Therapeutic Potential of Ajwa Dates (Phoenix dactylifera) Extract in Prevention of Benzo(a)pyrene-Induced Lung Injury through the Modulation of Oxidative Stress, Inflammation, and Cell Signalling Molecules
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Preparation of Ethanolic Extract of Ajwa Pulp (ADE)
2.3. Phytochemical Screening
2.4. Total Phenolic Content
2.5. Evaluation of the Amount of Total Flavonoids
2.6. Animals
2.7. Animal Ethics
2.8. Study Design and Treatment Plan
2.9. Measurement of Antioxidant Enzymes and Oxidative Stress Markers
2.10. Measurement of Malondialdehyde and Nitric Oxide Content
2.11. Quantification of Inflammatory Markers
2.12. Measurement of Lipid Profile
2.13. Measurement of VEGF Level
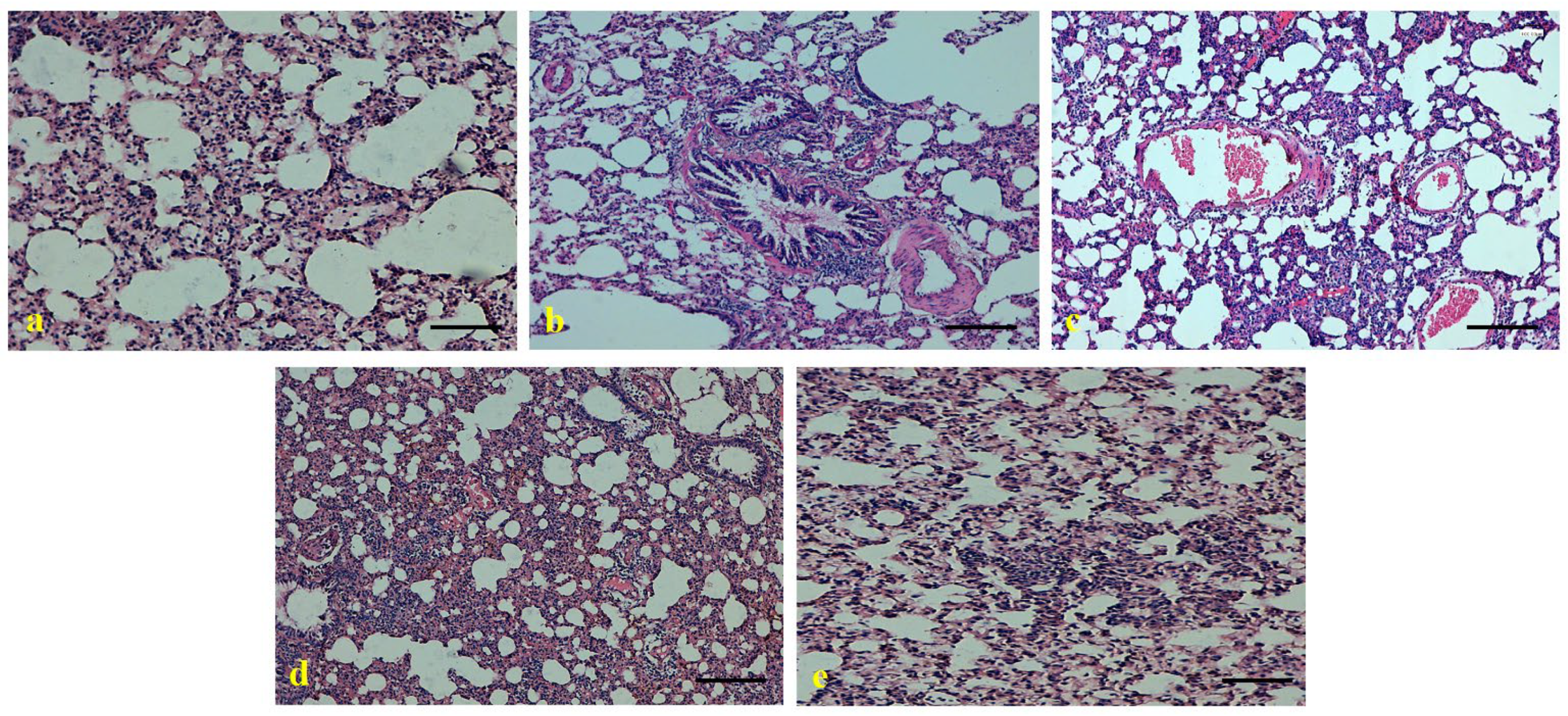
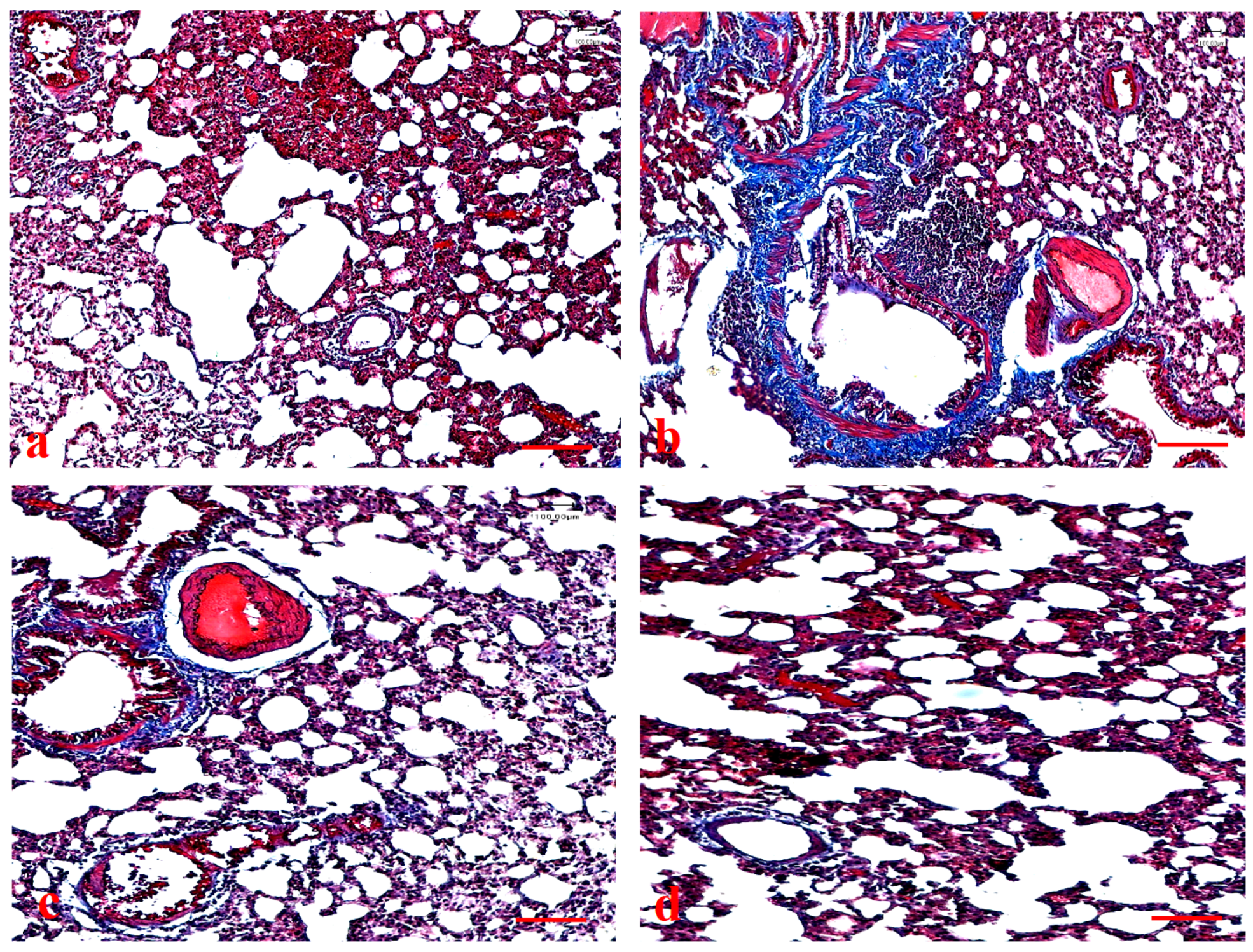
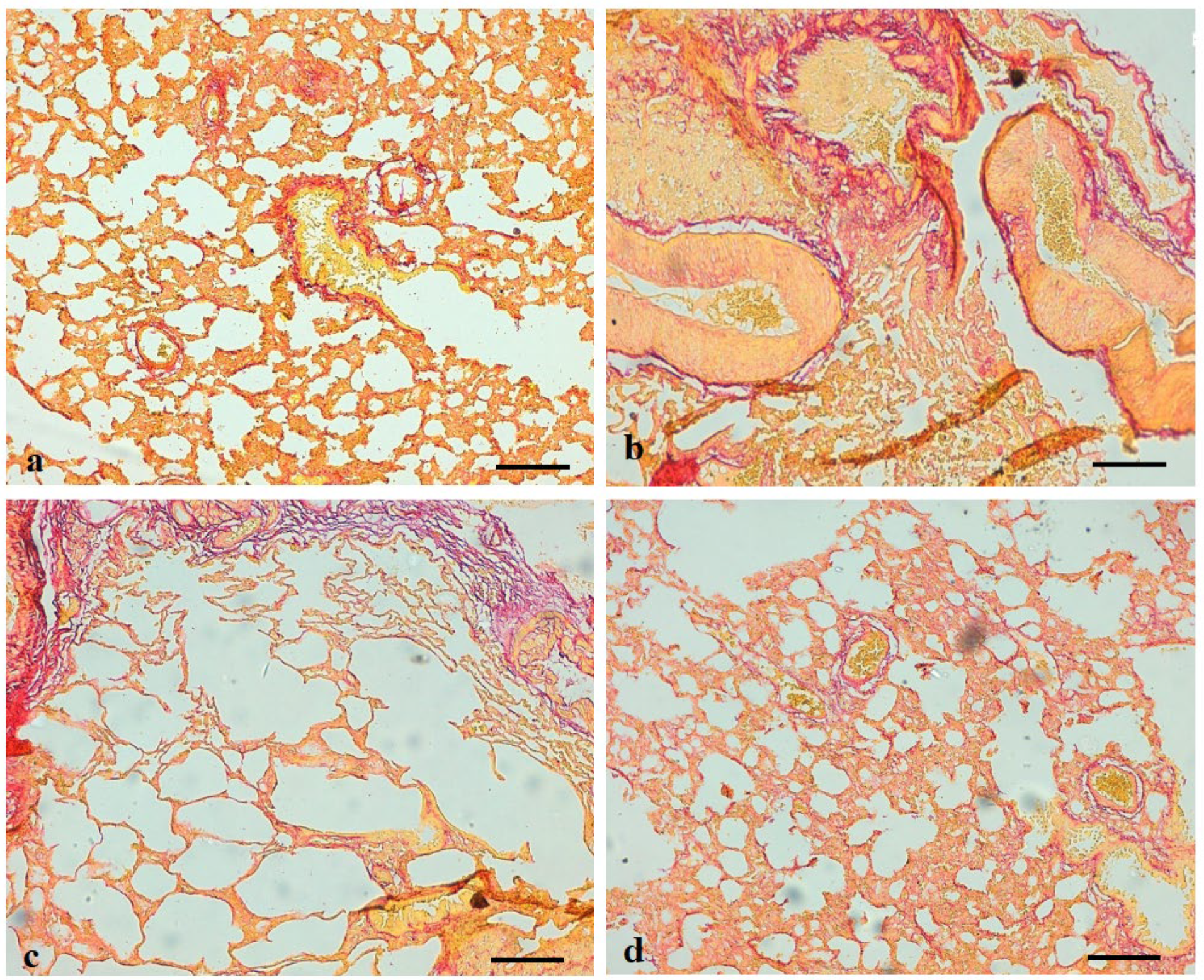
2.14. Assessment of Lung Histopathology
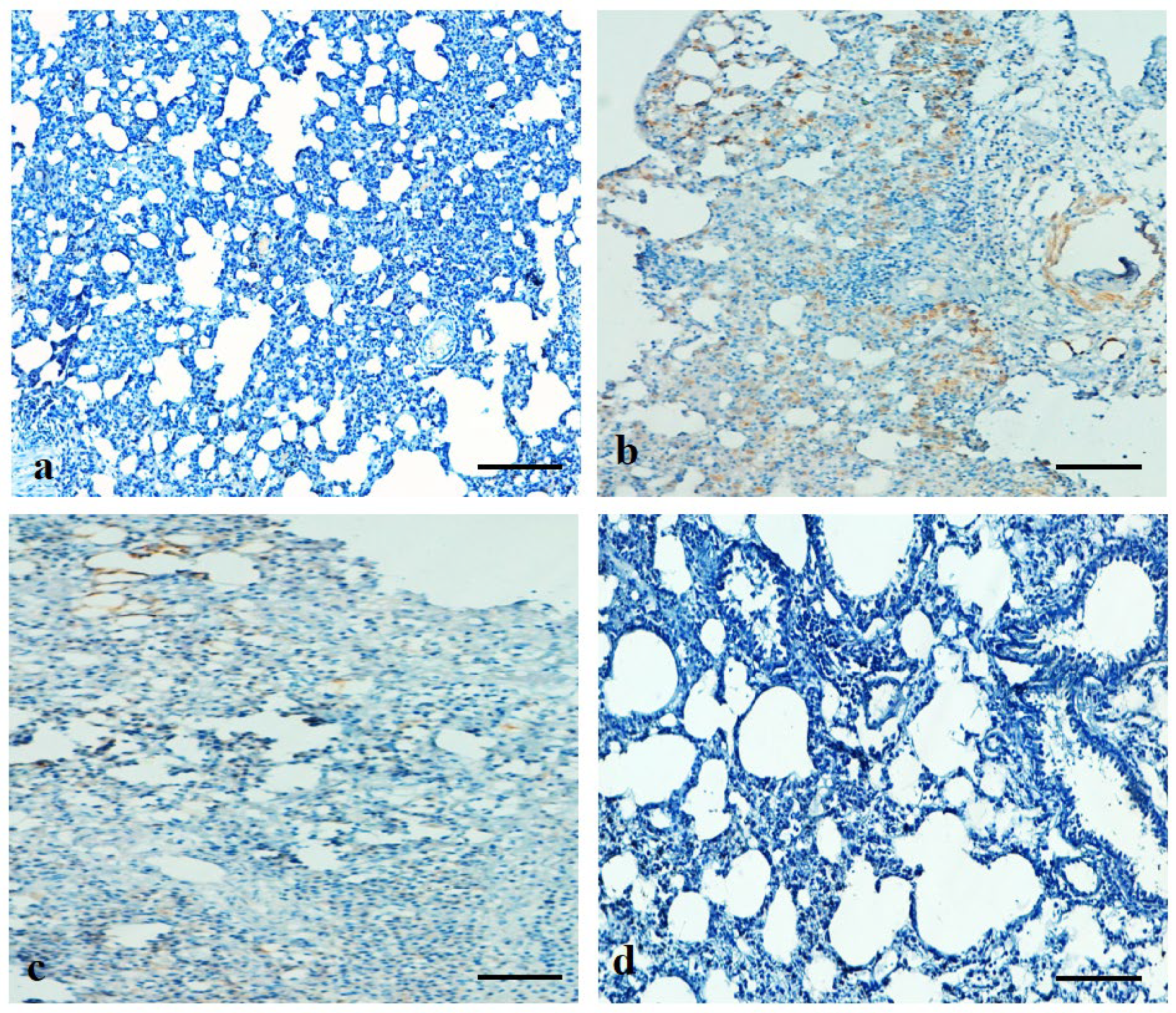
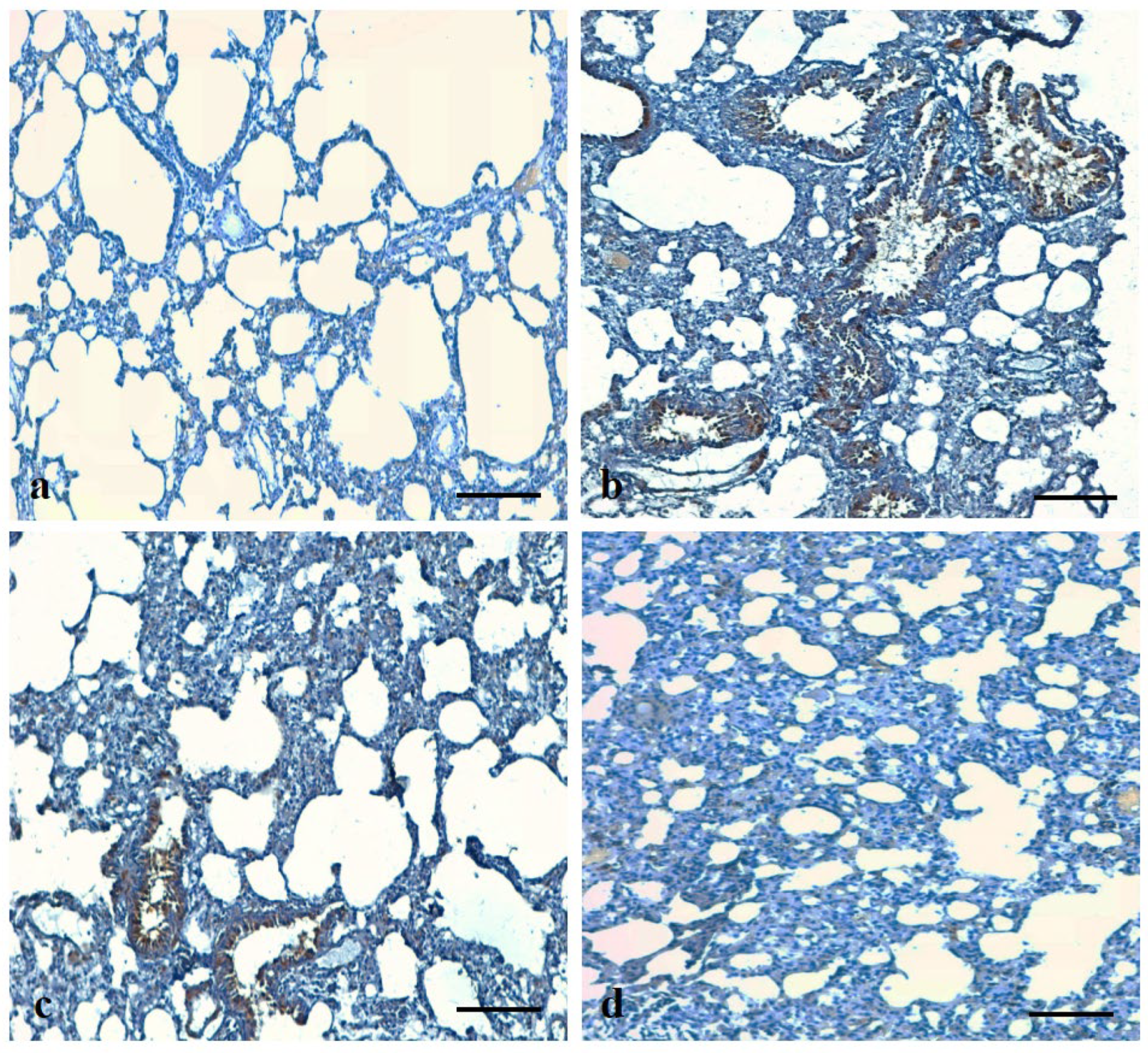
2.15. Immunohistochemical Analysis of Bax and VEGF Protein Expressions
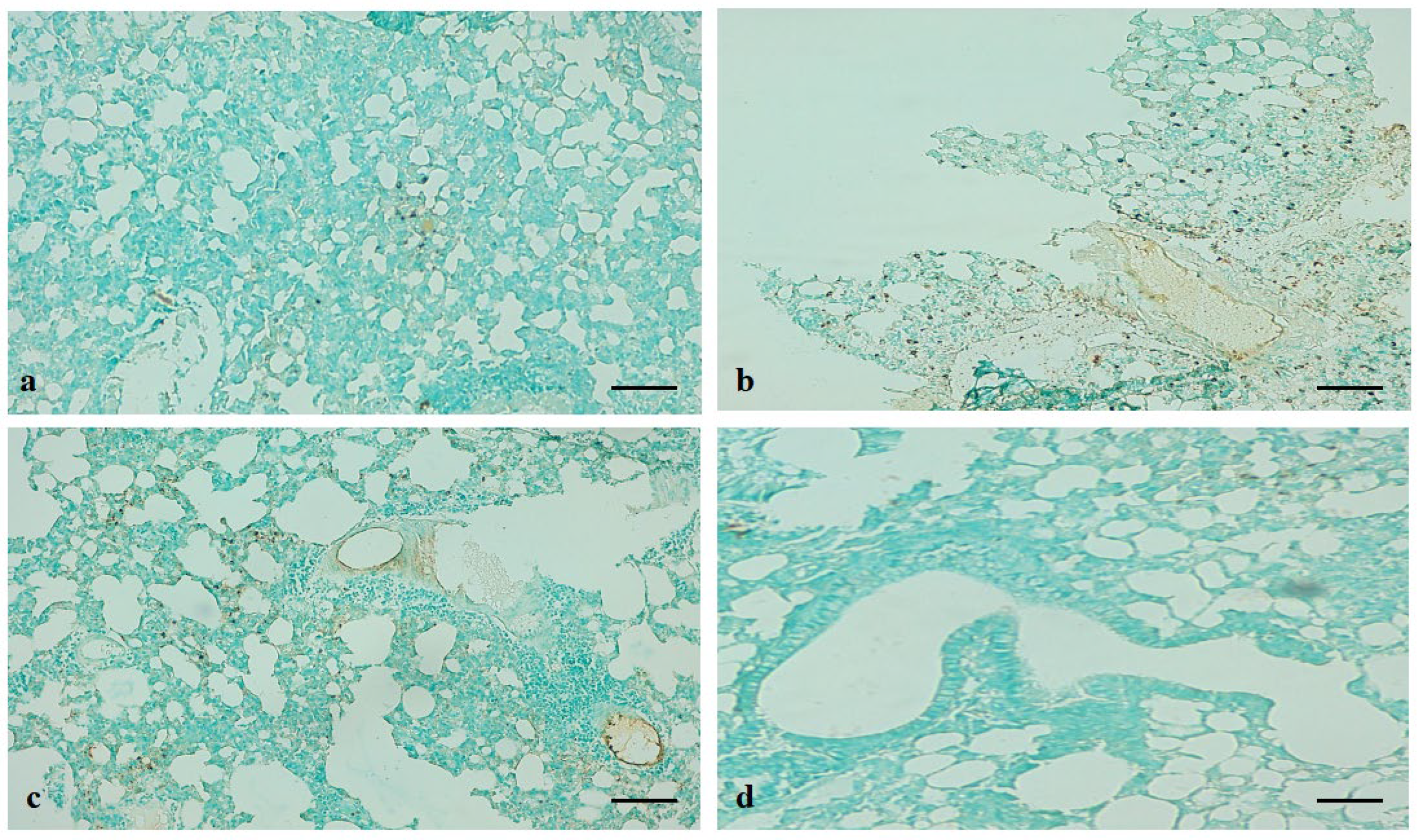
2.16. Terminal Deoxynucleotidyl Transferase-Mediated dUTP-Biotin Nick-End Labelling (TUNEL) Assay
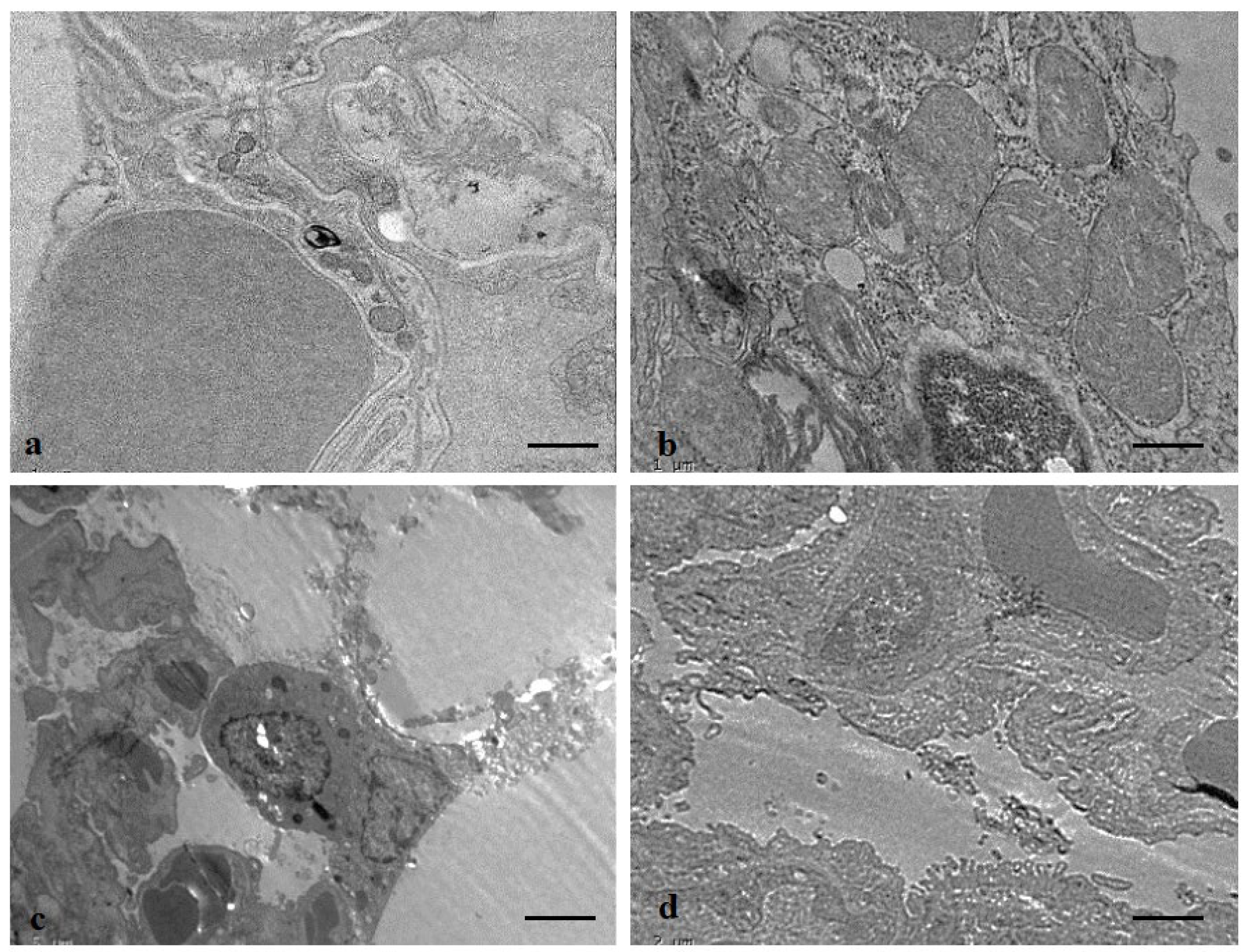
2.17. Transmission Electron Microscopy
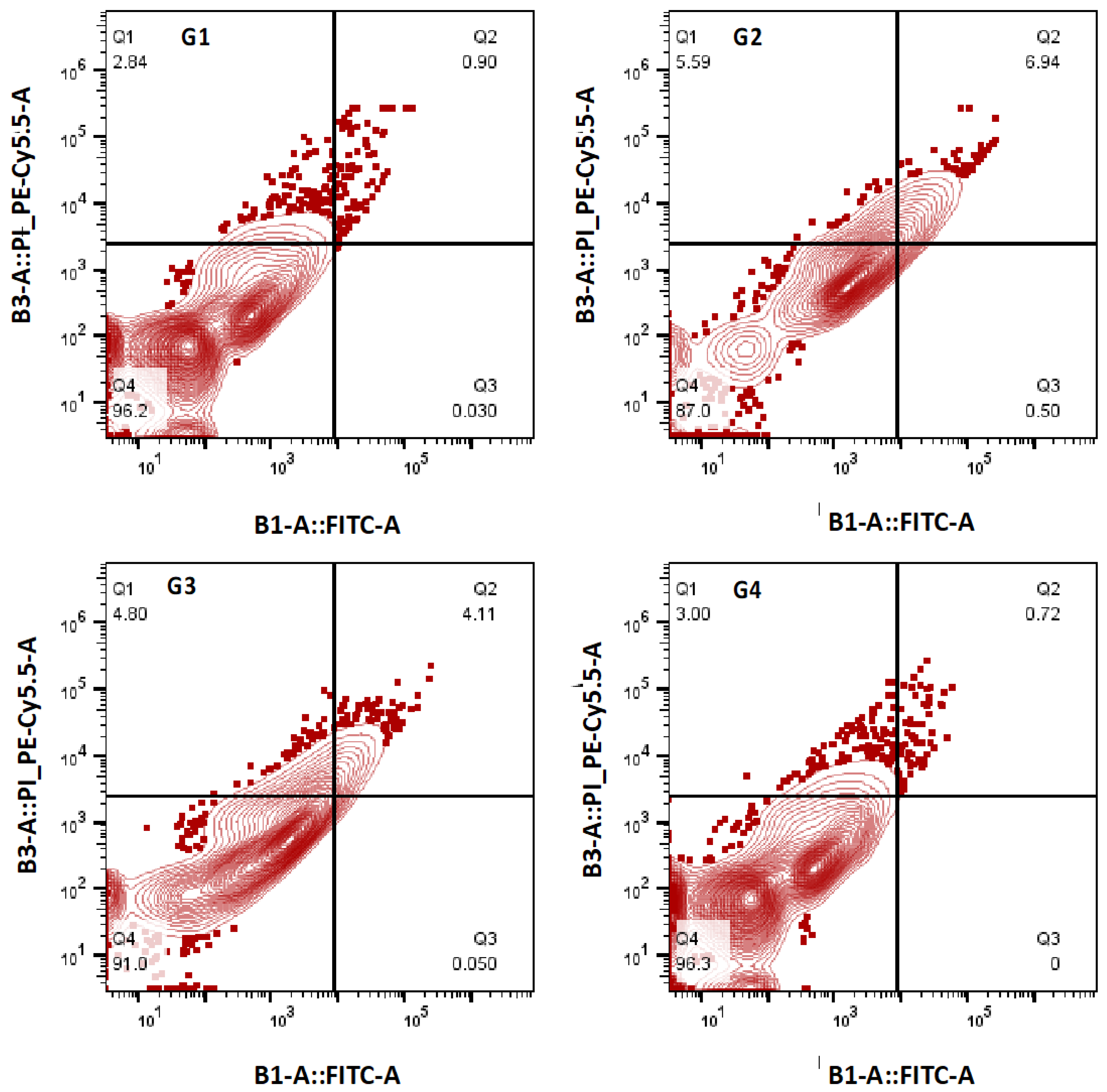
2.18. Apoptosis Analysis by Flow Cytometry
2.19. Statistical Analysis
3. Results
3.1. Preliminary Screening for the Presence of Flavonoid and Phenolic Compounds
3.2. Measurement of ADE Activity on Antioxidant Enzymes
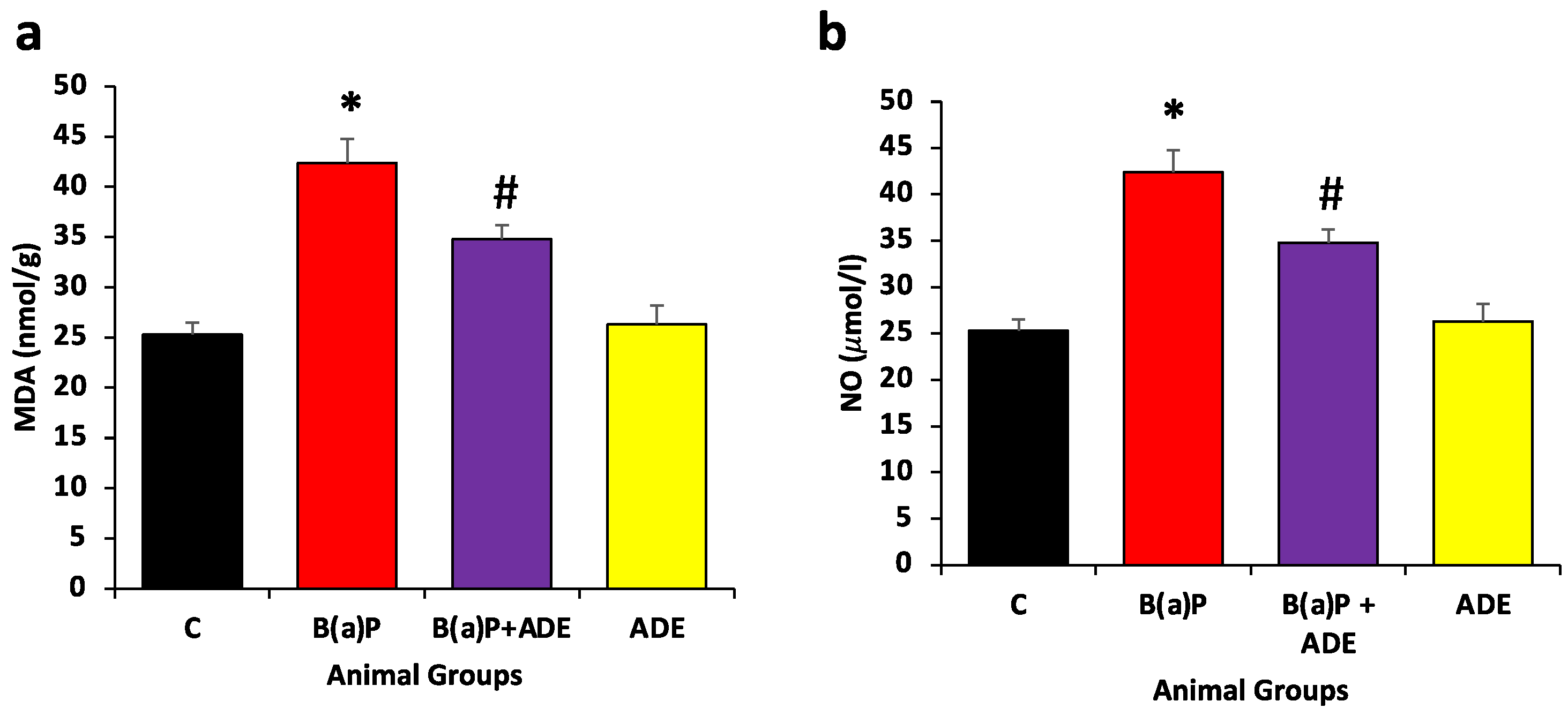
3.3. Measurement of ADE Effect on MDA and NO Contents
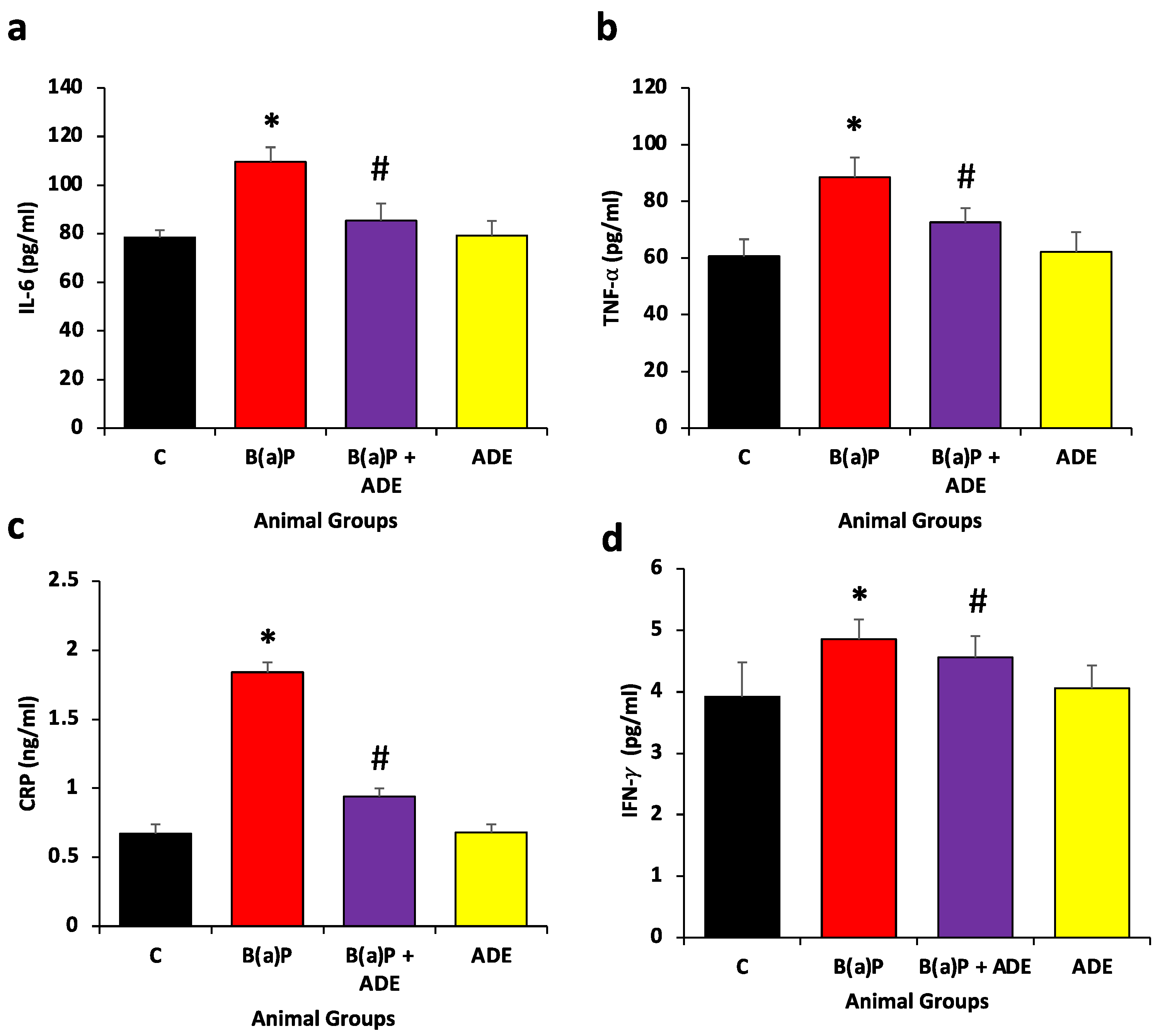
3.4. Effects of ADE on Inflammatory Markers
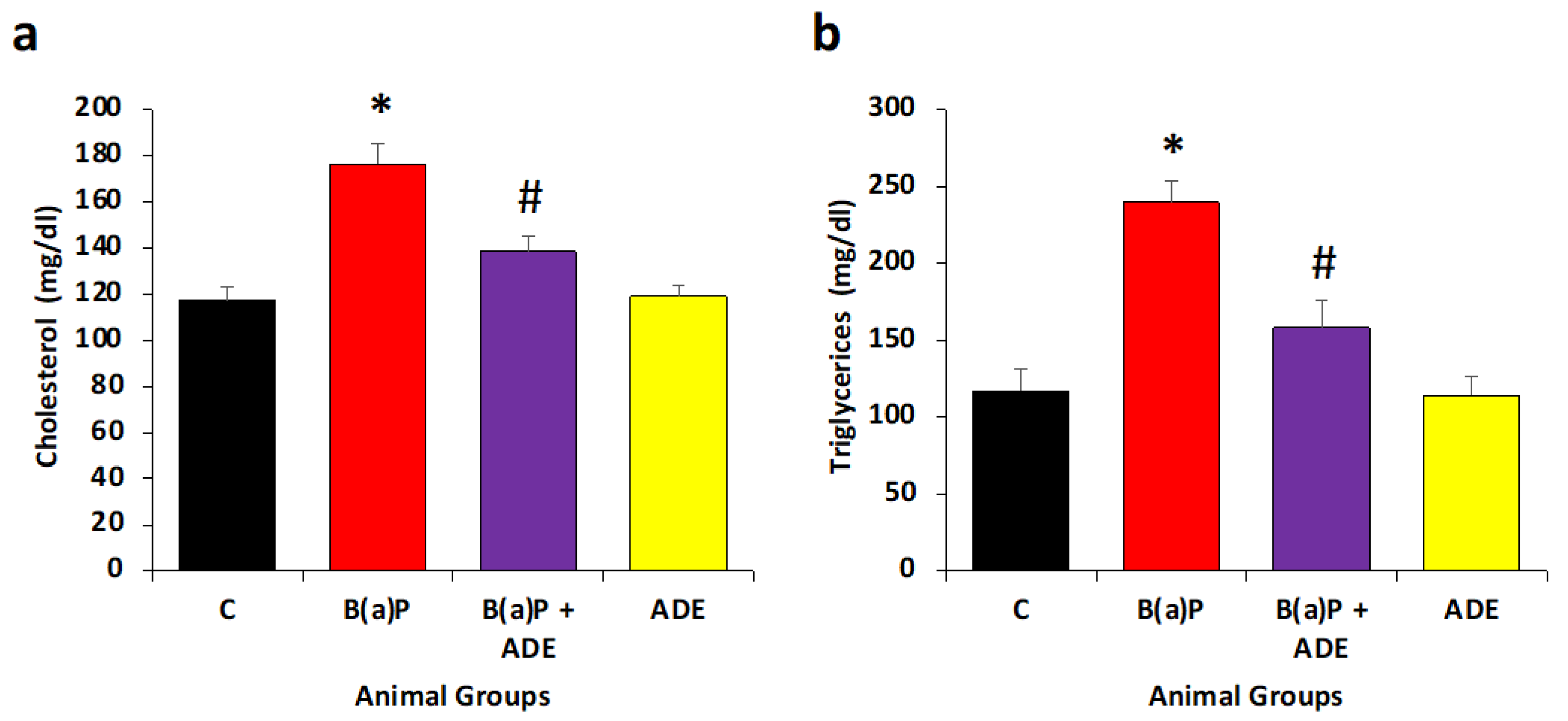
3.5. Measurement of Antihyperlipidemic Effects of ADE
3.6. Measurement of ADE Effect on Total Protein and Albumin Levels
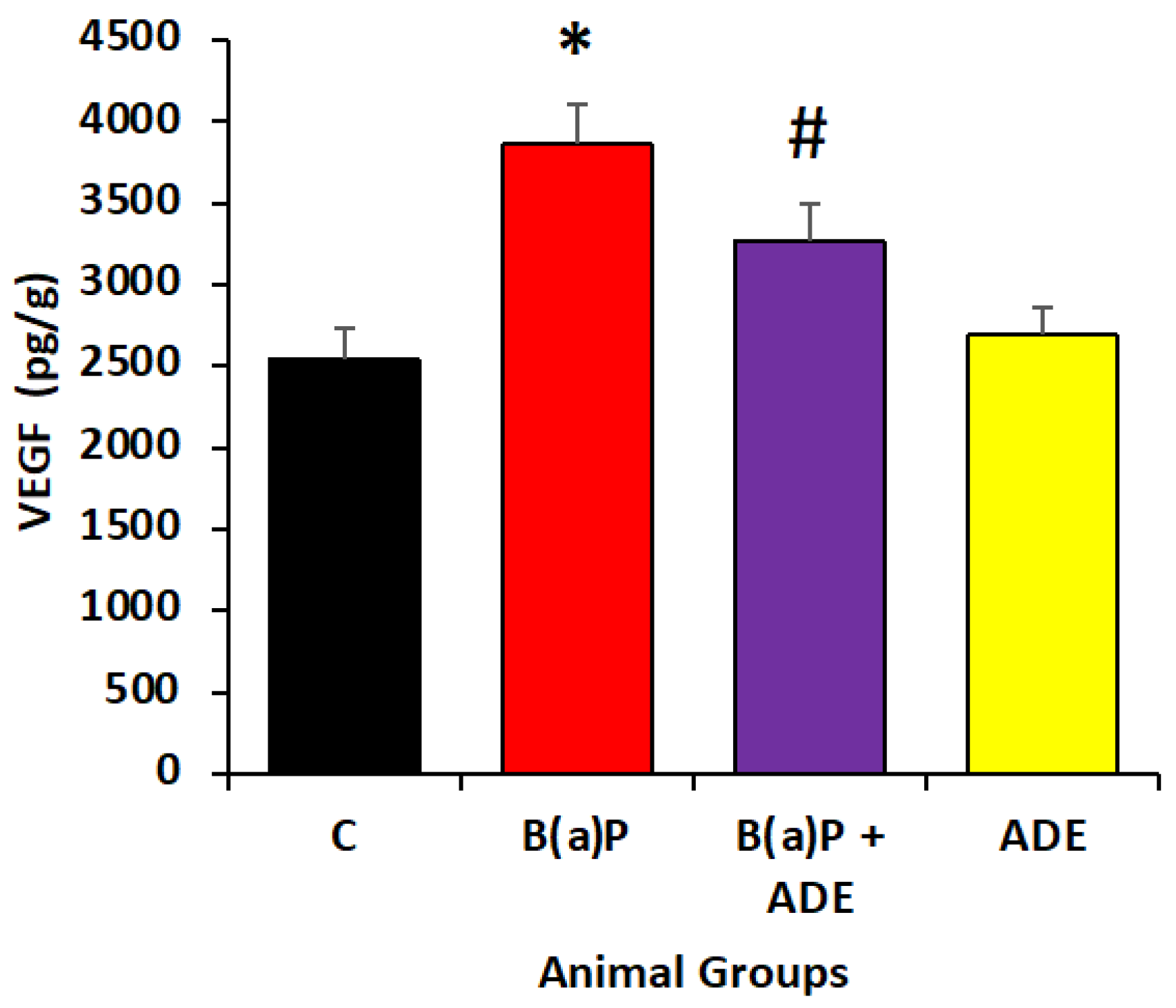
3.7. Effect of ADE on Angiogenic Marker Level
3.8. Role of Ajwa Date Extract on the Maintenance of Lung Architecture
3.9. Effects of Ajwa Date Extract on Lung Fibrosis Caused by B(a)P in Rats
3.10. Effects of ADE on Expression Pattern of Bax and VEGF Protein in Lung Tissue
3.11. ADE attenuates B(a)P-Induced Apoptosis in Lung Tissue
3.12. Protective Role of ADE on Lung Tissue Ultrastructure
3.13. Effect of ADE on Apoptotic Cell Death
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 354 Diseases and Injuries for 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Piccardo, M.T.; Stella, A.; Valerio, F. Is the smokers exposure to environmental tobacco smoke negligible? Environ. Health 2010, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Yeo, C.D.; Kim, J.W.; Ha, J.H.; Kim, S.J.; Lee, S.H.; Kim, I.K. Chemopreventive effect of phosphodieasterase-4 inhibition in ben-zo(a)pyrene-induced murine lung cancer model. Exp. Lung Res. 2014, 40, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Jahangir, T.; Sultana, S. Benzo(a)pyrene-induced genotoxicity: Attenuation by farnesol in a mouse model. J. Enzym. Inhib. Med. Chem. 2008, 23, 888–894. [Google Scholar] [CrossRef][Green Version]
- Kometani, T.; Yoshino, I.; Miura, N.; Okazaki, H.; Ohba, T.; Takenaka, T.; Shoji, F.; Yano, T.; Maehara, Y. Benzo[a]pyrene promotes proliferation of human lung cancer cells by accelerating the epidermal growth factor receptor signaling pathway. Cancer Lett. 2009, 278, 27–33. [Google Scholar] [CrossRef]
- Gelboin, H.V. Benzo[alpha]pyrene metabolism, activation and carcinogenesis: Role and regulation of mixed-function oxidases and related enzymes. Physiol. Rev. 1980, 60, 1107–1166. [Google Scholar] [CrossRef]
- Baer-Dubowska, W.; Kulinska, A.; Jarmuz, M.; Brauze, D.; Gnojkowski, J. DNA adducts detected by synchronous fluorescence spectrophotometry in rat liver, kidney, lung and spleen after intraperitoneal administration of benzo[a]pyrene. Acta Biochim. Pol. 1993, 40, 63–65. [Google Scholar] [CrossRef]
- Podechard, N.; Lecureur, V.; Le Ferrec, E.; Guenon, I.; Sparfel, L.; Gilot, D.; Gordon, J.R.; Lagente, V.; Fardel, O. Interleukin-8 induction by the environmental contaminant benzo(a)pyrene is aryl hydrocarbon receptor-dependent and leads to lung inflammation. Toxicol. Lett. 2008, 177, 130–137. [Google Scholar] [CrossRef]
- Lina, T.; Yang, M.S. Benzo[a]pyrene-induced elevation of GSH level protects against oxidative stress andenhances xenobioticdetoxificationinhumanHepG2 cells. Toxicology 2007, 235, 1–10. [Google Scholar] [CrossRef]
- Qamar, W.; Khan, A.Q.; Khan, R.; Lateef, A.; Tahir, M.; Rehman, M.U.; Ali, F.; Sultana, S. Benzo(a)pyrene-induced pulmonary inflammation, edema, surfactant dysfunction, and injuries in rats: Alleviation by farnesol. Exp. Lung Res. 2012, 38, 19–27. [Google Scholar] [CrossRef]
- Smerdova, L.; Neca, J.; Svobodova, J.; Topinka, J.; Schmuczerova, J.; Kozubik, A.; Machala, M.; Vondracek, J. Inflammatory mediators accelerate metabolism of benzo[a]pyrene in rat alveolar type II cells: The role of enhanced cytochrome P450 1B1 expression. Toxicology 2013, 314, 30–38. [Google Scholar] [CrossRef]
- Uno, S.; Makishima, M. Benzo [a] pyrene toxicity and inflammatory disease. Curr. Rheumatol. Rev. 2009, 5, 266–271. [Google Scholar] [CrossRef]
- Slader, C.A.; Reddel, H.K.; Jenkins, C.R.; Armour, C.L.; Bosnic-Anticevich, S.Z. Complementary and alternative medicine use in asthma: Who is using what? Respirology 2006, 11, 373–387. [Google Scholar] [CrossRef]
- Ragab, A.R.; Elkablawy, M.A.; Sheik, B.Y.; Baraka, H.N. Antioxidant and tissueprotective studies on Ajwa extract: Dates from al-Madinah al-Monwarah, Saudia Arabia. J. Environ. Anal. Toxicol. 2013, 3, 163. [Google Scholar]
- Zhang, C.R.; Aldosari, S.A.; Vidyasagar, P.S.; Nair, K.M.; Nair, M.G. Antioxidant and anti-inflammatory assays confirm bioactive compounds in Ajwa Date fruit. J. Agric. Food Chem. 2013, 61, 5834–5840. [Google Scholar] [CrossRef]
- Sheikh, B.Y.; Elsaed, W.M.; Samman, A.H.; Sheikh, B.Y.; Ladin, A.-M.M.B. Ajwa dates as a protective agent against liver toxicity in rat. Eur. Sci. J. 2014, 10, 358–368. [Google Scholar]
- Rahmani, A.H.; Khan, A.A.; Babiker, A.Y.; Khan, M.A.; Alzohairy, M.A. Therapeutic implications of ajwa dates (Phoenix dactylifera) in the inhibition of liver tissue alterations through the modulation of vascular endothelial growth factor and phosphatase, and tensin homolog gene. Pharmacogn. Res. 2018, 10, 151–155. [Google Scholar] [CrossRef]
- Ali, A.; Abdu, S. Antioxidant protection against pathological mycotoxins alterations on proximal tubules inrat kidney. Funct. Foods Health Dis. 2011, 1, 118–134. [Google Scholar] [CrossRef]
- Alqarni, M.M.; Osman, M.A.; Al-Tamimi, D.S.; Gassem, M.A.; Al-Khalifa, A.S.; Al-Juhaimi, F.; Mohamed Ahmed, I.A. Antioxidant and antihyperlipidemic effects of Ajwa date (Phoenix dactylifera L.) extracts in rats fed a cholesterol-rich diet. J. Food Biochem. 2019, 43, e12933. [Google Scholar] [CrossRef]
- Shahbaz, K.; Asif, J.A.; Liszen, T.; Nurul, A.A.; Alam, M.K. Cytotoxic and Antioxidant Effects of Phoenix dactylifera L. (Ajwa Date Extract) on Oral Squamous Cell Carcinoma Cell Line. BioMed Res. Int. 2022, 2022, 5792830. [Google Scholar] [CrossRef]
- Alsahli, M.A.; Anwar, S.; Alzahrani, F.M.; Almatroudi, A.; Alfheeaid, H.; Khan, A.A.; Allemailem, K.S.; Almatroodi, S.A.; Rahmani, A.H. Health Promoting Effect of Phyllanthus emblica and Azadiractha indica against Advanced Glycation End Products Formation. Appl. Sci. 2021, 11, 8819. [Google Scholar] [CrossRef]
- Anwar, S.; Almatroudi, A.; Allemailem, K.S.; Jacob Joseph, R.; Khan, A.A.; Rahmani, A.H. Protective Effects of Ginger Extract against Glycation and Oxidative Stress-Induced Health Complications: An In Vitro Study. Processes 2020, 8, 468. [Google Scholar] [CrossRef]
- Anwar, S.; Almatroodi, S.A.; Almatroudi, A.; Allemailem, K.S.; Joseph, R.J.; Khan, A.A.; Alrumaihi, F.; Alsahli, M.A.; Husain Rahmani, A. Biosynthesis of silver nanoparticles using Tamarix articulata leaf extract: An effective approach for attenuation of oxidative stress mediated diseases. Int. J. Food Prop. 2021, 24, 677–701. [Google Scholar] [CrossRef]
- Almatroodi, S.A.; Anwar, S.; Almatroudi, A.; Khan, A.A.; Alrumaihi, F.; Alsahli, M.A.; Rahmani, A.H. Hepatoprotective Effects of Garlic Extract against Carbon Tetrachloride (CCl4)-Induced Liver Injury via Modulation of Antioxidant, Anti-Inflammatory Activities and Hepatocyte Architecture. Appl. Sci. 2020, 10, 6200. [Google Scholar] [CrossRef]
- Hassan, S.K.; Mousa, A.M.; El-Sammad, N.M.; Abdel-Halim, A.H.; Khalil, W.K.; Elsayed, E.A.; Anwar, N.; Linscheid, M.W.; Moustafa, E.S.; Hashim, A.N.; et al. Antitumor activity of Cuphea ignea extract against benzo(a)pyrene-induced lung tumorigenesis in Swiss Albino mice. Toxicol. Rep. 2019, 6, 1071–1085. [Google Scholar] [CrossRef]
- Rahmani, A.; Alzohairy, M.; Khadri, H.; Mandal, A.K.; Rizvi, M.A. Expressional evaluation of vascular endothelial growth factor (VEGF) protein in urinary bladder carcinoma patients exposed to cigarette smoke. Int. J. Clin. Exp. Pathol. 2012, 5, 195–202. [Google Scholar]
- Babiker, A.Y.; Almatroudi, A.; Allemailem, K.S.; Husain, N.E.O.S.; Alsammani, M.A.; Alsahli, M.A.; Rahmani, A.H. Clinicopathologic Aspects of Squamous Cell Carcinoma of the Uterine Cervix: Role of PTEN, BCL2 and P53. Appl. Sci. 2018, 8, 2124. [Google Scholar] [CrossRef]
- Saad, B.; Azaizeh, H.; Abu-Hijleh, G.; Said, S. Safety of traditional Arab herbal medicine. Evid. Based Complement. Alternat. Med. 2006, 3, 433–439. [Google Scholar] [CrossRef]
- Akiibinu, O.M.; Ogunyemi, O.E.; Arinola, O.G.; Adenaike, A.F.; Adegoke, O.D. Assessment of antioxidants and nutritional status of pulmonary tuberculosis patients in Nigeria. Eur. J. Gen. Med. 2008, 5, 208–211. [Google Scholar]
- Almatroodi, S.A.; AlRumaihi, F.; Alsahli, M.A.; Alhommrani, M.F.; Khan, A.; Rahmani, A.H. Curcumin, an Active Constituent of Turmeric Spice: Implication in the Prevention of Lung Injury Induced by Benzo(a) Pyrene (BaP) in Rats. Molecules 2020, 25, 724. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.Q.; Ren, J.; Liu, J. Responses of antioxidant systems and LPO level to benzo(a)pyrene and benzo(k)fluoranthene in the haemolymph of the scallop Chlamys ferrari. Environ. Pollut. 2006, 141, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Kiruthiga, P.V.; Pandian, S.K.; Devi, K.P. Silymarin protects PBMC against B(a)P induced toxicity by replenishing redox status and modulating glutathione metabolizing enzymes—An in vitro study. Toxicol. Appl. Pharmacol. 2010, 247, 116–128. [Google Scholar] [CrossRef] [PubMed]
- El-Kott, A.F. Anti-angiogenic effectiveness of the pomegranate against benzo (a)pyrene induced lung carcinoma in mice. Cancer Res. 2015, 11, 164–174. [Google Scholar] [CrossRef]
- Elsadek, B.; El-Sayed, E.S.; Mansour, A.; Elazab, A. Abrogation of carbon tetrachloride-induced hepatotoxicity in Sprague-Dawley rats by Ajwa date fruit extract through ameliorating oxidative stress and apoptosis. Pak. J. Pharm. Sci. 2017, 30, 2183–2191. [Google Scholar]
- Gaston, B.; Kobzik, L.; Stamler, J.S. Distribution of nitric oxide synthase in the lung. In Nitric Oxide and the Lung; Zapol, W.M., Bloch, K.D., Eds.; Dekker: New York, NY, USA, 1997; pp. 75–86. [Google Scholar]
- Alzohairy, M.A.; Khan, A.A.; Alsahli, M.A.; Almatroodi, S.A.; Rahmani, A.H. Protective Effects of Thymoquinone, an Active Compound of Nigella sativa, on Rats with Benzo(a)pyrene-Induced Lung Injury through Regulation of Oxidative Stress and Inflammation. Molecules 2021, 26, 3218. [Google Scholar] [CrossRef]
- Baradaran Rahimi, V.; Rakhshandeh, H.; Raucci, F.; Buono, B.; Shirazinia, R.; Samzadeh Kermani, A.; Maione, F.; Mascolo, N.; Askari, V.R. Anti-Inflammatory and Anti-Oxidant Activity of Portulaca oleracea Extract on LPS-Induced Rat Lung Injury. Molecules 2019, 24, 139. [Google Scholar] [CrossRef]
- Wang, K.; Liu, C.T.; Wu, Y.H.; Feng, Y.L.; Bai, H.L. Budesonide/formoterol decreases expression of vascular endothelial growth factor (VEGF) and VEGF receptor 1 within airway remodelling in asthma. Adv. Ther. 2008, 25, 342–354. [Google Scholar] [CrossRef]
- Gomulka, K.; Liebhart, J.; Gladysz, U.; Medrala, W. VEGF serum concentration and irreversible bronchoconstriction in adult asthmatics. Adv. Clin. Exp. Med. 2019, 28, 759–763. [Google Scholar] [CrossRef]
- Alzohairy, M.A.; Khan, A.A.; Ansari, M.A.; Babiker, A.Y.; Alsahli, M.A.; Almatroodi, S.A.; Rahmani, A.H. Protective Effect of Quercetin, a Flavonol against Benzo(a)pyrene-Induced Lung Injury via Inflammation, Oxidative Stress, Angiogenesis and Cyclooxygenase-2 Signalling Molecule. Appl. Sci. 2021, 11, 8675. [Google Scholar] [CrossRef]
- Ali, R.; Shahid, A.; Ali, N.; Hasan, S.K.; Majed, F.; Sultana, S. Amelioration of benzo [a] pyrene-induced oxidative stress and pulmonary toxicity by naringenin in Wistar rats: A plausible role of COX-2 and NF-κB. Hum. Exp. Toxicol. 2017, 36, 349–364. [Google Scholar] [CrossRef]
- Shahid, A.; Ali, R.; Ali, N.; Kazim Hasan, S.; Barnwal, P.; Mohammad Afzal, S.; Vafa, A.; Sultana, S. Methanolic bark extract of Acacia catechu ameliorates benzo(a)pyrene induced lung toxicity by abrogation of oxidative stress, inflammation, and apop-tosis in mice. Environ. Toxicol. 2017, 32, 1566–1577. [Google Scholar] [CrossRef]
- Khan, F.; Khan, T.J.; Kalamegam, G.; Pushparaj, P.N.; Chaudhary, A.; Abuzenadah, A.; Kumosani, T.; Barbour, E.; Al-Qahtani, M. Anti-cancer effects of Ajwa dates (Phoenix dactylifera L.) in diethylnitrosamine induced hepatocellular carcinoma in Wistar rats. BMC Complement. Altern Med. 2017, 17, 418. [Google Scholar] [CrossRef]
- Barnwal, P.; Vafa, A.; Afzal, S.M.; Shahid, A.; Hasan, S.K.; Alpashree; Sultana, S. Benzo(a)pyrene induces lung toxicity and inflammation in mice: Prevention by carvacrol. Hum. Exp. Toxicol. 2018, 37, 752–761. [Google Scholar] [CrossRef]
- Khan, S.; Naseem, I. A comparative insight into the oxidative damage and cell death potential of photoilluminated aminophylline—Riboflavin system in normal and cancer lung cells of swiss albino mice. Toxicol. In Vitro 2019, 61, 104651. [Google Scholar] [CrossRef]
- Shahid, A.; Ali, R.; Ali, N.; Kazim Hasan, S.; Rashid, S.; Majed, F.; Sultana, S. Attenuation of genotoxicity, oxidative stress, apoptosis and inflammation by rutin in benzo(a)pyrene exposed lungs of mice: Plausible role of NF-kappaB, TNF-alpha and Bcl-2. J. Complement. Integr. Med. 2016, 13, 17–29. [Google Scholar] [CrossRef]
- Islam, J.; Shree, A.; Afzal, S.M.; Vafa, A.; Sultana, S. Protective effect of Diosmin against benzo(a)pyrene-induced lung injury in Swiss Albino Mice. Environ. Toxicol. 2020, 35, 747–757. [Google Scholar] [CrossRef]
- Sabbah, M.F.; Alshubali, F.; Baothman, O.A.; Zamzami, M.A.; Shash, L.; Hassan, I.A. Cardioprotective Effect of Ajwa Date Aqueous Extract on Doxorubicin-Induced Toxicity in Rats. Biomed. Pharmacol. J. 2018, 11, 1521–1536. [Google Scholar] [CrossRef]
- Al-Yahya, M.; Raish, M.; AlSaid, M.S.; Ahmad, A.; Mothana, R.A.; Al-Sohaibani, M.; Al-Dosari, M.S.; Parvez, M.K.; Rafatullah, S. ‘Ajwa’ dates (Phoenix dactylifera L.) extract ameliorates isoproterenol-induced cardiomyopathy through downregulation of oxidative, inflammatory and apoptotic molecules in rodent model. Phytomedicine 2016, 23, 1240. [Google Scholar] [CrossRef]
- Oren, M. Decision making by p53: Life, death and cancer. Cell Death Differ. 2003, 10, 431–442. [Google Scholar] [CrossRef]
- Schuler, M.; Green, D.R. Transcription, apoptosis and p53: Catch-22. Trends Genet. 2005, 21, 182–187. [Google Scholar] [CrossRef]
- Miyashita, T.; Reed, J.C. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell 1995, 80, 293–299. [Google Scholar]
- Nakano, K.; Vousden, K.H. PUMA, a novel proapoptotic gene, is induced by p53. Mol. Cell 2001, 7, 683–694. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, L. The transcriptional targets of p53 in apoptosis control. Biochem. Biophys. Res. Commun. 2005, 331, 851–858. [Google Scholar] [CrossRef]
- Zhu, W.; Cromie, M.M.; Cai, Q.; Lv, T.; Singh, K.; Gao, W. Curcumin and vitamin E protect against adverse effects of benzo[a]pyrene in lung epithelial cells. PLoS ONE 2014, 9, e92992. [Google Scholar] [CrossRef]
- Suzuki, K.; Yanagihara, T.; Yokoyama, T.; Maeyama, T.; Ogata-Suetsugu, S.; Arimura-Omori, M.; Mikumo, H.; Hamada, N.; Harada, E.; Kuwano, K.; et al. Bax-inhibiting peptide attenuates bleomycin-induced lung injury in mice. Biol. Open 2017, 6, 1869–1875. [Google Scholar] [CrossRef]


| Group Number | Short Name | Treatment Plan |
|---|---|---|
| Group I | C | The normal saline solution was given orally to rats |
| Group II | B(a)P | Benzopyrene (B(a)P) in corn oil (50 mg/kg b.w.) [25] was given orally twice a week for 6 successive weeks |
| Group III | B(a)P +ADE | 1 g/kg b.w. of ADE was given orally daily for 6 weeks and given B(a)P in olive oil (50 mg/kg b.w.) 2 times/week for 6 continuous weeks |
| Group IV | ADE | 1 g/kg b.w. ADE was given orally daily for 6 weeks. |
| Preliminary Screening | Ajwa Date Extract |
|---|---|
| Dry weight of Ajwa fruit | 50 g |
| Yield | 2.89% |
| Extract | Ethanol |
| Color | Reddish brown |
| Odour | No specific |
| Texture | Sticky |
| Phytochemical Constituents | Fruit Pulp |
|---|---|
| Saponins | + |
| Alkaloids | + |
| Tannins | ND |
| Glycosides | + |
| Flavonoids | + |
| Phenolic compounds | + |
| Terpenoids | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almatroodi, S.A.; Khan, A.A.; Aloliqi, A.A.; Ali Syed, M.; Rahmani, A.H. Therapeutic Potential of Ajwa Dates (Phoenix dactylifera) Extract in Prevention of Benzo(a)pyrene-Induced Lung Injury through the Modulation of Oxidative Stress, Inflammation, and Cell Signalling Molecules. Appl. Sci. 2022, 12, 6784. https://doi.org/10.3390/app12136784
Almatroodi SA, Khan AA, Aloliqi AA, Ali Syed M, Rahmani AH. Therapeutic Potential of Ajwa Dates (Phoenix dactylifera) Extract in Prevention of Benzo(a)pyrene-Induced Lung Injury through the Modulation of Oxidative Stress, Inflammation, and Cell Signalling Molecules. Applied Sciences. 2022; 12(13):6784. https://doi.org/10.3390/app12136784
Chicago/Turabian StyleAlmatroodi, Saleh A., Amjad Ali Khan, Abdulaziz A. Aloliqi, Mansoor Ali Syed, and Arshad Husain Rahmani. 2022. "Therapeutic Potential of Ajwa Dates (Phoenix dactylifera) Extract in Prevention of Benzo(a)pyrene-Induced Lung Injury through the Modulation of Oxidative Stress, Inflammation, and Cell Signalling Molecules" Applied Sciences 12, no. 13: 6784. https://doi.org/10.3390/app12136784
APA StyleAlmatroodi, S. A., Khan, A. A., Aloliqi, A. A., Ali Syed, M., & Rahmani, A. H. (2022). Therapeutic Potential of Ajwa Dates (Phoenix dactylifera) Extract in Prevention of Benzo(a)pyrene-Induced Lung Injury through the Modulation of Oxidative Stress, Inflammation, and Cell Signalling Molecules. Applied Sciences, 12(13), 6784. https://doi.org/10.3390/app12136784







