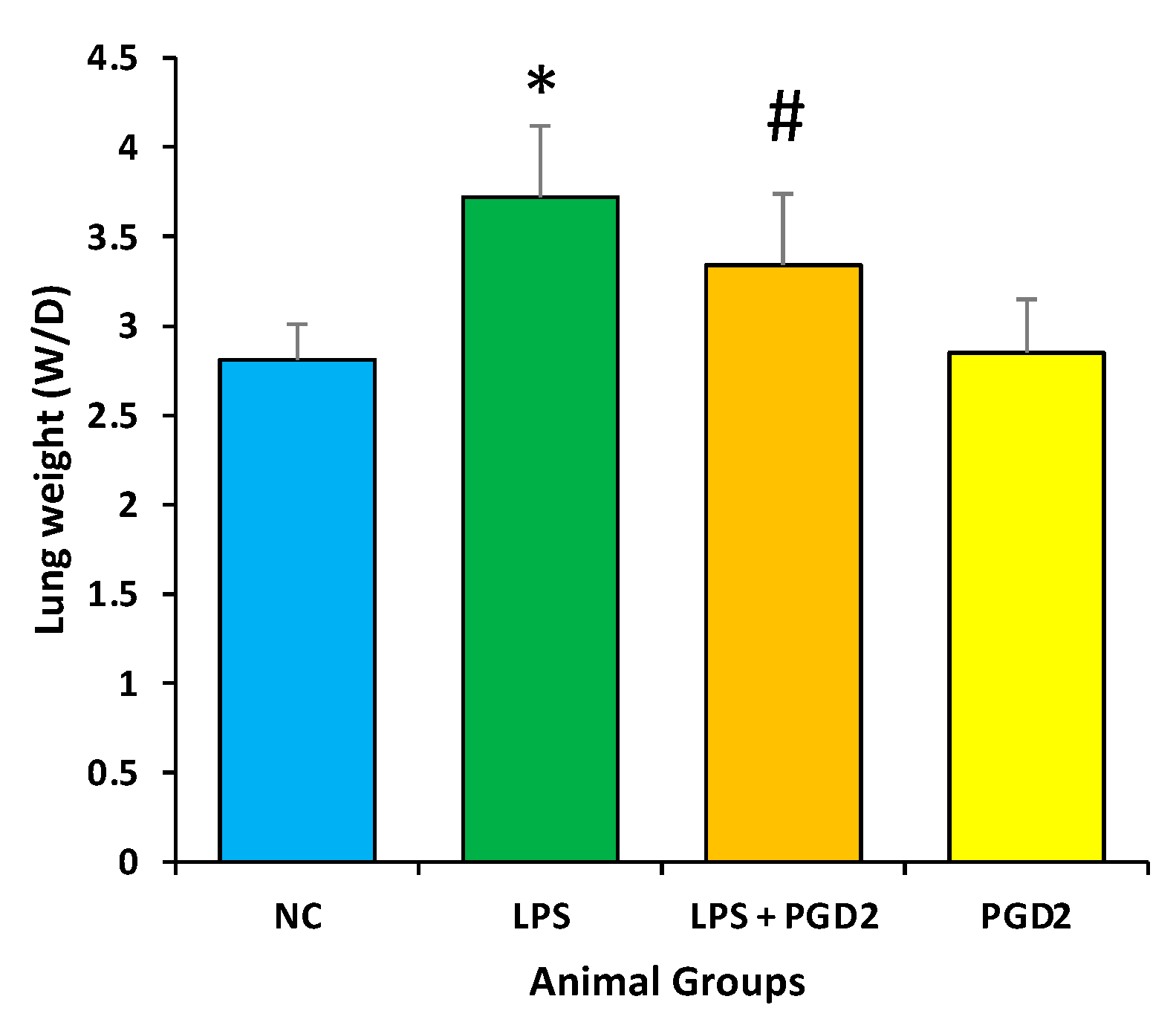
3.3. Effect of PGD2 on Weight (Wet/Dry) Ratio of Lungs
3.4. Effect of PGD2 on Inflammatory Markers
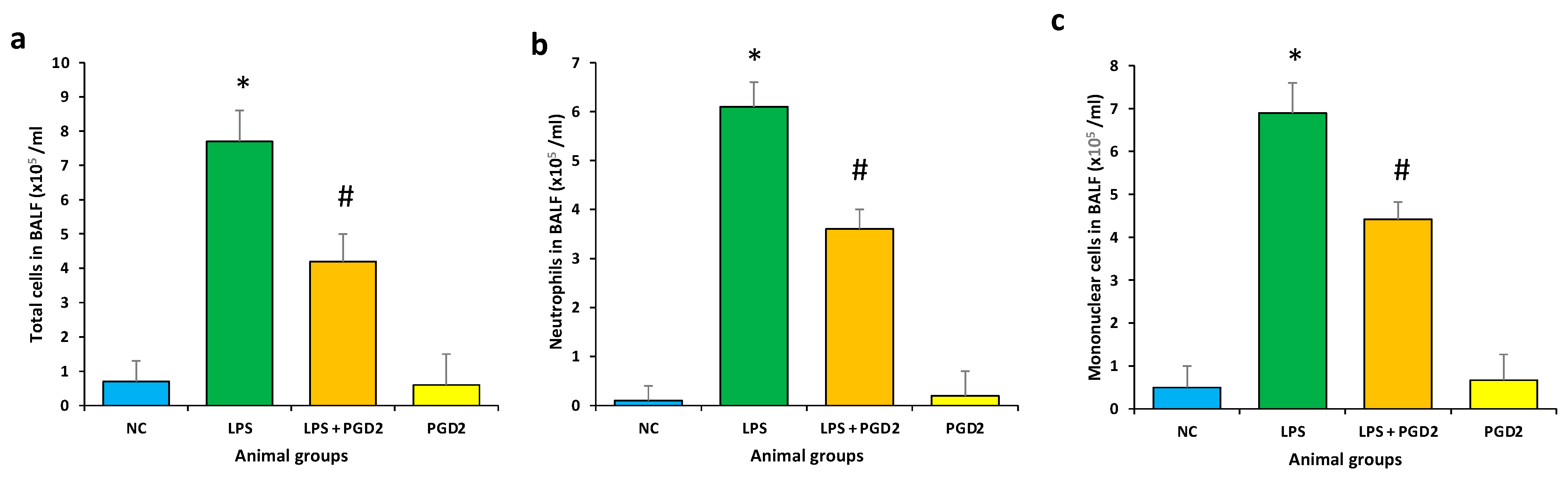
3.5. Effect of PGD2 on Inflammatory Cells Influx in BALF
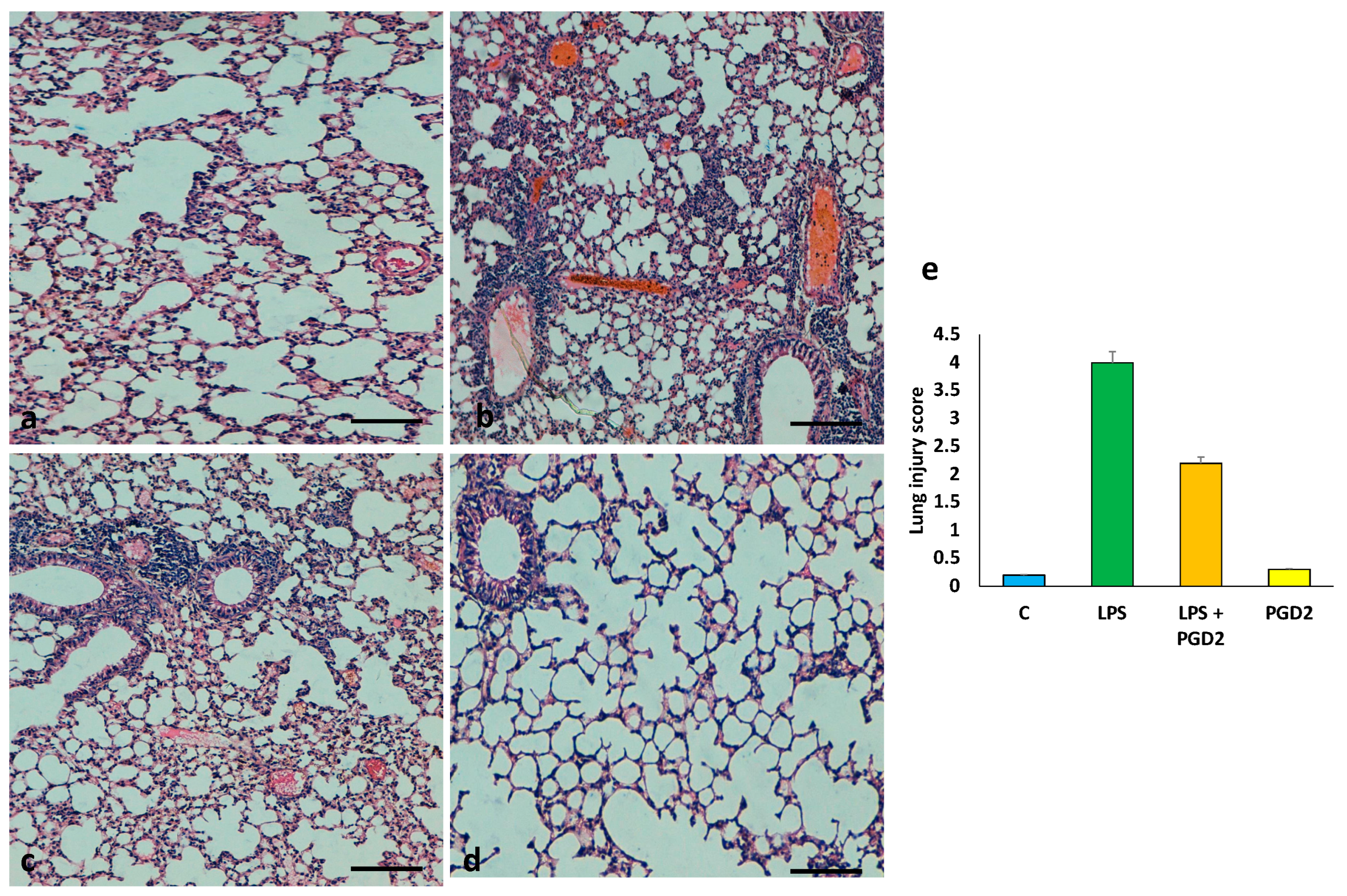
3.6. The Role of PGD2 on the Maintenance of Lung Tissue Architecture
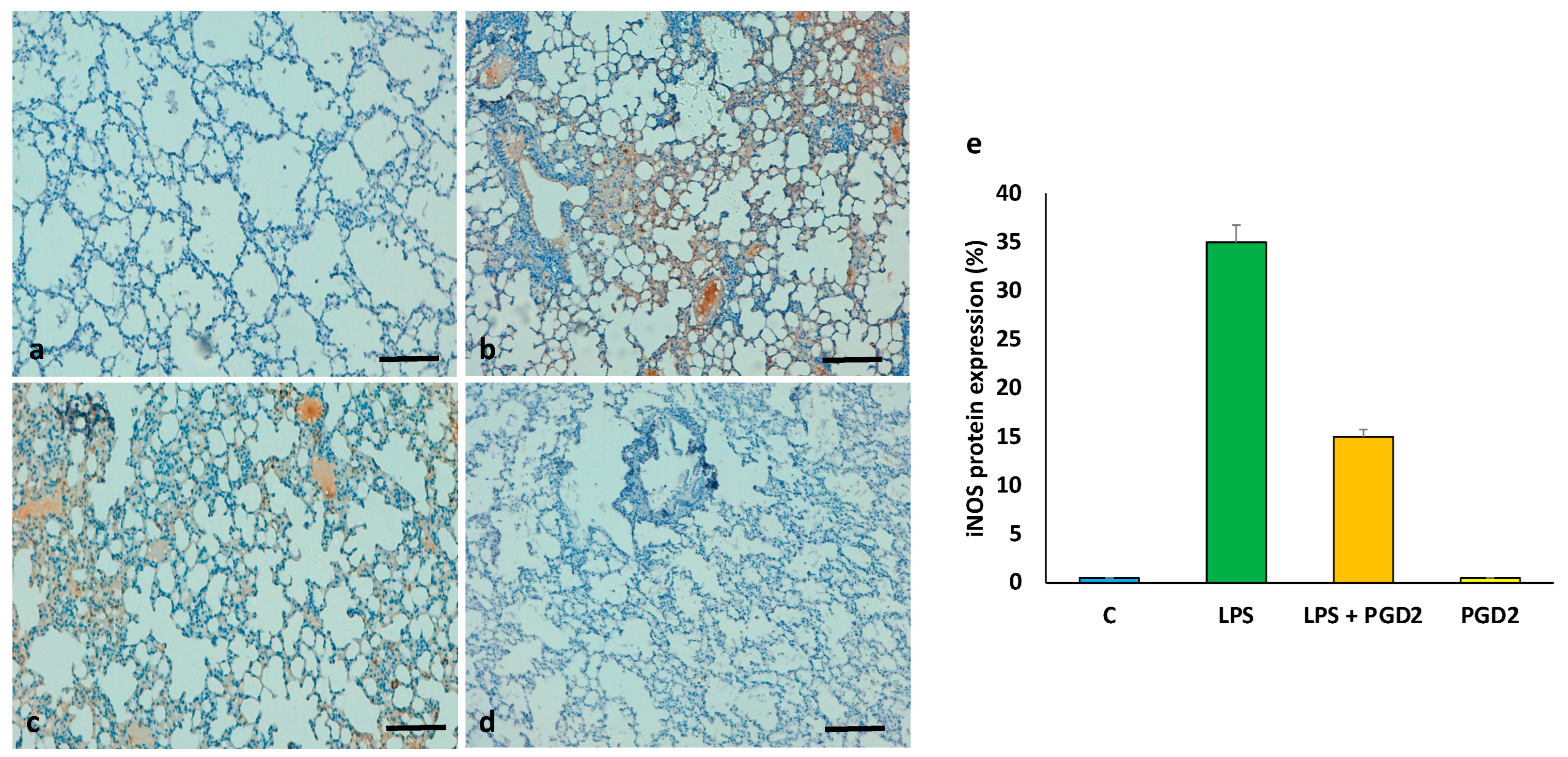
3.7. The Role of PGD2 on the Expression of iNOS
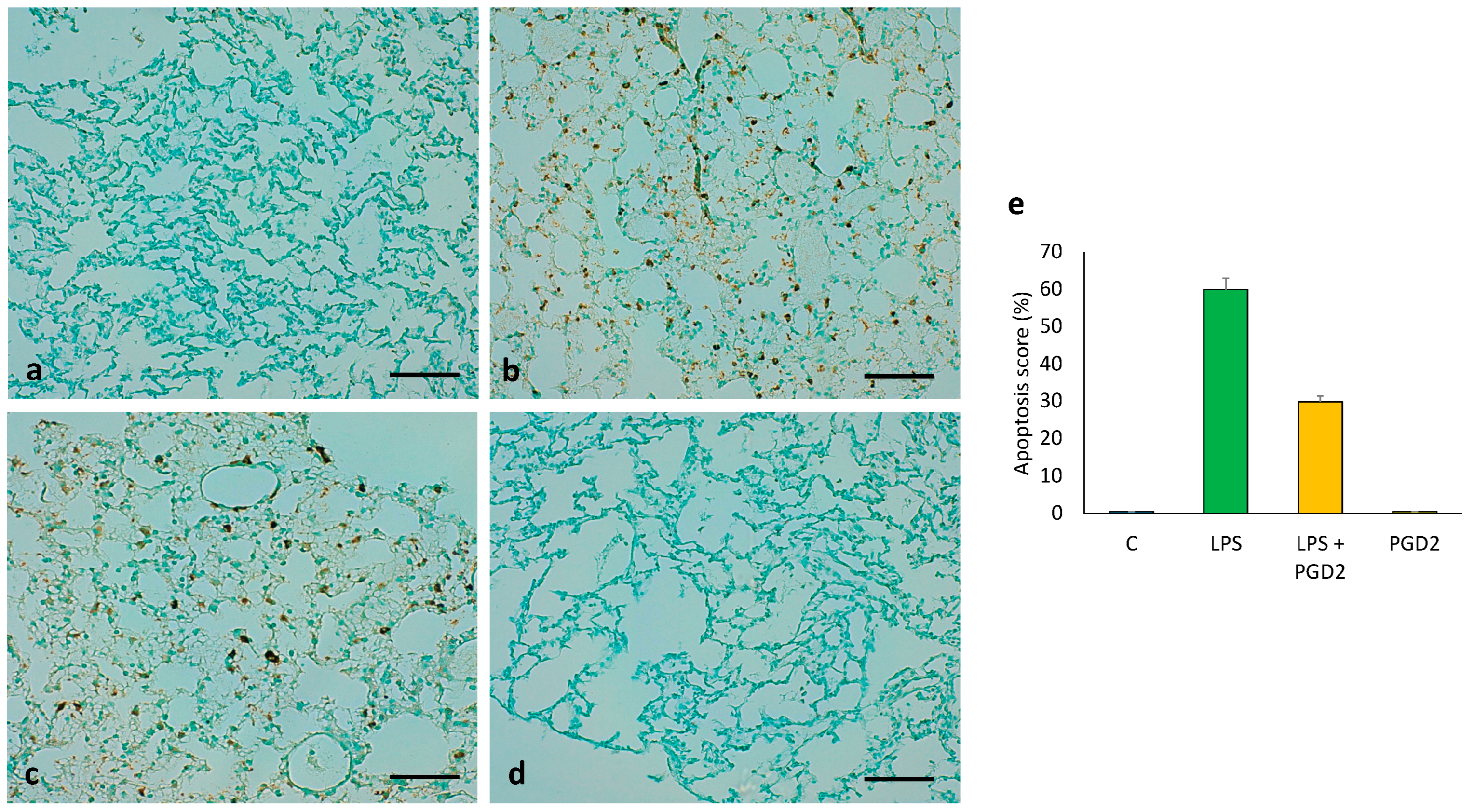
3.8. The Role of PGD2 on LPS-Treated Apoptotic Cell Death of Lung Tissue
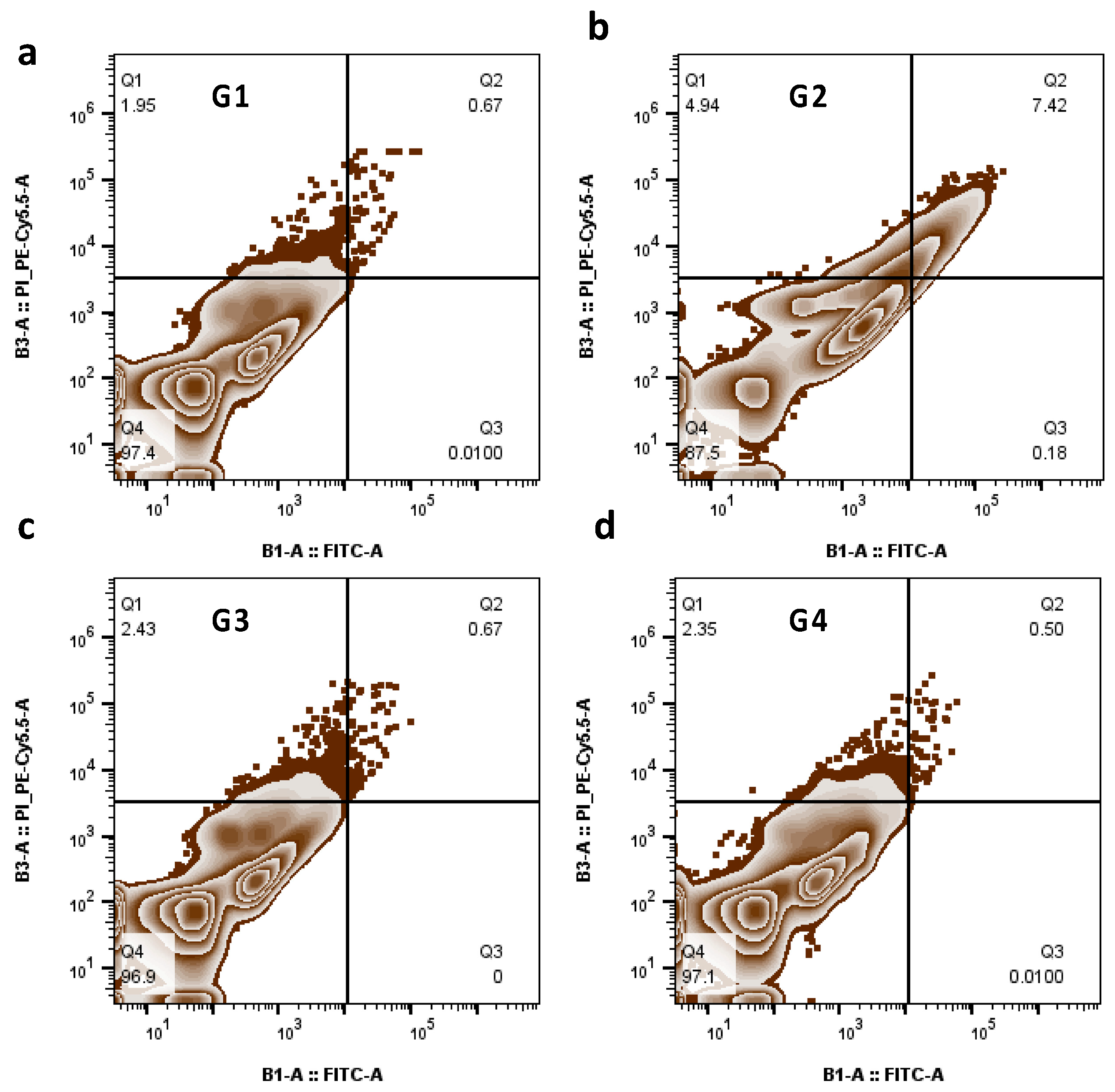
3.9. The Role of PGD2 Treatment on Apoptosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tao, W.; Su, Q.; Wang, H.; Guo, S.; Chen, Y.; Duan, J.; Wang, S. Platycodin D attenuates acute lung injury by suppressing apoptosis and inflammation in vivo and in vitro. Int. Immunopharmacol. 2015, 27, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Dushianthan, A.; Grocott, M.P.; Postle, A.D.; Cusack, R. Acute respiratory distress syndrome and acute lung injury. Postgrad. Med. J. 2011, 87, 612–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butt, Y.; Kurdowska, A.; Allen, T.C. Acute lung injury: A clinical and molecular review. Arch. Pathol. Lab. Med. 2016, 140, 345–350. [Google Scholar] [CrossRef] [Green Version]
- Mendez, J.L.; Hubmayr, R.D. New insights into the pathology of acute respiratory failure. Curr. Opin. Crit. Care 2005, 11, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Liu, Q.; Wen, Z.; Feng, H.; Deng, X.; Ci, X. Xanthohumol ameliorates lipopolysaccharide (LPS)-induced acute lung injury via induction of AMPK/GSK3β-Nrf2 signal axis. Redox Biol. 2017, 12, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Hu, S.; Su, Z.; Wang, Q.; Meng, G.; Guo, T.; Zhang, J.; Gao, P. Myricetin attenuates LPS-induced inflammation in RAW 264.7 macrophages and mouse models. Future Med. Chem. 2018, 10, 2253–2264. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.T.; Wu, Y.C.; Xie, X.X.; Zhou, X.; Wei, M.M.; Soromou, L.W.; Ci, X.X.; Wang, D.C. Phillyrin attenuates LPS-induced pulmonary inflammation via suppression of MAPK and NF-Κb activation in acute lung injury mice. Fitoterapia 2013, 90, 132–139. [Google Scholar] [CrossRef]
- Dias-Freitas, F.; Metelo-Coimbra, C.; Roncon-Albuquerque, R., Jr. Molecular mechanisms underlying hyperoxia acute lung injury. Respir. Med. 2016, 119, 23–28. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.L.; Wei, X.C.; Guo, L.Q.; Zhao, L.; Chen, X.H.; Cui, Y.D.; Yuan, J.; Chen, D.F.; Zhang, J. The therapeutic effects of jaceosidin on lipopolysaccharide-induced acute lung injury in mice. J. Pharmacol. Sci. 2019, 140, 228–235. [Google Scholar] [CrossRef]
- Kan, X.; Liu, B.; Guo, W.; Wei, L.; Lin, Y.; Guo, Y.; Gong, Q.; Li, Y.; Xu, D.; Cao, Y.; et al. Myricetin relieves LPS-induced mastitis by inhibiting inflammatory response and repairing the blood-milk barrier. J. Cel. Physiol. 2019, 234, 16252–16262. [Google Scholar] [CrossRef]
- Ulich, T.R.; Yin, S.; Remick, D.G.; Russell, D.; Eisenberg, S.P.; Kohno, T. Intratracheal administration of endotoxin and cytokines. IV. The soluble tumor necrosis factor receptor type I inhibits acute inflammation. Am. J. Pathol. 1993, 142, 1335–1338. [Google Scholar] [PubMed]
- Faffe, D.S.; Seidl, V.R.; Chagas, P.S.; de Moraes, V.G.; Capelozzi, V.L.; Rocco, P.R.; Zin, W.A. Respiratory effects of lipopolysaccharide-induced inflammatory lung injury in mice. Eur. Respir. J. 2000, 15, 85–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frank, J.A.; Matthay, M.A. Leukotrienes in acute lung injury: A potential therapeutic target? Am. J. Respir. Crit. Care Med. 2005, 172, 261–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinshaw, L.B.; Solomon, L.A.; Erdös, E.G.; Reins, D.A.; Gunter, B.J. Effects of acetylsalicylic acid on the canine response to endotoxin. J. Pharmacol. Exp. Ther. 1967, 157, 665–671. [Google Scholar] [PubMed]
- Cuzzocrea, S.; Mazzon, E.; Sautebin, L.; Dugo, L.; Serraino, I.; De Sarro, A.; Caputi, A.P. Protective effects of Celecoxib on lung injury and red blood cells modification induced by carrageenan in the rat. Biochem. Pharmacol. 2002, 63, 785–795. [Google Scholar] [CrossRef]
- Bernard, G.R.; Wheeler, A.P.; Russell, J.A.; Schein, R.; Summer, W.R.; Steinberg, K.P.; Fulkerson, W.J.; Wright, P.E.; Christman, B.W.; Dupont, W.D.; et al. The effects of ibuprofen on the physiology and survival of patients with sepsis. N. Engl. J. Med. 1997, 336, 912–918. [Google Scholar] [CrossRef]
- Syed, M.A.; Joo, M.; Abbas, Z.; Rodger, D.; Christman, J.W.; Mehta, D.; Sadikot, R.T. Expression of TREM-1 is inhibited by PGD2 and PGJ2 in macrophages. Exp. Cell Res. 2010, 316, 3140–3149. [Google Scholar] [CrossRef]
- Ali, H.; Khan, A.; Ali, J.; Ullah, H.; Khan, A.; Ali, H.; Irshad, N.; Khan, S. Attenuation of LPS-induced acute lung injury by continentalic acid in rodents through inhibition of inflammatory mediators correlates with increased Nrf2 protein expression. BMC Pharmacol. Toxicol. 2020, 21, 1–4. [Google Scholar] [CrossRef]
- Murata, T.; Aritake, K.; Tsubosaka, Y.; Maruyama, T.; Nakagawa, T.; Hori, M.; Hirai, H.; Nakamura, M.; Narumiya, S.; Urade, Y.; et al. Anti-inflammatory role of PGD2 in acute lung inflammation and therapeutic application of its signal enhancement. Proc. Natl. Acad. Sci. USA 2013, 110, 5205–5210. [Google Scholar] [CrossRef] [Green Version]
- Khalid, S.; Ullah, M.Z.; Khan, A.U.; Afridi, R.; Rasheed, H.; Khan, A.; Ali, H.; Kim, Y.S.; Khan, S. Antihyperalgesic properties of honokiol in inflammatory pain models by targeting of NF-κB and Nrf2 signaling. Front Pharmacol. 2018, 9, 140. [Google Scholar] [CrossRef]
- Ullah, M.Z.; Khan, A.U.; Afridi, R.; Rasheed, H.; Khalid, S.; Naveed, M.; Ali, H.; Kim, Y.S.; Khan, S. Attenuation of inflammatory pain by puerarin in animal model of inflammation through inhibition of pro-inflammatory mediators. Int. Immunopharmacol. 2018, 61, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Khan, S.; Ali, H.; Shah, K.U.; Ali, H.; Shehzad, O.; Onder, A.; Kim, Y.S. Anomalin attenuates LPS-induced acute lungs injury through inhibition of AP-1 signaling. Int. Immunopharmacol. 2019, 73, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.; Matsuda, A.; Yang, W.L.; Jacob, A.; Wang, P. Milk fat globule-epidermal growth factor-factor 8 attenuates neutrophil infiltration in acute lung injury via modulation of CXCR2. J. Immunol. 2012, 189, 393–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, T.; Miksa, M.; Wu, R.; Komura, H.; Zhou, M.; Dong, W.; Wang, Z.; Higuchi, S.; Chaung, W.; Blau, S.A.; et al. Milk fat globule epidermal growth factor 8 attenuates acute lung injury in mice after intestinal ischemia and reperfusion. Am. J. Respir. Crit. Care Med. 2010, 181, 238–246. [Google Scholar] [CrossRef] [Green Version]
- Rahmani, A.; Alzohairy, M.; Khadri, H.; Mandal, A.K.; Rizvi, M.A. Expressional evaluation of vascular endothelial growth factor (VEGF) protein in urinary bladder carcinoma patients exposed to cigarette smoke. Int. J. Clin. Exp. Pathol. 2012, 5, 195. [Google Scholar]
- Rahmani, A.H.; Babiker, A.Y.; AlWanian, W.M.; Elsiddig, S.A.; Faragalla, H.E.; Aly, S.M. Association of cytokeratin and vimentin protein in the genesis of transitional cell carcinoma of urinary bladder patients. Dis. Mark. 2015, 2015, 204759. [Google Scholar] [CrossRef] [Green Version]
- Almatroodi, S.A.; Almatroudi, A.; Anwar, S.; Yousif Babiker, A.; Khan, A.A.; Alsahli, M.A.; Rahmani, A.H. Antioxidant, anti-inflammatory and hepatoprotective effects of olive fruit pulp extract: In vivo and in vitro study. J Taibah Univ Sci. 2020, 14, 1660–1670. [Google Scholar] [CrossRef]
- Xie, K.; Yu, Y.; Huang, Y.; Zheng, L.; Li, J.; Chen, H.; Han, H.; Hou, L.; Gong, G.; Wang, G. Molecular hydrogen ameliorates lipopolysaccharide-induced acute lung injury in mice through reducing inflammation and apoptosis. Shock 2012, 37, 548–555. [Google Scholar] [CrossRef] [Green Version]
- Matthay, M.A.; Zimmerman, G.A.; Esmon, C.; Bhattacharya, J.; Coller, B.; Doerschuk, C.M.; Floros, J.; Gimbrone, M.A., Jr.; Hoffman, E.; Hubmayr, R.D.; et al. Future research directions in acute lung injury: Summary of a National Heart, Lung, and Blood Institute working group. Am. J. Respir. Crit. Care Med. 2003, 167, 1027–1035. [Google Scholar] [CrossRef] [Green Version]
- Gilroy, D.W.; Colville-Nash, P.R.; Willis, D.; Chivers, J.; Paul-Clark, M.J.; Willoughby, D.A. Inducible cyclooxygenase may have anti-inflammatory properties. Nat. Med. 1999, 5, 698–701. [Google Scholar] [CrossRef]
- Ishitsuka, Y.; Moriuchi, H.; Hatamoto, K.; Takase, J.; Irikura, M.; Irie, T.; Yang, C.; Golbidi, S. Involvement of thromboxane A2 (TXA2) in the early stages of oleic acid-induced lung injury and the preventive effect of ozagrel, a TXA2 synthase inhibitor, in guinea-pigs. J. Pharm. Pharmacol. 2004, 56, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Goggel, R.; Hoffman, S.; Nüsing, R.; Narumiya, S.; Uhlig, S. Platelet-activating factor- induced pulmonary edema is partly mediated by prostaglandin E(2), E-prostanoid 3-receptors, and potassium channels. Am. J. Respir. Crit. Care Med. 2002, 166, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, K.; Kohli, P.; Bonnans, C.; Fredenburgh, L.E.; Levy, B.D. Cyclooxygenase 2 plays a pivotal role in the resolution of acute lung injury. J. Immunol. 2005, 174, 5033–5039. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, M.; Ishii, T.; Itoh, K.; Iizuka, T.; Morishima, Y.; Kimura, T.; Kiwamoto, T.; Matsuno, Y.; Hegab, A.E.; Nomura, A.; et al. Role of 15-deoxy delta(12,14) prostaglandin J2 and Nrf2 pathways in protection against acute lung injury. Am. J. Respir. Crit. Care Med. 2005, 171, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Klebanoff, S.J. Myeloperoxidase: Friend and foe. J. Leukoc. Biol. 2005, 77, 598–625. [Google Scholar] [CrossRef]
- Goodman, R.B.; Pugin, J.; Lee, J.S.; Matthay, M.A. Cytokine-mediated inflammation in acute lung injury. Cytokine Growth Factor Rev. 2003, 14, 523–535. [Google Scholar] [CrossRef]
- Liu, Q.; Ci, X.; Wen, Z.; Peng, L. Diosmetin alleviates lipopolysaccharide-induced acute lung injury through activating the Nrf2 pathway and inhibiting the NLRP3 inflammasome. Biomol. Ther. 2018, 26, 157–166. [Google Scholar] [CrossRef] [Green Version]
- El-Agamy, D.S. Nilotinib ameliorates lipopolysaccharide-induced acute lung injury in rats. Toxicol. Appl. Pharmacol. 2011, 253, 153–160. [Google Scholar] [CrossRef]
- El-Agamy, D.S.; Mohamed, G.A.; Ahmed, N.; Elkablawy, M.A.; Elfaky, M.A.; Elsaed, W.M.; Mohamed, S.G.A.; Ibrahim, S.R.M. Protective anti-inflammatory activity of tovophyllin A against acute lung injury and its potential cytotoxicity to epithelial lung and breast carcinomas. Inflammopharmacology 2019, 28, 153–163. [Google Scholar] [CrossRef]
- Oyinloye, B.E.; Adenowo, A.F.; Kappo, A.P. Reactive oxygen species, apoptosis, antimicrobial peptides and human inflammatory diseases. Pharmaceuticals 2015, 8, 151. [Google Scholar] [CrossRef]
- Jing, W.; Chunhua, M.; Shumin, W. Effects of acteoside on lipopolysaccharide-induced inflammation in acute lung injury via regulation of NF-kappa B pathway in vivo and in vitro. Toxicol. Appl. Pharmacol. 2015, 285, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xiao, J.; Tan, Y.; Wang, J.; Zhang, Y.; Deng, X.; Luo, Y. Inhibition of PKR ameliorates lipopolysaccharide-induced acute lung injury by suppressing NF-kappa B pathway in mice. Immunopharmacol. Immunotoxicol. 2017, 39, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Galani, V.; Tatsaki, E.; Bai, M.; Kitsoulis, P.; Lekka, M.; Nakos, G.; Kanavaros, P. The role of apoptosis in the pathophysiology of Acute Respiratory Distress Syndrome (ARDS): An up-to- date cell-specific review. Pathol. Res. Pract. 2010, 206, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Oga, T.; Matsuoka, T.; Yao, C.; Nonomura, K.; Kitaoka, S.; Sakata, D.; Kita, Y.; Tanizawa, K.; Taguchi, Y.; Chin, K.; et al. Prostaglandin F(2alpha) receptor signaling facilitates bleomycin-induced pulmonary fibrosis independently of transforming growth factor-beta. Nat. Med. 2009, 15, 1426–1430. [Google Scholar] [CrossRef] [PubMed]
- Gasse, P.; Riteau, N.; Charron, S.; Girre, S.; Fick, L.; Petrilli, V.; Tschopp, J.; Lagente, V.; Quesniaux, V.F.J.; Ryffel, B.; et al. Uric acid is a danger signal activating NALP3 inflammasome in lung injury inflammation and fibrosis. Am. J. Respir. Crit. Care Med. 2009, 179, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Ayabe, S.; Kida, T.; Hori, M.; Ozaki, H.; Murata, T. Prostaglandin D2 inhibits collagen secretion from lung fibroblasts by activating the DP receptor. J. Pharmacol. Sci. 2013, 121, 312–317. [Google Scholar] [CrossRef] [Green Version]
- Van den Brule, S.; Wallemme, L.; Uwambayinema, F.; Huaux, F.; Lison, D. The D prostanoid receptor agonist BW245C [(4S)-(3-[(3R,S)-3-cyclohexyl-3-hydroxypropyl]-2,5-dioxo)-4-imidazolidine-heptanoi c acid] inhibits fibroblast proliferation and bleomycin-induced lung fibrosis in mice. J. Pharmacol. Exp. Ther. 2010, 335, 472–479. [Google Scholar] [CrossRef]
- Kida, T.; Ayabe, S.; Omori, K.; Nakamura, T.; Maehara, T.; Aritake, K.; Urade, Y.; Murata, T. Prostaglandin D2 attenuates bleomycin-induced lung inflammation and pulmonary fibrosis. PLoS ONE 2016, 11, e0167729. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; Li, Q.; Han, Y.; Liang, Y.; Xu, Z.; Ren, T. Prevention of LPS-induced acute lung injury in mice by progranulin. Mediat. Inflamm. 2012, 2012, 540794. [Google Scholar] [CrossRef]

| Group Number | Group Description | Short Name | Treatment Plan |
|---|---|---|---|
| 1 | Normal control | NC | The mice were treated with 50 μL of PBS i.p. as a vehicle |
| 2 | Disease control | LPS | LPS (5 mg/kg b.w. + vehicle) through i.p. injection [18] |
| 3 | Disease control + treatment | LPS + PGD2 | PGD2 (100 μg/kg b.w.) given 30 min before the LPS challenge (5 mg/kg b.w. + vehicle) [19] |
| 4 | Treatment only | PGD2 | Treatment with PGD2 (100 μg/kg b.w.) only |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almatroudi, A.; Alsahli, M.A.; Syed, M.A.; Khan, A.A.; Rahmani, A.H. Prostaglandin D2 Attenuates Lipopolysaccharide-Induced Acute Lung Injury through the Modulation of Inflammation and Macrophage Polarization. Appl. Sci. 2022, 12, 6076. https://doi.org/10.3390/app12126076
Almatroudi A, Alsahli MA, Syed MA, Khan AA, Rahmani AH. Prostaglandin D2 Attenuates Lipopolysaccharide-Induced Acute Lung Injury through the Modulation of Inflammation and Macrophage Polarization. Applied Sciences. 2022; 12(12):6076. https://doi.org/10.3390/app12126076
Chicago/Turabian StyleAlmatroudi, Ahmad, Mohammed A. Alsahli, Mansoor Ali Syed, Amjad Ali Khan, and Arshad Husain Rahmani. 2022. "Prostaglandin D2 Attenuates Lipopolysaccharide-Induced Acute Lung Injury through the Modulation of Inflammation and Macrophage Polarization" Applied Sciences 12, no. 12: 6076. https://doi.org/10.3390/app12126076







