Abstract
Echinacea purpurea (EP) has been widely used to treat upper respiratory infections, influenza, and the common cold. It can also exert various pharmacological activities, such as anti-inflammatory and anti-allergic effects. However, the potential of EP to modulate immune reactions remains unclear. Therefore, we evaluated the immunostimulatory effects of EP in cyclophosphamide (CP)-induced immunosuppressed mice. In this study, EP extract (12.5, 25, or 50 mg/kg) was orally administered to cyclophosphamide-induced immunosuppressed BALB/c mice. Then, indexes of immune organs, including the spleen and thymus, were recorded. Splenocyte proliferation and natural killer (NK) cell activities were measured by lactate dehydrogenase assay. Subsets of T cells, such as CD4+ and CD8+, were measured by flow cytometry, and immuno-cytokines, such as interleukin (IL)-2, tumor necrosis factor (TNF)-α, and interferon (IFN)-γ, were measured by enzyme-linked immunosorbent assay and real-time polymerase chain reaction. The immunosuppressed mice showed decreased thymus and spleen indexes and immune cell activities. Treatment of EP elevated the indexes of immune organs, splenocyte proliferation, and NK cell activities in CP-induced immunosuppressed mice. Simultaneously, administration of EP reversed the CP-induced decrease in T-lymphocyte subsets (CD4+ and CD8+) and immunocytokines (IL-2, TNF-α, and IFN-γ). Taken together, these findings suggest that EP could be used to enhance health and immunity in immunosuppressed conditions.
1. Introduction
Immunity is a physiological protective system of human bodies that distinguishes the ‘‘self” and ‘‘non-self” components thereof to maintain human health [1]. The largest lymphoid organ, which contains immune cells for immune responses, is the spleen [2,3,4]. Spleen is composed of lymphocytes, macrophages, dendritic cells, natural killer (NK) cells, and several immune cells, mediating immune responses [5]. Upon splenic lymphocytes’ activation, cytokines, such as IL-2, IL-6, and IFN-γ, as well as other inflammatory mediators, are released, initiating innate and adaptive immune responses [6]. NK cells, which act as primitive killers, play a major role in defending the host from cancer cells, bacteria, and virus-infected cells [7].
Generally, most anticancer chemotherapeutics drugs often cause many harmful side effects, such as immunosuppression and myelosuppression [8]. Cyclophosphamide (CP) is a commonly-used alkylating agent for the chemotherapeutic treatment of several cancers [9]. Administration of CP can generate excessive oxidative stresses via the disruption of redox signaling [10]. In addition, CP can cause an imbalance of Th1/Th2 responses and a reduction in the absolute number of T cells and B cells [11,12]. Therefore, many previous studies have used the Balb/c mice model with CP as an immunosuppressive model [13,14].
Many previous studies have approached new candidates for modulating immunoreaction [13,14,15] because the current clinical drugs or chemicals induce various adverse effects [16]. Thus, natural products and herbal medicines are receiving attention as alternative agents to regulate immunity or enhance people’s health [17,18]. Echinacea is a perennial flowering plant of the Asteraceae family, native to North America and commonly called purple coneflower [19]. Several plants of the Asteraceae family, including Cynara scolymus and Cichorium intybus, have already been reported to have immunostimulatory activities [20,21]. Echinacea purpurea (EP) is the most popular species in this genus and it has been used to treat the common cold, coughs, bronchitis and inflammation of the mouth and pharynx [22]. It also has been reported that the extracts of EP exert various biological functions, such as antibacterial, antioxidant, and anti-inflammatory activities [23,24,25]. However, no studies have reported on the immuno-enhancing activity of EP in CP-induced, immunosuppressed mice.
In this study, EP extract was investigated for its immunostimulatory activity in vivo. We evaluated the beneficial effects of EP against immune suppression by the chemotherapeutic agent CP in BALB/c mice and explored its mechanism of action.
2. Materials and Methods
2.1. Materials and Reagents
Cell culture products were from Gibco BRL (Grand Island, NE, USA). CP, concanavalin A (Con A) and lipopolysaccharide (LPS) were provided by Sigma-Aldrich (St. Louis, MO, USA). Cytokine ELISA kits and antibodies were purchased from eBioscience (San Diego, CA, USA).
2.2. Animals
All experiments were performed according to the protocols approved by the Animal Care Committee of Wonkwang University (WKU20-70). BALB/c mice (8 weeks of age) weighing 20 ± 2 g were purchased from OrientBio (Seongnam, KyongKiDo, Korea). They were bred and housed in standard shoebox cages in a climate-controlled room with an ambient temperature of 23 ± 2 °C and a 12-h light–dark cycle for 7 days. They were fed standard laboratory chow, provided with water ad libitum and randomly assigned to either control or experimental groups.
2.3. Preparation of EP Extract and High-Performance Liquid Chromatography (HPLC) Analysis
EP extract was supplied by NUON Co., Ltd., (Seongnam, Korea). The chemical standards analysis of EP extract was performed by HPLC using an OHAUS PIONNER (OHAUS, Parsippany, NJ, USA). The analytes were separated on a Waters Sunfire C18 (250 × 4.6-mm, I.D.S-5 μm, 12 nm) at 35 °C. The mobile phase was composed of 0.1% phosphoric acid in water (solvent A) and acetonitrile (solvent B). The flow rate was 1.5 mL/min and the injection volume was 10 μL. For quantitation, 10 mg of chicoric acid was dissolved in 20 mL of 70% ethanol. Then, 0.1 g of EP extract was diluted to 100 mL with 70% ethanol and filtered through a 0.45-mm membrane filter. We confirmed that the chicoric acid was included in the EP extract at approximately 2.0% by HPLC analysis.
2.4. CP-Induced Immunosuppression in Mice
A CP-induced immunosuppression model was established as previously described [26]. In brief, mice (n = 5 per group) were kept in specific pathogen-free animal facilities for one week before the start of experiments. One group, as the control group (normal group), was orally administered saline. The other groups were orally administered EP extract (12.5, 25 or 50 mg/kg), based on previous reports [27,28]. All groups were administered treatments once a day for 15 consecutive days. On days 10 and 12, all mice except the normal group intraperitoneally received 80 mg/kg of CP (Sigma-Aldrich, Burlington, MA, USA) once a day following the beginning of administration, and mice were sacrificed at 96 h after their last CP treatment. Mice were sacrificed via CO2 asphyxiation. The CO2 flow rate displaced 50% of the cage volume per minute. To ensure death following CO2 asphyxiation, cervical dislocation was also performed.
2.5. Immune Organ Indices
The spleens and thymuses of five mice were cleaned and weighed with an electronic balance, and the following index was calculated for each organ: index (mg/g) = immune organ weight (mg)/body weight (g).
2.6. Preparation of Spleen Cell Suspension
The spleens of the mice were minced in iced HBSS and then pressed gently through a 200-mesh metal sieve to remove major tissue aggregates. The filtrates were lysed with hemolysis solution (7 g/L NH4Cl and 2.6 g/L Tris-HCl) and centrifuged at 180× g for 10 min to remove erythrocytes, from which the spleen cell suspensions were finally obtained. The spleen cells were suspended in 1 mL of cell-culture medium (RPMI-1640 complete medium containing 10% v/v FBS, 100 U/mL of penicillin and 0.1 mg/mL of streptomycin), and an MTT assay was performed to determine cell viability [29].
2.7. Lymphocyte Proliferation Assay
An aliquot of 100 μL of the splenocyte suspension (5 × 105 cells/mL) was seeded into each well of a 96-well plate, along with either Con A (5 μg/mL) or LPS (10 μg/mL). After 24 h of preincubation at 37 °C in a humidified 5%-CO2 incubator, cell viability was determined by lactate dehydrogenase (LDH) assay [30].
2.8. Splenic Natural Killer (NK) Cell Activity
The activity of NK cells as effector cells in spleen was evaluated using YAC-1 cells as target cells (Korean Cell Line Bank, Seoul, Korea). Splenocytes and YAC-1 cells were co-cultured to gain an effector-to-target cell ratio of 100:1. After 4 h of co-culturing, the cultured supernatant was centrifuged at 400× g to remove contaminated cells. By using a LDH assay, the cultured supernatant was then mixed [30]. The absorbance at 490 nm of each sample was tested using a microplate reader (Molecular devices, San Jose, CA, USA), and NK cell activity was calculated according to the manufacturer’s instructions.
2.9. Splenic T-Lymphocyte Subpopulation Assay
For the splenic T-lymphocyte subpopulation assay, the splenocyte suspension was adjusted to a concentration of 1 × 106 cells/mL and mixed with 10 μL of either anti-CD4 or anti-CD8 antibody. After 1 h of incubation at 4 °C, the cells were washed twice with PBS, and then resuspended in 1% paraformaldehyde (PFA). CD4+ and CD8+ T-lymphocyte counts were determined using a flow cytometer (BD FACSCalibur, San Jose, CA, USA) and expressed as the percentages of the total counts of T lymphocytes [31].
2.10. Determination of Serum Cytokines
Blood samples were obtained from the samples by centrifugation at 3000 rpm for 3 min. The interleukin (IL)-2, tumor necrosis factor (TNF)-α, and interferon (IFN)-γ contents in the serum were assayed using ELISA kits from eBioscience (San Diego, CA, USA).
2.11. RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total RNA was isolated from the spleen using Trizol (Invitrogen, Carlsbad, CA, USA). The extracted concentration was measured by nanophotometer (Implen, München, Germany). The isolated RNA was used as a template to produce first-strand cDNA, using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster city, CA, USA). Subsequently, cDNA was amplified with a SYBR Premix kit (Applied Biosystems, USA) using a StepOne Plus Real-Time PCR system (Applied Biosystems, Foster city, CA, USA). The sequences of the primers used in this experiment were as follows: IL-2—forward (5′-CCT GAG CAG GGA GAA TTA CA-3′), reverse (5′-TCC AGA ACA TGC CGC AGA-3′); TNF-α—forward (5′-GTG GAA CTG GCA GAA GAG GC-3′), reverse (5′-AGA CAG AAG AGC GTG GTG GC-3′); IFN-γ—forward (5′-CGG CAC AG T CAT TGA AAG CCT A-3′), reverse (5′-CTT GTC TTT GAC CCA GTA GC-3′); and glyceraldehyde 3-phosphate dehydrogenase (GAPDH)—forward (5′-TTC ACC ACC ATG GAG AAG GC-3′), reverse (5′-GTT GCT GAT GGC CTG ATT GTC-3′). The amplification conditions included 30 s at 95 °C; 50 cycles at 95 °C for 5 s and 60 °C for 60 s each; dissociation for 15 s at 95 °C and 30 s at 60 °C; and then 15 s at 95 °C on a T ABI StepOnePlus. StepOne software (Applied Biosystems, Foster city, CA, USA) was used for data analysis. Relative gene expression (target gene expression normalized to the expression of the endogenous control gene) was calculated using the comparative Ct method (2−ΔΔCt). The analysis was conducted three times, independently.
2.12. Statistical Analysis
The results are expressed as means ± standard error of deviation (SD). The statistical significance of intergroup differences was evaluated using one-way analysis of variance, with dose as the variable, followed by a Duncan’s post-hoc test. All statistical analyses were performed using SPSS. Values of p < 0.05 were considered statistically significant. All experiments were carried out in triplicate.
3. Results
3.1. Fingerprint of EP Extract
To determine the standard composition of EP extract, we detected the chicoric acid content using HPLC. As shown in Figure 1, the chicoric acid content in the EP extract was approximately 2%, confirmed by comparison with the chromatogram of the standard (Figure 1). The optimized EP extract was used in all subsequent experiments.
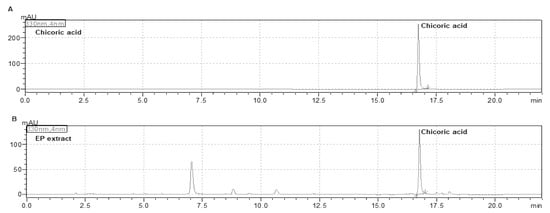
Figure 1.
High-performance liquid chromatography (HPLC) analysis of EP extract. HPLC chromatograms of (A) chicoric acid and (B) EP extract.
3.2. Effects of EP on Spleen and Thymus Indexes in the CP-Induced Immunosuppression Model
To examine the immunostimulatory effects of EP on an immunosuppressive mice model, mice were administered EP extract for 15 days and subsequently challenged with CP (Figure 2A). We found that indexes of representative immune organs, such as those of the spleen and thymus, were significantly decreased by intraperitoneal injection of CP (Figure 2B,C). However, these decreases in the CP-treated mice were significantly reversed by oral administration of EP extract in a dose-dependent manner (Figure 2B,C).
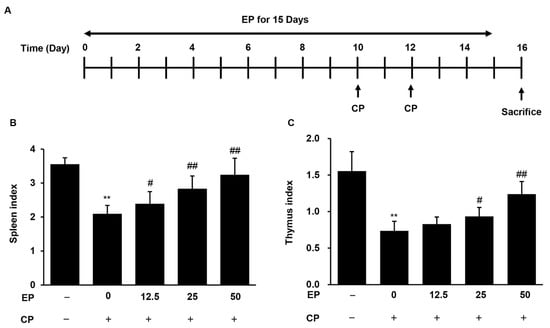
Figure 2.
Effects of EP on spleen and thymus indexes in CP-induced immunosuppressive mice. (A) The experimental scheme. The indexes of (B) spleen, and (C) thymus. Results are expressed as the mean ± SD of at least three independent experiments (n = 5). ** p < 0.01 vs. control group; # p < 0.05 and ## p < 0.01 vs. CP alone.
3.3. Effect of EP on Splenic Lymphocyte Proliferation in the CP-Induced Immunosuppression Model
To evaluate the proliferation of splenic lymphocytes, splenocytes were stimulated by either Con A or LPS for 24 h. Compared with the normal group, splenic lymphocyte proliferation, in response to both Con A and LPS, significantly decreased in the CP-treated group (Figure 3). However, the CP treatment enhanced the proliferation of splenic lymphocytes against Con A and LPS (Figure 3).
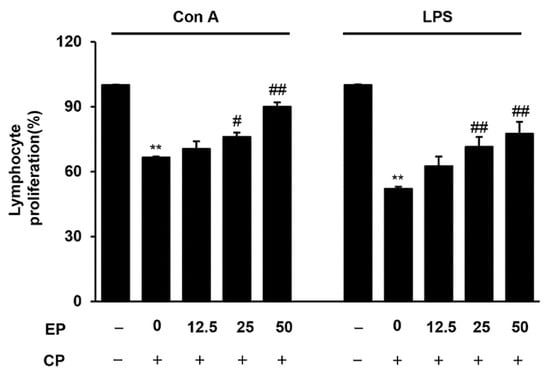
Figure 3.
Effects of EP on Con A or LPS-induced splenocyte proliferation in CP-induced immunosuppressive mice. The splenocytes were stimulated with Con A (5 μg/mL) or LPS (10 μg/mL) for 24 h. Then, proliferation was measured from cell viability with LDH assay. Results are expressed as the mean ± SD of at least three independent experiments (n = 5). ** p < 0.01 vs. control group; # p < 0.05 and ## p < 0.01 vs. CP alone.
3.4. Effect of EP on Splenic NK Cell Activity in the CP-Induced Immunosuppression Model
To evaluate the splenic NK cell activity, we used a co-culturing system of YAC-1 cells. The cultured cell supernatants were used to determine cytotoxicity via LDH assay. As shown in Figure 4, splenic NK cell activity was suppressed by CP treatment as compared with normal mice. However, EP treatment markedly recovered the NK cell activity of splenocytes in CP-treated mice (Figure 4).
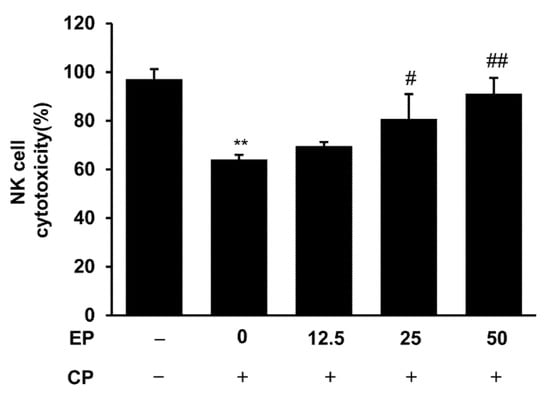
Figure 4.
Effects of EP on splenic NK cell cytotoxic activity in CP-induced immunosuppressive mice. Splenocytes were co-cultured with YAK-1 cells for the measurement of NK cell activity. Then, the cytotoxic activity of NK cells was calculated as the survival rate of YAK-1 cells compared with that of control cells. Results are expressed as the mean ± SD of at least three independent experiments (n = 5). ** p < 0.01 vs. control group; # p < 0.05 and ## p < 0.01 vs. CP alone.
3.5. Effect of EP on Splenic T-Lymphocyte Subpopulations in the CP-Induced Immunosuppression Model
As EP could regulate splenic lymphocyte cell proliferation (Figure 3), we also examined the splenic T-lymphocyte subsets using flow cytometry. As seen in Figure 5, the percentages of CD4+ T cells (helper T lymphocytes, Th cells) and CD8+ T cells (killer T lymphocytes, Tc cells) were strikingly decreased in the CP-treated group. However, treatment with EP significantly recovered the percentages of CD4+ and CD8+ cells (Figure 5).
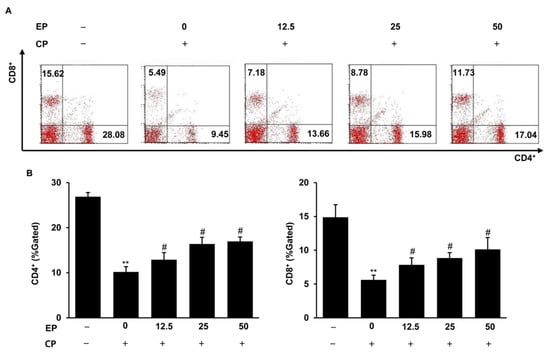
Figure 5.
Effects of EP on splenic T-lymphocyte subsets in CP-induced immunosuppressive mice. (A) Splenic T-lymphocyte subsets (CD4+/CD8+) were detected by flow cytometry. (B) Splenic T-lymphocyte subpopulation, analyzed by flow cytometry in the CP-treated mice. Results are expressed as the mean ± SD of at least three independent experiments (n = 5).** p < 0.01 vs. control group; # p < 0.05 vs. CP alone.
3.6. Effect of EP on Production of Immuno-Cytokines in the CP-Induced Immunosuppression Model
The production of immune-associated cytokines, such as IL-2, TNF-α and IFN-γ, in the serum and spleen were evaluated by ELISA and qRT-PCR. Figure 6 shows that the circulating levels and splenic mRNA levels of IL-2, TNF-α and IFN-γ were significantly reduced in the CP-treated group (Figure 6A–C). However, EP administration increased the immuno-cytokines in both serum and spleen against CP challenges (Figure 6).
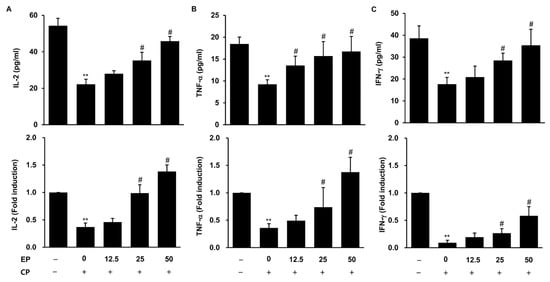
Figure 6.
Effects of EP on the production of immuno-cytokines in CP-induced immunosuppressive mice. The serum levels of cytokines, quantified by ELISA, and splenic cytokine expressions were measured by qRT-PCR. (A) Serum and splenic IL-2. (B) Serum and splenic TNF-α. (C) Serum and splenic IFN-γ. Results are expressed as the mean ± SD of at least three independent experiments (n = 5). ** p < 0.01 vs. control group; # p < 0.05 vs. CP alone.
4. Discussion
Immunosuppression is a temporal or permanent lack of immunity and can sensitize the body to outer pathogens [13]. Thus, it is an active area of interest for discovering immune-enhancing agents of medicinal plant origins for the treatment of immunosuppressive diseases [32]. Clinically, immunostimulators could be used in infectious diseases, tumors, immunodeficiency diseases and so on [33]. In this study, we focused on EP as an immune-enhancing agent against immunosuppression. From the previous reports of its anti-inflammatory and anti-allergic effects [34,35], we could speculate on the immunomodulatory properties of EP. In this study, we firstly showed that EP treatment could recover the immunosuppression caused by CP. In detail, EP treatment reversed CP-induced reductions in immuno-organ indexes, splenic lymphocyte proliferation and NK cell activity. Furthermore, serum and splenic cytokines were recovered by EP treatment against CP-induced immunosuppression. These findings indicate that EP could restore the immune response caused by CP.
CP is an important chemotherapeutic drug used widely in tumor treatment, but it is not beneficial for healthy cells and has pronounced side effects, such as bone marrow suppression, immunosuppression and oxidative stress [36]. In addition, CP challenge typically can cause damage and weight loss in the spleen and thymus, which, as representative immuno-organs, play an important role in maintaining the body’s immune homeostasis [37]. Thus, to evaluate the immunostimulatory activity of EP in a CP-induced immunosuppressive mice model, we examined the increase in the weights of these immuno-organs. In this study, in accordance with previous reports [38], the indexes of spleen and thymus were decreased by CP (Figure 2). Several studies have considered whether immune system activators could increase spleen and thymus weight in CP-induced immunosuppressive mice [39,40]. Similarly, the reduction of weight in both organs was alleviated by EP treatment, which suggests that EP could resist immunosuppressive effects in immune-organ development (Figure 2).
Lymphocyte proliferation is a fatal event in the activation cascade of immune responses [41]. However, lymphocyte proliferation could be interfered with by CP treatment to abolish the immune responses [42]. The consequence of the side effects of CP on the monocytes and macrophages that regulate the functions of lymphocytes leads to the reduced proliferative capacities of T- and B-lymphocyte populations [43]. Thus, to assess the immunostimulatory activity of EP on lymphocytes, we investigated the ConA- and LPS-induced splenocyte proliferation to evaluate T- and B-lymphocyte proliferation activities. In the present study, in accordance with previous reports [41], the proliferation of T and B lymphocytes was up- or downregulated by Con A and LPS, respectively (Figure 3). However, treatment of EP promoted T- and B-lymphocyte proliferation against the CP challenge, which suggests EP exhibits immunostimulatory activity in splenic lymphocytes, especially in T and B lymphocytes (Figure 3).
Another type of splenic lymphocyte, NK cells, can kill foreign and abnormal cells, and plays a pivotal role in the immune response [44]. NK cells negatively regulate tumor growth and metastasis, as well as infection by harmful viruses [44]. Thus, assessment of NK cell activity could be a useful method for measuring immune response capacity of [39]. In this study, splenic NK cell cytotoxic activity was significantly decreased by CP, which means CP caused the malfunction of NK cells (Figure 4). However, treatment with EP restored NK cell activity against the CP challenge, which suggests EP could contribute to the regulation of NK cell activity (Figure 4).
T lymphocytes are the essential regulatory cells of the adaptive immune system [45]. T cells are defined by their surface receptor expressions and are comprise several subtypes, such as T helper (Th) cells, cytotoxic T cells, and so on. CD4+ T cells and CD8+ T cells are the T-helper (Th) and T-cytotoxic (Tc) lymphocytes, respectively, that regulate the immune system via the release of immuno-cytokines or via direct cytotoxic effects [46]. Helper CD4+ T cells could be divided into two subsets by determining if and how other parts of the immune system respond to a specific, recognized risk. Type 1 T helper (Th1) cells mainly control the cell-mediated immune responses, while Type 2 T helper (Th2) cells mainly manage humoral or allergic responses by releasing immuno-cytokines [45]. Immuno-cytokines can not only determine the functions of cell subsets but also participate in the activation and proliferation of the corresponding cell subsets and have mutually antagonistic functions [45]. Generally, Th1 cells control the regulation of IL-2, IFN-γ and TNF-α, while Th2 cells control the regulation of IL-4 and IL-13 [47]. Thus, to evaluate the immunostimulatory activity of EP in a CP-induced immunosuppressive mice model, we examined the T-cell subsets and production of immuno-cytokines. Our results show that EP was able to significantly reverse the CP-induced decrease of CD4+ and CD8+ T cells (Figure 5), or the decline of Th1-type cytokines (IL-2, TNF-α and IFN-γ) (Figure 6), indicating that EP possesses the capability to modulate T-cell activation. Although we did not examine the Th2 cytokines here, we could speculate that EP could regulate allergic responses and strengthen the Th2 immune responses from a previous study [35]. Therefore, we confirmed that EP could increase T-cell proliferation, and, subsequently, stimulated Th cytokines to regulate and restore immunosuppression.
The Echinacea genus is valuable for its healthy properties, such as anti-inflammation and immunostimulation [48]. Among them, EP is the one of the most known medical plant because of its unique chemical composition, including volatile chemical components, caffeic acid derivates (chicoric acid), sesquiterpenes and polysaccharides, which have been reported to play pharmacological roles, having immunostimulatory, anti-inflammatory and antioxidant effects [49]. Thus, these compounds probably contributed to the immunostimulating effects of EP in the immunosuppressed mice. Chicoric acid, the standard compound used in this paper (Figure 1), is reported to have a strong immunostimulatory activity in chronically stressed mice [50]. Therefore, we could suggest that chicoric acid might be responsible for the immunostimulatory activity of EP, at least partially. However, because this is just a hypothesis, further investigation is needed to determine whether chicoric acid/or other constituents are actually responsible, synergistically, in this model.
5. Conclusions
In summary, we evaluated the immunomodulatory effect of EP in a CP-induced immunosuppressed mice model. EP treatment improved the spleen and thymus indexes against a CP challenge. In addition, EP treatment increased splenocyte proliferation and NK cell activity. More importantly, EP administration increased the T-cell population and cytokines in the CP-treated mice. These findings suggest that EP could function as an effective immunostimulatory agent in in patients with immunosuppression induced by the chemotherapy.
Author Contributions
G.-S.B. and K.-B.K. designed the study. H.-R.K., Y.-S.K., D.-R.L. and B.-K.C. performed the experiments and analyzed the data. G.-S.B. and K.-B.K. drafted the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Wonkwang University in 2020.
Institutional Review Board Statement
All experiments were performed according to the protocols approved by the Animal Care Committee of Wonkwang University (WKU20-70).
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Viladomiu, M.; Hontecillas, R.; Bassaganya-Riera, J. Modulation of inflammation and immunity by dietary conjugated linoleic acid. Eur. J. Pharmacol. 2016, 785, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Balogh, P.; Horvath, G.; Szakal, A.K. Immunoarchitecture of distinct reticular fibroblastic domains in the white pulp of mouse spleen. J. Histochem. Cytochem. 2004, 52, 1287–1298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nolte, M.A.; Hamann, A.; Kraal, G.; Mebius, R.E. The strict regulation of lymphocyte migration to splenic white pulp does not involve common homing receptors. Immunology 2002, 106, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Lori, A.; Perrotta, M.; Lembo, G.; Carnevale, D. The spleen: A hub connecting nervous and immune systems in cardiovascular and metabolic diseases. Int. J. Mol. Sci. 2017, 18, 1216. [Google Scholar] [CrossRef] [PubMed]
- Mebius, R.E.; Kraal, G. Structure and function of the spleen. Nat. Rev. Immunol. 2005, 5, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Im, S.A.; Kim, K.; Lee, C.K. Immunomodulatory activity of polysaccharides isolated from Salicornia herbacea. Int. Immunopharmacol. 2006, 6, 1451–1458. [Google Scholar] [CrossRef]
- Spiering, M.J. Primer on the immune system. Alcohol. Res. 2015, 37, 171–175. [Google Scholar]
- Pass, G.J.; Carrie, D.; Boylan, M.; Lorimore, S.; Wright, E.; Houston, B.; Henderson, C.J.; Wolf, C.R. Role of hepatic cytochrome p450s in the pharmacokinetics and toxicity of cyclophosphamide: Studies with the hepatic cytochrome p450 reductase null mouse. Cancer Res. 2005, 65, 4211–4217. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, D.N.; Jena, G.B. Intervention of astaxanthin against cyclophosphamide-induced oxidative stress and DNA damage: A study in mice. Chem. Biol. Interact. 2009, 80, 398–406. [Google Scholar] [CrossRef]
- Pratheeshkumar, P.; Kuttan, G. Ameliorative action of Vernonia cinerea L. on cyclophosphamide-induced immunosuppression and oxidative stress in mice. Inflammopharmacology 2010, 18, 197–207. [Google Scholar] [CrossRef]
- Rabinovitch, A.; Sorensen, O.; Suarez-Pinzon, W.L.; Power, R.F.; Rajotte, R.V.; Bleackley, R.C. Analysis of cytokine mRNA expression in syngeneic islet grafts of NOD mice: Interleukin 2 and interferon gamma mRNA expression correlate with graft rejection and interleukin 10 with graft survival. Diabetologia 1994, 37, 833–837. [Google Scholar] [CrossRef]
- Yu, Q.; Nie, S.P.; Wang, J.Q.; Huang, D.F.; Li, W.J.; Xie, M.Y. Molecular mechanism underlying chemoprotective effects of Ganoderma atrum polysaccharide in cyclophosphamide-induced immunosuppressed mice. J. Funct. Foods 2015, 15, 52–60. [Google Scholar] [CrossRef]
- Qi, Q.; Dong, Z.; Sun, Y.; Li, S.; Zhao, Z. Protective Effect of Bergenin against Cyclophosphamide-Induced Immunosuppression by Immunomodulatory Effect and Antioxidation in Balb/c Mice. Molecules 2018, 23, 2668. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.E.; Monmai, C.; Rod-In, W.; Jang, A.Y.; You, S.G.; Lee, S.M.; Park, W.J. Immune Enhancement Effects of Codium fragile Anionic Macromolecules Combined with Red Ginseng Extract in Immune-Suppressed Mice. J. Microbiol. Biotechnol. 2019, 17, 1213–1216. [Google Scholar] [CrossRef]
- Gao, H.Y.; Li, G.Y.; Huang, J.; Han, Y.; Sun, F.Z.; Du, X.W.; An, L.J.; Wang, H.Y.; Wang, J.H. Protective effects of Zhuyeqing liquor on the immune function of normal and immunosuppressed mice in vivo. BMC Complement. Altern. Med. 2013, 13, 252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markman, M. Chemotherapy-associated neurotoxicity: An important side effect-impacting on quality, rather than quantity, of life. J. Cancer Res. Clin. Oncol. 1996, 122, 511–512. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.; Silván, B.; Entrialgo-Cadierno, R.; Villar, C.J.; Capasso, R.; Uranga, J.A.; Lombó, F.; Abalo, R. Antiproliferative and palliative activity of flavonoids in colorectal cancer. Biomed. Pharmacother. 2021, 143, 112241. [Google Scholar] [CrossRef]
- Küpeli Akkol, E.; Genç, Y.; Karpuz, B.; Sobarzo-Sánchez, E.; Capasso, R. Coumarins and Coumarin-Related Compounds in Pharmacotherapy of Cancer. Cancers 2020, 12, 1959. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.; Anderson, L.A.; Gibbons, S.; Phillipson, J.D. Echinacea species (Echinacea angustifolia (DC.) Hell., Echinacea pallida (Nutt.) Nutt., Echinacea purpurea (L.) Moench): A review of their chemistry, pharmacology and clinical properties. J. Pharm. Pharmacol. 2005, 57, 929–954. [Google Scholar] [CrossRef] [Green Version]
- Hueza, I.M.; Gotardo, A.T.; da Silva Mattos, M.I.; Górniak, S.L. Immunomodulatory effect of Cynara scolymus (artichoke) in rats. Phytother. Res. 2019, 33, 167–173. [Google Scholar] [CrossRef] [Green Version]
- Karimi, M.H.; Ebrahimnezhad, S.; Namayandeh, M.; Amirghofran, Z. The effects of cichorium intybus extract on the maturation and activity of dendritic cells. DARU J. Pharm. Sci. 2014, 22, 28. [Google Scholar] [CrossRef] [Green Version]
- Hohmann, J.; R’edei, D.; Forgo, P.; Szab´o, P.; Freund, T.F.; Haller, J.; Bojnik, E.; Benyhe, S. Alkamides and a neolignan from Echinacea purpurea roots and the interaction of alkamides with G-protein-coupled cannabinoid receptors. Phytochemistry 2011, 72, 1848–1853. [Google Scholar] [CrossRef]
- Woelkart, K.; Bauer, R. The role of alkamides as an active principle of echinacea. Planta Med. 2007, 73, 615–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, M.; Anderson, S.A.; Schoop, R.; Hudson, J.B. Induction of multiple pro-inflammatory cytokines by respiratory viruses and reversal by standardized Echinacea, a potent antiviral herbal extract. Antivir. Res. 2009, 83, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Todd, D.A.; Gulledge, T.V.; Britton, E.R.; Oberhofer, M.; Leyte-Lugo, M.; Moody, A.N.; Shymanovich, T.; Grubbs, L.F.; Juzumaite, M.; Tyler, N.G.; et al. Ethanolic Echinacea purpurea extracts contain a mixture of cytokine-suppressive and cytokine-inducing compounds, including some that originate from endophytic bacteria. PLoS ONE 2015, 10, e0124276. [Google Scholar]
- Lee, H.H.; Cho, Y.; Kim, G.H.; Cho, H. Undaria pinnatifida Fucoidan-Rich Extract Recovers Immunity of Immunosuppressed Mice. J. Microbiol. Biotechnol. 2020, 30, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Abouelella, A.M.; Shahein, Y.E.; Tawfik, S.S.; Zahran, A.M. Phytotherapeutic effects of Echinacea purpurea in gamma-irradiated mice. J. Vet. Sci. 2007, 8, 341–351. [Google Scholar] [CrossRef] [Green Version]
- Raso, G.M.; Pacilio, M.; Di Carlo, G.; Esposito, E.; Pinto, L.; Meli, R. In-vivo and in-vitro anti-inflammatory effect of Echinacea purpurea and Hypericum perforatum. J. Pharm. Pharmacol. 2002, 54, 1379–1383. [Google Scholar] [CrossRef]
- Gao, T.; Bi, H.; Ma, S.; Lu, J. The antitumor and immunostimulating activities of water soluble polysaccharides from Radix Aconiti, Radix Aconiti Lateralis and Radix Aconiti Kusnezoffii. Nat. Prod. Commun. 2010, 5, 447–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.R.; Lee, H.S.; Cho, S.Y.; Kim, Y.S.; Shin, K.S. Antimetastatic effect of polysaccharide isolated from Colocasia esculenta is exerted through immunostimulation. Int. J. Mol. Med. 2013, 31, 361–368. [Google Scholar] [CrossRef] [Green Version]
- Qi, Z.; Chen, L.; Li, Z.; Shao, Z.; Qi, Y.; Gao, K.; Liu, S.; Sun, Y.; Li, P.; Liu, J. Immunomodulatory effects of (24R)-Pseudo-Ginsenoside HQ and (24S)-Pseudo- Ginsenoside HQ on cyclophosphamide-induced immunosuppression and their anti-tumor effects study. Int. J. Mol. Sci. 2019, 20, 836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.; Conlon, M.; Ren, W.; Chen, B.B.; Baczek, T. Natural products as targeted modulators of the immune system. J. Immunol. Res. 2018, 2018, 7862782. [Google Scholar] [CrossRef] [PubMed]
- Asherson, R.A.; Gunter, K.; Daya, D.; Shoenfeld, Y. Multiple Autoimmune Diseases in a Young Woman: Tuberculosis and Splenectomy as Possible Triggering Factors? Another Example of the “Mosaic” of Autoimmunity. J. Rheumatol. 2008, 35, 1224–1227. [Google Scholar] [PubMed]
- Cheng, Z.Y.; Sun, X.; Liu, P.; Lin, B.; Li, L.Z.; Yao, G.D.; Huang, X.X.; Song, S.J. Sesquiterpenes from Echinacea purpurea and their anti-inflammatory activities. Phytochemistry 2020, 179, 112503. [Google Scholar] [CrossRef]
- Zorig, A.; Toko, R.; Sukhbold, E.; Takasugi, M.; Arai, H. Echinacea purpurea water extracts suppress the release of chemical mediators from mast cells. Biosci. Biotechnol. Biochem. 2021, 85, 931–940. [Google Scholar] [CrossRef]
- Wang, H.; Wang, M.; Chen, J.; Tang, Y.; Dou, J.; Yu, J.; Xi, T.; Zhou, C. A polysaccharide from Strongylocentrotus nudus eggs protects against myelosuppression and immunosuppression in cyclophosphamide-treated mice. Int. Immunopharmacol. 2011, 11, 1946–1953. [Google Scholar] [CrossRef] [PubMed]
- Tanahashi, T.; Sekiguchi, N.; Matsuda, K.; Matsumoto, A.; Ito, T.; Nakazawa, H.; Ishida, F. A screening method with lymphocyte percentage and proportion of granular lymphocytes in the peripheral blood for large granular lymphocyte (LGL) leukemia. Int. J. Hematol. 2017, 105, 87–91. [Google Scholar] [CrossRef]
- Cui, H.L.; Chen, Y.; Wang, S.S.; Kai, G.Q.; Fang, Y.M. Isolation, partial characterisation and immunomodulatory activities of polysaccharide from Morchella esculenta. J. Sci. Food Agric. 2011, 91, 2180–2185. [Google Scholar] [CrossRef]
- Meng, Y.; Li, B.; Jin, D.; Zhan, M.; Lu, J.; Huo, G. Immunomodulatory activity of Lactobacillus plantarum KLDS1.0318 in cyclophosphamide-treated mice. Food Nutr. Res. 2018, 62, 1269. [Google Scholar] [CrossRef]
- Kawabata, T.T.; White, K.L. Suppression of the vitro humoral immune response of mouse splenocytes by benzo(a)pyrene metabolites and inhibition of benzo(a)pyrene-induced immunosuppression by alpha-naphthoflavone. Cancer Res. 1987, 47, 2317–2322. [Google Scholar]
- Wang, J.; Tong, X.; Li, P.; Cao, H.; Su, W. Immuno-enhancement effects of shenqi fuzheng injection on cyclophosphamide-induced immunosuppression in BALB/c mice. J. Ethnopharmacol. 2014, 139, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Lu, Y.; Wang, D.; Liu, J.; Song, X.; Zhang, W.; Zhao, X.; Nguyen, T.L.; Hu, Y. Effect of epimedium polysaccharide-propolis flavone immunopotentiator on immunosuppression induced by cyclophosphamide in chickens. Cell. Immunol. 2013, 281, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Duggina, P.; Kalla, C.M.; Varikasuvu, S.R.; Bukke, S.; Tartte, V. Protective effect of centella triterpene saponins against cyclophosphamide-induced immune and hepatic system dysfunction in rats: Its possible mechanisms of action. J. Physiol. Biochem. 2015, 71, 435–454. [Google Scholar] [CrossRef]
- Kos, F.J.; Engleman, E.G. Immune regulation: A critical link between NK cells and CTLs. Immunol. Today 1996, 17, 174–176. [Google Scholar] [CrossRef]
- Constant, S.L.; Bottomly, K. Induction of Th1 and Th2 CD4+ T cell responses: The alternative approaches. Annu. Rev. Immunol. 1997, 15, 297–322. [Google Scholar] [CrossRef]
- Zhu, X.L.; Chen, A.F.; Lin, Z.B. Ganoderma lucidum polysaccharides enhance the function of immunological effector cells in immunosuppressed mice. J. Ethnopharmacol. 2007, 111, 219–226. [Google Scholar] [CrossRef]
- Mosmann, T.R.; Coffman, R.L. TH1 and TH2 cells: Different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 1989, 7, 145–173. [Google Scholar] [CrossRef] [PubMed]
- Erenler, R.; Telci, I.; Ulutas, M.; Demirtas, I.; Gul, F.; Elmastas, M.; Kayir, O. Chemical constituents, quantitative analysis and antioxidant activities of Echinacea purpurea (L.) Moench and Echinacea pallida (Nutt.) Nutt. J. Food Biochem. 2015, 39, 622–630. [Google Scholar] [CrossRef]
- Chiellini, C.; Maida, I.; Maggini, V.; Bosi, E.; Mocali, S.; Emiliani, G.; Perrin, E.; Firenzuoli, F.; Mengoni, A.; Fani, R. Preliminary data on antibacterial activity of Echinacea purpurea-associated bacterial communities against Burkholderia cepacia complex strains, opportunistic pathogens of Cystic Fibrosis patients. Microbiol. Res. 2017, 196, 34–43. [Google Scholar] [CrossRef]
- Park, S.; Lee, M.S.; Jung, S.; Lee, S.; Kwon, O.; Kreuter, M.H.; Perrinjaquet-Moccetti, T.; Min, B.; Yun, S.H.; Kim, Y. Echinacea purpurea Protects Against Restraint Stress-Induced Immunosuppression in BALB/c Mice. J. Med. Food 2018, 21, 261–268. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).