A Conventional VOC-PID Sensor for a Rapid Discrimination among Aromatic Plant Varieties: Classification Models Fitted to a Rosemary Case-Study
Abstract
:1. Introduction
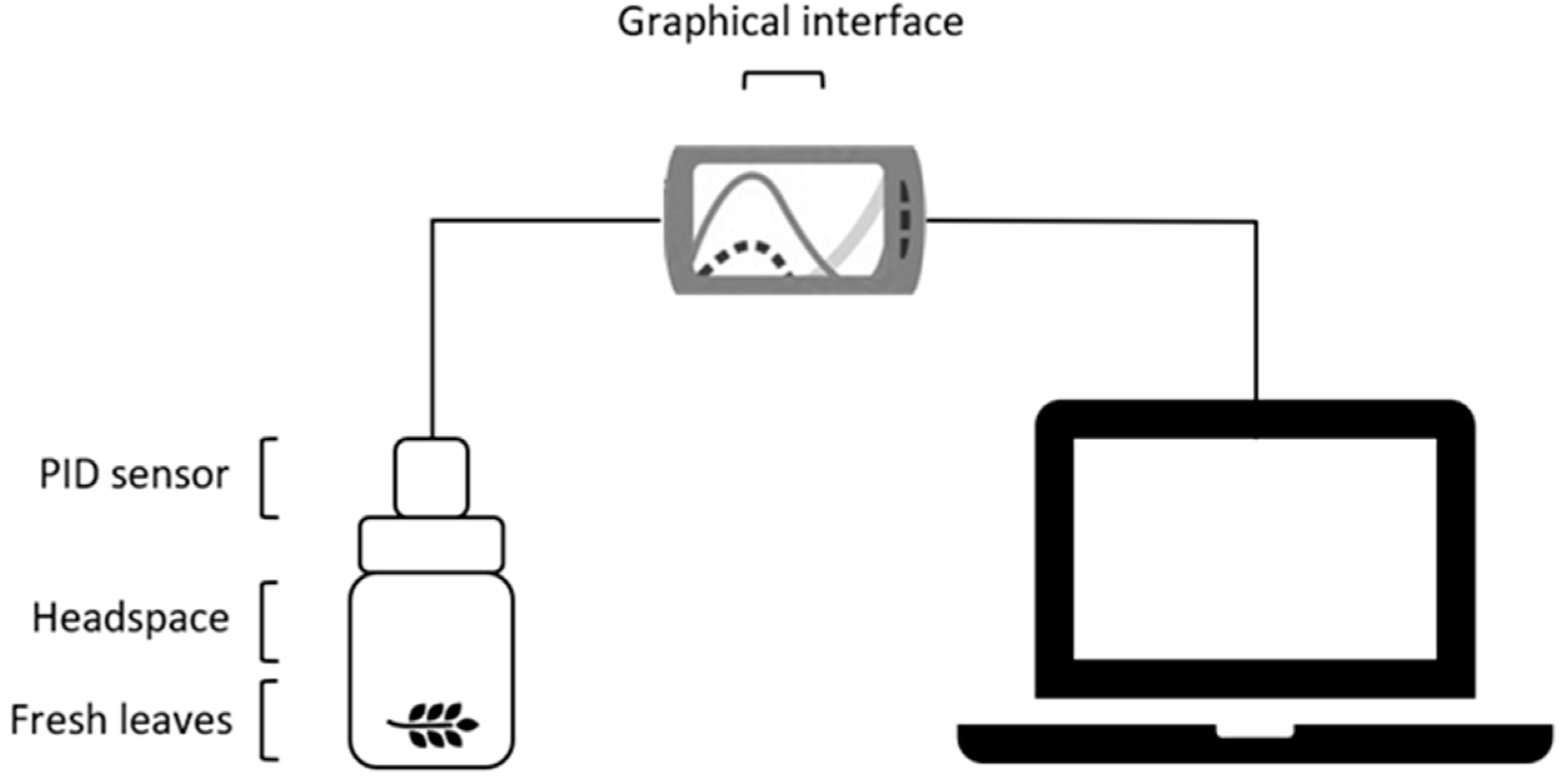
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. GC-MS Analysis
2.4. Statistical Analysis
- -
- α, β, and γ (the exponential model parameters)
- -
- GM (the grand mean of readings computed over a data series acquisition time)
- -
- GMsd (standard deviation of readings computed over a data series acquisition time)
- -
- Max (the maximum signal reading)
- -
- AUC (the area under the curve).
3. Results
3.1. Essential Oil Characterization
3.2. PID Measurements
3.3. Data Analysis
3.3.1. Principal Component Analysis
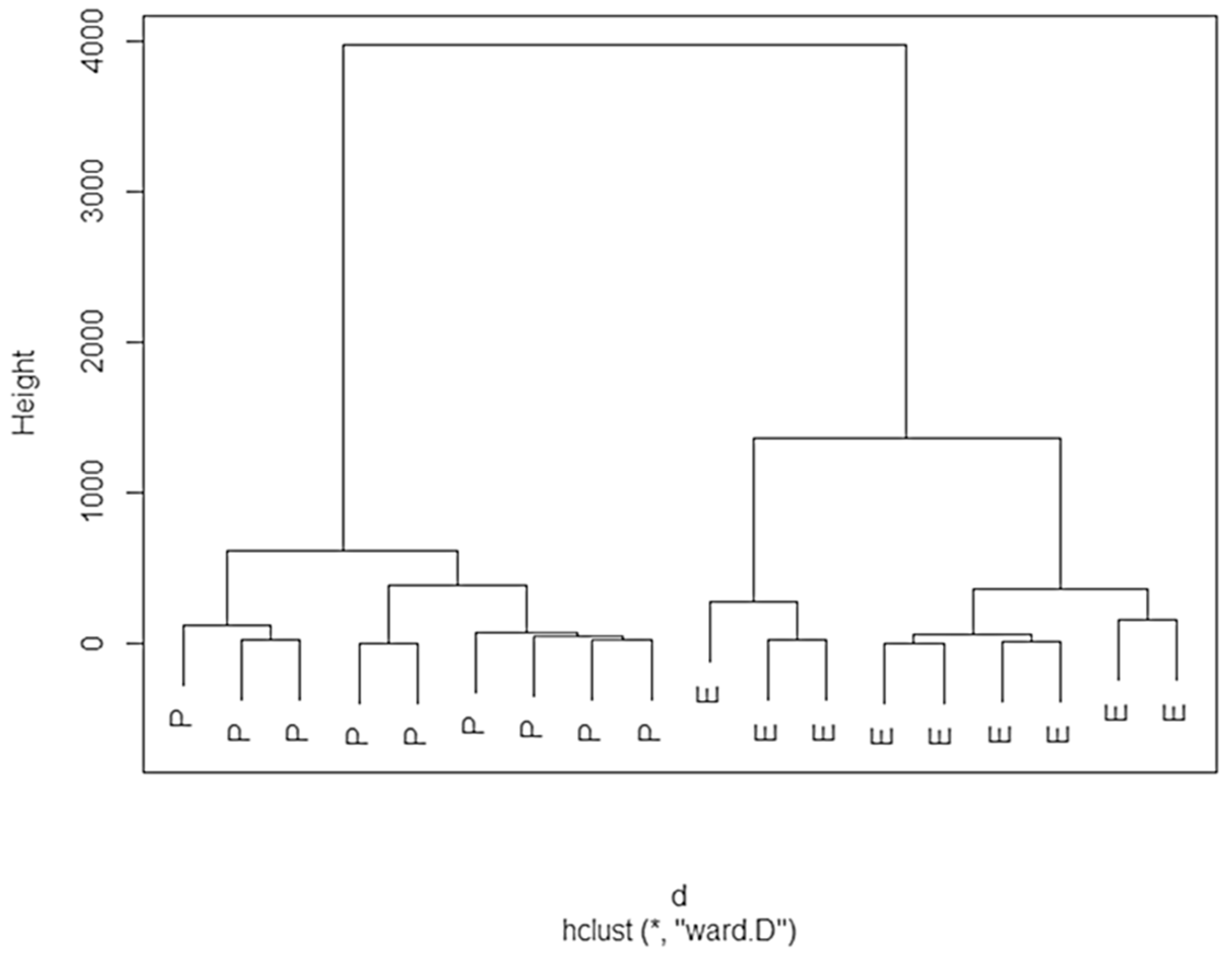
3.3.2. Cluster Analysis
3.4. Support Vector Machine
3.5. Artificial Neural Networks
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Werker, E. Trichome Diversity and Development Department of Botany, The Hebrew University of Jerusalem. Adv. Bot. Res. 2000, 31, 1–35. [Google Scholar]
- Wheatley, R.E. The Consequences of Volatile Organic Compound Mediated Bacterial and Fungal Interactions. Antonie Van Leeuwenhoek 2002, 81, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Borges, R.S.; Ortiz, B.L.S.; Pereira, A.C.M.; Keita, H.; Carvalho, J.C.T. Rosmarinus Officinalis Essential Oil: A Review of Its Phytochemistry, Anti-Inflammatory Activity, and Mechanisms of Action Involved. J. Ethnopharmacol. 2019, 229, 29–45. [Google Scholar] [CrossRef]
- Ribeiro-Santos, R.; Carvalho-Costa, D.; Cavaleiro, C.; Costa, H.S.; Albuquerque, T.G.; Castilho, M.C.; Ramos, F.; Melo, N.R.; Sanches-Silva, A. A Novel Insight on an Ancient Aromatic Plant: The Rosemary (Rosmarinus Officinalis L.). Trends Food Sci. Technol. 2015, 45, 355–368. [Google Scholar] [CrossRef]
- Boutekedjiret, C.; Bentahar, F.; Belabbes, R.; Bessiere, J.M. The Essential Oil from Rosmarinus Officinalis L. in Algeria. J. Essent. Oil Res. 1998, 10, 680–682. [Google Scholar] [CrossRef]
- Sharma, Y.; Schaefer, J.; Streicher, C.; Stimson, J.; Fagan, J. Qualitative Analysis of Essential Oil from French and Italian Varieties of Rosemary (Rosmarinus Officinalis L.) Grown in the Midwestern United States. Anal. Chem. Lett. 2020, 10, 104–112. [Google Scholar] [CrossRef]
- Zaouali, Y.; Bouzaine, T.; Boussaid, M. Essential Oils Composition in Two Rosmarinus Officinalis L. Varieties and Incidence for Antimicrobial and Antioxidant Activities. Food Chem. Toxicol. 2010, 48, 3144–3152. [Google Scholar] [CrossRef]
- Fadel, O.; El Kirat, K.; Morandat, S. The Natural Antioxidant Rosmarinic Acid Spontaneously Penetrates Membranes to Inhibit Lipid Peroxidation in Situ. Biochim. Biophys. Acta Biomembr. 2011, 1808, 2973–2980. [Google Scholar] [CrossRef] [Green Version]
- Benbelaïd, F.; Khadir, A.; Bendahou, M.; Zenati, F.; Bellahsene, C.; Muselli, A.; Costa, J. Antimicrobial Activity of Rosmarinus Eriocalyx Essential Oil and Polyphenols: An Endemic Medicinal Plant from Algeria. J. Coast. Life Med. 2016, 4, 39–44. [Google Scholar] [CrossRef]
- Agbroko, S.O.; Covington, J. A Novel, Low-Cost, Portable PID Sensor for the Detection of Volatile Organic Compounds. Sens. Actuators B Chem. 2018, 275, 10–15. [Google Scholar] [CrossRef] [Green Version]
- Laothawornkitkul, J.; Moore, J.P.; Taylor, J.E.; Possell, M.; Gibson, T.D.; Hewitt, C.N.; Paul, N.D. Discrimination of Plant Volatile Signatures by an Electronic Nose: A Potential Technology for Plant Pest and Disease Monitoring. Environ. Sci. Technol. 2008, 42, 8433–8439. [Google Scholar] [CrossRef] [PubMed]
- Kiani, S.; Minaei, S.; Ghasemi-Varnamkhasti, M. Application of Electronic Nose Systems for Assessing Quality of Medicinal and Aromatic Plant Products: A Review. J. Appl. Res. Med. Aromat. Plants 2016, 3, 1–9. [Google Scholar] [CrossRef]
- Capone, S.; Epifani, M.; Quaranta, F.; Siciliano, P.; Taurino, A.; Vasanelli, L. Monitoring of Rancidity of Milk by Means of an Electronic Nose and a Dynamic PCA Analysis. Sens. Actuators B Chem. 2001, 78, 174–179. [Google Scholar] [CrossRef]
- Costache, G.N.; Corcoran, P.; Puslecki, P. Combining PCA-Based Datasets without Retraining of the Basis Vector Set. Pattern Recognit. Lett. 2009, 30, 1441–1447. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, J. Discrimination of Different Types Damage of Rice Plants by Electronic Nose. Biosyst. Eng. 2011, 109, 250–257. [Google Scholar] [CrossRef]
- Sanaeifar, A.; Mohtasebi, S.S.; Ghasemi-Varnamkhasti, M.; Ahmadi, H.; Lozano, J. Development and Application of a New Low Cost Electronic Nose for the Ripeness Monitoring of Banana Using Computational Techniques (PCA, LDA, SIMCA, and SVM). Czech J. Food Sci. 2014, 32, 538–548. [Google Scholar] [CrossRef] [Green Version]
- Huang, R.S.P.; Nedelcu, E.; Bai, Y.; Wahed, A.; Klein, K.; Tint, H.; Gregoric, I.; Patel, M.; Kar, B.; Loyalka, P.; et al. Post-Operative Bleeding Risk Stratification in Cardiac Pulmonary Bypass Patients Using Artificial Neural Network. Ann. Clin. Lab. Sci. 2015, 45, 181–186. [Google Scholar]
- Lozano, J.; Santos, J.P.; Horrillo, M.C. Classification of White Wine Aromas with an Electronic Nose. Talanta 2005, 67, 610–616. [Google Scholar] [CrossRef]
- Mazzocchi, C.; Corsi, S.; Sali, G. Agricultural Land Consumption in Periurban Areas: A Methodological Approach for Risk Assessment Using Artificial Neural Networks and Spatial Correlation in Northern Italy. Appl. Spat. Anal. Policy 2017, 10, 3–20. [Google Scholar] [CrossRef]
- Xu, W.; Cai, Y.; Gao, S.; Hou, S.; Yang, Y.; Duan, Y.; Fu, Q.; Chen, F.; Wu, J. New Understanding of Miniaturized VOCs Monitoring Device: PID-Type Sensors Performance Evaluations in Ambient Air. Sens. Actuators B Chem. 2021, 330, 129285. [Google Scholar] [CrossRef]
- Khorramifar, A.; Karami, H.; Wilson, A.D.; Sayyah, A.H.A.; Shuba, A.; Lozano, J. Grape Cultivar Identification and Classification by Machine Olfaction Analysis of Leaf Volatiles. Chemosensors 2022, 10, 125. [Google Scholar] [CrossRef]
- Khorramifar, A.; Rasekh, M.; Karami, H.; Malaga-Toboła, U.; Gancarz, M. A Machine Learning Method for Classification and Identification of Potato Cultivars Based on the Reaction of MOS Type Sensor-Array. Sensors 2021, 21, 5836. [Google Scholar] [CrossRef] [PubMed]
- Gorji-Chakespari, A.; Nikbakht, A.M.; Sefidkon, F.; Ghasemi-Varnamkhasti, M.; Valero, E.L. Classification of Essential Oil Composition in Rosa Damascena Mill. Genotypes Using an Electronic Nose. J. Appl. Res. Med. Aromat. Plants 2017, 4, 27–34. [Google Scholar] [CrossRef]
- Tholl, D.; Hossain, O.; Weinhold, A.; Röse, U.S.R.; Wei, Q. Trends and Applications in Plant Volatile Sampling and Analysis. Plant J. 2021, 106, 314–325. [Google Scholar] [CrossRef]
- Li, G.; Cervelli, C.; Ruffoni, B.; Shachter, A.; Dudai, N. Volatile Diversity in Wild Populations of Rosemary (Rosmarinus Officinalis L.) from the Tyrrhenian Sea Vicinity Cultivated under Homogeneous Environmental Conditions. Ind. Crops Prod. 2016, 84, 381–390. [Google Scholar] [CrossRef]
- Carrubba, A.; Abbate, L.; Sarno, M.; Sunseri, F.; Mauceri, A.; Lupini, A.; Mercati, F. Characterization of Sicilian Rosemary (Rosmarinus Officinalis L.) Germplasm through a Multidisciplinary Approach. Planta 2020, 251, 37. [Google Scholar] [CrossRef]
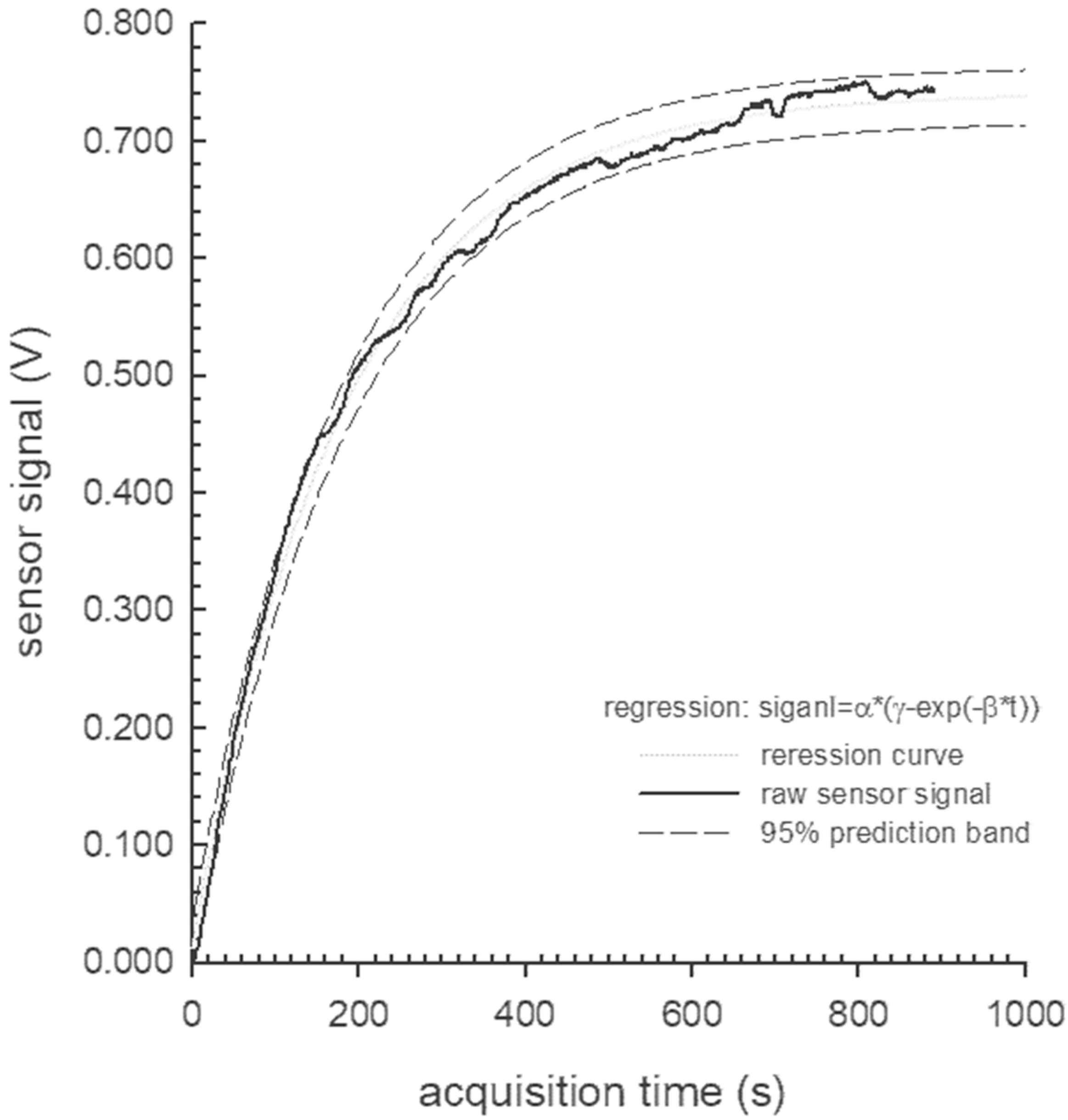




| Dynamic Fit Options | |||||
|---|---|---|---|---|---|
| Total Number of Fits | 200 | ||||
| Maximum Number of Iterations | 200 | ||||
| Parameter Ranges for Initial Estimates | |||||
| Minimum | Maximum | ||||
| α | −1.0000 | 3.0000 | |||
| β | 0.0000 | 3.0000 | |||
| γ | −1.0000 | 3.0000 | |||
| Summary of Fit Results | |||||
| Converged | 87.0% | ||||
| Singular Solutions | 15.0% | ||||
| Ill-Conditioned Solutions | 14.5% | ||||
| Iterations Exceeding 200 | 13.0% | ||||
| Results for the Overall Best-Fit Solution | |||||
| R | R2 | Adj R2 | Standard Error of Estimate | ||
| 0.9978 | 0.9957 | 0.9956 | 0.0119 | ||
| Coefficient | Std. Error | t | P | ||
| α | 0.7282 | 0.0018 | 413.2867 | <0.0001 | |
| β | 0.0055 | 2.9060 × 10−5 | 188.1148 | <0.0001 | |
| γ | 1.0166 | 0.0026 | 387.0196 | <0.0001 | |
| Analysis of Variance | |||||
| Uncorrected for the mean of the observations | |||||
| DF | SS | MS | |||
| Regression | 3 | 341.3877 | 113.7959 | ||
| Residual | 889 | 0.1268 | 0.0001 | ||
| Total | 892 | 341.5145 | 0.3829 | ||
| Corrected for the mean of the observations | |||||
| DF | SS | MS | F | P | |
| Regression | 2 | 29.0691 | 14.5345 | 101,941.7917 | <0.0001 |
| Residual | 889 | 0.1268 | 0.0001 | ||
| Total | 891 | 29.1958 | 0.0328 | ||
| Compound. | Rt (min) | Variety (%) | |
|---|---|---|---|
| “Prostratus” | “Erectus” | ||
| alpha-pinene | 8.6 | 35.30 ± 1.50 a | 13.17 ± 0.85 b |
| camphene | 9.3 | 8.07 ± 0.30 b | 10.02 ± 0.70 a |
| cis-pinocamphone | 12.2 | 1.74 ± 0.31 b | 2.51 ± 0.22 a |
| (+)-4-Carene | 12.5 | 1.78 ± 0.11 a | 0.70 ± 0.05 b |
| limonene | 13.3 | 1.77 ± 0.17 a | 0.56 ± 0.14 b |
| eucalyptol | 13.4 | 2.57 ± 1.50 a | 1.29 ± 0.36 a |
| alpha-terpinene | 15.2 | 1.09 ± 0.19 a | 0.04 ± 0.05 b |
| alpha-phellandrene | 15.9 | 1.98 ± 0.40 a | 0.42 ± 0.07 b |
| p-cymene | 18.1 | 3.48 ± 1.82 a | 2.18 ± 0.90 a |
| camphor | 21.9 | 8.28 ± 0.42 a | 8.10 ± 0.35 b |
| linalool | 22.2 | 1.49 ± 0.38 a | 0.11 ± 0.15 b |
| humulene | 25.8 | 1.42 ± 0.21 a | 1.29 ± 0.15 b |
| 4-ol-terpinen | 26.1 | 2.31 ± 0.27 b | 4.40 ± 1.10 a |
| bornyl acetate | 26.1 | 13.31 ± 0.62 b | 30.40 ± 1.71 a |
| endo-borneol | 26.2 | 2.65 ± 0.71 b | 8.78 ± 0.52 a |
| alpha-terpineol | 28.6 | 7.41 ± 1.26 a | 7.26 ± 0.61 a |
| eugenol | 33.1 | 1.52 ± 0.66 b | 3.99 ± 0.86 a |
| compound identified (%) | 96.17 | 95.22 | |
| From\to | E | P | Total | % Correct |
|---|---|---|---|---|
| E | 6 | 0 | 6 | 100.00% |
| P | 0 | 6 | 6 | 100.00% |
| Total | 6 | 6 | 12 | 100.00% |
| From\to | E | P | Total | % Correct |
|---|---|---|---|---|
| E | 3 | 0 | 3 | 100.00% |
| P | 1 | 2 | 3 | 66.67% |
| Total | 4 | 2 | 6 | 83.33% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spadi, A.; Angeloni, G.; Guerrini, L.; Corti, F.; Maioli, F.; Calamai, L.; Parenti, A.; Masella, P. A Conventional VOC-PID Sensor for a Rapid Discrimination among Aromatic Plant Varieties: Classification Models Fitted to a Rosemary Case-Study. Appl. Sci. 2022, 12, 6399. https://doi.org/10.3390/app12136399
Spadi A, Angeloni G, Guerrini L, Corti F, Maioli F, Calamai L, Parenti A, Masella P. A Conventional VOC-PID Sensor for a Rapid Discrimination among Aromatic Plant Varieties: Classification Models Fitted to a Rosemary Case-Study. Applied Sciences. 2022; 12(13):6399. https://doi.org/10.3390/app12136399
Chicago/Turabian StyleSpadi, Agnese, Giulia Angeloni, Lorenzo Guerrini, Ferdinando Corti, Francesco Maioli, Luca Calamai, Alessandro Parenti, and Piernicola Masella. 2022. "A Conventional VOC-PID Sensor for a Rapid Discrimination among Aromatic Plant Varieties: Classification Models Fitted to a Rosemary Case-Study" Applied Sciences 12, no. 13: 6399. https://doi.org/10.3390/app12136399
APA StyleSpadi, A., Angeloni, G., Guerrini, L., Corti, F., Maioli, F., Calamai, L., Parenti, A., & Masella, P. (2022). A Conventional VOC-PID Sensor for a Rapid Discrimination among Aromatic Plant Varieties: Classification Models Fitted to a Rosemary Case-Study. Applied Sciences, 12(13), 6399. https://doi.org/10.3390/app12136399








