The Effect of Soil Amendments on Trace Elements’ Bioavailability and Toxicity to Earthworms in Contaminated Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.1.1. Soil Amendments
- Zabrze compost (ZC): Produced in Zabrze in pile-composting process, based on a range of biodegradable-waste types (green waste, kitchen waste, and municipal biosolids) (dry compost dose equal to 10% of soil mass);
- GWDA compost (GC): Produced by GWDA Piła in a pile-composting process using mixture of biosolids, food waste, municipal green waste, and agricultural biodegradable waste as a substrate (dry compost dose equal to 10% of soil weight);
- Bełchatów biosolids (BB): Produced in a municipal sewage treatment plant in Bełchatów (dry sludge dose equal to 10% of the soil mass);
- Grabów biosolids (GB): Produced in the small municipal sewage treatment in Grabów on Pilica river (dry sludge dose equal to 10% of soil mass);
- Calcium phosphate (CP): Added to soil as reagent-grade calcium phosphate CaHPO4 (dose of phosphorus equal to 2% of soil weight);
- Iron oxide (IO): Added to soil as amorphous iron oxide in the form of reagent grade Fe(OH)3 (a dose of iron equal to 2% of soil weight);
- Bentonite (BE): A fossil aluminium phyllosilicate clay, consisting mostly of montmorillonite (dose of dry matter equal to 5% of soil weight);
- Rock waste (RW): Rock waste from mining process with a high content of clay minerals (dry matter dose of material equal to 10% of soil mass);
- Limestone (CC): Added to soil as reagent-grade calcium carbonate (a dose of calcium carbonate equal to 10% of the weight of the soil).
2.1.2. Earthworms
2.2. Sample Analysis
2.2.1. Soil Analysis
2.2.2. Earthworm Analysis
2.3. Statistical Analysis
3. Results and Discussion
3.1. Earthworm Survival and Growth in Amended Soils
3.2. Effect of Applied Amendments on Trace-Element Accumulation in Earthworms
3.3. Influence of Amendments and Earthworm Activity on Soil pH
3.4. Solubility of Trace Elements in Soils Driven by Applied Amendments and Earthworm Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shen, F.; Liao, R.; Ali, A.; Mahar, A.; Guo, D.; Li, R.; Xining, S.; Awasthi, M.K.; Wang, Q.; Zhang, Z. Spatial distribution and risk assessment of heavy metals in soil near a Pb/Zn smelter in Feng County; China. Ecotoxicol. Environ. Saf. 2017, 139, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Kumpiene, J.; Lagerkvist, A.; Maurice, C. Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments—A review. J. Waste Manag. 2008, 28, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Bolan, N.; Kunhikrishnan, A.; Thangarajan, R.; Kumpiene, J.; Park, J.; Makino, T.; Kirkham, M.B.; Scheckel, K. Remediation of heavy metal(loid)s contaminated soils-to mobilize or to immobilize? J. Hazard. Mater. 2014, 266, 141–166. [Google Scholar] [CrossRef] [PubMed]
- NRC Committee (National Research Council Committee on Bioavailability of Contaminants in Soils and Sediments). Bioavailability of Contaminants in Soils and Sediments: Processes, Tools, and Applications; The National Academic Press: Washington, DC, USA, 2003. [Google Scholar]
- US EPA (United States Environment Protection Agency). Ecological Soil Screening Level (Eco-SSL) Guidance and Documents. Washington DC. 2005. Available online: http://www.epa.gov/oswer/riskassessment/ecorisk/ecossl.htm (accessed on 24 November 2014).
- JoL 2016 item 1395. Regulation of Minister of Environmental Protection from 1th September 2016 on Assessment of Soil Surface Pollution. Available online: http://prawo.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20160001395 (accessed on 17 June 2020).
- Ding, Q.; Cheng, G.; Wang, Y.; Zhuang, D.F. Effects of natural factors on the spatial distribution of heavy metals in soils surrounding mining regions. Sci. Total Environ. 2017, 578, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Lanno, R.; Wells, J.; Conder, J.; Bradham, K.; Basta, N. The bioavailability of chemicals in soil for earthworms. Ecotoxicol. Environ. Saf. 2004, 57, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Semple, K.T.; Doick, K.J.; Jones, K.C.; Burauel, P.; Craven, A.; Harms, H. Defining bioavailability and bioaccessibility of contaminated soil and sediment is complicated. Environ. Sci. Technol. 2004, 38, 228A–231A. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradham, K.; Dayton, E.; Basta, N.; Schroder, J.; Payton, M.; Lanno, R. Effect of soil properties on lead bioavailability and toxicity to earthworms. Environ. Toxicol. Chem. 2006, 25, 769–775. [Google Scholar] [CrossRef]
- Violante, A.; Pigna, M. Sorption-Desorption Processes of Metals and Metalloids in Soil Environments. In Proceedings of the 5th International Symposium ISMOM 2008, Pucón, Chile, 24–28 November 2008. [Google Scholar]
- Lemtiri, A.; Liénard, A.; Alabi, T.; Brostaux, Y.; Cluzeau, D.; Francis, F.; Colinet, G. Earthworms Eisenia fetida affect the uptake of heavy metals by plants Vicia faba and Zea mays in metal-contaminated soils. Appl. Soil Ecol. 2016, 104, 67–78. [Google Scholar] [CrossRef]
- Wang, K.; Qiao, Y.; Zhang, H.; Yue, S.; Li, H.; Ji, X.; Liu, L. Bioaccumulation of heavy metals in earthworms from field contaminated soil in a subtropical area of China. Ecotoxicol. Environ. Saf. 2018, 148, 876–883. [Google Scholar] [CrossRef]
- Kiikkila, O. Remediation through Mulching with Organic Matter of Soil Polluted by a Copper-Nickel Smelter; Academic Dissertation in Environmental Protection Science Faculty of Agriculture and Forestry University of Helsinki: Helsinki, Finland, 2002. [Google Scholar]
- Tandy, S.; Healey, J.; Nason, M.; Williamson, J.; Jones, D. Remediation of metal polluted mine soil with compost: Co-composting versus incorporation. Environ. Pollut. 2009, 157, 690–697. [Google Scholar] [CrossRef]
- Tao, X.; Li, A.; Yang, H. Immobilization of metals in contaminated soils using natural polymer-based stabilizers. Environ. Pollut. 2017, 222, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Núnez, R.; Alba, M.D.; Orta, M.M.; Vidal, M.; Rigol, A. Remediation of metal-contaminated soils with the addition of materials e Part I: Characterization and viability studies for the selection of non-hazardous waste materials and silicates. Chemosphere 2011, 85, 1511–1517. [Google Scholar] [CrossRef] [PubMed]
- Sahariah, B.; Goswami, L.; Kim, K.H.; Bhattacharyya, P.; Bhattacharya, S.S. Metal remediation and biodegradation potential of earthworm species on municipal solid waste: A parallel analysis between Metaphire posthuma and Eisenia fetida. Bioresour. Technol. 2015, 180, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.M.; Chaney, R.L.; Siebielec, G.; Kershner, B.A. Response of four turfgrass cultivars to limestone and biosolids compost amendment of a zinc and cadmium contaminated soil at Palmerton. J. Environ. Qual. 2000, 29, 1440–1447. [Google Scholar] [CrossRef]
- Beesley, L.; Moreno-Jimenez, E.; Gomez-Eyles, J.L.; Harris, E.; Robinson, B.; Sizmur, T. A review of biochars’ potential role in the remediation, revegetation and restoration of contaminated soils. Environ. Pollut. 2011, 159, 3269–3282. [Google Scholar] [CrossRef]
- Xiao, R.; Ali, A.; Xu, Y.; Abdelrahman, H.; Li, R.; Lin, Y.; Bolan, N.; Shaheen, S.; Rinklebe, J.; Zhang, Z. Earthworms as candidates for remediation of potentially toxic elements contaminated soils and mitigating the environmental and human health risks: A review. Environ. Int. 2022, 158, 106924. [Google Scholar] [CrossRef]
- Hamon, R.E.; McLaughlin, M.J.; Cozens, G. Mechanisms of attenuation of metal availability in in situ remediation treatments. Environ. Sci. Technol. 2002, 36, 3991–3996. [Google Scholar] [CrossRef]
- Sun, Y.B.; Sun, G.H.; Xu, Y.M.; Wang, L.; Lin, D.S.; Liang, X.F.; Shi, X. In situ stabilization remediation of cadmium contaminated soils of wastewater irrigation region using sepiolite. J. Environ. Sci. China 2012, 24, 1799–1805. [Google Scholar] [CrossRef]
- Kavehei, A.; Hose, G.; Gore, D. Effects of red earthworms (Eisenia fetida) on leachability of lead minerals in soil. Environ. Pollut. 2018, 237, 851–857. [Google Scholar] [CrossRef]
- Sizmur, T.; Hodson, M.E. Do earthworms impact metal mobility and availability in soil?—A review. Environ. Pollut. 2009, 157, 1981–1989. [Google Scholar] [CrossRef] [Green Version]
- Buatier, M.D.; Sobanska, S.; Elsass, F. TEM–EDX investigation on Zn- and Pb-contaminated soils. Appl. Geochem. 2001, 16, 1165–1177. [Google Scholar] [CrossRef]
- Degryse, F.; Smolders, E.; Zang, H.; Davison, W. Predicting availability of mineral elements to plants with the DGT technique: A review of experimental data and interpretation by modeling. Environ. Chem. 2009, 6, 198–218. [Google Scholar] [CrossRef] [Green Version]
- Cortet, J.; Gomot-De Vauflery, A.; Poinsot-Balaguer, N.; Gomot, L.; Texier, C.; Cluzeau, D. The use of invertebrate soil fauna in monitoring pollutant effects. Eur. J. Soil Biol. 1999, 35, 115–134. [Google Scholar] [CrossRef]
- Suthar, S.; Singh, S.; Dhawan, S. Earthworms as bioindicator of metals (Zn, Fe, Mn, Cu, Pb and Cd) in soils: Is metal bioaccumulation affected by their ecological category? Ecol. Eng. 2008, 32, 99–107. [Google Scholar] [CrossRef]
- Jeyanthi, V.; Paul, J.A.J.; Selvi, B.K.; Karmegam, N. Comparative Study of Biochemical Responses in Three Species of Earthworms Exposed to Pesticide and Metal Contaminated Soil. Environ. Process. 2016, 3, 167–178. [Google Scholar] [CrossRef]
- Hobbelen, P.H.F.; Koolhaas, J.E.; van Gestel, C.A.M. Bioaccumulation of heavy metals in the earthworms Lumbricus rubellus and Apporectodea caliginosa in relation tototal and available metal concentrations in field soils. Environ. Pollut. 2006, 144, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Verma, F.; Singh, S.; Singh, J.; Dhaliwal, S.S.; Parkash, C.; Kumar, V.; Kumar, R. Assessment of heavy metal contamination and its effect on earthworms in different types of soils. Int. J. Environ. Sci. Technol. 2021, 19, 4337–4350. [Google Scholar] [CrossRef]
- Ruan, H.; Zhang, Y.; Wang, Q.; Wang, C.; Wang, T. A novel earthworm-inspired smart lubrication material with self-healing function. Tribol. Int. 2022, 165, 107303. [Google Scholar] [CrossRef]
- Bakht, F.; Khan, S.; Muhammad, S.; Khan, M. Heavy metal bioavailability in the earthworm-assisted soils of different land types of Pakistan. Arab. J. Geosci. 2022, 15, 186. [Google Scholar] [CrossRef]
- Yuvaraj, A.; Govarthanan, M.; Karmegam, N.; Biruntha, M.; Kumar, D.; Arthanari, M.; Govindarajan, R.; Tripathi, S.; Ghosh, S.; Kumar, P.; et al. Metallothionein dependent-detoxification of heavy metals in the agricultural field soil of industrial area: Earthworm as field experimental model system. Chemosphere 2021, 267, 129240. [Google Scholar] [CrossRef]
- Xiao, L.; Li, M.; Dai, J.; Motelica-Heino, M.; Chen, X.; Wu, J.; Zhao, L.; Liu, K.; Zhang, C. Assessment of earthworm activity on Cu, Cd, Pb and Zn bioavailability in contaminated soils using biota to soil accumlation factor and DTPA extraction. Ecotoxicol. Environ. Saf. 2020, 195, 110513. [Google Scholar] [CrossRef] [PubMed]
- Kauschke, E.; Mohrig, W.; Cooper, E.L. Coelomic fluid proteins as basic components of innate immunity in earthworms. Eur. J. Soil Biol. 2007, 43, 110–114. [Google Scholar] [CrossRef]
- Lee, M.R.; Hodson, M.E.; Langworthy, G.N. Earthworms produce granules of intricately zoned calcite. Geology 2008, 36, 943–946. [Google Scholar] [CrossRef]
- Cheng, J.; Wong, M.H. Effects of earthworms on Zn fractionation in soils. Biol. Fertil. Soils 2002, 36, 72–78. [Google Scholar] [CrossRef]
- Karaca, A. Biology of Earthworms; Springer Science and Business Media: Berlin/Heidelberg, Germany, 2008; pp. 51–68. ISBN 978-3-642-14635-0. [Google Scholar]
- Bandara, T.; Chathurika, J.; Franks, A.; Xu, J.; Tang, C. Interactive effects of biochar type and pH on the bioavailability of As and Cd and microbial activities in co-contaminated soils. Environ. Technol. Innov. 2021, 23, 101767. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, C.; Wang, G.; Xiong, B.; Zhou, W.; Xue, F.; Qi, W.; Qui, C.; Liu, Z. Mechanism underlying earthworm on the remediation of cadmium-contaminated soil. Sci. Total Environ. 2020, 728, 138904. [Google Scholar] [CrossRef]
- Nejad, Z.; Rezania, S.; Jung, C.; Al-Ghamdi, A.; Mustafa, A.; Elshikh, S. Effects of fine fractions of soil organic, semiorganic, and inorganic amendments on the mitigation of heavy metal (loid) s leaching and bioavailability in a post-mining area. Chemosphere 2021, 271, 129538. [Google Scholar] [CrossRef]
- Podolak, A.; Kostecka, J.; Mazur-Pączka, A.; Garczyńska, M.; Pączka, G.; Szura, R. Life Cycle of the Eisenia fetida and Dendrobaena veneta Earthworms (Oligohaeta, Lumbricidae). J. Ecol. Eng. 2020, 21, 40–45. [Google Scholar] [CrossRef]
- Nannoni, F.; Rossi, S.; Protano, G. Soil properties and metal accumulation byearthworms in the Siena urban area (Italy). Appl. Soil Ecol. 2014, 77, 9–17. [Google Scholar] [CrossRef]
- Wen, B.; Hu, X.; Liu, Y.; Wang, W.; Feng, M.; Shan, X. The role of earthworms (Eisenia fetida) in influencing bioavailability of heavy metals in soils. Biol. Fertil. Soils 2004, 40, 181–187. [Google Scholar] [CrossRef]
- Hodson, M.E.; Benning, L.G.; Demarchi, B.; Penkman, K.E.H.; Rodriguez-Blanco, J.D.; Schofield, P.F.; Versteegh, E.A.A. Biomineralisation by earthworms—An investigation into the stability and distribution of amorphous calcium carbonate. Geochem. Trans. 2015, 16, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brinza, L.; Schofield, P.F.; Mosselmans, J.F.W.; Donner, E.; Lombi, E.; Paterson, D.; Hodson, M.E. Can earthworm-secreted calcium carbonate immobilise Zn in contaminated soils? Soil Biol. Biochem. 2014, 74, 1–10. [Google Scholar] [CrossRef]
- Reeves, P.G.; Chaney, R.L. Marginal nutritional status of zinc; iron; and calcium increases cadmium retention in the duodenum and other organs of rats fed rice-based diets. Environ. Res. 2004, 96, 311–322. [Google Scholar] [CrossRef]
- Nannoni, F.; Protano, G.; Riccobono, F. Uptake and bioaccumulation of heavy elements by two earthworm species from a smelter contaminated area in northern Kosovo. Soil Biol. Biochem. 2011, 43, 2359–2367. [Google Scholar] [CrossRef]
- Maity, S.; Bhattacharya, S.; Chaudhury, S. Metallothionein response in earthworms Lampito mauritii (Kinberg) exposed to fly ash. Chemosphere 2009, 77, 319–324. [Google Scholar] [CrossRef]
- Basta, N.T.; Ryan, J.A.; Chaney, R.L. Trace element chemistry in residua treated soil: Key concepts and metal bioavailability. J. Environ. Qual. 2005, 34, 49–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruemmer, G.W.; Gerth, J.; Tiller, K.G. Reaction kinetics of the adsorption and desorption of nickel, zinc, and cadmium by goethite. I. Adsorption and diffusion of metals. Eur. J. Soil Sci. 1988, 39, 37–52. [Google Scholar] [CrossRef]
- Siebielec, G.; Chaney, R.L. Testing amendments for remediation of military range contaminated soil. J. Environ. Manag. 2012, 108, 8–13. [Google Scholar] [CrossRef]
- Siebielec, S.; Siebielec, G.; Stuczyński, T.; Sugier, P.; Grzęda, E.; Grządziel, J. Long term insight into biodiversity of a smelter wasteland reclaimed with biosolids and by-product lime. Sci. Total Environ. 2018, 636, 1048–1057. [Google Scholar] [CrossRef]


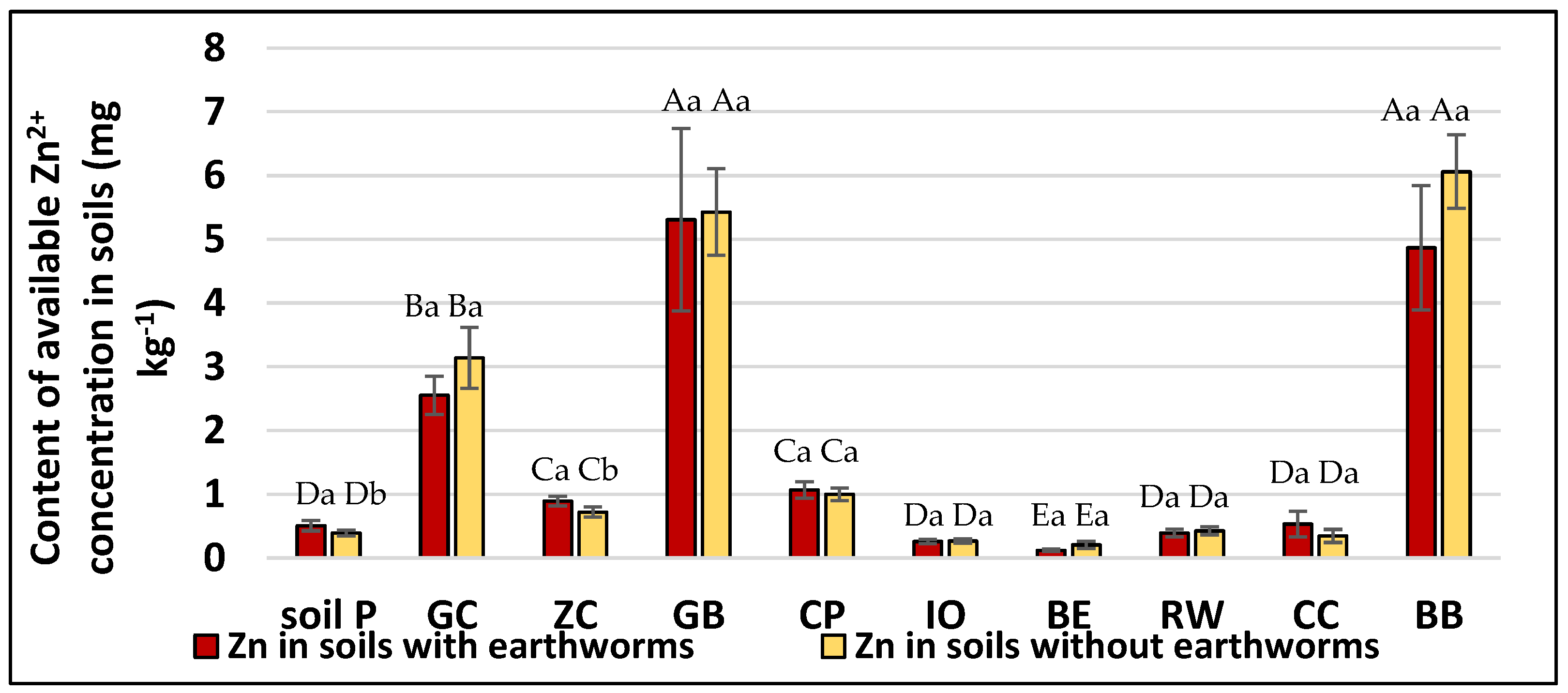
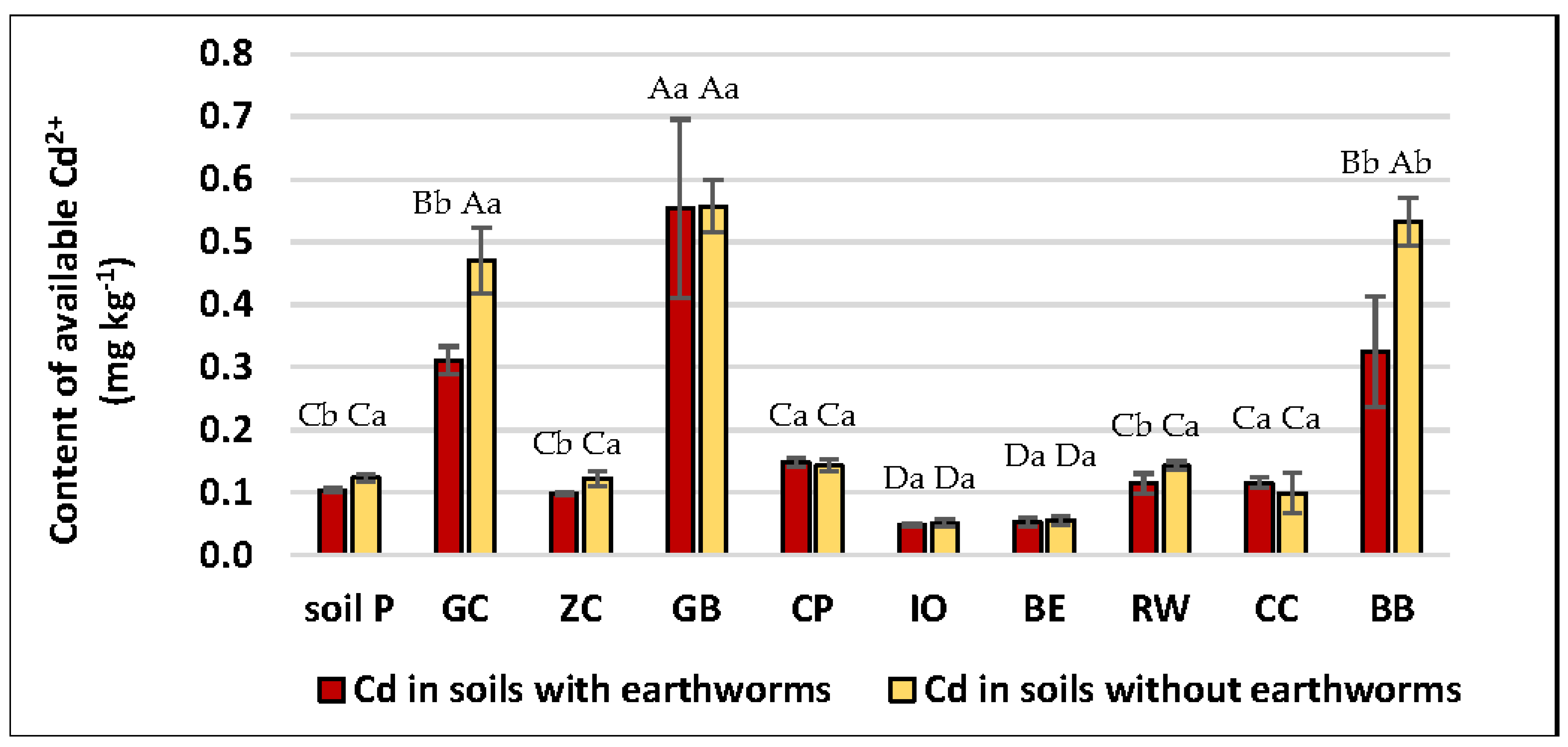
| Soil Amendments | pH | Total Concentration | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Abbrev. | OM | P | Fe | Mn | Cd | Zn | Pb | ||
| [%] | [%] | [mg kg−1] | |||||||
| Zabrze compost | ZC | 7.4 | 36.3 | 0.60 | 1.79 | 1290 | 4.1 | 816 | 350 |
| GWDA compost | GC | 5.3 | 43.9 | 0.20 | 2.58 | 500 | 0.9 | 1600 | 77 |
| Bełchatów biosolids | BB | 6.7 | 54.3 | 0.21 | 3.54 | 490 | 5.5 | 1890 | 91 |
| Grabów biosolids | GB | 6.1 | 75.1 | 0.39 | 0.84 | 450 | 1.7 | 1650 | 29 |
| Bentonite | BE | 9.0 | - | 0.30 | 0.81 | 189 | 0.05 | 85 | 35 |
| Rock waste | RW | 6.5 | - | 0.13 | 0.92 | 110 | <0.05 | 89 | 36 |
| TE Concentrations in Earthworm Tissue (mg kg−1) | ||||||
| Zn ET | Pb ET | Cd ET | Cu ET | Ni ET | Cr ET | |
| Soil UP | 114.39 ± 28.19 | 10.45 ± 9.76 | 10.11 ± 1.45 | 11.28 ± 1.2 | 1.12 ± 0.02 | 0.3 ± 0.04 |
| Soil P | 290.3 ± 31.7 | 408.5 ± 105.8 | 112.5 ± 25.8 | 16.0 ± 4.1 | 0.9 ± 0.3 | 0.6 ± 0.1 |
| GC | 267.8 ± 131.7 | 311.7 ± 219.5 | 50.8 ± 9.3 | 24.8 ± 27.3 | 1.6 ± 0.7 | 1.4 ± 0.1 |
| ZC | 367.9 ± 109.8 | 335.9 ± 64.0 | 48.3 ± 11.9 | 18.9 ± 2.2 | 1.8 ± 1.5 | 2.5 ± 3.0 |
| GB | - | - | - | - | - | - |
| CP | 394.6 ± 338.7 | 351.8 ± 177.8 | 66.10.53 ± | 18.2 ± 6.7 | 1.3 ± 1.3 | 1.4 ± 1.1 |
| IO | 358.6 ± 149.5 | 226.4 ± 75.4 | 70.5 ± 19.3 | 17.3 ± 1.1 | 0.8 ± 0.4 | 0.7 ± 0.5 |
| BE | 336.4 ± 161.6 | 332.6 ± 90.8 | 82.7 ± 13.3 | 20.5 ± 24.3 | 0.9 ± 0.5 | 0.8 ± 0.9 |
| RW | 246.7 ± 50.9 | 382.1 ± 50.1 | 93.8 ± 29.0 | 19.2 ± 4.1 | 1.0 ± 0.1 | 0.3 ± 0.1 |
| CC | 270.7 ± 22.2 | 332.0 ± 24.9 | 94.3 ± 29.9 | 20.5 ± 3.4 | 0.7 ± 0.1 | 0.1 ± 0.1 |
| BB | - | - | - | - | - | - |
| BSAF Values | ||||||
| Zn BSAF | Pb BSAF | Cd BSAF | Cu BSAF | Ni BSAF | Cr BSAF | |
| Soil P | 0.07 ± 0.01a | 0.19 ± 0.05a | 0.77 ± 0.18a | 0.55 ± 0.14a | 0.07 ± 0.03a | 0.05 ± 0.01b |
| GC | 0.07 ± 0.02a | 0.14 ± 0.10ab | 0.35 ± 0.06c | 0.66 ± 0.95a | 0.13 ± 0.06a | 0.13 ± 0.01a |
| ZC | 0.09 ± 0.03a | 0.15 ± 0.03ab | 0.33 ± 0.08c | 0.66 ± 0.08a | 0.15 ± 0.13a | 0.23 ± 0.20abc |
| GB | - | - | - | - | - | - |
| CP | 0.10 ± 0.07a | 0.16 ± 0.08ab | 0.45 ± 0.07bc | 0.63 ± 0.23a | 0.11 ± 0.11a | 0.13 ± 0.10abc |
| IO | 0.09 ± 0.04a | 0.10 ± 0.03b | 0.48 ± 0.13bc | 0.60 ± 0.04a | 0.07 ± 0.03a | 0.06 ± 0.04b |
| BE | 0.08 ± 0.04a | 0.15 ± 0.04ab | 0.57 ± 0.09ab | 0.71 ± 0.84a | 0.07 ± 0.04a | 0.07 ± 0.06abc |
| RW | 0.06 ± 0.01a | 0.17 ± 0.02ab | 0.64 ± 0.20ab | 0.67 ± 0.14a | 0.08 ± 0.01a | 0.03 ± 0.01c |
| CC | 0.07 ± 0.01a | 0.15 ± 0.01ab | 0.65 ± 0.20ab | 0.71 ± 0.12a | 0.06 ± 0.01a | 0.01 ± 0.01c |
| BB | - | - | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ukalska-Jaruga, A.; Siebielec, G.; Siebielec, S.; Pecio, M. The Effect of Soil Amendments on Trace Elements’ Bioavailability and Toxicity to Earthworms in Contaminated Soils. Appl. Sci. 2022, 12, 6280. https://doi.org/10.3390/app12126280
Ukalska-Jaruga A, Siebielec G, Siebielec S, Pecio M. The Effect of Soil Amendments on Trace Elements’ Bioavailability and Toxicity to Earthworms in Contaminated Soils. Applied Sciences. 2022; 12(12):6280. https://doi.org/10.3390/app12126280
Chicago/Turabian StyleUkalska-Jaruga, Aleksandra, Grzegorz Siebielec, Sylwia Siebielec, and Monika Pecio. 2022. "The Effect of Soil Amendments on Trace Elements’ Bioavailability and Toxicity to Earthworms in Contaminated Soils" Applied Sciences 12, no. 12: 6280. https://doi.org/10.3390/app12126280






