Abstract
Under polycyclic aromatic hydrocarbon (PAH) pollution conditions (149.17–187.54 mg/kg), we had found the dominant flora of PAHs by observing the response of the soil microbial community after planting purple coneflower (Echinacea purpurea (L.) Moench). In this study, pot experiments were conducted in a growth chamber to explore the changes in the rhizosphere microbial community structure during remediation of heavily PAH-contaminated soil using purple coneflower (Echinacea purpurea (L.) Moench). The phospholipid fatty acid (PLFA) content in the soil was measured during four periods before and after planting, and the results showed that: (i) at 120 days, E. purpurea can regulate the microbial community structure but had no significant effect on soil microbial diversity, (ii) at 120 days, the number of PLFAs characterizing actinomycetes, bacteria, and fungi increased, and both Gram-negative bacteria and Arbuscular mycorrhizal fungi (AMF) were significant with the observed PLFA level (p < 0.05), and (iii) the results indicated that AMF and Gram-negative bacteria represent some of the main factors that can promote the degradation of PAHs. The results obtained in this work are important to future research on PAH-degradation-functional genes and degradation mechanisms of the selection of flora.
1. Introduction
PAHs are widely distributed in the human living environment such as water, atmosphere and soil. Leakage during oil exploration and transportation is an important source of PAH soil pollution [1]. PAHs are compounds that have carcinogenic, teratogenic, and mutagenic properties and consist of two or more benzene rings connected in the form of fused rings [2,3]. Because of their low water solubility and hydrophobicity, PAHs become strongly distributed in nonaqueous phases and adsorbed on particulate matter, and soil represents one of the main environmental fates of these compounds [4]. Under certain conditions, PAHs in the soil will re-enter other environmental media in various forms and cause “secondary pollution”. The pollution concentration of PAHs in the soil environment of China has increased from µg/kg to mg/kg levels [5], which has seriously affected soil production, ecological functions, and human health. A national survey report on soil pollution showed that the proportion of PAHs in heavily polluted cultivated land, forestland, grassland, and unused land in China has reached 1.1%, 1.3%, 0.7%, and 1.0%, respectively, and these values are on the rise [6]. Therefore, the removal of PAHs from the soil has become an important topic in the field of environmental research.
Phytoremediation has developed in recent years as a safe and effective green remediation technology. Phytoremediation uses the synergistic action of plants and rhizosphere microorganisms to remediate contaminated soil. Compared with physical and chemical methods, its energy consumption and cost are much lower. Compared with ryegrass, white clover, and other plants, E. purpurea has a short life-cycle and high tolerance to high concentrations of PAHs [7,8]. Under high concentration stress, it exhibits the potential to remediate soil contaminated with high concentrations of PAHs. The removal rates of the four PAHs of pyrene (Pyr), chrysene (CHR), benzo(b)fluoranthene (BbF), and benzo(k)fluoranthene (BkF) were 66.2%, 70.3%, 40.6%, and 65.4%, and the total removal rates reached 56.93% [9]. The removal of organic pollutants by plants includes direct degradation and compound degradation. On the one hand, it relies on the direct degradation of enzymes produced by the root system. On the other hand, plants can synthesize various natural products and carry out the joint degradation of rhizosphere microorganisms when subjected to biological or abiotic stress. These two actions are based on the interaction of microorganisms and enzymes [10]. Therefore, the dynamic change in microorganisms can be regarded as an indicator of PAH degradation.
Microorganisms in the soil are the most sensitive and rapid indicators for reflecting disturbances and biochemical changes in the soil ecosystem [11,12], and the quantitative description of their community structure and diversity can be used as a potential tool for soil quality evaluation [13]. Analysis of PLFAs is a common method for the quantitative detection of soil microbial biomass [14]. The characteristic PLFAs of various bacterial groups are different, and these profiles are highly specific. Accordingly, PLFAs can be used as markers for different groups in the microbial community. Changes in the composition of phospholipids can reflect the changes in the microbial community structure in environmental samples [15]. Quantitative description of microorganisms provides relevant information for further research. In recent years, this approach has been widely used in the study of soil microbial community structure [16].
Our previous studies have shown that purple coneflower (Echinacea purpurea (L.) Moench). was able to remediate PAHs in contaminated soil [17]. The aims of this study were (i) to examine the changes in soil microbial community after planting E. purpurea, (ii) to look for the dominant bacterial flora to degrade polycyclic aromatic hydrocarbons under this condition, and (iii) through the discovery of the dominant bacterial group for PAH degradation, provide a direction for future research on functional genes.
2. Materials and Methods
2.1. Experimental Design
The aged PAH-contaminated soil used in this research was obtained at Shengli Oilfield, Dongying City, Shandong Province (sampling depth of 250 mm) with the approval of the Ministry of Safety and Environmental Protection of Shengli Oilfield. Soil analysis was performed by the Key Laboratory of Terrestrial Ecological Processes of the Institute of Applied Ecology, Chinese Academy of Sciences. The physicochemical properties of contaminated soil were: pH, 7.66; C, 45.77 g/kg; p, 0.65 g/kg; n, 0.73 g/kg; and available phosphorus, 0.002 g/kg, respectively. Uncontaminated soil samples were collected in Shenyang Wanliutang Park for dilution. The physicochemical properties of uncontaminated soil were: pH, 6.7; C, 12.82 g/kg; p, 0.44 g/kg; n, 0.8 g/kg; and available phosphorus, 0.011 g/kg. The concentration of PAHs in the contaminated soil ranged between 228 mg/kg and 398 mg/kg. The soil samples were sieved through a 4.00 mm sieve to ensure homogeneity. According to the previous results, none of the plants tested was able to grow in the aged PAH-contaminated soil. We added uncontaminated reference soil at a ratio of approximately 1:1, and diluted the contaminated soil to 149.17–187.54 mg/kg. The tested plant known as E. purpurea was screened from 14 plants after four-year-long experiments and had a short life cycle and grew logarithmically from 100 to 120 days. Therefore, we choose to sample at 60, 120, and 150 days to observe the overall changes in the microbial community.
The experiment was divided into two treatments—an experimental treatment (E. purpurea planted in PAH-contaminated soil, labeled as S) and a control treatment (no crop planted in PAH-contaminated soil, labeled as C). Each treatment has 24 pots (Φ = 20 cm, h = 18 cm, and soil = 2.5 kg), including 12 blank control pots. A disc of filter paper was placed at the bottom of each 20 cm pot to prevent the dry soil from escaping through the drainage holes, and the pots were placed on saucers. Each pot was initially filled with 2.3 kg of PAH-contaminated soil, then a layer of uncontaminated soil (0.3 kg) was placed on the surface of the contaminated soil. Purple coneflower seeds (20 seeds per pot) were sown on the surface of the uncontaminated soil, and finally the seeds were covered by 0.2 kg of PAH-contaminated soil. After the germination of the plants, 15 healthy seedlings were left in each pot. Corresponding control treatments were in PAH-contaminated soil without planting of plant seeds. They were placed in a growth chamber (41°48′ N, 123°26′ E). After 60, 120, or 150 days of cultivation, E. purpurea grown in the experimental soil (149.17–187.54 mg/kg) was harvested. The soil in the C group was watered in the same way, and all treatments were processed within 150 days. The cycle was 16 h/25 °C during the day and 8 h/15 °C at night. These plants were watered every other day to maintain a moisture content of approximately 60% by weight [18]. The experiments were performed from 28 April 2009 to 29 September 2009 and lasted for 150 days.
2.2. Extraction and Analysis of PLFAs
To extract lipids from the soil, the soil samples were lyophilized, and then, 5 g was extracted with a single-phase chloroform–methanol–water buffer system [19]. The total lipid extract was fractionated by silicic acid chromatography into neutral lipids, glycolipids, and polar lipids and polar lipid components containing phospholipids [20]. The phospholipid component was methylated with methanolic HCl and measured on a GC-MSD system equipped with an HP-5 column (30.0 m × 320 µm × 0.25 µm). Chromatographic conditions: HP-5 column (30.0 m × 320 µm × 0.25 µm), injection volume 1 μL, split ratio 100:1, carrier gas (N2) flow rate 0.8 mL/min. The initial temperature was maintained for 3 min at 140 °C and then programmed in four stages: 140–190 °C, 4 °C/min, for 1 min; 190–230 °C, 3 °C/min, for 1 min; 230–250 °C, 2 °C/min, hold for 2 min; and 250–300 °C, 10 °C/min, hold for 1 min. A flame ionization detector (FID) was used [21].
2.3. Data Analysis
The PLFA detection range was between ECL 9.000 (FAME 9:0) and ECL 20.000 (FAME 20:0). For data processing, we refer to the methods provided by other research institutes. PLFAs were organized by biomarker group. Peaks with multiple possible FAME identifications were grouped as summed features, and unknown peaks were grouped and named by their respective ECL [14]. The biomarker panel is described in Table 1.

Table 1.
Biomarker concentrations in soil at 0, 60, 120, and 150 days before and after planting E. purpurea (nmol/g).
All treatments were replicated three times in the experiments. Considering that the data obeyed normal distribution, statistical analysis was performed using the SPSS 20.0 with independent-sample t-test. Principal component analysis (PCA) was performed to analyze the correlation between soil samples and different microbial marker levels.
The Shannon–Wiener diversity index (H) was used to calculate the PLFA diversity of soil microorganisms in the analysis samples at different periods. The specific formula is as follows:
where , represents the i-th fatty acid content, and represents the fatty acid content.
2.4. PLFA Nomenclature
The PLFA convention was specified as X:YwZ according to the Omega Reference Standard, where “X” represents the total number of carbon atoms in the molecule, “Y” represents the number of double bonds, “Z” represents the position of the first double bond or cyclopropane ring, and w represents a position molecule from the methyl terminus [14]. The prefix “i” means iso branch, “a” means anteiso branch, “cy” means cyclopropane ring, the suffixes “c” and “t” indicate cis and trans configurations, respectively, and a number followed by “I” means middle. The position of the chain methyl group is branched with respect to the carboxyl terminus. The number before the OH refers to the end of the molecule at the position of the hydroxyl group relative to the carboxyl group.
3. Results
Generally, transformation of land-use has a great impact on the soil microbial community structure. The change of total microorganisms in soil is generally indicated by the content of total PLFA in rhizosphere soil. The total PLFAs shown in Table 2 confirm that the soil had a higher PLFA biomass after planting E. purpurea, and the total amount of microorganisms in the soil changed significantly. Compared with the soil in which E. purpurea was not planted, the total amount of PLFAs was obviously higher, with an average increase of approximately 70.90% (p < 0.01). After planting E. purpurea, the Shannon–Wiener diversity index was mainly stable at 2.62 during the period of 0–150 days. Planting E. purpurea did not significantly change microbial diversity of heavily PAH-contaminated soil (p > 0.05).

Table 2.
Defined biomarker groups.
The characteristic PLFAs were as follows: (1) actinomycetes (ACT): 16:0 10-methyl and 18:0 10-methyl; (2) Gram-positive bacteria (G+): 14:0 iso, 15:0 iso, 16:0 iso, 17:0 iso, 17:0 anteiso, 15:0 anteiso; (3) Gram-negative bacteria (G−): 16:1 w7c, 18:1 w7c, 17:1 w8c; (4) bacteria (BAC): Gram-positive and Gram-negative biomarkers, 14:0, 15:0, 17:0, 18:0, 16:0, 18:1 w7c 10-methyl, 17:1 iso w9c, 19:0 cyclo w7c, 17:0 cyclo w7c; (5) fungi (FUN): 18:1w9c, 18:2w6c; and (6) arbuscular mycorrhizal fungi (AMF): 16:1w5c. For each biomarker group at all different periods, the content of the main PLFAs is described in Table 1.
PCA was performed on the samples using the identified PLFAs at different periods before and after planting. Two principal components were extracted in this experiment, namely, the first principal component PC1 (86.3%) and the second principal component PC2 (9.1%), which explained 95.4% of the variability.
As shown in Figure 1, biomarkers of different microorganisms had good correlation with S120 and S150 in all soil samples. This is consistent with the results that the biomarkers of different microbes were at a higher level at 120 and 150 days after planting E. purpurea.
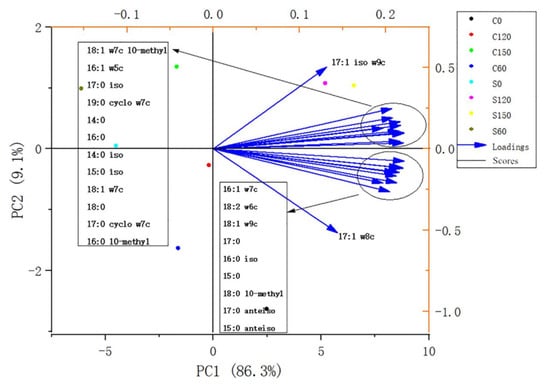
Figure 1.
PCA analysis chart. The horizontal axis is the first principal component (PC1), and the vertical axis is the second principal component (PC2). C indicates that E. purpurea was not planted, and S indicates that E. purpurea was planted. C0, C60, C120, C150, S0, S60, S120, and S150 represent soil samples at 0, 60, 120, and 150 days before and after planting, respectively.
We can see in Figure 2 the total amount of phospholipid fatty acids associated with AMF(A) and Gram-negative bacteria (B) in rhizosphere soil before and after planting under high PAH pollution. From 0 to 60 days, the total amount of phospholipid fatty acids diagnostic of AMF decreased from 0.15 nmol/g to 0.11 nmol/g. By 120 days, the amount rose significantly to 0.47 nmol/g. At 150 days, the amount was 0.46 nmol/g. The content of AMF in the soil in which E. purpurea was planted was significantly changed year-on-year and without planting, and it was most significant at 60–120 days (p < 0.05).
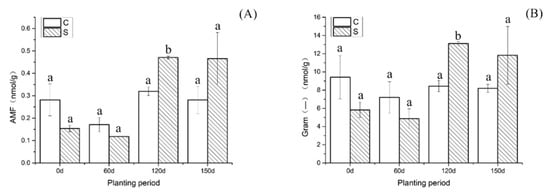
Figure 2.
Changes in the total amount of phospholipid fatty acids associated with AMF (A) and Gram-negative bacteria (B) in the rhizosphere soil under high levels of PAH pollution. C, nonplanted; S, planted. Bars (means ± SD, n = 3) with different letters indicate the difference between samples in the same period (p < 0.05).
From 0 to 60 days, the concentration of associated Gram-negative bacteria in the soil decreased from 9.42 nmol/g to 7.21 nmol/g. By 120 days, the concentration rose to 8.44 nmol/g. After 150 days, the value was 8.21 nmol/g. This result shows that a high concentration of PAHs can slightly inhibit the growth of Gram-negative bacteria in soil.
From 0 to 60 days after planting E. purpurea, the concentration of phospholipid fatty acids associated with Gram-negative bacteria in the soil decreased from 5.82 nmol/g to 4.24 nmol/g. By 120 days, the concentration rose to 13.11 nmol/g. By 150 days, this concentration had dropped to 11.82 nmol/g. Compared with the unplanted control, between 60 and 120 days, the concentration of Gram-negative bacteria was greater in the soil where E. purpurea was grown, and the change trend was more significant (p < 0.05).
4. Discussion
The purpose of this work was to investigate and analyze the response of microbial community structure under heavy PAH pollution stress by planting E. purpurea.
The contents of total PLFA in C and S groups decreased in 0–60 days. This was due to the inhibitory effect of PAHs on microorganisms. For the inhibition effect, E. purpurea relieved the stress of PAHs and increased the total amount of PLFAs in the soil, and the effect was significant at 120 days (p < 0.05). The inhibitory effect of PAHs on microorganisms was mainly due to their strong adsorption capacity in the soil and low solubility, which limits the life activities of soil microorganisms. This is consistent with the view of Wolf et al. (2019) [27]. E. purpurea promotes microbial growth through plant roots. This is because the root system of plants is an important factor that affects the changes in the rhizosphere microbial community. Especially during the development of plants, changes in the rhizosphere will lead to the rapid growth of certain microbial populations and affect the choice of survival opportunities for other species [28]. Studies by Suresh et al. (2004) and Gao et al. (2011) confirmed that under contaminated soil conditions, the plant roots can create more favorable living conditions for microorganisms to perform degradation, due to maintaining aerobic conditions in the rhizosphere, thereby promoting the degradation of organic matter [29,30]. The highest total amount of PLFAs appeared at 120 days. We believe that this result is closely related to the degradation of PAHs by E. purpurea. Relevant research shows that E. purpurea has a good remediation effect on PAHs in contaminated soil. After 120 days, the degradation rate of PAHs was the highest, reaching 86.57% to 99.39%, which was 33.24% to 61.20% higher than that of unplanted soil [9].
It was found in the experiment that the counts of bacteria and fungi changed to different degrees before and after planting E. purpurea and showed significant differences at 120 days (p < 0.05). Analysis of changes in bacteria and fungi showed that E. purpurea can alleviate the inhibitory effect of PAHs and stimulate them through rhizosphere exudates. With the degradation of PAHs, the available effective carbon sources in the soil increase, which is conducive to the growth of bacteria. Relevant research shows that the degradation of rhizosphere microorganisms is the main factor in the removal of PAHs. Microbial populations use organic compounds as a carbon substrate to grow, and this process is usually stimulated by plant rhizosphere secretions [31]. The fact is supported by Pagé et al. (2015) [32], who reported that fast-growing willow trees had the ability to stimulate indigenous bacteria in soil (Actinomycetales, Burkholderiales, and Rhizobiales) to produce cytochrome P450 monooxygenases and laccase/polyphenol oxidases with the capability to degrade, for instance, fluoranthene, anthracene, and benzo[a]pyrene, in soil. Willow root exudates in the soil may cause the desorption of PAHs adsorbed on soil particles and enhance the use of PAHs by soil microorganisms [33,34]. When plants are under biotic or abiotic stress, they often secrete active metabolites and hormones from the root, which will cause positive plant–soil feedback [35,36,37]. E. purpurea has a short growth cycle and the most active life activities is around 120 days [38]. This is also one of the reasons why the level of PLFAs represented by bacteria and fungi changed significantly in 120 days.
Arbuscular mycorrhizal fungi (AMF) can form mutually beneficial symbioses with more than 90% of vascular plants on the planet. A large number of reports indicate that AMF can significantly improve the efficiency of plant rhizosphere remediation of PAH-contaminated soil and can affect plant uptake of PAHs [29,39]. Studies by Suresh et al. (2011) proposed that AMF can be used to remediate organically contaminated soil [40]. AMF can promote the degradation and transformation of toxic organic pollutants, reduce the amount of residual organic pollutants in the soil, and have a good effect on the remediation of toxic organic pollution. Therefore, it is important to study the relative biomass changes of PLFAs characteristic of AMF in the soil. It is not difficult to find that the relative biomass of PLFAs characteristic of AMF in the soil changes significantly before and after planting (Figure 2A). The activity of AMF in the rhizosphere of group S was higher than that of group C. The activity was strong and reached a peak at 120 days and a decrease at 150 days, which was consistent with the change in the total amount of fungal-associated phospholipid fatty acids and related to the degradation efficiency of E. purpurea against PAHs. In organically contaminated soil, plant mycorrhiza promote the absorption of mineral nutrients and water in the soil by plants. This is conducive to plant growth and phytoremediation [41]. Many studies have pointed out that the presence of mycorrhizae can often promote plant growth and PAH degradation. Joner et al. (2003) studied the effect of mycorrhizal fungi on the removal of PAHs in contaminated soil. After planting clover and ryegrass for a period of time, it was found that the presence of mycorrhizae promoted plant growth and PAH degradation, and the degradation rate of rhizosphere PAHs was higher than that of non-sterile rhizospheres [42]. Research by Liu (2014) showed that in an experiment on remediating PAH-contaminated soil with E. purpurea, the removal rate of the four PAHs showed an increasing trend with increasing time after planting, and the growth rate gradually decreased [43]. At 120 days, the removal rate increased the most. The removal rates of tetracyclic pyrene (Pyr) and chrysene (CHR) reached 46.11% and 71.91%, respectively, and those of pentacyclic benzo(b]fluoranthene (BbF) and benzo(k)luoranthene (BkF) were 30.00% and 46.72%, respectively. At this time, the soil microbial community was the most active, and the PLFA level reached its peak. At 150 days, the removal rate increased, that is, the microbial community activity decreased, the PLFA content decreased, and the removal rate appeared to reach the maximum. The removal rates of Pyr and CHR were 66.10% and 70.30%, respectively. The removal rates of BbF and BkF were 40.50% and 60.40%, respectively. The main reason for this is related to the growth period of the plant. At 120 days, the plant grew vigorously, and the rhizosphere secretions (including polyphenol oxidase, dehydrogenase, peroxidase, etc.) contributed to the degradation of PAHs [18,43]. These secretions are conducive to the life activities of AMF, that promotes the growth of plant roots [44] and the degradation of PAHs. In addition, we speculate that rhizosphere exudates of E. purpurea may contain factors that activate genes of AMF to efficiently degrade PAHs, but the specific mechanism needs further study.
After planting E. purpurea, the growth trend of Gram-positive bacteria was not significant compared with the non-planted case. It may be that Gram-positive bacteria have poor tolerance under this condition, and the activated carbon source required by Gram-positive bacteria cannot be adequately supplied.
In contrast, the content of Gram-negative bacteria in the soil increased significantly at 60 to 120 days after planting, indicating that E. purpurea has a significant promotion effect on this type of bacteria. We consider that the cultivation of E. purpurea can promote the growth of Gram-negative bacteria in the soil. As the plants develop, changes in the physiological state of the plants affect the growth of different microbiota, mediated by changes in the composition of root exudates in the rhizosphere [35]. For annual and perennial plant species, the vegetative stage is an important factor leading to changes in the composition of microbial rhizosphere communities under field and greenhouse conditions [36,37,44]. Gram-negative bacteria can not only use relatively unstable plant-derived carbon sources [45] but also exhibit strong rhizosphere secretions from 60–120 days, good degradation of PAHs in the soil, and provide Gram-negative bacteria more organic carbon sources. With more organic carbon sources, the content of Gram-negative bacteria changes significantly. Studies have shown that quorum sensing of acylated homoserine lactone (AHL) is widely conserved among Gram-negative bacteria and plays a vital role in regulating many biological processes. The acyl-homoserine lactone (AHL)-mediated density-sensing mechanism endows Gram-negative bacteria with quorum sensing (QS) to enhance their bioremediation potential and is a promising strategy for remediating PAH contamination [46]. Therefore, we theorize that in the process of PAH degradation, Gram-negative bacteria are one of the important links in this process. The microbe cooperates with the rhizosphere secretions of E. purpurea to promote the degradation of PAHs.
The change in the ratio of Gram-negative to Gram-positive phospholipid fatty acid supplements indicated that the regulatory effect of E. purpurea on the microbial community can alleviate the inhibitory effect of PAHs on Gram-positive and Gram-negative bacteria. This result is consistent with previous discussions.
It is well known that, except for annual and perennial plant species, different growth stages of plants are important factors that lead to changes in the composition of the microbial rhizosphere community (under field or greenhouse conditions) [47,48,49]. Our results show that the ratio of the population of other species except Gram-positive bacteria to that when no E. purpurea was planted was significantly different. This result also fits the characteristics of E. purpurea as a short-term plant. Therefore, E. purpurea is closely related to the degradation of PAHs and plays a regulatory role in the microbial community.
5. Conclusions
Through analysis of the experimental results, the following conclusions can be obtained: (i) E. purpurea can relieve the stress of PAHs (149.17–187.54 mg/kg) on soil microorganisms and regulate the microbial community structure but has no significant effect on soil microbial diversity, (ii) at 120 days, the number of PLFA-characterizing actinomycetes, bacteria, and fungi increases to varying degrees, of which Gram-negative bacteria and AMF contribute significantly (p < 0.05), (iii) high concentration of PAHs can slightly inhibit the growth of Gram-negative bacteria in soil, and (iv) E. purpurea may be the key to stimulating the mechanism or functional genes of AMF and Gram-negative bacteria in soil to degrade PAHs. In the future, we want to provide a scientific basis for strengthening microbial remediation through studying at the molecular level.
Author Contributions
Conceptualization, R.L. and X.W.; methodology, R.L.; software, K.L., T.D., Y.W. and Y.X.; formal analysis, X.D. and M.S.; investigation, R.L.; resources, R.L.; writing—original draft preparation, K.L.; writing—review and editing, R.L. and K.L.; visualization, K.L.; supervision, R.L.; project administration, R.L.; funding acquisition, R.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (Grant No: 31470547, 31770545).
Data Availability Statement
Not applicable.
Acknowledgments
This work was financially supported by two general projects of the National Natural Science Foundation of China (Grant No: 31470547, 31770545).
Conflicts of Interest
We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
References
- Tao, Y.; Li, Y. Study on the sources and monitoring analysis methods of polycyclic aromatic hydrocarbons (PAHs) in the environment. Environ. Dev. 2019, 31, 96–98. (In Chinese) [Google Scholar]
- Meudec, A.; Dussauze, J.; Jourdin, M.; Deslandes, E.; Poupart, N. Gas chorolatographic-mass spectrometric method for polycyclic aromatic hydrocarbo n analysis in plant biota. J. Chromatogr. A 2006, 1108, 240–247. [Google Scholar] [CrossRef]
- Zhou, Q.X.; Song, Y. Contaminated Soil Remediation: Principles and Methods; Science Press: Beijing, China, 2004. (In Chinese) [Google Scholar]
- Zhou, Q.X.; Sun, F.H.; Liu, R. Joint chemical flushing of soils contaminated with petroleum hydrocarbons. Environ. Int. 2005, 31, 835–839. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Li, P.; He, N.; Ren, W.; Zhang, H.; Xu, H. Research of Phytoremediation on Contaminated Soil with Polycyclic Aromatic Hydrocarbons (PAHs). J. Agro-Environ. Sci. 2007, 26, 2007–2013. [Google Scholar]
- Chen, N.; Zheng, Y.; He, X.; Li, X.; Zhang, X. Analysis of “National Survey Bulletin of Soil Pollution Status”. J. Agric. Environ. Sci. 2017, 36, 1689–1692. [Google Scholar]
- Liu, R.; Jadeja, R.N.; Zhou, Q.; Liu, Z. Treatment and remediation of petroleum-contaminated soils using selective orna mental plants. Environ. Eng. Sci. 2012, 29, 494–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J. The Effects of Plants, Carbon Sources and Bacteria on the Remedation of PAHs Contaminated Soils and Water. Master’s Thesis, Zhejiang University, Hangzhou, China, 2012. (In Chinese). [Google Scholar]
- Zhang, X.; Xu, L.; Qi, Y.; Sun, L.; Zhang, J. Effect of purple pine nut chrysanthemum on remediation of soil contaminated with polycyclic aromatic hydrocarbons. J. Ecol. 2018, 37, 492–497. [Google Scholar]
- Abbaspour, A.; Zohrabi, F.; Dorostkar, V.; Faz, A.; Acosta, J.A. Remediation of an oil-contaminated soil by two native plants treated with biochar and mycorrhizae. J. Environ. Manag. 2020, 254, 109755. [Google Scholar] [CrossRef]
- Frenk, S.; Hadar, Y.; Minz, D. Resilience of soil bacterial community to irrigation with water of different qualities under Mediterranean climate. J. Environ. Microbiol. 2014, 16, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Zornoza, R.; Guerrero, C.; Mataix-Solera, J.; Scow, K.M.; Arcenegui, V.; Mataix-Beneyto, J. Changes in soil microbial community structure following the abandonment of agricultural terraces in mountainous areas of Eastern Spain. Appl. Soil Ecol. 2009, 42, 315–323. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Liu, Y.; Tao, Q.; Hou, Q.; Wu, K.; Song, Y.; Liu, Y.; Guo, X.; Li, J.; Muhammad, L.R.H.; et al. Successive phytoextraction alters ammonia oxidation and associated microbial communities in heavy metal contaminated agricultural soils. Sci. Total Environ. 2019, 664, 616–625. [Google Scholar] [CrossRef]
- Francisco, R.; Stone, D.; Creamer, R.E.; Sousa, J.P.; Morais, P.V. European scale analysis of phospholipid fatty acid composition of soils to establish operating ranges. Appl. Soil Ecol. 2016, 97, 49–60. [Google Scholar] [CrossRef]
- Ganzert, L.; Lipski, A.; Hubberten, H.-W.; Wagner, D. The impact of different soil parameters on the community structure of dominant bacteria from nine different soils located on Livingston Island, South Shetland Archipelago, Antarctica. FEMS Microbiol. Ecol. 2011, 76, 476–491. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Rajkumar, M.; Oliveira, R.S.; Zhang, C.; Freitas, H. Potential of plant beneficial bacteria and arbuscular mycorrhizal fungi in phytoremediation of metal-contaminated saline soils. J. Hazard. Mater. 2019, 379, 120813. [Google Scholar] [CrossRef]
- Xiao, N.; Liu, R.; Jin, C.; Dai, Y. Efficiency of five ornamental plant species in the phytoremediation of polycyclic aromatic hydrocarbon (PAH)-contaminated soil. Ecol. Eng. 2015, 75, 384–391. [Google Scholar] [CrossRef]
- Dai, Y.; Liu, R.; Zhou, Y.; Li, N.; Ma, Q.; Gao, B. Fire Phoenix facilitates phytoremediation of PAH-Cd co-contaminated soil through promotion of beneficial rhizosphere bacterial communities. Environ. Int. 2019, 136, 105421. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ding, N.; Wang, G.; Xu, J.; Wu, J.; Philip, C.B. Effects of different soil weights, storage times and extraction methods on soil phospholipid fatty acid analyses. Geoderma 2009, 150, 171–178. [Google Scholar] [CrossRef]
- Frostegård, A.; Tunlid, A.; Bååth, E. Use and misuse of PLFA measurements in soils. Soil Biol. Biochem. 2010, 43, 1621–1625. [Google Scholar] [CrossRef]
- Piotrowska-Seget, Z.; Cycoń, M.; Kozdrój, J. Metal-tolerant bacteria occurring in heavily polluted soil and mine spoil. Appl. Soil Ecol. 2005, 28, 237–246. [Google Scholar] [CrossRef]
- Kaiser, C.; Koranda, M.; Kitzler, B.; Fuchslueger, L.; Schnecker, J.; Schweiger, P.; Rasche, F.; Zechmeister-Boltenstern, S.; Sessitsch, A.; Richter, A. Belowground carbon allocation by trees drives seasonal patterns of extracellular enzyme activities by altering microbial community composition in a beech forest soil. New Phytol. 2010, 187, 843–858. [Google Scholar] [CrossRef] [Green Version]
- Ruess, L.; Chamberlain, P.M. The fat that matters: Soil food web analysis using fatty acids and their carbon stable isotope signature. Soil Biol. Biochem. 2010, 42, 1898–1910. [Google Scholar] [CrossRef]
- Buyer, J.S.; Teasdale, J.R.; Roberts, D.P.; Zasada, I.A.; Maul, J.E. Factors affecting microbial community structure in tomato cropping systems. Soil Biol. Biochem. 2010, 42, 831–841. [Google Scholar] [CrossRef]
- Kaur, A.; Chaudhary, A.; Kaur, A.; Choudhary, R.; Kaushik, R. Phospholipid fatty acid—A bioindicator of environment monitoring and assessment in soil ecosystem. Curr. Sci. 2005, 89, 1103–1112. [Google Scholar]
- Feng, Y.; Motta, A.C.; Reeves, D.W.; Burmester, C.H.; van Santen, E.; Osborne, J.A. Soil microbial communities under conventional-till and no-till continuous cotton systems. Soil Biol. Biochem. 2003, 35, 1693–1703. [Google Scholar] [CrossRef]
- Wolf, D.C.; Cryder, Z.; Gan, J. Soil bacterial community dynamics following surfactant addition and bioaugmentation in pyrene-contaminated soils. Chemosphere 2019, 231, 93–102. [Google Scholar] [CrossRef]
- Mustafa, S.; Kabir, S.; Shabbir, U.; Batool, R. Plant growth promoting rhizobacteria in sustainable agriculture: From theoretical to pragmatic approach. Symbiosis 2019, 78, 115–123. [Google Scholar] [CrossRef]
- Suresh, B.; Ravishankar, G.A. Phytoremediation—A novel and promising approach for environmental clean-up. Crit. Rev. Biotechnol. 2004, 24, 97–124. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, Q.; Ling, W.; Zhu, X. Arbuscular mycorrhizal phytoremediation of soils contaminated with phenanthrene and pyrene. J. Hazard. Mater. 2011, 185, 703–709. [Google Scholar] [CrossRef]
- Slađana, Č.A.; Biljana, S.M.; Vesna, B.R. How can plants manage polycyclic aromatic hydrocarbons? May these effects represent a useful tool for an effective soil remediation? A review. Clean Technol. Environ. Policy 2015, 17, 597–614. [Google Scholar]
- Pagé, A.P.; Yergeau, E.; Greer, C.W. Salix purpurea stimulates the expression of specific bacterial xenobiotic degradation genes in a soil contaminated with hydrocarbons. PLoS ONE 2015, 10, e0132062. [Google Scholar] [CrossRef] [Green Version]
- Phillips, L.A.; Greer, C.W.; Farell, R.E.; Germida, J.J. Plant root exudates impact the hydrocarbon degradation potential of a weathered-hydrocarbon contaminated soil. Appl. Soil Ecol. 2012, 52, 56–64. [Google Scholar] [CrossRef] [Green Version]
- Oleszczuk, P.; Rakowska, M.; Bucheli, T.D.; Godlewska, P.; Reible, D.D. Combined effects of plant cultivation and sorbing carbon amendments on freely dissolved PAHs in contaminated soil. Environ. Sci. Technol. 2019, 53, 4860–4868. [Google Scholar] [CrossRef] [PubMed]
- Bakker, P.A.H.M.; Pieterse, C.M.J.; de Jonge, R.; Berendsen, R.L. The Soil-Borne Legacy. Cell 2018, 172, 1178–1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berendsen, R.L.; Vismans, G.; Yu, K.; Song, Y.; de Jonge, R.; Burgman, W.P.; Burmølle, M.; Herschend, J.; Bakker, P.A.H.M.; Pieterse, C.M.J. Disease-induced assemblage of a plant-beneficial bacterial consortium. ISME J. 2018, 12, 1496–1507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pineda, A.; Kaplan, I.; Bezemer, T.M. Steering Soil Microbiomes to Suppress Aboveground Insect Pests. Trends Plant Sci. 2017, 22, 770–778. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L. New Favorite of Root Flower—Pine Cone. Chin. Flower Hortic. 2013, 24. (In Chinese) [Google Scholar]
- Cheng, Z.; Ling, W.; Gao, Y.; Wang, J. Impacts of arbuscular mycorrhizal on plant uptake and phytoremediation of pyrene in soils. Plant Nutr. Fert. Sci. 2008, 14, 1178–1185. [Google Scholar]
- Joner, E.J.; Leyval, C. Rhizosphere gradients of polycyclic aromatic hydrocarbon (PAH) dissipation in two industrial soils and the impact of arbuscular mycorrhiza. Environ. Sci. Technol. 2003, 37, 2371–2375. [Google Scholar] [CrossRef]
- Chen, R.; Lin, X.; Yin, R. Effect of mycorrhizae on bioremediation of soil polluted by organic matters. Chin. J. Ecol. 2005, 24, 176–180. [Google Scholar]
- Liu, R.; Zhao, L.; Jin, C.; Xiao, N.; Jadeja, R.N.; Sun, T. Enzyme responses to phytoremediation of PAH-contaminated soil using Echinacea purpurea. Water Air Soil Pollut. 2014, 225, 2230.48. [Google Scholar] [CrossRef]
- Xia, J.; Li, J. Advances in Increasing Plant Drought Resistance by AM Fungi. Chin. Agric. Sci. Bull. 2005, 2, 625014. [Google Scholar]
- Sasse, J.; Martinoia, E.; Northen, T. Feed your friends: Do plant exudates shape the root microbiome? Trends Plant Sci. 2018, 23, 25–41. [Google Scholar] [CrossRef] [Green Version]
- Fanin, N.; Kardol, P.; Farrell, M.; Nilsson, M.C.; Gundale, M.J.; Wardle, D.A. The ratio of Gram-positive to Gram-negative bacterial PLFA markers as an indicator of carbon availability in organic soils. Soil Biol. Biochem. 2019, 128, 111–114. [Google Scholar] [CrossRef]
- Kumari, S.; Mangwani, N.; Das, S. Synergistic effect of quorum sensing genes in biofilm development and PAHs degradation by a marine bacterium. Bioengineered 2016, 7, 205–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houlden, A.; Timms-Wilson, T.M.; Day, M.J.; Bailey, M.J. Influence of plant developmental stage on microbial community structure and activity in the rhizosphere of three field crops. FEMS Microbiol. Ecol. 2008, 65, 193–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Micallef, S.A.; Channer, S.; Shiaris, M.P.; Colón-Carmona, A. Plant age and genotype impact the progression of bacterial community succession in the Arabidopsis rhizosphere. Plant Signal. Behav. 2009, 4, 777–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Na, X.; Li, X.; Zhang, Z.; Li, M.; Kardol, P.; Xu, T.; Wang, M.; Cao, X.; Ma, F. Bacterial community dynamics in the rhizosphere of a long-lived, leguminous shrub across a 40-year age sequence. J. Soils Sediments 2017, 18, 76–84. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).