Abstract
An atmospheric pressure microwave plasma source operating on water vapor has many potential applications. To avoid the corrosion of metal electrodes in a traditional water vapor microwave plasma system, we propose a simple water vapor electrodeless microwave plasma device. By introducing a ceramic tube, the device can work directly with liquid water without complex evaporation equipment. This study examined the relationship between microwave power and water vapor torch plasma duration. When the microwave power is greater than 800 W, the plasma torch can be excited permanently and stably without the loss of ceramic. The excitation of the oxygen atom, hydroxyl radical, and hydrogen atom was found using optical spectroscopy, confirming the water vapor’s decomposition. In addition, it was also found that the crystallinity of the ceramic was improved after microwave discharge. This work enriches the microwave plasma techniques for water vapor for various applications, such as electric propulsion, hydrogen production, and surface treatment.
1. Introduction
Non-thermal plasma is a weakly ionized plasma that can work at room temperature and atmospheric pressure while still producing highly active species and electrons. The electronic energy works well to excite molecular and atomic species and destroy chemical bonds, so the non-thermal plasma is widely used in various applications, such as surface modification [1,2], air-cleaning [3] and assisted combustion [4], etc. When water vapor is used as the supporting gas of atmospheric pressure non-thermal plasma, it can produce reducing and oxidizing free radicals such as H, OH, and O so that the plasma can more effectively treat various hydrogen-rich compounds such as methane, natural gas, ethanol, and coal and use them as raw materials to produce hydrogen [5,6,7,8,9,10,11]. The commonly used atmospheric pressure non-thermal plasma sources include glow discharge, corona discharge, dielectric barrier discharge (DBD), radio frequency (RF) discharge, and microwave discharge [12,13,14]. Among these plasma sources, the microwave discharge is used to generate water vapor plasma, which has many advantages, because the strong interaction between 2.45 GHz microwave and water molecules can produce higher electron temperature and higher plasma density, realize high-level dissociation, and realize high-efficiency microwave power transmission from the microwave generator to the plasma [15,16,17,18]. For example, Canadian researchers have studied a method based on a microwave plasma source, which uses microwaves to generate plasma to decompose water vapor into hydrogen. The energy efficiency can reach 53% [15].
However, some issues still exist in producing continuous and lasting water vapor plasma with high microwave power. One of the problems is that the strong chemical reaction between the active atoms produced by microwave plasma and the surface of the metal electrodes will lead to electrode corrosion, which will limit the service life of plasma devices [18,19]. Therefore, many studies have proposed electrodeless discharge devices to overcome the problem of electrode poisoning caused by electrode discharge [20], such as surface-wave-sustained discharges [21,22,23], microwave plasma jets [24], plasma guns [25], etc. However, when we apply liquid water to these devices, many additional devices are often added to generate water vapor plasma, such as heating and evaporation equipment or vacuum sealing device. Which undoubtedly greatly aggravates the use restriction of the device itself, and there is also the risk of water vapor condensation [15,20,26].
Based on the above problems, a stable and simple water vapor electrodeless microwave plasma device is developed and designed in this paper. In this device, the ceramic tube can quickly absorb water after heating, and the water in the ceramic can soon evaporate and ionize on the ceramic tube top to form a water vapor plasma torch.
2. Experimental Setup
Figure 1 shows a schematic view of the water vapor microwave plasma system. It consists of the 2.45 GHz microwave generator, waveguide components, water container, ceramic tube, and wick. The microwave power supply provides a maximum power of 1000 W and is connected to a magnetron (LG, M246, Seoul, South Korea) to generate microwaves. The water-cooling system is a circulating water tank to prevent the microwave generator from overheating. The waveguide cavity is composed of a rectangular waveguide (WR340) and a dual-port tapered waveguide. The quartz tube is vertically inserted into the opening of the upper and lower surfaces of the dual-port conical waveguide. It is directly connected with the water container to guide and generate a plasma torch. The quartz tube has an inner diameter of 24 mm, an outer diameter of 28 mm, and a length of 600 mm. The ceramic tube is placed inside the quartz tube, its lower end is fixed at the bottom of the water container, and its upper end is in the center of the dual-port tapered waveguide. The main component of ceramics is silicon dioxide, which is produced from pressed powder material. The dielectric permittivity of the ceramic tube is 3.86, and its melting temperature is about 1200 °C. The wick is placed inside the ceramic tube, partially exposing the nozzle. The characteristics of water vapor microwave plasma are detected by the infrared thermometer (Guide infrared, PS400, Wuhan, China) with a high-temperature lens (GD-20), a high-speed camera (Phantom, V1612, Dallas, TX, USA) matching with a microscopic zoom lens (Navitar 12-X), a high-definition camera (Sony, A7M3, Tokyo, Japan), and optical emission spectroscopy (Ocean Optics, HR4000CG-UV-NIR spectrometer, Orlando, FL, USA).
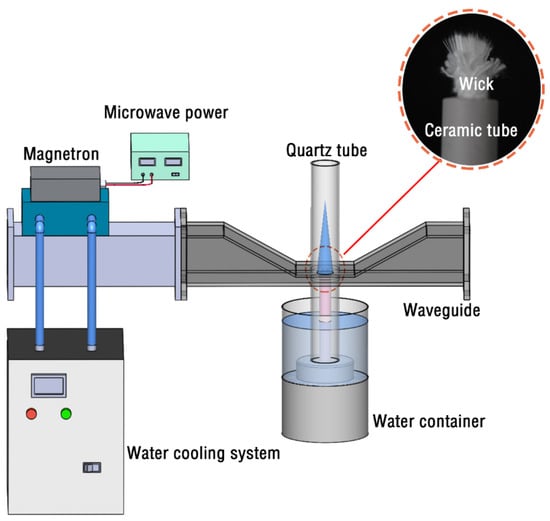
Figure 1.
Schematic of the water vapor microwave plasma setup.
The dual-port tapered waveguide in the experiment can concentrate the microwave energy at its center, as shown in Figure 2. However, the microwave energy in the experiment is not enough to directly ionize water vapor to produce plasma. Therefore, we will ignite the wick in the ceramic tube and then excite the high-temperature flame through the microwave. Flames already contain a high concentration of plasma species, so they can easily produce plasma by focusing relatively low-power microwaves into a high-temperature flame. When the plasma generated by the flame is excited, it will have an extremely high temperature, which can heat the ceramic tube, evaporate the water at the bottom of the ceramic tube and fill the whole quartz tube. Subsequently, water vapor becomes the ion source of the latest plasma, forming a water vapor plasma torch. The inner diameter of the ceramic tube in the experiment is 0.8 cm and the outer diameter is 1 cm; the inner diameter of the quartz tube is 2.4 cm and the outer diameter is 3 cm; the main components of the wick are cotton yarn.
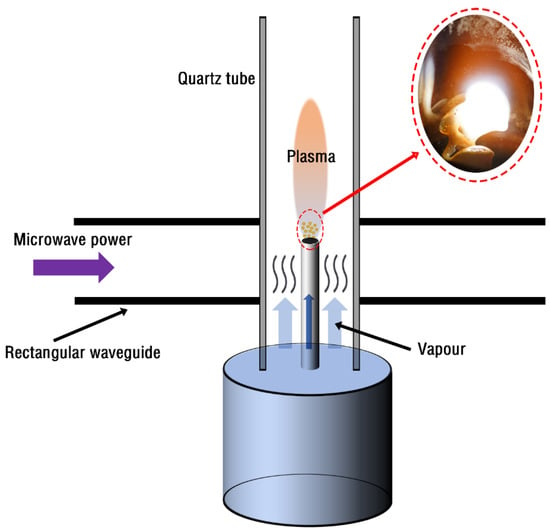
Figure 2.
Water vapor plasma is excited by microwave discharge; the left is the actual image of the torch flame on the ceramic tube.
3. Results and Discussion
Images of the plasma torch are shown in Figure 3a for when the water vapor is heated and evaporated, reaches the center of the waveguide and is excited. The figure shows the torch images under 300, 500, 700, and 900 W microwave power, respectively. It can be seen that the brightness and height of the torch increase with the increase in power. Figure 3b shows in detail the variation of the height and duration of the plasma torch with power. It can be seen here that the minimum excitation power of the torch is 300 W, and its torch height is 25 mm. With the increase in power, its height can reach 90 mm at 1000 W. It can also be seen from the figure that the duration of the flare is different under different powers. When the power is 300 W, the duration is only 2 min. With the increase in power by 800 W, it can be continuously and stably excited and no longer extinguished. The reason is that when the microwave power is low, the evaporation of water is too small to provide enough free radicals to excite the plasma torch continuously and stably.
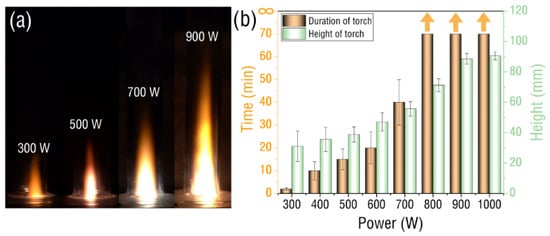
Figure 3.
(a) Water vapor plasma torch image captured by a high-definition camera with different power (multimedia view: Video S1). (b) The torch height and duration of water vapor plasma vary with power.
The process of exciting water vapor plasma in the experiment is as follows: first, add a little alcohol to the wick to burn it well, use the igniter to ignite the wick, and turn on the microwave power supply. At this time, the ignited wick burns rapidly under the action of the microwave and carbonizes, as shown in Figure 4a. In the figure, 0 s indicates that the microwave has just been turned on, and 20 s indicates that the wick is carbonizing. Then the carbonized wick begins to discharge, which makes the ceramic tube rapidly heated and absorbs the water in the container. Finally, the water at the top of the ceramic tube begins to evaporate and ionize, and the discharge position begins to transfer to the ceramic tube. After the water vapor plasma torch is formed on the top of the ceramic tube, the top of the ceramic is quickly heated to a molten state. The water vapor at the lower part continuously comes to the top of the ceramic tube. It is excited by microwaves, resulting in periodic expansion and contraction of the ceramic, as shown in Figure 4b. The above description can be confirmed by the temperature measurement of the infrared camera. Figure 4c shows the infrared image of the torch and the change of its maximum temperature with time. In the absence of microwaves, the maximum temperature of the flame is concentrated in the flame center, and the maximum temperature of the flame is 360 °C. As the microwave begins to act on the flame, the flame is enhanced, and its high-temperature increases to 420 °C. Then the wick began to discharge in a small range, the core temperature of the device was concentrated at the discharge of the wick, and the temperature changed significantly. At about 60 s, the discharge position is finally transferred to the ceramic tube. At this time, the maximum temperature in the device reaches the maximum temperature of 2000 ℃ that can be tested by the infrared camera, and a continuous plasma torch begins to appear. After each hour of operation, the weight of the ceramic tube is measured after drying. It is found that the weight of the whole ceramic remains about 18.5 g, with little change, as shown in Figure 4d, which shows that the water can be ionized on the top of the ceramic to form a plasma torch without losing the quality of the ceramic.
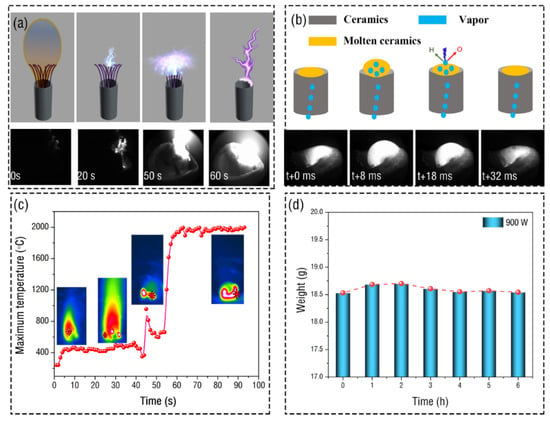
Figure 4.
The excitation process of water vapor microwave plasma torch. (a) The wick with flame discharges under the action of the microwave, causing evaporation and discharge of water at the top of the ceramic tube (multimedia view: Video S2, the upper part is the schematic diagram). (b) Process of water vapor plasma excited by microwave (multimedia view: Video S3, the upper part is the schematic diagram). (c) Temperature changes at the beginning of the excitation of water vapor microwave plasma. (d) Change of mass of ceramic tube after discharge with time.
After the top of the ceramic tube is discharged, the image of the ceramic tube before and after discharge is compared and analyzed. It can be found that the molten part on the top becomes very transparent. An XRD analysis of the ceramics can be seen in Figure 5a. The results show that the ceramics have a similar structure before and after discharge. To further explore the composition of the ceramic, we analyzed the ceramic material by energy dispersive spectroscopy (EDS) and found that the main elements are Si and O. Therefore, it can be found that the peak intensity of SiO2 after discharge is significantly stronger than that before discharge, which indicates that the high temperature generated by discharge can promote the crystallinity of the crystal. Finally, we also carried out experiments with alumina ceramic tubes and obtained similar results, which shows that this device can also be used for the crystal growth of non-metallic materials.
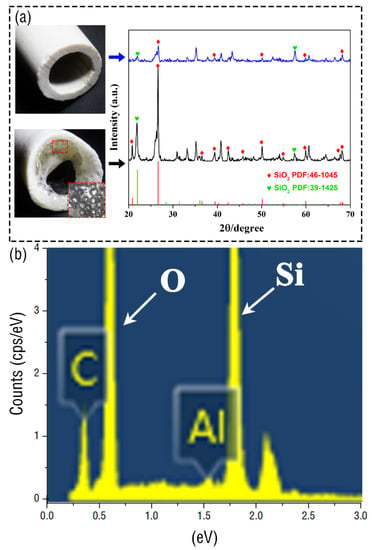
Figure 5.
(a) Image comparison and XRD of the ceramic tube before and after discharge. (b) Analysis of the surface composition of ceramic materials.
For different values of plasma input power, the emission spectrum of water vapor plasma of the device is obtained in the wavelength range of 250 to 1000 nm. The two-second integration time is applied, and the software PLASUA SpecLine (ver. 2.1, Plasus SpecLine, Kissing, Germany) is used to analyze and allocate the emission line. Shown in Figure 6 are the emission spectra of water vapor microwave plasma obtained by excitation at three different microwave powers of 900, 700, and 500 W, respectively. It illustrates that with the increase in power, the peak value of the spectrum is enhanced, and the emission line increases gradually. In this spectrum, the OH band is clearly identified with intense peaks in the range 302.1–308.9 nm [8]. Hα at 656.3 nm is also clearly identified [27]. In particular, the atomic oxygen line at 777 nm and 844 nm was remarkably enhanced. The strongest spectral line with a peak at 589.59 nm in the spectrum is most likely Na [28], which mainly comes from Na ions in water. In short, the emission spectrum confirms the dissociation of water molecules.
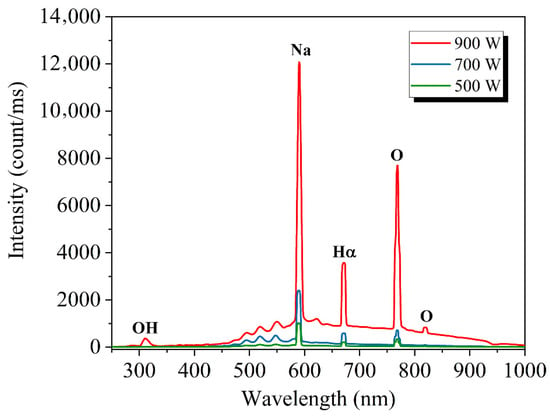
Figure 6.
Spectral data of water vapor plasma with different power.
4. Conclusions
In conclusion, we experimentally demonstrate a simple and stable electrodeless microwave plasma device as an atmospheric pressure water vapor microwave plasma source. The water vapor microwave plasma torch combustion process is investigated systematically. The results show that the microwave plasma torch can excite lasting and stably when the microwave power is greater than 800 W, and the height of the plasma torch increases with the increase in power. At the same time, it is also found that the plasma torch will make the top of the ceramic in a molten state instead of corroding the electrode during the excitation process. The XRD test confirmed that the crystallinity of the ceramic was improved after discharge. In addition, the dissociation of water vapor by microwave was confirmed by an emission spectrum. With the introduced ceramic, it is possible to design an even simpler and smaller water vapor microwave plasma torch for special applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app12136813/s1. Video S1. Water vapor plasma torch at 700 W. Video S2. Wick discharge under microwave excitation. Video S3. Discharge process of water vapor on ceramic tube.
Author Contributions
Data curation, Q.T., Z.H., X.C. and Z.T.; Formal analysis, Z.H.; Funding acquisition, Q.T. and J.T.; Investigation, Q.T. and Z.H.; Methodology, Z.H.; Project administration, J.T.; Software, Z.T. and J.T.; Writing—original draft, Q.T., Z.H., X.C., Z.T. and J.T.; Writing—review & editing, Q.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by [National Key Scientific Facility] grant number [51727901] and the APC was funded by [Qiang Tang].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Morent, R.; De Geyter, N.; Verschuren, J.; De Clerck, K.; Kiekens, P.; Leys, C. Non-thermal plasma treatment of textiles. Surf. Coat. Technol. 2008, 202, 3427–3449. [Google Scholar] [CrossRef]
- Yanling, C.; Yingkuan, W.; Chen, P.; Deng, S.; Ruan, R. Non-thermal plasma assisted polymer surface modification and synthesis: A review. Int. J. Agric. Biol. Eng. 2014, 7, 1–9. [Google Scholar]
- Matsumoto, T.; Wang, D.; Namihira, T.; Akiyama, H. Non-thermal plasma technic for air pollution control. In Air Pollution—A Comprehensive Perspective; IntechOpen: London, UK, 2012; p. 215. [Google Scholar]
- Ju, Y.; Sun, W. Plasma assisted combustion: Dynamics and chemistry. Prog. Energy Combust. Sci. 2015, 48, 21–83. [Google Scholar] [CrossRef]
- Czylkowski, D.; Hrycak, B.; Jasiński, M.; Dors, M.; Mizeraczyk, J. Microwave plasma-based method of hydrogen production via combined steam reforming of methane. Energy 2016, 113, 653–661. [Google Scholar] [CrossRef]
- Du, C.; Li, H.; Zhang, L.; Wang, J.; Huang, D.; Xiao, M.; Cai, J.; Chen, Y.; Yan, H.; Xiong, Y. Hydrogen production by steam-oxidative reforming of bio-ethanol assisted by Laval nozzle arc discharge. Int. J. Hydrogen Energy 2012, 37, 8318–8329. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Tsai, C.-H.; Chang, W.-Y.; Kuo, Y.-M. Methane steam reforming for producing hydrogen in an atmospheric-pressure microwave plasma reactor. Int. J. Hydrogen Energy 2010, 35, 135–140. [Google Scholar] [CrossRef]
- Nguyen, S.V.T.; Foster, J.E.; Gallimore, A.D. Operating a radio-frequency plasma source on water vapor. Rev. Sci. Instrum. 2009, 80, 083503. [Google Scholar] [CrossRef]
- Lytle, S.; Siddiqui, O.; Chehade, G.; Dincer, I. Analysis and modelling of microwave plasma hydrogen production utilizing water vapor and tungsten electrodes. Int. J. Hydrogen Energy 2019, 44, 25319–25334. [Google Scholar] [CrossRef]
- Tamošiūnas, A.; Valatkevičius, P.; Gimžauskaitė, D.; Jeguirim, M.; Mėčius, V.; Aikas, M. Energy recovery from waste glycerol by utilizing thermal water vapor plasma. Environ. Sci. Pollut. Res. 2017, 24, 10030–10040. [Google Scholar] [CrossRef]
- Grigaitienė, V.; Snapkauskienė, V.; Valatkevičius, P.; Tamošiūnas, A.; Valinčius, V. Water vapor plasma technology for biomass conversion to synthetic gas. Catal. Today 2011, 167, 135–140. [Google Scholar] [CrossRef]
- Chen, H.; Mu, Y.; Xu, S.; Xu, S.; Hardacre, C.; Fan, X. Recent advances in non-thermal plasma (NTP) catalysis towards C1 chemistry. Chin. J. Chem. Eng. 2020, 28, 2010–2021. [Google Scholar] [CrossRef]
- Brandenburg, R. Dielectric barrier discharges: Progress on plasma sources and on the understanding of regimes and single filaments. Plasma Sources Sci. Technol. 2017, 26, 053001. [Google Scholar] [CrossRef]
- Karatum, O.; Deshusses, M.A. A comparative study of dilute VOCs treatment in a non-thermal plasma reactor. Chem. Eng. J. 2016, 294, 308–315. [Google Scholar] [CrossRef]
- Chehade, G.; Lytle, S.; Ishaq, H.; Dincer, I. Hydrogen production by microwave based plasma dissociation of water. Fuel 2020, 264, 116831. [Google Scholar] [CrossRef]
- Radoiu, M.; Hussain, S. Microwave plasma removal of sulphur hexafluoride. J. Hazard. Mater. 2009, 164, 39–45. [Google Scholar] [CrossRef]
- Yubero, C.; Garcia, M.C.; Calzada, M.D. On the use of the Hα spectral line to determine the electron density in a microwave (2.45 GHz) plasma torch at atmospheric pressure. Spectrochim. Acta Part B At. Spectrosc. 2006, 61, 540–544. [Google Scholar] [CrossRef]
- Prokisch, C.; Bilgic, A.M.; Voges, E.; Broekaert, J.A.C.; Jonkers, J.; Van Sande, M.; Van der Mullen, J.A.M. Photographic plasma images and electron number density as well as electron temperature mappings of a plasma sustained with a modified argon microwave plasma torch (MPT) measured by spatially resolved Thomson scattering. Spectrochim. Acta Part B At. Spectrosc. 1999, 54, 1253–1266. [Google Scholar] [CrossRef]
- Sun, H.; Lee, J.; Bak, M.S. Experiments and modeling of atmospheric pressure microwave plasma reforming of a methane-carbon dioxide mixture. J. CO2 Util. 2021, 46, 101464. [Google Scholar] [CrossRef]
- Oh, J.-S.; Kawamura, K.; Pramanik, B.K.; Hatta, A. Investigation of water-vapor plasma excited by microwaves as ultraviolet light source. IEEE Trans. Plasma Sci. 2008, 37, 107–112. [Google Scholar] [CrossRef] [Green Version]
- Christova, M.; Castanos-Martinez, E.; Calzada, M.D.; Kabouzi, Y.; Luque, J.M.; Moisan, M. Electron density and gas temperature from line broadening in an argon surface-wave-sustained discharge at atmospheric pressure. Appl. Spectrosc. 2004, 58, 1032–1037. [Google Scholar] [CrossRef]
- Benova, E.; Marinova, P.; Tafradjiiska-Hadjiolova, R.; Sabit, Z.; Bakalov, D.; Valchev, N.; Traikov, L.; Hikov, T.; Tsonev, I.; Bogdanov, T. Characteristics of 2.45 GHz Surface-Wave-Sustained Argon Discharge for Bio-Medical Applications. Appl. Sci. 2022, 12, 969. [Google Scholar] [CrossRef]
- Rachdi, L.; Sushkov, V.; Hofmann, M. Optical emission spectroscopy diagnostics for plasma parameters investigation in a Duo-Plasmaline surface-wave sustained discharge. Spectrochim. Acta Part B At. Spectrosc. 2022, 194, 106432. [Google Scholar] [CrossRef]
- Vecten, S.; Wilkinson, M.; Martin, A.; Dexter, A.; Bimbo, N.; Dawson, R.; Herbert, B. Experimental study of steam and carbon dioxide microwave plasma for advanced thermal treatment application. Energy 2020, 207, 118086. [Google Scholar] [CrossRef]
- Robert, E.; Barbosa, E.; Dozias, S.; Vandamme, M.; Cachoncinlle, C.; Viladrosa, R.; Pouvesle, J.M. Experimental study of a compact nanosecond plasma gun. Plasma Processes Polym. 2009, 6, 795–802. [Google Scholar] [CrossRef]
- Lee, B.-J.; Jo, S.-I.; Heo, S.-G.; Lee, W.-Y.; Jeong, G.-H. Structure-controllable synthesis of ZnO nanowires using water vapor in an atmospheric-pressure microwave plasma system. Curr. Appl. Phys. 2021, 28, 52–58. [Google Scholar] [CrossRef]
- Umetsu, J.; Koga, K.; Inoue, K.; Matsuzaki, H.; Takenaka, K.; Shiratani, M. Discharge power dependence of Hα intensity and electron density of Ará+ áH2 discharges in H-assisted plasma CVD reactor. Surf. Coat. Technol. 2008, 202, 5659–5662. [Google Scholar] [CrossRef]
- Stojadinovic, S.; Vasilic, R.; Belca, I.; Petkovic, M.; Kasalica, B.; Nedic, Z.; Zekovic, L. Characterization of the plasma electrolytic oxidation of aluminium in sodium tungstate. Corros. Sci. 2010, 52, 3258–3265. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).