Featured Application
Two strains from high-altitude lakes showed an exciting capacity to produce carbohydrates, proteins, and lipids under an optimized C/N/P ratio.
Abstract
This study evaluated the role of C/N/P in the increase in the synthesis of carbohydrates, proteins, and lipids in two high-mountain strains of algae (Chlorella sp. UFPS019 and Desmodesmus sp. UFPS021). Three carbon sources (sodium acetate, sodium carbonate, and sodium bicarbonate), and the sources of nitrogen (NaNO3) and phosphate (KH2PO4 and K2HPO4) were analyzed using a surface response (3 factors, 2 levels). In Chlorella sp. UFPS019, the optimal conditions to enhance the synthesis of carbohydrates were high sodium carbonate content (3.53 g/L), high KH2PO4 and K2HPO4 content (0.06 and 0.14 g/L, respectively), and medium-high NaNO3 (0.1875 g/L). In the case of lipids, a high concentration of sodium acetate (1.19 g/L) coupled with high KH2PO4 and K2HPO4 content (0.056 and 0.131 g/L, respectively) and a low concentration of NaNO3 (0.075 g/L) drastically induced the synthesis of lipids. In the case of Desmodesmus sp. UFPS021, the protein content was increased using high sodium acetate (2 g/L), high KH2PO4 and K2HPO4 content (0.056 and 0.131 g/L, respectively), and high NaNO3 concentration (0.25 g/L). These results demonstrate that the correct adjustment of the C/N/P ratio can enhance the capacity of high-mountain strains of algae to produce high concentrations of carbohydrates, proteins, and lipids.
1. Introduction
Rapid population growth has caused a rise in demand for food and energy [1], thus keeping greenhouse gas emissions (CH4, CO2, CO, NOX, SOX, and others) at high levels [2,3] and adding to the severe threat of climate change [4,5]. Therefore, to achieve the sustainable development of human society, it is necessary to identify and exploit new renewable sources that will contribute to the development of food and energy processes [6,7,8].
In recent years, microalgae and cyanobacteria have been established as sustainable sources of carbohydrates, proteins, lipids, secondary metabolites, and minerals of great interest to the cosmetic, energy, pharmaceutical, and food industries [9,10,11,12,13,14]. Microalgae cultivation is a promising alternative to combine anthropogenic emissions with the production of raw materials for the energy, food, pharmaceutical, and cosmetic industries [15]. The use of microalgae presents significant advantages over terrestrial crops, including high content of target products: up to 60% protein [16,17,18,19], between 20–40% carbohydrates (mainly starch) [20,21,22], and between 20–50% lipids [23,24,25,26]. Similarly, the ability of these systems to use wastewater as a nutrient source, along with their low soil requirements and high cell growth rates, have positioned them as promising alternatives to producing multiple feedstocks [27,28,29,30,31]. This is due to the rapid maturation of production technologies and the international market for specialized metabolites [32].
The number of industrially produced strains is limited to a handful of genera and species such as Chlorella sp. (Chlorophyta) [22,33,34,35,36], Dunaliella salina (Chlorophyta) [37,38,39], Haematococcus lacustris (formerly Haematococcus pluvialis) (Chlorophyta) [40,41,42,43], Nannochloropsis sp. (Ochrophyta, Eustigmatophyceae) [44,45,46,47,48], Phaeodactylum tricornutum (Bacillariophyta) [49,50,51,52,53,54,55,56], Porphyridium sp. (Rhodophyta) [57,58,59], Scenedesmus sp. (Chlorophyta) [60,61,62,63], Spirulina (Arthorspira) sp. (Cyanobacteria) [62,63,64,65,66,67,68,69,70], Tisochrysis lutea (Haptophyta, Coccolithophyceae) [71,72,73,74,75], and Tetraselmis sp. (Chlorophyta) [72,73,74,75,76,77,78,79,80]. However, the most popular genus is Spirulina (Arthorspira) sp. According to Araújo et al. [81], in Europe, 222 different companies (57% of the total worldwide) are devoted to the production of Spirulina biomass, with an annual production of up to 143 tons.
The proper selection of microalgae strains is a key aspect in the utilization and cost reduction of biotechnological processes since native strains present greater adaptation to the environmental conditions of local ecosystems [82,83]. High mountain lakes are remote and extreme ecosystems subject to harsh climatic conditions, characterized by low temperatures and high solar and ultraviolet radiation (UVR) [84]. These extreme conditions can give them unique characteristics such as tolerance to high radiation, high plasticity to sudden temperature changes, and the ability to deposit high concentrations of metabolites (carbohydrates, proteins, lipids, etc.). Studies investigating species diversity in continuous water bodies such as rivers, wetlands, and high mountain lakes have increased, reflecting the interest in and recognition of these habitats [85,86,87]. However, these types of strains are poorly represented in the scientific literature.
Culture parameter optimization is a key point in the improvement of industrial algal production. Both culture media and operational conditions must be “tailored-made” for individual strains to maximize their productivity [88]. One available tool is the application of the design of experiments (DoE) [88,89,90,91,92] to improve concentrations of specific metabolites. This type of experiment has been successfully applied to improve the deposition of specific metabolites in algae and cyanobacteria before [93,94,95,96,97,98], especially in the selection of the carbon and nitrogen sources and their specific concentrations. However, to the best of our knowledge, there is no scientific literature on the optimization of culture media for strains from high-mountain environments. Therefore, the present study aimed to determine the optimal C/N/P ratio that enhances the concentration of carbohydrates, proteins, lipids, and carotenoids in two algal strains isolated from high-mountain lakes.
2. Materials and Methods
2.1. Strains
Chlorella sp. (CHLO_UFPS019) and two strains of Desmodesmus sp. (DESM_UFPS020 and UFPS021) were isolated from lakes located at 3300–3900 M.A.S.L. in the region of Norte de Santander (Colombia) and have shown an interesting capability to produce large concentrations of carbohydrates, proteins, lipids, and carotenoids (data not shown).
The identities of the strains were determined by internal transcribed spacer (ITS) gene analysis. DNA was extracted according to the CTAB-NaCl protocol [99]. The ITS gene was amplified by polymerase chain reaction (PCR) using ITS1F ITS4R primers [100] following the conditions described by Fei et al. [101]. The sequences were compared with ITS gene sequences available in GenBank databases, showing that all the strains had a high percentage of identity with their respective genera (99.2% for Desmodesmus sp. UFPS16, 99.46% for Desmodesmus sp. UFPS18, and 98.85% for Chlorella sp. UFPS14).
The strains are kept in the INNOValgae collection (Universidad Francisco de Paula Santander, Colombia), and were cultured in a 2 L tubular glass flask with 1.3 L of Bold’s basal medium [102] at pH 7.0. Each flask was mixed through the injection of filtered air with 1% (v/v) CO2 at a flow rate of 0.78 L min−1, with a light:dark cycle of 12:12 h at 100 µmol m−2 s−1, at 27 °C for 30 days.
2.2. Experimental Design
The optimization of carbohydrates (analyzed in Chlorella sp. UFPS019), proteins (analyzed in Desmodesmus sp. UFPS021), lipids (analyzed in Chlorella sp. UFPS019), and carotenoids (analyzed in Desmodesmus sp UFPS020) through the adjustment of the carbon/nitrogen/phosphate ratio was evaluated (in triplicate) using a central non-factorial response surface design with three central points and three blocks in the software STATISTICA 7.0 (Statsoft). The central points permitted a statistical check for the goodness-of-fit of the factorial model. Sodium carbonate (Na2CO3), sodium bicarbonate (NaHCO3), and sodium acetate (NaC2H3O2) were tested to identify the carbon source that maximized the production of the metabolites. The variables and their levels can be found in Table 1. The concentrations of NaNO3 (0.25 g/L), K2HPO4 (0.075 g/L), and KH2PO4 (0.175 g/L) were adjusted following the content of each nutrient in Bold’s basal medium [102]. The final concentration of each carbon source was calculated according to its relative carbon content.

Table 1.
Variables evaluated with their respective levels.
For each experiment, the C/N/P ratio was adjusted in 300 mL of Bold’s basal medium [103] at pH 7.0 according to the resolved design (Table 2). Each flask was attached to a gas line with an airflow of 0.18 L min−1 (Resun, LP-100, Aarau, Switzerland) without the addition of extra CO2, and a light:dark cycle of 12:12 h at 100 µmol m−2 s−1 at 27 °C. To produce carbohydrates and proteins, the strains were grown for 20 days, while to produce lipids and carotenoids, the strains were cultured for up to 40 days. In the case of carotenoids, the light intensity was increased up to 250 µmol m−2 s−1, with a light:dark cycle of 12:12 h at 27 °C. The pH was monitored twice a day and adjusted to pH 7.0 ± 0.5 on a laminar flow cabinet using sterile solutions of either NaOH (1 M) or HCl (1 M).

Table 2.
Resolved design of experiments.
2.3. Quantification of Biomass and Metabolites
The biomass produced was concentrated using a lab-scale electroflotation device [104], washed thrice with distilled water, reconcentrated by centrifugation (2876× g, 20 °C, 20 min), lyophilized, and stored (4 °C) until use. Finally, the different components of the strains were measured in triplicate.
For carbohydrates, 5 mg of grounded biomass were mixed with 5 mL of 1 M H2SO4 in acid-resistant test tubes, vortexed (Multi Reax, Heidoplh, Schwabach, Germany) at 1500 rpm for 10 min, and incubated (100 °C, 60 min). The mixture was cooled down at room temperature (30 min) and centrifuged (2876× g, 20 min). Two milliliters of the supernatant was mixed with 1 mL of phenol solution (5% w/v) and 5 mL of concentrated H2SO4 and cooled down at room temperature (30 min). The sample was measured at 485 nm, and the final content was calculated in % w/w. [103].
The total lipids were measured using the method described by Mishra et al. [105]. Briefly, 5 mg of ground biomass were mixed with 2 mL of concentrated H2SO4 in acid-resistant test tubes and vortexed (Multi Reax, Heidoplh) at 1500 rpm for 5 min. The sample was then incubated (100 °C, 10 min) and cooled down in an ice bath for 5 min. Five milliliters of fresh phospho-vanillin reagent was added to the sample and incubated for a second time (37 °C, 15 min). The final sample was centrifuged (2876× g, 20 min), the supernatant was measured at 530 nm, and the final content was calculated in % w/w.
The total content of proteins was measured using the method described by Mota et al. [106]. Briefly, 5 mg of grounded biomass was mixed with 0.5 mL of distilled water and 0.5 mL of 10% w/v of SDS solution and vortexed (Multi Reax, Heidoplh) at 1500 rpm for 10 min. The sample was centrifuged (8000× g, 5 min), and 1 mL of the supernatant was diluted in 5 mL of reagent C using the Lowry method. Finally, 0.5 mL of Folin reagent (diluted 3 times) was added to the mixture and incubated at room temperature for 30 min. The sample was measured at 750 nm, and the final content was calculated in % w/w.
Finally, the content of total carotenoids was measured according to Hynstova et al. [107]. Briefly, 100 mg of grounded biomass was mixed with 1 mL of acetone and 10 mg of MgCO3. The mixture was vortexed (Multi Reax, Heidoplh) at 2000 rpm for 10 min. The sample was then centrifuged (8000× g, 20 min). The supernatant was diluted 4 times in acetone and measured at 470 nm, and the final content was calculated in % w/w.
3. Results
3.1. Production of Carbohydrates under Different C/N/P
The experimental data obtained from the C/N/P ratio on the production of carbohydrates were fitted on two models (linear (L) and quadratic (Q) with a p-value of 0.05 (p = 0.05)). The statistical software provided a number to identify them; in this case NaNO3 was tagged with the number 1, followed by the phosphate buffer and carbon source: (NaNO3 (1), K2HPO4 + KH2PO4 (2), and carbon source (3). This tag is used by the software to identify possible interactions between two or more variables (example: 1L_by_2L represents and interaction between the lineal model of NaNO3 and K2HPO4 + KH2PO4). In the Y-axis of each Pareto chart can be found the analysis for every single factor adjusted by the two models (Q and L) with the numbers of the three variables evaluated (1, 2 or 3), and on the right side of each row, the assigned number allows us to understand which level has the most effect. Lower numbers (-) indicate that lower levels of that specific variable are positively or negatively affecting the response.
According to the Pareto chart (Figure 1a), when sodium carbonate (Sc) is used as a carbon source, the carbohydrate synthesis is significantly affected by all three factors of the C/N/P ratio (p = 0.05). The surface response (Figure 1b) shows that high concentrations of NaNO3 (0.15–0.25 g/L) and high concentrations of the carbon source (2.64–4.41 g/L of Na2CO3) positively affect the synthesis of carbohydrates up to 50% w/w of the total biomass. In the case when sodium bicarbonate (Sb) is employed, the phosphate buffer and the carbon source significantly affect the synthesis of carbohydrates (Figure 1c). The surface response (Figure 1d) shows that high concentrations of K2HPO4 and KH2PO4 (0.075 and 0.175 g/L, respectively), and high concentrations of the carbon source (2.1–3.49 g/L of NaHCO3) positively affect the synthesis of carbohydrates up to 60% (w/w) of the total biomass. Finally, the Pareto chart of sodium acetate (Sa) (Figure 1e) shows that none of the analyzed nutrients in the designated concentrations significantly affects (p = 0.05) the synthesis of carbohydrates; however, the surface response (Figure 1f) shows that high concentrations of K2HPO4 and KH2PO4 (0.075 and 0.175 g/L, respectively), and high concentrations of the carbon source (0.85–1.36 of C2H3NaO2) can increase the concentration of carbohydrates up to 45% (w/w) of the total biomass in Chlorella sp. UFPS019.
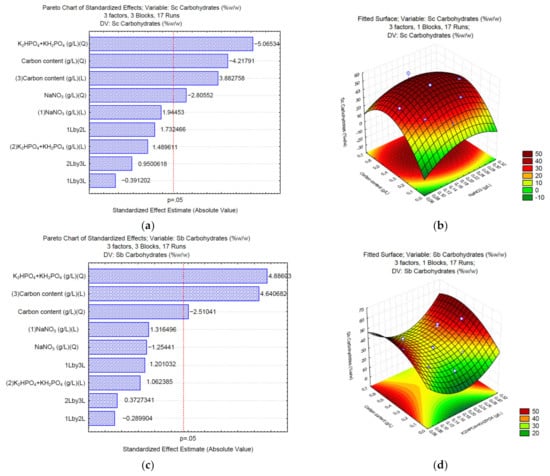
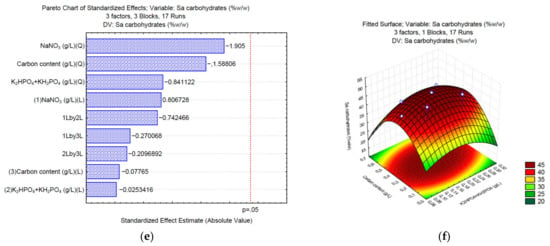
Figure 1.
Pareto charts and surface response of carbohydrate production using Na2CO3 (a,b), NaHCO3 (c,d), and C2H3NaO2 (e,f).
3.2. Production of Proteins under Different C/N/P
In the case of protein synthesis, when sodium carbonate (Sc) is used, the Pareto chart (Figure 2a) shows no significant effect for any of the analyzed variables; however, according to the surface response (Figure 2b), high NaNO3 (>0.25 g/L) and low concentrations of the carbon source (<1.76 g/L of Na2CO3) might increase the total content of proteins. In the case of sodium bicarbonate (Sb), the phosphate buffer and the carbon source significantly affect the synthesis of proteins (Figure 2c). The surface response (Figure 2d) shows that medium concentrations of K2HPO4 and KH2PO4 (0.0375 and 0.0875 g/L, respectively), and medium concentrations of the carbon source (1.39–3.49 g/L of NaHCO3) positively affect the synthesis of proteins up to 40% w/w of the total biomass. Finally, the Pareto chart of sodium acetate (Sa) (Figure 2e) shows that the phosphate buffer, sodium nitrate, and the interaction between NaNO3 and sodium acetate affect the synthesis of proteins. The surface response (Figure 2f) shows that high NaNO3 (>0.25 g/L) and low concentrations of the carbon source (<1.5 g/L of C2H3NaO2) might increase the total content of proteins in Desmodesmus sp UFPS021.
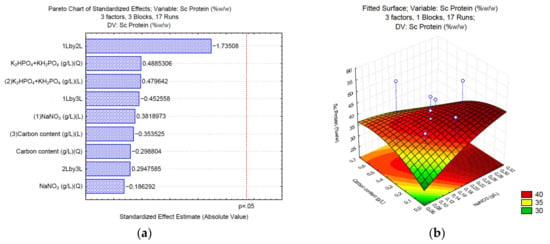
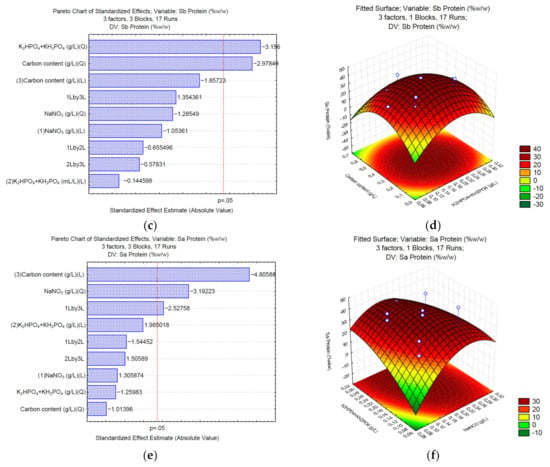
Figure 2.
Pareto charts and surface response of protein production using Na2CO3 (a,b), NaHCO3 (c,d), and C2H3NaO2 (e,f).
3.3. Production of Lipids under Different C/N/P
To produce lipids, the use of sodium carbonate (Sa) as a carbon source shows that none of the analyzed variables significantly (p = 0.05) affects the final content of lipids (Figure 3a). The surface response (Figure 3b) shows that medium concentrations of K2HPO4 and KH2PO4 (0.0375 and 0.0875 g/L, respectively) and the carbon source (3 g/L of Na2CO3) might increase the total content of lipids. In the case of sodium bicarbonate (Sb), NaNO3, and the interaction between the phosphate buffer and the carbon source significantly affect the synthesis of lipids (Figure 3c). The surface response (Figure 3d) shows that high concentrations of K2HPO4 and KH2PO4 (>0.075 and 0.175 g/L, respectively), and low concentrations of the carbon source (<0.7 g/L of NaHCO3) positively affect the synthesis of lipids up to 30% w/w of the total biomass. Finally, the Pareto chart of sodium acetate (Sa) (Figure 3e) shows that sodium nitrate affects the synthesis of lipids. The surface response (Figure 3f) shows that low NaNO3 (<0.075 g/L) and medium concentrations of the carbon source (0.68–1.7 g/L of C2H3NaO2) might increase the total content of lipids in Chlorella sp. UFPS019.
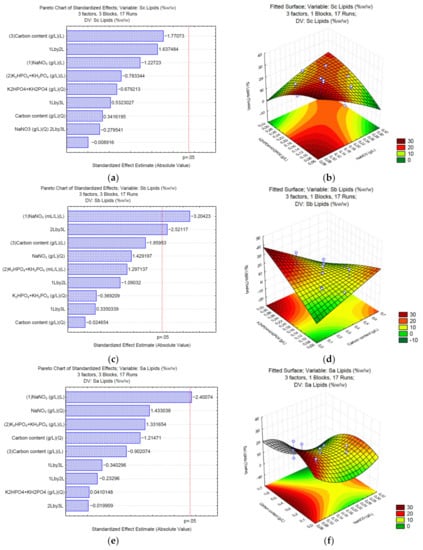
Figure 3.
Pareto charts and surface response of lipid production using Na2CO3 (a,b), NaHCO3 (c,d), and C2H3NaO2 (e,f).
3.4. Production of Total Carotenoids under Different C/N/P
In the case of total carotenoids, none of the analyzed variables in any of the different carbon sources studied (Figure 4a,c,e) showed a significant effect. In the case of Na2CO3 (Sa), the surface response (Figure 4b) shows that lower concentrations of the carbon source (<0.9 g/L of Na2CO3) and K2HPO4, and KH2PO4 (<0.0375 and 0.0875 g/L, respectively) might increase the content of total carotenoids. In the case of NaHCO3 (Sb) (Figure 4d), lower content of carbon source (<0.7 g/L of NaHCO3) and high levels of K2HPO4, and KH2PO4 (>0.075 and 0.175 g/L, respectively) can increase the concentration of total carotenoids. Finally, when sodium acetate (Sa) is used (Figure 4f), high levels of NaNO3 (>0.25 g/L) and K2HPO4 and KH2PO4 (>0.075 and 0.175 g/L, respectively) can increase the final content of total carotenoids in Desmodesmus sp. UFPS020.
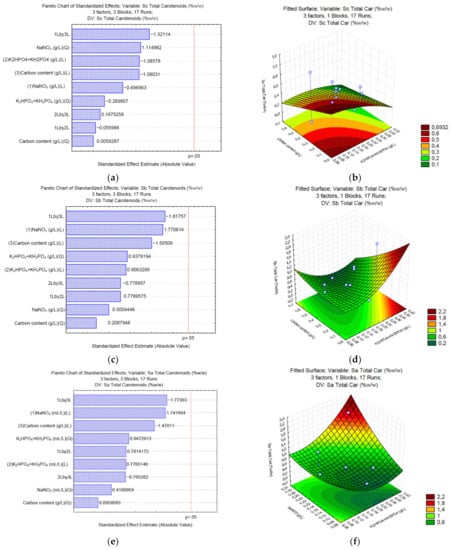
Figure 4.
Pareto charts and surface response of total carotenoids production using Na2CO3 (a,b), NaHCO3 (c,d), and C2H3NaO2 (e,f).
By analyzing the results from the interactions in the C/N/P ratio, the values of the three variables were adjusted to increase the production of carbohydrates, proteins, and lipids in Chlorella sp. UFPS019 and Desmodesmus sp. UFPS021. Table 3 represents the highest scenarios for increasing each metabolite in the designated strains. Both algae were grown in a 10 L flask (0.6 L/min of filtered air, 12:12 h light/dark cycle at 100 µmol·m−2·s−1) for 20 days for carbohydrates and proteins, and 40 days for lipids. As a control, both strains were grown in Bold’s basal medium without any modification. The biomass produced was concentrated by electroflocculation [104]. The culture media were filtered on 0.45 µm GF-C (Sartorius, Germany) to remove excess cells, and the media were analyzed for the final content of NO3 and PO4 using HANNA test kits (HI 93728-01, and HI 93713-01).

Table 3.
Variables for optimal biomass concentration in both strains were studied.
The experimental data for each of the metabolites were adjusted to the exponential or logarithmic phase of microalgal growth, in which the maximum specific speed (µmax), saturation, and affinity constants for NO3 and PO4 (), as well as the yield coefficient, define the biomass production rate from a mass measure (). Table 4 presents the data obtained for each strain under optimal conditions.

Table 4.
Growth parameters for production of carbohydrates, proteins, and lipids.
Figure 5 shows that to produce both carbohydrates and proteins, the two strains were still in the exponential growth phase, while the algae had to be in the late stationary phase to produce lipids. On the other hand, it was shown that for all experiments, NO3 was consumed entirely after 20 days, while the concentration of PO4 varied. In the case of the optimized medium for carbohydrate production, 55% of the phosphate was consumed, while protein and lipid consumption was higher (70%).

Figure 5.
Biomass production and consumption of NO3 and PO4 in the optimized C/N/P ratio for production of carbohydrates (a), proteins (b) and lipids (c).
The results obtained for the different metabolites were analyzed using one-way ANOVA (Figure 6). This result shows that the concentrations of carbohydrates, proteins, and lipids under the optimized conditions were statistically significant in comparison with the results expected from the surface response and the control. The latter indicated that the proposed method effectively enhances the production of the different metabolites in the studied strains.
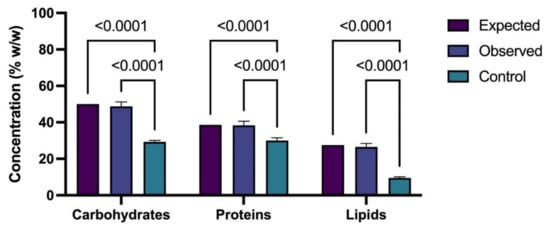
Figure 6.
One-way ANOVA between the expected and obtained results to produce carbohydrates and lipids in Chlorella sp. UFPS019 and proteins in Scenedesmus sp. UFPS021.
4. Discussion
The optimization of culture media is considered one of the key processes to improve the production of biomass and target metabolites, whereby it is possible to adjust the concentrations of certain nutrients (both macro and micronutrients) depending on the strain. The enhancement of the different metabolites can be manipulated through growth conditions; therefore, the main strategies include nutritional factors such as nutrient limitation/supplementation (nitrogen, phosphorus, sulfur, iron), and continuous addition of carbon source, as well as factors such as light intensity, pH, temperature, and salinity [108]. In recent years, different researchers have demonstrated the efficiency of this approach, especially using one-factor-at-a-time (OFAT) experiments, which involve the adjustment of one factor while the others are kept constant. Examples of this approach can be found in the scientific literature with a wide range of strains including (but not limited to) Botryococcus braunii [109], Chlorella vulgaris [110], Chlorococcum sp. (Chlorophyta) [111], Vischeria vischeri (formerly Eustigmatos vischeri) (Ochrophyta, Eustigmatophyceae) [112], Isochrysis galbana (Haptophyta, Coccolithophyceae) [113], Lobosphaera incisa (Chlorophyta) [114], and Picochlorum soloecismus (Chlorophyta) [115]. However, OFAT is considered time-consuming and labor-intensive when several variables may affect the outcome. In this case, due to their intrinsic nature of combining multiple salts in different concentrations, algal culture media are a perfect candidate for more robust and advanced methodologies. In recent years, the optimization of culture media and extraction of different metabolites through the application of design of experiments (DoE) has become more common [116,117,118,119,120]. Table 5 present a list of the most recent papers where the improvement of a specific metabolite was achieved through the application of DoE.
The most evaluated metabolites are lipids (including hydrocarbons) [23,93,97,121,122,123,124,125,126,127,128,129,130]. In the second position can be found the optimization of carbohydrate synthesis [93,131,132], followed by the enhancement of protein synthesis [97,98]. The latter has occurred due to the international interest in the application of algal biomass as a replacement for biofuel production, which remains a strong alternative within the international scientific community [32]. In the quest to improve the synthesis of each of the algal metabolites, the most studied components are the nitrogen and carbon sources [121,122,123,124,125,126,127,128,129]. According to de Farias Silva et al. [133], nitrogen limitation causes an interruption in amino acid synthesis, and photosynthetically fixed carbon in the Calvin cycle is then converted to carbohydrates. Another nutrient of great importance is phosphate, which is essential for starch and sucrose synthesis; however, high concentrations of P inhibit the action of enzymes such as ADP-glucose phosphorylase [134].

Table 5.
List of reported algal strains with culture media optimized via design of experiments.
Table 5.
List of reported algal strains with culture media optimized via design of experiments.
| Strain | Biomass (g/L) | Productivity (g/L·d−1) | Carbohydrates (% w/w) | Proteins (% w/w) | Lipids (% w/w) | References |
|---|---|---|---|---|---|---|
| Ankistrodesmus falcatus KJ671624 | 1.74 | 0.124 | n/a | 59.6 | [121] | |
| Auxenochlorella protothecoides (formerly Chlorella protothecoides) | 1.06 | 0.71 | n/a | 43.9 | [122] | |
| Auxenochlorella pyrenoidosa (formerly Chlorella pyrenoidosa) | 0.89 | n/a | [98] | |||
| Botryococcus sp. | 0.28 | n/a | 73 | [123] | ||
| B. braunii | 2 | 0.133 | n/a | 70 | [24] | |
| B. braunii LB572 | 4.57 | 0.18 | n/a | 64.9 | [124] | |
| Chlorella sp. | 0.44 | n/a | 55 | [123] | ||
| Chlorella protothecoides UTEX 250 | 1.19 | n/a | 12.9 | [125] | ||
| C. sorokiniana 211-32 | 1.18 | 0.039 | n/a | 38 | [126] | |
| C. sorokiniana UTEX 1602 | 0.68 | n/a | 9 | [127] | ||
| C. sorokiniana UTEX 2805 | 0.66 | n/a | 52 | n/a | [131] | |
| C. vulgaris UTEX 2714 | 0.24 | n/a | 59 | n/a | ||
| C. vulgaris UTEX 1803 | 3.7 | n/a | 60 | n/a | [95] | |
| C. vulgaris FSP-E | 5.51 | 51.3 | [132] | |||
| Chlorococcum oleofaciens | 1.6 | 20 | [97] | |||
| Dunaliella salina | n/a | 0.035 | n/a | 22 | [128] | |
| D. tertiolecta | n/a | 0.044 | n/a | 23 | ||
| D. parva | n/a | 0.045 | n/a | 39 | [129] | |
| Desmodesmus armatus | 1.65 | n/a | 53.6 | n/a | [96] | |
| Scenedesmus sp. ASK22 | 4.67 | 31.06 | 37.1 | [130] | ||
| S. obliquus | n/a | 10–17 | 50–56 | 12–14 | [128] | |
| 2.63 | n/a | 40 | n/a | [132] | ||
| 0.685 | n/a | 39.6 | [93] | |||
| n/a | 66 | [23] | ||||
| S. vacuolatus | 2 | n/a | 4% | [90] | ||
| Chlorella sp. UFPS019 | 0.72 | 0.036 | 48.8 | n/a | This research | |
| 1.5 | 0.048 | n/a | 26.5 | |||
| Desmodesmus sp. UFPS021 | 0.95 | 0.038 | n/a | 38.4 | n/a | |
In microalgae, carbohydrates are generally found in different concentrations depending on the species and culture conditions; however, few species can synthesize a high carbohydrate content [134]. Porphyridium cruentum can accumulate carbohydrates up to 57% of its dry weight, while Chlamydomonas sp. normally does not exceed 17% [135]. According to Dragone et al. [136], C. vulgaris can accumulate between 9 and 41% of its total weight (in dry weight), while S. obliquus can accumulate between 10 and 47% (in dry weight). According to Muthuraj et al. [137], in Chlorella sp., nitrogen reduction in the culture medium enhanced the accumulation of carbohydrates (up to 66% w/w). Under sufficient inorganic phosphate levels, Chlorella sp. FC2 IITG presented high carbohydrate content (up to 47.35% w/w), whereas under deficient conditions, the content was significantly reduced (32.21% w/w). The results obtained in this paper are in accordance with Tourang et al. [138], where the interaction between the carbon (low to medium content) and phosphate source (medium to high content) can effectively enhance the final concentration of carbohydrates in Chlorella sp. UFPS019.
In the case of lipids, a method focused on the enhancement of lipid and TAG biosynthesis through the selective deficiency of specific nutrients (such as phosphorus and nitrogen) coupled with salinity stress is the most widely used protocol [128], since N and P availability in the media will play an essential role in the synthesis of amino acids and proteins [139]. Examples such as the optimization of Dunaliella parva via response surface methodology prove that a high content of NaNO3 (0.63 g/L), NaCl (1.61 M), and low KH2PO4 (0.02 g/L) will increase the final content of lipids up to 1.4 times [128]. In the specific context of this research, the concentration of nitrate employed in the optimization was 0.075 g/L of NaNO3, while the concentrations of KH2PO4 and K2HPO4 were 0.056, and 0.131 g/L, respectively. These results are similar to those reported using Selenastrum sp. GA66 (0.06 g/L of NaNO3 and 2-fold increase in total lipids) [140]. However, the evaluation of the C-N-P ratio is a rare approach that has not been studied in-depth.
Since CO2 is the most common carbon source, the analysis of other inorganic sources and even some organic carbon sources has been limited over the years. Our results prove that the proper adjustment of not only N and P, but also the specific addition of an organic source, will effectively increase the final content of total lipids in this Chlorella sp. UFPS019 strain. However, in the long run, this method (especially when nitrogen is reduced) will substantially reduce the final content of proteins and the overall biomass produced in the selected strains. Regarding carotenoids, the results demonstrated that all the different experiments were successful in maximizing carotenogenesis. The latter may be due to the lack of other stressful conditions such as light and salinity [141,142]. Finally, to produce proteins, both algae and cyanobacteria require adequate concentrations of carbon, nitrogen, and phosphate sources, since proteins and amino acids are the building blocks for the synthesis of multiple enzymes and different cellular processes [143,144]. Therefore, excesses of N and P will allow the fast synthesis of different proteins, which is reflected in the optimization conditions obtained in this research (0.25 g/L of NaNO3 and 1.875 g/L of KH2PO4 + K2HPO4).
5. Conclusions
The optimal C/N/P ratios for the synthesis of carbohydrates, proteins, and lipids in two high-mountain algal strains, Chlorella sp. UFPS019, and Desmodesmus sp. UFPS021, were determined. In Chlorella sp. UFPS019, the optimal conditions to enhance the synthesis of carbohydrates were high sodium carbonate content (3.53 g/L), high KH2PO4 and K2HPO4 content (0.06 and 0.14 g/L, respectively), and medium-high NaNO3 (0.1875 g/L). In the case of lipids, a high concentration of sodium acetate (1.19 g/L) coupled with high KH2PO4 and K2HPO4 content (0.056 and 0.131 g/L, respectively) and a low concentration of NaNO3 (0.075 g/L) drastically improved the synthesis of lipids. In the case of Desmodesmus sp. UFPS021, the protein content was increased using high sodium acetate (2 g/L), high KH2PO4 and K2HPO4 content (0.056 and 0.131 g/L, respectively), and a high NaNO3 concentration (0.25 g/L). Further studies should focus on the possible interactions between other stress-inducing nutrients (Na, Mg, Ca, etc.) and LEDs that can eventually maximize the final content of these metabolites.
Author Contributions
Conceptualization, R.O.G.-R., A.Z. and A.F.B.-S.; methodology, W.H.S.Q., J.B.G.-M. and G.L.L.-B.; software, A.F.B.-S., and A.Z.; validation, N.A.U.-S., and G.L.L.-B.; formal analysis, W.H.S.Q., J.B.G.-M. and A.Z.; investigation, W.H.S.Q. and A.F.B.-S.; resources, A.F.B.-S. and A.Z.; data curation, A.Z.; writing—original draft preparation, W.H.S.Q. and R.O.G.-R.; writing—review and editing, A.F.B.-S., A.Z. and G.L.L.-B.; visualization, G.L.L.-B.; supervision, J.B.G.-M.; project administration, A.F.B.-S. and J.B.G.-M.; funding acquisition, A.F.B.-S. and R.O.G.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This paper was supported by Newton-Caldas Fund Institutional Links, with the project “Algalcolor: Bio-Platform for the Sustainable Production of Cyanobacterial-Based Colours and Fine Chemicals” ID 527624805, and The Colombian Ministry of Science Technology and Innovation MINCIENCIAS for the support of national Ph.D. Doctorates through the Francisco José de Caldas scholarship program (753-2016).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to express our sincere gratitude to Universidad Francisco de Paula Santander (Colombia) for providing the equipment for this research and the Colombian Ministry of Science Technology and Innovation MINCIENCIAS for the support to national Ph.D. Doctorates through the Francisco José de Caldas scholarship program.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Matos, Â.P. The Impact of Microalgae in Food Science and Technology. JAOCS J. Am. Oil Chem. Soc. 2017, 94, 1333–1350. [Google Scholar] [CrossRef]
- Amini, Z.; Ilham, Z.; Ong, H.C.; Mazaheri, H.; Chen, W.-H. State of the Art and Prospective of Lipase-Catalyzed Transesterification Reaction for Biodiesel Production. Energy Convers. Manag. 2017, 141, 339–353. [Google Scholar] [CrossRef]
- Tekin, K.; Karagöz, S.; Bektaş, S. A Review of Hydrothermal Biomass Processing. Renew. Sustain. Energy Rev. 2014, 40, 673–687. [Google Scholar] [CrossRef]
- Baykara, S.Z. Hydrogen: A Brief Overview on Its Sources, Production and Environmental Impact. Int. J. Hydrgen Energy 2018, 43, 10605–10614. [Google Scholar] [CrossRef]
- Kosourov, S.; Murukesan, G.; Seibert, M.; Allahverdiyeva, Y. Evaluation of Light Energy to H2 Energy Conversion Efficiency in Thin Films of Cyanobacteria and Green Alga under Photoautotrophic Conditions. Algal Res. 2017, 28, 253–263. [Google Scholar] [CrossRef]
- Abbas, J.; Sağsan, M. Impact of Knowledge Management Practices on Green Innovation and Corporate Sustainable Development: A Structural Analysis. J. Clean. Prod. 2019, 229, 611–620. [Google Scholar] [CrossRef]
- Nagarajan, D.; Lee, D.-J.; Kondo, A.; Chang, J.-S. Recent Insights into Biohydrogen Production by Microalgae—From Biophotolysis to Dark Fermentation. Bioresour. Technol. 2017, 227, 373–387. [Google Scholar] [CrossRef]
- Show, P.L.; Tang, M.S.Y.; Nagarajan, D.; Ling, T.C.; Ooi, C.-W.; Chang, J.-S. A Holistic Approach to Managing Microalgae for Biofuel Applications. Int. J. Mol. Sci. 2017, 18, 215. [Google Scholar] [CrossRef]
- Bleakley, S.; Hayes, M. Algal Proteins: Extraction, Application, and Challenges Concerning Production. Foods 2017, 6, 33. [Google Scholar] [CrossRef]
- Koyande, A.K.; Chew, K.W.; Rambabu, K.; Tao, Y.; Chu, D.-T.; Show, P.-L. Microalgae: A Potential Alternative to Health Supplementation for Humans. Food Sci. Hum. Wellness 2019, 8, 16–24. [Google Scholar] [CrossRef]
- Kusmayadi, A.; Leong, Y.K.; Yen, H.-W.; Huang, C.-Y.; Chang, J.-S. Microalgae as Sustainable Food and Feed Sources for Animals and Humans—Biotechnological and Environmental Aspects. Chemosphere 2021, 271, 129800. [Google Scholar] [CrossRef]
- Del Mondo, A.; Smerilli, A.; Sané, E.; Sansone, C.; Brunet, C. Challenging Microalgal Vitamins for Human Health. Microb. Cell Fact. 2020, 19, 201. [Google Scholar] [CrossRef]
- Fu, Y.; Chen, T.; Chen, S.H.Y.; Liu, B.; Sun, P.; Sun, H.; Chen, F. The Potentials and Challenges of Using Microalgae as an Ingredient to Produce Meat Analogues. Trends Food Sci. Technol. 2021, 112, 188–200. [Google Scholar] [CrossRef]
- Kent, M.; Welladsen, H.M.; Mangott, A.; Li, Y. Nutritional Evaluation of Australian Microalgae as Potential Human Health Supplements. PLoS ONE 2015, 10, e0118985. [Google Scholar] [CrossRef]
- Quintero-Dallos, V.; García-Martínez, J.B.; Contreras-Ropero, J.E.; Barajas-Solano, A.F.; Barajas-Ferrerira, C.; Lavecchia, R.; Zuorro, A. Vinasse as a Sustainable Medium for the Production of Chlorella Vulgaris UTEX 1803. Water 2019, 11, 1526. [Google Scholar] [CrossRef]
- da Silva, M.E.T.; Correa, K.d.P.; Martins, M.A.; da Matta, S.L.P.; Martino, H.S.D.; Coimbra, J.S. dos R. Food Safety, Hypolipidemic and Hypoglycemic Activities, and in Vivo Protein Quality of Microalga Scenedesmus Obliquus in Wistar Rats. J. Funct. Foods 2020, 65, 103711. [Google Scholar] [CrossRef]
- Amorim, M.L.; Soares, J.; Vieira, B.B.; Batista-Silva, W.; Martins, M.A. Extraction of Proteins from the Microalga Scenedesmus Obliquus BR003 Followed by Lipid Extraction of the Wet Deproteinized Biomass Using Hexane and Ethyl Acetate. Bioresour. Technol. 2020, 307, 123190. [Google Scholar] [CrossRef]
- Gateau, H.; Blanckaert, V.; Veidl, B.; Burlet-Schiltz, O.; Pichereaux, C.; Gargaros, A.; Marchand, J.; Schoefs, B. Application of Pulsed Electric Fields for the Biocompatible Extraction of Proteins from the Microalga Haematococcus Pluvialis. Bioelectrochemistry 2021, 137, 107588. [Google Scholar] [CrossRef]
- Grossmann, L.; Wörner, V.; Hinrichs, J.; Weiss, J. Mechanism of the Formation of Insoluble Structures in a Protein Extract of the Microalga Chlorella Protothecoides at PH 3. Food Biosci. 2019, 28, 140–142. [Google Scholar] [CrossRef]
- Cheng, D.; Li, D.; Yuan, Y.; Zhou, L.; Li, X.; Wu, T.; Wang, L.; Zhao, Q.; Wei, W.; Sun, Y. Improving Carbohydrate and Starch Accumulation in Chlorella sp. AE10 by a Novel Two-Stage Process with Cell Dilution. Biotechnol. Biofuels 2017, 10, 75. [Google Scholar] [CrossRef]
- Mathimani, T.; Baldinelli, A.; Rajendran, K.; Prabakar, D.; Matheswaran, M.; Pieter van Leeuwen, R.; Pugazhendhi, A. Review on Cultivation and Thermochemical Conversion of Microalgae to Fuels and Chemicals: Process Evaluation and Knowledge Gaps. J. Clean. Prod. 2019, 208, 1053–1064. [Google Scholar] [CrossRef]
- Barajas-Solano, A.F.; Gonzalez-Delgado, A.D.; Kafarov, V. Effect of Thermal Pre-Treatment On Fermentable Sugar Production of Chlorella Vulgaris. Chem. Eng. Trans. 2014, 37, 655–660. [Google Scholar] [CrossRef]
- Cuellar García, D.J.; Rangel-Basto, Y.A.; Barajas-Solano, A.F.; Muñoz-Peñalosa, Y.A.; Urbina-Suarez, N.A. Towards the Production of Microalgae Biofuels: The Effect of the Culture Medium on Lipid Deposition. Biotechnologia 2019, 100, 273–278. [Google Scholar] [CrossRef]
- Barajas-Solano, A.F.; Guzmán-Monsalve, A.; Kafarov, V. Effect of Carbon-Nitrogen Ratio for the Biomass Production, Hydrocarbons and Lipids on Botryoccus Braunii UIS 003. Chem. Eng. Trans. 2016, 49, 247–252. [Google Scholar] [CrossRef]
- Zuorro, A.; Malavasi, V.; Cao, G.; Lavecchia, R. Use of Cell Wall Degrading Enzymes to Improve the Recovery of Lipids from Chlorella Sorokiniana. Chem. Eng. J. 2019, 377, 120325. [Google Scholar] [CrossRef]
- Zuorro, A.; Lavecchia, R.; Maffei, G.; Marra, F.; Miglietta, S.; Petrangeli, A.; Familiari, G.; Valente, T. Enhanced lipid extraction from unbroken microalgal cells using enzymes. Chem. Eng. Trans. 2015, 43, 211–216. [Google Scholar] [CrossRef]
- Chew, K.W.; Yap, J.Y.; Show, P.L.; Suan, N.H.; Juan, J.C.; Ling, T.C.; Lee, D.-J.; Chang, J.-S. Microalgae Biorefinery: High Value Products Perspectives. Bioresour. Technol. 2017, 229, 53–62. [Google Scholar] [CrossRef]
- Kumar, G.; Shobana, S.; Chen, W.-H.; Bach, Q.-V.; Kim, S.-H.; Atabani, A.E.; Chang, J.-S. A Review of Thermochemical Conversion of Microalgal Biomass for Biofuels: Chemistry and Processes. Green Chem. 2017, 19, 44–67. [Google Scholar] [CrossRef]
- Brigljević, B.; Liu, J.; Lim, H. Green Energy from Brown Seaweed: Sustainable Polygeneration Industrial Process via Fast Pyrolysis of S. Japonica Combined with the Brayton Cycle. Energy Convers. Manag. 2019, 195, 1244–1254. [Google Scholar] [CrossRef]
- Xu, X.; Gu, X.; Wang, Z.; Shatner, W.; Wang, Z. Progress, Challenges and Solutions of Research on Photosynthetic Carbon Sequestration Efficiency of Microalgae. Renew. Sustain. Energy Rev. 2019, 110, 65–82. [Google Scholar] [CrossRef]
- Yu, K.L.; Show, P.L.; Ong, H.C.; Ling, T.C.; Chen, W.-H.; Salleh, M.A.M. Biochar Production from Microalgae Cultivation through Pyrolysis as a Sustainable Carbon Sequestration and Biorefinery Approach. Clean Technol. Environ. Policy 2018, 20, 2047–2055. [Google Scholar] [CrossRef]
- Zuorro, A.; García-Martínez, J.B.; Barajas-Solano, A.F. The Application of Catalytic Processes on the Production of Algae-Based Biofuels: A Review. Catalysts 2021, 11, 22. [Google Scholar] [CrossRef]
- Mohamed, A.G.; Abo-El-Khair, B.E.; Shalaby, S.M. Quality of novel healthy processed cheese analogue enhanced with marine microalgae Chlorella vulgaris biomass. World Appl. Sci. J. 2013, 23, 914–925. [Google Scholar]
- Furbeyre, H.; van Milgen, J.; Mener, T.; Gloaguen, M.; Labussière, E. Effects of Dietary Supplementation with Freshwater Microalgae on Growth Performance, Nutrient Digestibility and Gut Health in Weaned Piglets. Animal 2017, 11, 183–192. [Google Scholar] [CrossRef]
- Oh, S.T.; Zheng, L.; Kwon, H.J.; Choo, Y.K.; Lee, K.W.; Kang, C.W.; An, B.K. Effects of Dietary Fermented Chlorella Vulgaris (CBT®) on Growth Performance, Relative Organ Weights, Cecal Microflora, Tibia Bone Characteristics, and Meat Qualities in Pekin Ducks. Asian-Australas. J. Anim. Sci. 2015, 28, 95–101. [Google Scholar] [CrossRef]
- Kang, H.K.; Salim, H.M.; Akter, N.; Kim, D.W.; Kim, J.H.; Bang, H.T.; Kim, M.J.; Na, J.C.; Hwangbo, J.; Choi, H.C.; et al. Effect of Various Forms of Dietary Chlorella Supplementation on Growth Performance, Immune Characteristics, and Intestinal Microflora Population of Broiler Chickens. J. Appl. Poult. Res. 2013, 22, 100–108. [Google Scholar] [CrossRef]
- El-Baz, F.K.; Abdo, S.M.; Hussein, A.M.S. Microalgae Dunaliella salina for use as food supplement to improve pasta quality. Int. J. Pharm. Sci. Rev. Res. 2017, 46, 45–51. [Google Scholar]
- Xu, Y.; Ibrahim, I.M.; Wosu, C.I.; Ben-Amotz, A.; Harvey, P.J. Potential of New Isolates of Dunaliella Salina for Natural β-Carotene Production. Biology 2018, 7, 14. [Google Scholar] [CrossRef]
- Sui, Y.; Mazzucchi, L.; Acharya, P.; Xu, Y.; Morgan, G.; Harvey, P.J. A Comparison of β-Carotene, Phytoene and Amino Acids Production in Dunaliella salina DF 15 (CCAP 19/41) and Dunaliella salina CCAP 19/30 Using Different Light Wavelengths. Foods 2021, 10, 2824. [Google Scholar] [CrossRef]
- Chan, K.; Chen, S.; Chen, P. Astaxanthin Attenuated Thrombotic Risk Factors in Type 2 Diabetic Patients. J. Funct. Foods 2019, 53, 22–27. [Google Scholar] [CrossRef]
- Sheikhzadeh, N.; Tayefi-Nasrabadi, H.; Khani Oushani, A.; Najafi Enferadi, M.H. Effects of Haematococcus Pluvialis Supplementation on Antioxidant System and Metabolism in Rainbow Trout (Oncorhynchus Mykiss). Fish Physiol. Biochem. 2012, 38, 413–419. [Google Scholar] [CrossRef]
- Do, T.-T.; Ong, B.-N.; Le, T.-L.; Nguyen, T.-C.; Tran-Thi, B.-H.; Thu Hien, B.T.; Melkonian, M.; Tran, H.-D. Growth of Haematococcus pluvialis on a Small-Scale Angled Porous Substrate Photobioreactor for Green Stage Biomass. Appl. Sci. 2021, 11, 1788. [Google Scholar] [CrossRef]
- Rizzo, A.; Ross, M.E.; Norici, A.; Jesus, B. A Two-Step Process for Improved Biomass Production and Non-Destructive Astaxanthin and Carotenoids Accumulation in Haematococcus pluvialis. Appl. Sci. 2022, 12, 1261. [Google Scholar] [CrossRef]
- Neves, M.; Ferreira, A.; Antunes, M.; Laranjeira Silva, J.; Mendes, S.; Gil, M.M.; Tecelão, C. Nannochloropsis oceanica as a Sustainable Source of n-3 Polyunsaturated Fatty Acids for Enrichment of Hen Eggs. Appl. Sci. 2021, 11, 8747. [Google Scholar] [CrossRef]
- du Preez, R.; Majzoub, M.E.; Thomas, T.; Panchal, S.K.; Brown, L. Nannochloropsis oceanica as a Microalgal Food Intervention in Diet-Induced Metabolic Syndrome in Rats. Nutrients 2021, 13, 3991. [Google Scholar] [CrossRef]
- Saito, T.; Ichihara, T.; Inoue, H.; Uematsu, T.; Hamada, S.; Watanabe, T.; Takimura, Y.; Webb, J. Comparison of Areal Productivity of Nannochloropsis Oceanica Between Lab-Scale and Industrial-Scale Raceway Pond. Mar. Biotechnol. 2020, 22, 836–841. [Google Scholar] [CrossRef]
- Zhang, R.; Parniakov, O.; Grimi, N.; Lebovka, N.; Marchal, L.; Vorobiev, E. Emerging Techniques for Cell Disruption and Extraction of Valuable Bio-Molecules of Microalgae Nannochloropsis sp. Bioprocess Biosyst. Eng. 2019, 42, 173–186. [Google Scholar] [CrossRef]
- Mitra, M.; Mishra, S. A Biorefinery from Nannochloropsis spp. Utilizing Wastewater Resources BT—Application of Microalgae in Wastewater Treatment: Volume 2: Biorefinery Approaches of Wastewater Treatment; Gupta, S.K., Bux, F., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 123–145. [Google Scholar] [CrossRef]
- Branco-Vieira, M.; San Martin, S.; Agurto, C.; Santos, M.A.d.; Freitas, M.A.V.; Mata, T.M.; Martins, A.A.; Caetano, N.S. Potential of Phaeodactylum tricornutum for Biodiesel Production under Natural Conditions in Chile. Energies 2018, 11, 54. [Google Scholar] [CrossRef]
- Afonso, C.; Bragança, A.R.; Rebelo, B.A.; Serra, T.S.; Abranches, R. Optimal Nitrate Supplementation in Phaeodactylum tricornutum Culture Medium Increases Biomass and Fucoxanthin Production. Foods 2022, 11, 568. [Google Scholar] [CrossRef]
- Neumann, U.; Derwenskus, F.; Flaiz Flister, V.; Schmid-Staiger, U.; Hirth, T.; Bischoff, S.C. Fucoxanthin, A Carotenoid Derived from Phaeodactylum tricornutum Exerts Antiproliferative and Antioxidant Activities In Vitro. Antioxidants 2019, 8, 183. [Google Scholar] [CrossRef]
- Eilers, U.; Bikoulis, A.; Breitenbach, J.; Büchel, C.; Sandmann, G. Limitations in the Biosynthesis of Fucoxanthin as Targets for Genetic Engineering in Phaeodactylum Tricornutum. J. Appl. Phycol. 2016, 28, 123–129. [Google Scholar] [CrossRef]
- Kwon, D.Y.; Vuong, T.T.; Choi, J.; Lee, T.S.; Um, J.-I.; Koo, S.Y.; Hwang, K.T.; Kim, S.M. Fucoxanthin Biosynthesis Has a Positive Correlation with the Specific Growth Rate in the Culture of Microalga Phaeodactylum Tricornutum. J. Appl. Phycol. 2021, 33, 1473–1485. [Google Scholar] [CrossRef]
- Li, Y.; Ma, Q.; Pan, Y.; Chen, Q.; Sun, Z.; Hu, P. Development of an Effective Flocculation Method by Utilizing the Auto-Flocculation Capability of Phaeodactylum Tricornutum. Algal Res. 2021, 58, 102413. [Google Scholar] [CrossRef]
- Pereira, H.; Sá, M.; Maia, I.; Rodrigues, A.; Teles, I.; Wijffels, R.H.; Navalho, J.; Barbosa, M. Fucoxanthin Production from Tisochrysis Lutea and Phaeodactylum Tricornutum at Industrial Scale. Algal Res. 2021, 56, 102322. [Google Scholar] [CrossRef]
- Kadalag, N.L.; Pawar, P.R.; Prakash, G. Co-Cultivation of Phaeodactylum Tricornutum and Aurantiochytrium Limacinum for Polyunsaturated Omega-3 Fatty Acids Production. Bioresour. Technol. 2022, 346, 126544. [Google Scholar] [CrossRef]
- Han, S.-I.; Jeon, M.S.; Park, Y.H.; Kim, S.; Choi, Y.-E. Semi-Continuous Immobilized Cultivation of Porphyridium Cruentum for Sulfated Polysaccharides Production. Bioresour. Technol. 2021, 341, 125816. [Google Scholar] [CrossRef]
- Medina-Cabrera, E.V.; Gansbiller, M.; Rühmann, B.; Schmid, J.; Sieber, V. Rheological Characterization of Porphyridium Sordidum and Porphyridium Purpureum Exopolysaccharides. Carbohydr. Polym. 2021, 253, 117237. [Google Scholar] [CrossRef]
- Seemashree, M.H.; Chauhan, V.S.; Sarada, R. Phytohormone Supplementation Mediated Enhanced Biomass Production, Lipid Accumulation, and Modulation of Fatty Acid Profile in Porphyridium Purpureum and Dunaliella Salina Cultures. Biocatal. Agric. Biotechnol. 2022, 39, 102253. [Google Scholar] [CrossRef]
- da Silva, M.E.T.; Leal, M.A.; Resende, M.d.O.; Martins, M.A.; Coimbra, J.S.d.R. Scenedesmus Obliquus Protein Concentrate: A Sustainable Alternative Emulsifier for the Food Industry. Algal Res. 2021, 59, 102468. [Google Scholar] [CrossRef]
- Tao, R.; Lakaniemi, A.-M.; Rintala, J.A. Cultivation of Scenedesmus Acuminatus in Different Liquid Digestates from Anaerobic Digestion of Pulp and Paper Industry Biosludge. Bioresour. Technol. 2017, 245, 706–713. [Google Scholar] [CrossRef]
- Singh, D.V.; Upadhyay, A.K.; Singh, R.; Singh, D.P. Implication of Municipal Wastewater on Growth Kinetics, Biochemical Profile, and Defense System of Chlorella Vulgaris and Scenedesmus Vacuolatus. Environ. Technol. Innov. 2022, 26, 102334. [Google Scholar] [CrossRef]
- Tavanandi, H.A.; Raghavarao, K.S.M.S. Ultrasound-Assisted Enzymatic Extraction of Natural Food Colorant C-Phycocyanin from Dry Biomass of Arthrospira Platensis. LWT 2020, 118, 108802. [Google Scholar] [CrossRef]
- İlter, I.; Akyıl, S.; Demirel, Z.; Koç, M.; Conk-Dalay, M.; Kaymak-Ertekin, F. Optimization of Phycocyanin Extraction from Spirulina Platensis Using Different Techniques. J. Food Compos. Anal. 2018, 70, 78–88. [Google Scholar] [CrossRef]
- Ayekpam, C.; Hamsavi, G.K.; Raghavarao, K.S.M.S. Efficient Extraction of Food Grade Natural Blue Colorant from Dry Biomass of Spirulina Platensis Using Eco-Friendly Methods. Food Bioprod. Process. 2021, 129, 84–93. [Google Scholar] [CrossRef]
- Ferreira-Santos, P.; Nunes, R.; De Biasio, F.; Spigno, G.; Gorgoglione, D.; Teixeira, J.A.; Rocha, C.M.R. Influence of Thermal and Electrical Effects of Ohmic Heating on C-Phycocyanin Properties and Biocompounds Recovery from Spirulina Platensis. LWT 2020, 128, 109491. [Google Scholar] [CrossRef]
- Tavanandi, H.A.; Mittal, R.; Chandrasekhar, J.; Raghavarao, K.S.M.S. Simple and Efficient Method for Extraction of C-Phycocyanin from Dry Biomass of Arthospira Platensis. Algal Res. 2018, 31, 239–251. [Google Scholar] [CrossRef]
- Devi, A.C.; Tavanandi, H.A.; Govindaraju, K.; Raghavarao, K.S.M.S. An Effective Method for Extraction of High Purity Phycocyanins (C-PC and A-PC) from Dry Biomass of Arthrospira Maxima. J. Appl. Phycol. 2020, 32, 1141–1151. [Google Scholar] [CrossRef]
- Carullo, D.; Pataro, G.; Donsì, F.; Ferrari, G. Pulsed Electric Fields-Assisted Extraction of Valuable Compounds from Arthrospira Platensis: Effect of Pulse Polarity and Mild Heating. Front. Bioeng. Biotechnol. 2020, 8, 1–15. [Google Scholar] [CrossRef]
- Khandual, S.; Sanchez, E.O.L.; Andrews, H.E.; de la Rosa, J.D.P. Phycocyanin Content and Nutritional Profile of Arthrospira Platensis from Mexico: Efficient Extraction Process and Stability Evaluation of Phycocyanin. BMC Chem. 2021, 15, 1–13. [Google Scholar] [CrossRef]
- del Pilar Sánchez-Saavedra, M.; Maeda-Martínez, A.N.; Acosta-Galindo, S. Effect of Different Light Spectra on the Growth and Biochemical Composition of Tisochrysis Lutea. J. Appl. Phycol. 2016, 28, 839–847. [Google Scholar] [CrossRef]
- Matsui, H.; Intoy, M.M.B.; Waqalevu, V.; Ishikawa, M.; Kotani, T. Suitability of Tisochrysis Lutea at Different Growth Phases as an Enrichment Diet for Brachionus Plicatilis sp. Complex Rotifers. J. Appl. Phycol. 2020, 32, 3933–3947. [Google Scholar] [CrossRef]
- Marchetti, J.; da Costa, F.; Bougaran, G.; Quéré, C.; Soudant, P.; Robert, R. The Combined Effects of Blue Light and Dilution Rate on Lipid Class and Fatty Acid Composition of Tisochrysis Lutea. J. Appl. Phycol. 2018, 30, 1483–1494. [Google Scholar] [CrossRef]
- Gao, F.; Cabanelas, I.T.D.; Wijffels, R.H.; Barbosa, M.J. Fucoxanthin and Docosahexaenoic Acid Production by Cold-Adapted Tisochrysis Lutea. New Biotechnol. 2022, 66, 16–24. [Google Scholar] [CrossRef]
- Mohamadnia, S.; Tavakoli, O.; Faramarzi, M.A. Production of Fucoxanthin from the Microalga Tisochrysis Lutea in the Bubble Column Photobioreactor Applying Mass Transfer Coefficient. J. Biotechnol. 2022, 348, 47–54. [Google Scholar] [CrossRef]
- Hernández-López, I.; Benavente Valdés, J.R.; Castellari, M.; Aguiló-Aguayo, I.; Morillas-España, A.; Sánchez-Zurano, A.; Acién-Fernández, F.G.; Lafarga, T. Utilisation of the Marine Microalgae Nannochloropsis sp. and Tetraselmis sp. as Innovative Ingredients in the Formulation of Wheat Tortillas. Algal Res. 2021, 58, 102361. [Google Scholar] [CrossRef]
- Magpusao, J.; Giteru, S.; Oey, I.; Kebede, B. Effect of High Pressure Homogenization on Microstructural and Rheological Properties of A. Platensis, Isochrysis, Nannochloropsis and Tetraselmis Species. Algal Res. 2021, 56, 102327. [Google Scholar] [CrossRef]
- Khatoon, H.; Penz, K.R.; Banerjee, S.; Rahman, M.R.; Minhaz, T.M.; Islam, Z.; Mukta, F.A.; Nayma, Z.; Sultana, R.; Amira, K.I. Immobilized Tetraselmis sp. for Reducing Nitrogenous and Phosphorous Compounds from Aquaculture Wastewater. Bioresour. Technol. 2021, 338, 125529. [Google Scholar] [CrossRef]
- Farahin, A.W.; Natrah, I.; Nagao, N.; Katayama, T.; Imaizumi, Y.; Mamat, N.Z.; Yusoff, F.M.; Shariff, M. High Intensity of Light: A Potential Stimulus for Maximizing Biomass by Inducing Photosynthetic Activity in Marine Microalga, Tetraselmis Tetrathele. Algal Res. 2021, 60, 102523. [Google Scholar] [CrossRef]
- Souto, M.; Saavedra, M.; Pousão-Ferreira, P.; Herrero, C. Riboflavin Enrichment throughout the Food Chain from the Marine Microalga Tetraselmis Suecica to the Rotifer Brachionus Plicatilis and to White Sea Bream (Diplodus Sargus) and Gilthead Sea Bream (Sparus Aurata) Larvae. Aquaculture 2008, 283, 128–133. [Google Scholar] [CrossRef]
- Araújo, R.; Vázquez Calderón, F.; Sánchez López, J.; Azevedo, I.C.; Bruhn, A.; Fluch, S.; Garcia Tasende, M.; Ghaderiardakani, F.; Ilmjärv, T.; Laurans, M.; et al. Current Status of the Algae Production Industry in Europe: An Emerging Sector of the Blue Bioeconomy. Front. Mar. Sci. 2021, 7, 626389. [Google Scholar] [CrossRef]
- Nascimento, I.A.; Marques, S.S.I.; Cabanelas, I.T.D.; de Carvalho, G.C.; Nascimento, M.A.; de Souza, C.O.; Druzian, J.I.; Hussain, J.; Liao, W. Microalgae Versus Land Crops as Feedstock for Biodiesel: Productivity, Quality, and Standard Compliance. BioEnergy Res. 2014, 7, 1002–1013. [Google Scholar] [CrossRef]
- Neofotis, P.; Huang, A.; Sury, K.; Chang, W.; Joseph, F.; Gabr, A.; Twary, S.; Qiu, W.; Holguin, O.; Polle, J.E.W. Characterization and Classification of Highly Productive Microalgae Strains Discovered for Biofuel and Bioproduct Generation. Algal Res. 2016, 15, 164–178. [Google Scholar] [CrossRef]
- Hobbs, W.O.; Telford, R.J.; Birks, H.J.B.; Saros, J.E.; Hazewinkel, R.R.O.; Perren, B.B.; Saulnier-Talbot, É.; Wolfe, A.P. Quantifying Recent Ecological Changes in Remote Lakes of North America and Greenland Using Sediment Diatom Assemblages. PLoS ONE 2010, 5, e10026. [Google Scholar] [CrossRef] [PubMed]
- Dunck, B.; Felisberto, S.A.; de Souza Nogueira, I. Effects of Freshwater Eutrophication on Species and Functional Beta Diversity of Periphytic Algae. Hydrobiologia 2019, 837, 195–204. [Google Scholar] [CrossRef]
- Schuster, K.F.; Tremarin, P.I.; de Souza-Franco, G.M. Alpha and beta diversity of phytoplankton in two subtropical eutrophic streams in southern Brazil. Acta Bot. Bras. 2015, 29, 597–607. [Google Scholar] [CrossRef][Green Version]
- Gunkel, G.; Casallas, J. Limnology of an Equatorial High Mountain Lake—Lago San Pablo, Ecuador: The Significance of Deep Diurnal Mixing for Lake Productivity. Limnologica 2002, 32, 33–43. [Google Scholar] [CrossRef]
- Zuorro, A.; Leal-Jerez, A.G.; Morales-Rivas, L.K.; Mogollón-Londoño, S.O.; Sanchez-Galvis, E.M.; García-Martínez, J.B.; Barajas-Solano, A.F. Enhancement of Phycobiliprotein Accumulation in Thermotolerant Oscillatoria sp. through Media Optimization. ACS Omega 2021, 6, 10527–10536. [Google Scholar] [CrossRef]
- Sánchez-Zurano, A.; Morillas-España, A.; Gómez-Serrano, C.; Ciardi, M.; Acién, G.; Lafarga, T. Annual Assessment of the Wastewater Treatment Capacity of the Microalga Scenedesmus Almeriensis and Optimisation of Operational Conditions. Sci. Rep. 2021, 11, 21651. [Google Scholar] [CrossRef]
- Lv, J.-M.; Cheng, L.-H.; Xu, X.-H.; Zhang, L.; Chen, H.-L. Enhanced Lipid Production of Chlorella Vulgaris by Adjustment of Cultivation Conditions. Bioresour. Technol. 2010, 101, 6797–6804. [Google Scholar] [CrossRef]
- Mandik, Y.I.; Cheirsilp, B.; Boonsawang, P.; Prasertsan, P. Optimization of Flocculation Efficiency of Lipid-Rich Marine Chlorella sp. Biomass and Evaluation of Its Composition in Different Cultivation Modes. Bioresour. Technol. 2015, 182, 89–97. [Google Scholar] [CrossRef]
- Cuéllar-García, D.J.; Rangel-Basto, Y.A.; Urbina-Suarez, N.A.; Barajas-Solano, A.F.; Muñoz-Peñaloza, Y.A. Lipids production from Scenedesmus obliquus through carbon/nitrogen ratio optimization. J. Phys. Conf. Ser. 2019, 1388, 012043. [Google Scholar] [CrossRef]
- Barajas-Solano, A.F. Optimization of Phycobiliprotein Solubilization from a Thermotolerant Oscillatoria sp. Processes 2022, 10, 836. [Google Scholar] [CrossRef]
- González-Delgado, A.D.; Barajas-Solano, A.F.; Ardila-Álvarez, A.M. Biomass and Protein Production of Chlorella vulgaris Beyerinck (Chlorellales: Chlorellaceae) via the Design of Selective Culture Media. Corpoica Cienc. Tecnol. Agropecu. 2017, 18, 451–461. [Google Scholar] [CrossRef]
- Wang, S.; Cao, M.; Wang, B.; Deng, R.; Gao, Y.; Liu, P. Optimization of growth requirements and scale-up cultivation of freshwater algae Desmodesmus armatus using response surface methodology. Aquacult. Res. 2019, 50, 3313–3325. [Google Scholar] [CrossRef]
- Pauline, J.M.N.; Achary, A. Novel media for lipid production of Chlorococcum oleofaciens: A RSM approach. Acta Protozool. 2019, 58, 31–41. [Google Scholar] [CrossRef]
- Mubarak, M.; Shaija, A.; Suchithra, T.V. Cost effective approach for production of Chlorella pyrenoidosa: A RSM based study. Waste Biomass Valorization 2019, 10, 3307–3319. [Google Scholar] [CrossRef]
- Fawley, M.W.; Fawley, K.P. A Simple and Rapid Technique for The Isolation Of DNA From Microalgae. J. Phycol. 2004, 40, 223–225. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.D.; Lee, S.B.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Proto- Cols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., Whire, T.J., Eds.; Academic Press: London, UK, 1990; pp. 315–322. [Google Scholar]
- Fei, C.; Zou, S.; Wang, T.; Wang, C.; Kemuma, N.D.; He, M.; Amin, S.A.; Wang, C. A Quick Method for Obtaining High-Quality DNA Barcodes without DNA Extraction in Microalgae. J. Appl. Phycol. 2020, 32, 1165–1175. [Google Scholar] [CrossRef]
- Andersen, R.A.; Berges, J.A.; Harrison, P.J.; Watanabe, M.M. Appendix A—Recipes for Freshwater and Seawater Media. In Algal Culturing Techniques; Andersen, R.A., Ed.; Elsevier Academic Press: Burlington, MA, USA, 2005; pp. 429–538. [Google Scholar]
- Sanchez-Galvis, E.M.; Cardenas-Gutierrez, I.Y.; Contreras-Ropero, J.E.; García-Martínez, J.B.; Barajas-Solano, A.F.; Zuorro, A. An Innovative Low-Cost Equipment for Electro-Concentration of Microalgal Biomass. Appl. Sci. 2020, 10, 4841. [Google Scholar] [CrossRef]
- Mishra, S.K.; Suh, W.I.; Farooq, W.; Moon, M.; Shrivastav, A.; Park, M.S.; Yang, J.W. Rapid Quantification of Microalgal Lipids in Aqueous Medium by a Simple Colorimetric Method. Bioresour. Technol. 2014, 155, 330–333. [Google Scholar] [CrossRef]
- García-Martínez, J.B.; Ayala-Torres, E.; Reyes-Gómez, O.; Zuorro, A.; Barajas-Solano, A.F.; Barajas-Ferreira, C. Evaluation of a Two-Phase Extraction System of Carbohydrates and Proteins from Chlorella Vulgaris Utex 1803. Chem. Eng. Trans. 2016, 49, 355–360. [Google Scholar] [CrossRef]
- Mota, M.F.S.; Souza, M.F.; Bon, E.P.S.; Rodrigues, M.A.; Freitas, S.P. Colorimetric Protein Determination in Microalgae (Chlorophyta): Association of Milling and SDS Treatment for Total Protein Extraction. J. Phycol. 2018, 54, 577–580. [Google Scholar] [CrossRef]
- Hynstova, V.; Sterbova, D.; Klejdus, B.; Hedbavny, J.; Huska, D.; Adam, V. Separation, Identification and Quantification of Carotenoids and Chlorophylls in Dietary Supplements Containing Chlorella Vulgaris and Spirulina Platensis Using High-Performance Thin Layer Chromatography. J. Pharm. Biomed. Anal. 2018, 148, 108–118. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Zhao, X.-Q.; Yen, H.-W.; Ho, S.-H.; Cheng, C.-L.; Lee, D.-J.; Bai, F.-W.; Chang, J.-S. Microalgae-Based Carbohydrates for Biofuel Production. Biochem. Eng. J. 2013, 78, 1–10. [Google Scholar] [CrossRef]
- Ruangsomboon, S. Effects of Different Media and Nitrogen Sources and Levels on Growth and Lipid of Green Microalga Botryococcus Braunii KMITL and Its Biodiesel Properties Based on Fatty Acid Composition. Bioresour. Technol. 2015, 191, 377–384. [Google Scholar] [CrossRef]
- Whangchai, K.; Mathimani, T.; Sekar, M.; Shanmugam, S.; Brindhadevi, K.; Van Hung, T.; Chinnathambi, A.; Alharbi, S.A.; Pugazhendhi, A. Synergistic Supplementation of Organic Carbon Substrates for Upgrading Neutral Lipids and Fatty Acids Contents in Microalga. J. Environ. Chem. Eng. 2021, 9, 105482. [Google Scholar] [CrossRef]
- Rehman, Z.U.; Anal, A.K. Enhanced Lipid and Starch Productivity of Microalga (Chlorococcum sp. TISTR 8583) with Nitrogen Limitation Following Effective Pretreatments for Biofuel Production. Biotechnol. Rep. 2019, 21, e00298. [Google Scholar] [CrossRef]
- Xu, J.; Li, T.; Li, C.-L.; Zhu, S.-N.; Wang, Z.-M.; Zeng, E.Y. Lipid Accumulation and Eicosapentaenoic Acid Distribution in Response to Nitrogen Limitation in Microalga Eustigmatos Vischeri JHsu-01 (Eustigmatophyceae). Algal Res. 2020, 48, 101910. [Google Scholar] [CrossRef]
- Zarrinmehr, M.J.; Farhadian, O.; Heyrati, F.P.; Keramat, J.; Koutra, E.; Kornaros, M.; Daneshvar, E. Effect of Nitrogen Concentration on the Growth Rate and Biochemical Composition of the Microalga, Isochrysis Galbana. Egypt. J. Aquat. Res. 2020, 46, 153–158. [Google Scholar] [CrossRef]
- Kokabi, K.; Gorelova, O.; Ismagulova, T.; Itkin, M.; Malitsky, S.; Boussiba, S.; Solovchenko, A.; Khozin-Goldberg, I. Metabolomic Foundation for Differential Responses of Lipid Metabolism to Nitrogen and Phosphorus Deprivation in an Arachidonic Acid-Producing Green Microalga. Plant Sci. 2019, 283, 95–115. [Google Scholar] [CrossRef]
- Chakravarty, S.; Mallick, N. Engineering a Cultivation Strategy for Higher Lipid Accretion and Biodiesel Production by the Marine Microalga Picochlorum Soloecismus. Sustain. Chem. Pharm. 2022, 26, 100635. [Google Scholar] [CrossRef]
- Zuorro, A. Enhanced Lycopene Extraction from Tomato Peels by Optimized Mixed-Polarity Solvent Mixtures. Molecules 2020, 25, 2038. [Google Scholar] [CrossRef] [PubMed]
- Zuorro, A.; Lavecchia, R. Polyphenols and Energy Recovery from Spent Coffee Grounds. Chem. Eng. Trans. 2011, 25, 285–290. [Google Scholar] [CrossRef]
- Zuorro, A.; Iannone, A.; Natali, S.; Lavecchia, R. Green Synthesis of Silver Nanoparticles Using Bilberry and Red Currant Waste Extracts. Processes 2019, 7, 193. [Google Scholar] [CrossRef]
- Montanaro, D.; Lavecchia, R.; Petrucci, E.; Zuorro, A. UV-Assisted Electrochemical Degradation of Coumarin on Boron-Doped Diamond Electrodes. Chem. Eng. J. 2017, 323, 512–519. [Google Scholar] [CrossRef]
- Zuorro, A.; Maffei, G.; Lavecchia, R. Kinetic Modeling of Azo Dye Adsorption on Non-Living Cells of Nannochloropsis Oceanica. J. Environ. Chem. Eng. 2017, 5, 4121–4127. [Google Scholar] [CrossRef]
- Singh, P.; Guldhe, A.; Kumari, S.; Rawat, I.; Bux, F. Investigation of Combined Effect of Nitrogen, Phosphorus and Iron on Lipid Productivity of Microalgae Ankistrodesmus Falcatus KJ671624 Using Response Surface Methodology. Biochem. Eng. J. 2015, 94, 22–29. [Google Scholar] [CrossRef]
- Polat, E.; Yüksel, E.; Altınbaş, M. Mutual Effect of Sodium and Magnesium on the Cultivation of Microalgae Auxenochlorella Protothecoides. Biomass Bioenergy 2020, 132, 105441. [Google Scholar] [CrossRef]
- Vishwakarma, R.; Dhar, D.W.; Pabbi, S. Formulation of a Minimal Nutritional Medium for Enhanced Lipid Productivity in Chlorella sp. and Botryococcus sp. Using Response Surface Methodology. Water Sci. Technol. 2018, 77, 1660–1672. [Google Scholar] [CrossRef]
- Tran, H.-L.; Kwon, J.-S.; Kim, Z.-H.; Oh, Y.; Lee, C.-G. Statistical Optimization of Culture Media for Growth and Lipid Production of Botryococcus Braunii LB572. Biotechnol. Bioprocess Eng. 2010, 15, 277–284. [Google Scholar] [CrossRef]
- Cheng, K.C.; Ren, M.; Ogden, K.L. Statistical Optimization of Culture Media for Growth and Lipid Production of Chlorella protothecoides UTEX 250. Bioresour. Technol. 2013, 128, 44–48. [Google Scholar] [CrossRef]
- León-Vaz, A.; León, R.; Díaz-Santos, E.; Vigara, J.; Raposo, S. Using Agro-Industrial Wastes for Mixotrophic Growth and Lipids Production by the Green Microalga Chlorella Sorokiniana. New Biotechnol. 2019, 51, 31–38. [Google Scholar] [CrossRef]
- Li, T.; Zheng, Y.; Yu, L.; Chen, S. Mixotrophic Cultivation of a Chlorella Sorokiniana Strain for Enhanced Biomass and Lipid Production. Biomass Bioenergy 2014, 66, 204–213. [Google Scholar] [CrossRef]
- Ra, C.H.; Kang, C.-H.; Kim, N.K.; Lee, C.-G.; Kim, S.-K. Cultivation of Four Microalgae for Biomass and Oil Production Using a Two-Stage Culture Strategy with Salt Stress. Renew. Energy 2015, 80, 117–122. [Google Scholar] [CrossRef]
- Fawzy, M.A.; Alharthi, S. Use of Response Surface Methodology in Optimization of Biomass, Lipid Productivity and Fatty Acid Profiles of Marine Microalga Dunaliella Parva for Biodiesel Production. Environ. Technol. Innov. 2021, 22, 101485. [Google Scholar] [CrossRef]
- Pandey, A.; Gupta, A.; Sunny, A.; Kumar, S.; Srivastava, S. Multi-Objective Optimization of Media Components for Improved Algae Biomass, Fatty Acid and Starch Biosynthesis from Scenedesmus sp. ASK22 Using Desirability Function Approach. Renew. Energy 2020, 150, 476–486. [Google Scholar] [CrossRef]
- Choix, F.J.; De-Bashan, L.E.; Bashan, Y. Enhanced Accumulation of Starch and Total Carbohydrates in Alginate-Immobilized Chlorella Spp. Induced by Azospirillum Brasilense: I. Autotrophic Conditions. Enzyme Microb. Technol. 2012, 51, 294–299. [Google Scholar] [CrossRef]
- Ho, S.-H.; Chen, C.-Y.; Yeh, K.-L.; Chen, W.-M.; Lin, C.-Y.; Chang, J.-S. Characterization of Photosynthetic Carbon Dioxide Fixation Ability of Indigenous Scenedesmus Obliquus Isolates. Biochem. Eng. J. 2010, 53, 57–62. [Google Scholar] [CrossRef]
- de Farias Silva, C.E.; Sforza, E.; Bertucco, A. Stability of Carbohydrate Production in Continuous Microalgal Cultivation under Nitrogen Limitation: Effect of Irradiation Regime and Intensity on Tetradesmus Obliquus. J. Appl. Phycol. 2018, 30, 261–270. [Google Scholar] [CrossRef]
- McClain, A.M.; Sharkey, T.D. Triose Phosphate Utilization and beyond: From Photosynthesis to End Product Synthesis. J. Exp. Bot. 2019, 70, 1755–1766. [Google Scholar] [CrossRef]
- Ghosh, A.; Samadhiya, K.; Kashyap, M.; Anand, V.; Sangwan, P.; Bala, K. The Use of Response Surface Methodology for Improving Fatty Acid Methyl Ester Profile of Scenedesmus Vacuolatus. Environ. Sci. Pollut. Res. 2020, 27, 27457–27469. [Google Scholar] [CrossRef] [PubMed]
- González-Fernández, C.; Ballesteros, M. Linking Microalgae and Cyanobacteria Culture Conditions and Key-Enzymes for Carbohydrate Accumulation. Biotechnol. Adv. 2012, 30, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Dragone, G.; Fernandes, B.D.; Abreu, A.P.; Vicente, A.A.; Teixeira, J.A. Nutrient Limitation as a Strategy for Increasing Starch Accumulation in Microalgae. Appl. Energy 2011, 88, 3331–3335. [Google Scholar] [CrossRef]
- Muthuraj, M.; Kumar, V.; Palabhanvi, B.; Das, D. Evaluation of Indigenous Microalgal Isolate Chlorella sp. FC2 IITG as a Cell Factory for Biodiesel Production and Scale up in Outdoor Conditions. J. Ind. Microbiol. Biotechnol. 2014, 41, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Tourang, M.; Baghdadi, M.; Torang, A.; Sarkhosh, S. Optimization of Carbohydrate Productivity of Spirulina Microalgae as a Potential Feedstock for Bioethanol Production. Int. J. Environ. Sci. Technol. 2019, 16, 1303–1318. [Google Scholar] [CrossRef]
- Li-Beisson, Y.; Beisson, F.; Riekhof, W. Metabolism of Acyl-Lipids in Chlamydomonas Reinhardtii. Plant J. 2015, 82, 504–522. [Google Scholar] [CrossRef]
- Chakravarty, S.; Mallick, N. Optimization of Lipid Accumulation in an Aboriginal Green Microalga Selenastrum sp. GA66 for Biodiesel Production. Biomass Bioenergy 2019, 126, 1–13. [Google Scholar] [CrossRef]
- Schüler, L.M.; Santos, T.; Pereira, H.; Duarte, P.; Katkam, N.G.; Florindo, C.; Schulze, P.S.C.; Barreira, L.; Varela, J.C.S. Improved Production of Lutein and β-Carotene by Thermal and Light Intensity Upshifts in the Marine Microalga Tetraselmis sp. CTP4. Algal Res. 2020, 45, 101732. [Google Scholar] [CrossRef]
- Aburai, N.; Sumida, D.; Abe, K. Effect of Light Level and Salinity on the Composition and Accumulation of Free and Ester-Type Carotenoids in the Aerial Microalga Scenedesmus sp. (Chlorophyceae). Algal Res. 2015, 8, 30–36. [Google Scholar] [CrossRef]
- de Souza da Silva, S.P.; Perrone, D.; do Valle, A.F. Optimization of Arthrospira Maxima Cultivation for Biomass and Protein Production and Biomass Technological Treatment to Color, Flavor, and Aroma Masking for Addition to Food Products. J. Appl. Phycol. 2022, 34, 65–80. [Google Scholar] [CrossRef]
- Kumaran, J.; Poulose, S.; Joseph, V.; Bright Singh, I.S. Enhanced Biomass Production and Proximate Composition of Marine Microalga Nannochloropsis Oceanica by Optimization of Medium Composition and Culture Conditions Using Response Surface Methodology. Anim. Feed Sci. Technol. 2021, 271, 114761. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).