Holistic Approach to the Restoration of a Vandalized Monument: The Cross of the Inquisition, Seville City Hall, Spain
Abstract
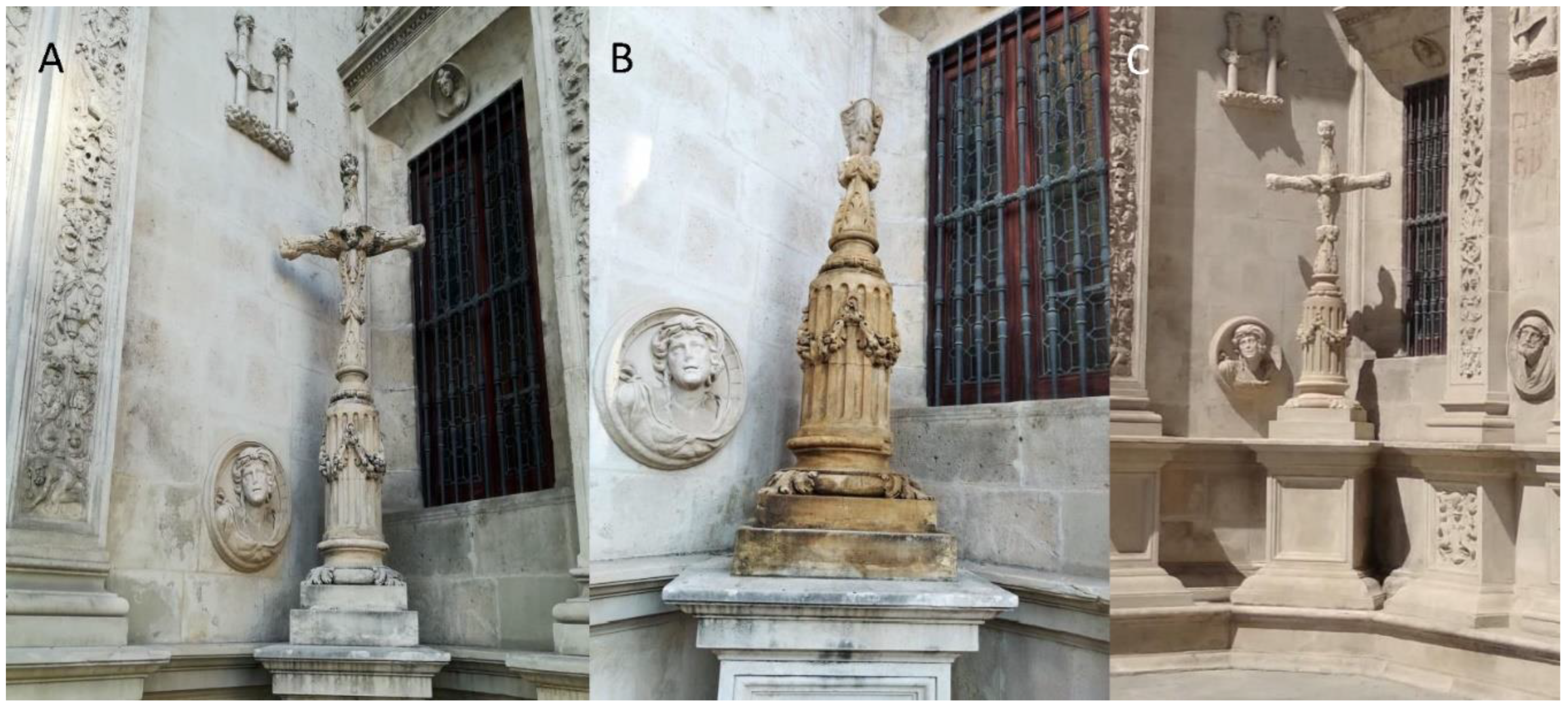
:1. Introduction
2. Materials and Methods
3. Results and Discussion
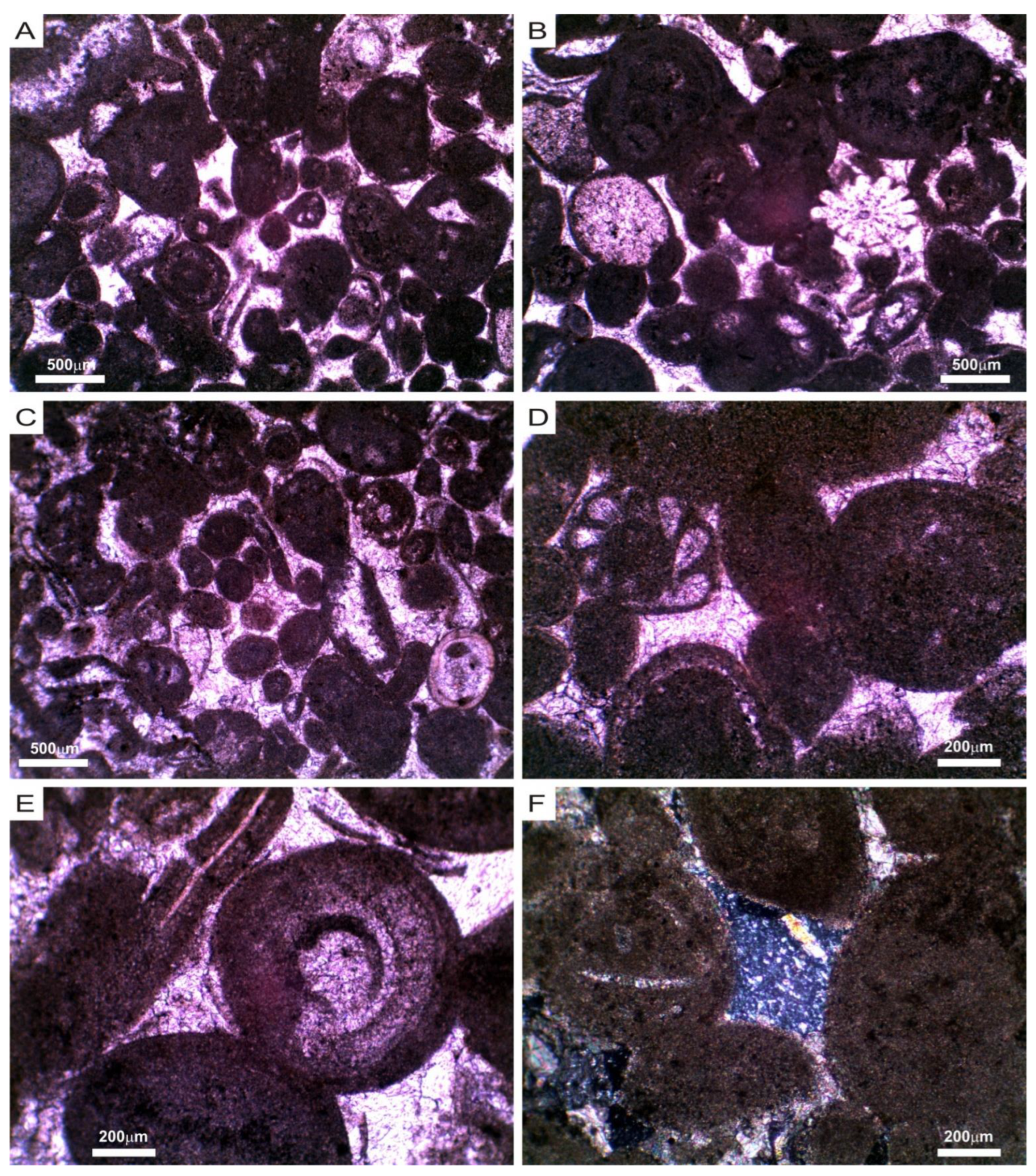
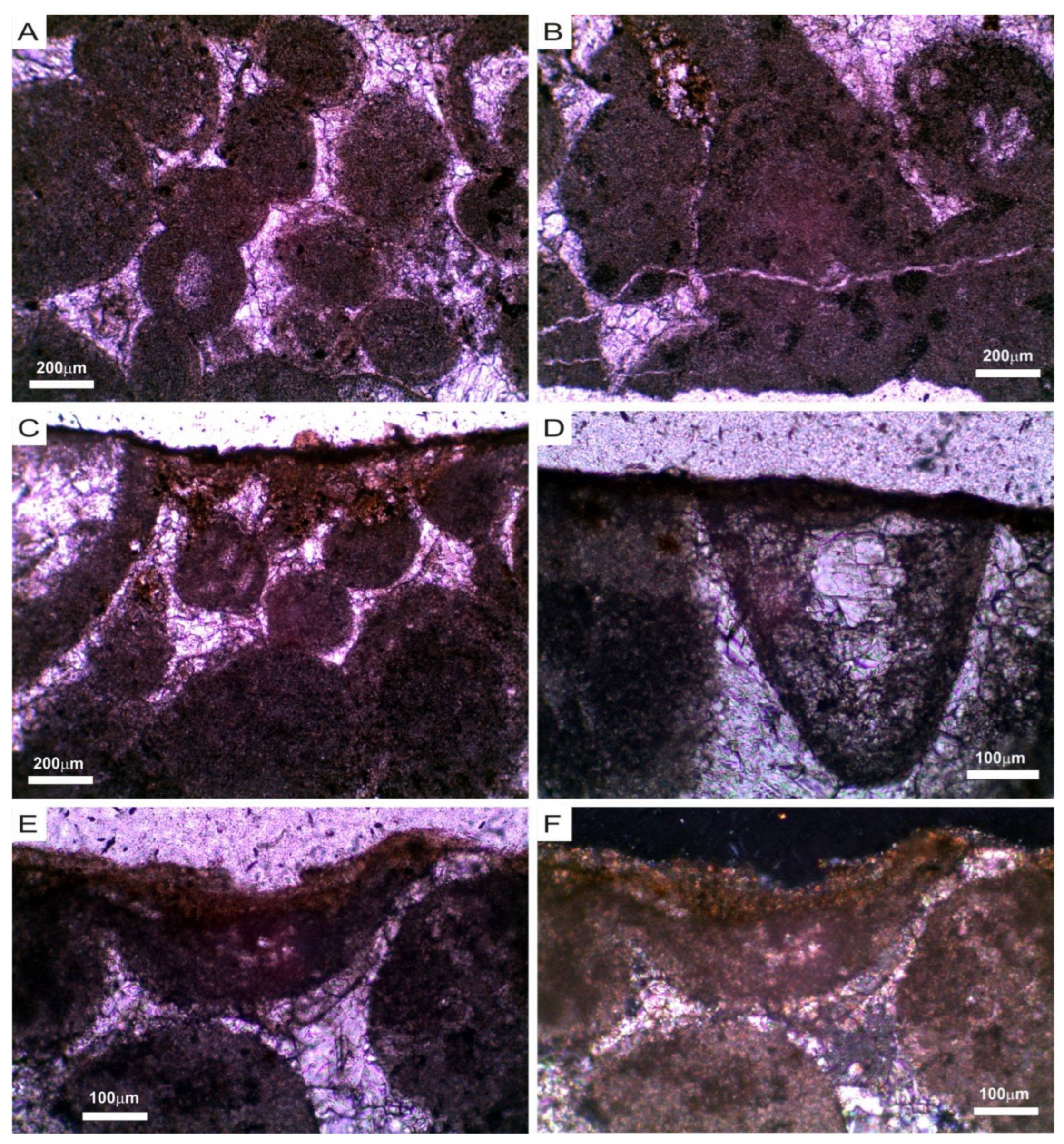
3.1. Mineralogical, Chemical and Petrophysical Characteristics of the Stone
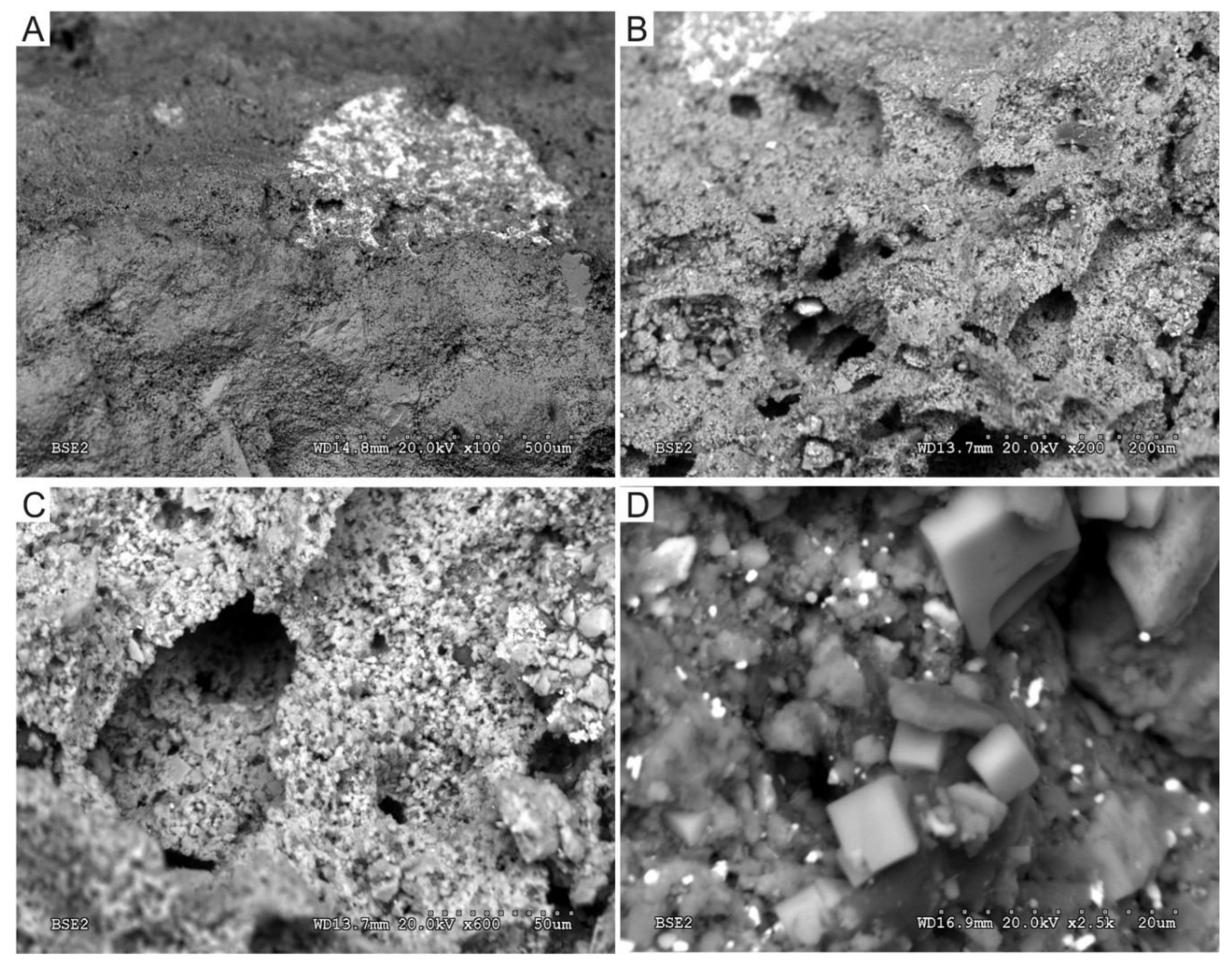
3.2. Scanning Electron Microscopy and Microanalysis (SEM-EDS)
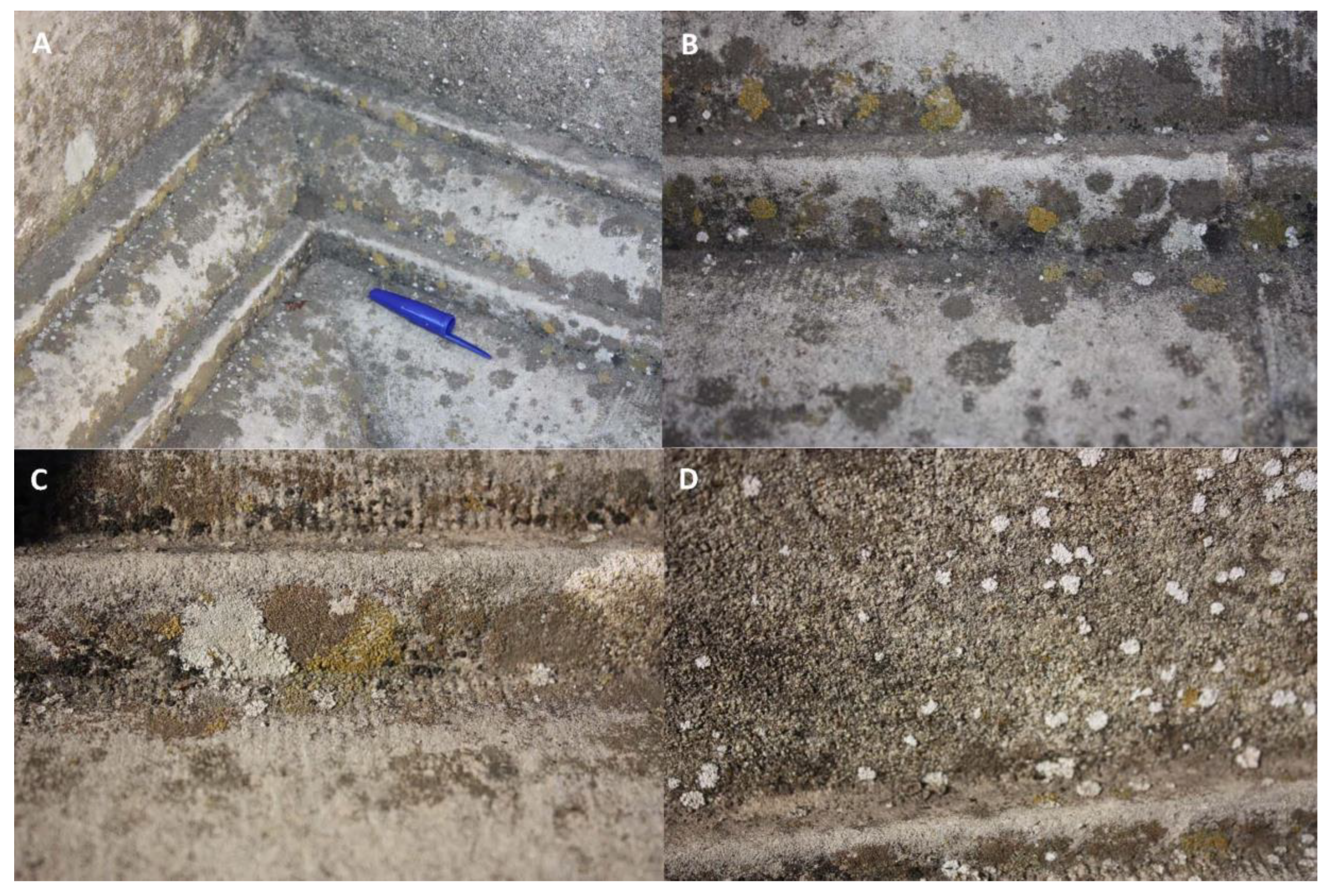
3.3. Organisms on the Cross of the Inquisition limestone
3.4. Restoration of the Cross of the Inquisition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lindholm, R.C.V.; Finkelman, R.B. Calcite staining: Semiquantitative determination of ferrous iron. J. Sediments Res. 1972, 42, 239–242. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Yarza, P.; Yilmaz, P.; Pruesse, E.; Glöckner, F.O.; Ludwig, W.; Schleifer, K.-H.; Whitman, W.B.; Euzéby, J.; Amann, R.; Rosselló-Móra, R. Uniting the classification of cultured and uncultured bacteria and archaea using 16S rRNA gene sequences. Nat. Rev. 2014, 12, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Folk, R.L. Spectral subdivision of limestone types. In Classification of Carbonates Rocks—A Syposium; Ham, W.E., Ed.; American Association of Petroleum Geologist: Tulsa, OK, USA, 1962; pp. 62–84. [Google Scholar]
- Dunham, R.J. Classification of carbonate rocks according to depositional texture. In Classification of Carbonates Rocks—A Syposium; Ham, W.E., Ed.; American Association of Petroleum Geologist: Tulsa, OK, USA, 1962; pp. 108–121. [Google Scholar]
- Rodriguez-Navarro, C.; Doehne, E. Salt weathering: Influence of evaporation rate, supersaturation and crystallization pattern. Earth Surf. Process. Landf. J. Br. Geomorphol. Res. Group 1999, 24, 191–209. [Google Scholar] [CrossRef]
- Sabbioni, C. Mechanisms of air pollution damage of stone. In The Effects of Air Pollution on the Built Environment; Brimblecombe, P., Ed.; Air Pollution Reviews; Imperial College Press: London, UK, 2003; Volume 2, pp. 63–106. [Google Scholar]
- Benavente, D.; Cueto, N.; Martinez-Martinez, J.; Garcia-del-Cura, M.A.; Cañaveras, J.C. The influence of petrophysical properties on the salt weathering of porous building rocks. Environ. Geol. 2007, 52, 197–206. [Google Scholar] [CrossRef]
- Benavente, D.; Sanchez-Moral, S.; Fernandez-Cortes, A.; Cañaveras, J.C.; Elez, J.; Saiz-Jimenez, C. Salt damage and microclimate in the Postumius Tomb, Roman Necropolis of Carmona, Spain. Environ. Earth Sci. 2011, 63, 1529–1543. [Google Scholar] [CrossRef] [Green Version]
- Cachier, H.; Sarda-Esteve, R.; Oikonomou, K.; Sciare, J.; Bonazza, A.; Sabbioni, C.; Greco, M.; Reyes, J.; Hermosin, B.; Saiz-Jimenez, C. Aerosol characterization and sources in different European atmospheres: Paris, Seville, Florence and Milan. In Air Pollution and Cultural Heritage; Saiz-Jimenez, C., Ed.; Balkema: Leiden, The Netherlands, 2004; pp. 3–14. [Google Scholar]
- Reyes, J.; Hermosin, B.; Saiz-Jimenez, C. Organic analysis of aerosols in Seville atmosphere. In Air Pollution and Cultural Heritage; Saiz-Jimenez, C., Ed.; Balkema: Leiden, The Netherlands, 2004; pp. 15–20. [Google Scholar]
- Reyes, J.; Hermosin, B.; Saiz-Jimenez, C. Organic composition of Seville aerosols. Org. Geochem. 2006, 37, 2019–2025. [Google Scholar] [CrossRef]
- Caneva, G.; Nugari, M.P.; Ricci, S.; Salvadori, O. Pitting of marble Roman monuments and the related microflora. In Proceedings of the 7th International Congress on Deterioration and Conservation of Stone, Lisbon, Portugal, 15–18 June 1992; Delgado Rodrigues, J., Henriques, F., Telmo Jeremias, F., Eds.; NLCE: Lisbon, Portugal, 1992; pp. 521–529. [Google Scholar]
- Sterflinger, K.; Krumbein, W.E. Dematiaceous fungi as a major agent of biopitting for Mediterranean marbles and limestones. Geomicrobiol. J. 1997, 14, 219–230. [Google Scholar] [CrossRef]
- De Leo, F.; Antonelli, F.; Pietrini, A.M.; Ricci, S.; Urzì, C. Study of the euendolithic activity of black meristematic fungi isolated from a marble statue in the Quirinale Palace’s Gardens in Rome, Italy. Facies 2019, 65, 18. [Google Scholar] [CrossRef]
- Ahmadjian, V. The Lichen Symbiosis; John Wiley: New York, NY, USA, 1993. [Google Scholar]
- Cordeiro, L.M.C.; Reis, R.A.; Cruz, L.M.; Stocker-Wörgotter, E.; Grube, M.; Iacomini, M. Molecular studies of photobionts of selected lichens from the coastal vegetation of Brazil. FEMS Microbiol. Ecol. 2005, 54, 381–390. [Google Scholar] [CrossRef] [Green Version]
- Petrzik, K.; Vondrák, J.; Kvíderová, J.; Lukavský, J. Platinum anniversary: Virus and lichen alga together more than 70 years. PLoS ONE 2015, 10, e0120768. [Google Scholar] [CrossRef] [PubMed]
- Sanders, W.B.; Masumoto, H. Lichen algae: The photosynthetic partners in lichen symbioses. Lichenologist 2021, 53, 347–393. [Google Scholar] [CrossRef]
- Sommer, V.; Mikhailyuk, T.; Glaser, K.; Karsten, U. Uncovering unique green algae and cyanobacteria isolated from biocrusts in highly saline potash tailing pile habitats, using an integrative approach. Microorganisms 2020, 8, 1667. [Google Scholar] [CrossRef]
- Lewis, L.A.; Flechtner, V.R. Green algae (Chlorophyta) of desert microbiotic crusts: Diversity of North American taxa. Taxon 2002, 51, 443–451. [Google Scholar] [CrossRef]
- Voytsekhovich, A.; Beck, A. Lichen photobionts of the rocky outcrops of Karadag massif (Crimean Peninsula). Symbiosis 2016, 68, 9–24. [Google Scholar] [CrossRef]
- Casas, C.; Brugués, M.; Cros, R.M.; Sérgio, C. Handbook of Mosses of the Iberian Peninsula and the Balearic Islands: Illustrated Keys to Genera and Species; Institut d’Estudis Catalans: Barcelona, Spain, 2020. [Google Scholar]
- Kosior, G.; Prell, M.; Samecka-Cymerman, A.; Stankiewicz, A.; Kolon, K.; Kryza, R.; Brudzinska-Kosior, A.; Frontasyeva, M.; Kempers, A.J. Metals in Tortula muralis from sandstone buildings in an urban agglomeration. Ecol. Indic. 2015, 58, 122–131. [Google Scholar] [CrossRef]
- Isermann, M. Diversity of bryophytes in an urban area of NW Germany. Lindbergia 2007, 32, 75–81. [Google Scholar]
- Ekwealor, J.T.B.; Fisher, K.M. Life under quartz: Hypolithic mosses in the Mojave Desert. PLoS ONE 2020, 15, e0235928. [Google Scholar] [CrossRef]
- Gorbushina, A.A. Life on the rocks. Environ. Microbiol. 2007, 9, 1613–1631. [Google Scholar] [CrossRef]
- Sterflinger, K. Fungi: Their role in deterioration of cultural heritage. Fungal Biol. Rev. 2010, 24, 47–55. [Google Scholar] [CrossRef]
- Diederich, P.; Kocourkova, J.; Etayo, J.; Zhurbenko, M. The lichenicolous Phoma species (coelomycetes) on Cladonia. Lichenologist 2007, 39, 153–163. [Google Scholar] [CrossRef] [Green Version]
- Aveskamp, M.M.; de Gruyter, J.; Woudenberg, J.H.C.; Verkley, G.J.M.; Crous, P.W. Highlights of the Didymellaceae: A polyphasic approach to characterise Phoma and related pleosporalean genera. Stud. Mycol. 2010, 65, 1–60. [Google Scholar] [CrossRef] [PubMed]
- Lawrey, J.D.; Diederich, P.; Nelsen, M.P.; Freebury, C.; Van den Broeck, D.; Sikaroodi, M.; Ertz, D. Phylogenetic placement of lichenicolous Phoma species in the Phaeosphaeriaceae (Pleosporales, Dothideomycetes). Fungal Divers. 2012, 55, 195–213. [Google Scholar] [CrossRef]
- Tosi, S.; Casado, B.; Gerdol, R.; Caretta, G. Fungi isolated from Antarctic mosses. Polar Biol. 2002, 25, 262–268. [Google Scholar] [CrossRef]
- Buzzini, P.; Lachance, M.-A.; Yurkov, A. Yeasts in Natural Ecosystems: Diversity; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Woudenberg, J.H.C.; Seidl, M.F.; Groenewald, J.Z.; de Vries, M.; Stielow, J.B.; Thomma, B.P.H.J.; Crous, P.W. Alternaria section Alternaria: Species, formae speciales or pathotypes? Stud. Mycol. 2015, 82, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jurado, V.; Gonzalez-Pimentel, J.L.; Hermosin, B.; Saiz-Jimenez, C. Biodeterioration of Salón de Reinos, Museo Nacional del Prado, Madrid, Spain. Appl. Sci. 2021, 11, 8858. [Google Scholar] [CrossRef]
- Tsuneda, A.; Thormann, M.N.; Currah, R.S. Characteristics of a disease of Sphagnum fuscum caused by Scleroconidioma sphagnicola. Can. J. Bot. 2001, 78, 1294–1298. [Google Scholar]
- Davey, M.L.; Currah, R.S. Interactions between mosses (Bryophyta) and fungi. Can. J. Bot. 2006, 84, 1509–1519. [Google Scholar] [CrossRef]
- Phillips, A.J.L.; Alves, A.; Abdollahzadeh, J.; Slippers, B.; Wingfield, M.J.; Groenewald, J.Z.; Crous, P.W. The Botryosphaeriaceae: Genera and species known from culture. Stud. Mycol. 2013, 76, 51–167. [Google Scholar] [CrossRef] [Green Version]
- Dissanayake, A.J.; Phillips, A.J.L.; Li, X.H.; Hyde, K.D. Botryosphaeriaceae: Current status of genera and species. Mycosphere 2016, 7, 1001–1073. [Google Scholar] [CrossRef]
- Lugauskas, A.; Sveistyte, L.; Ulevicius, V. Concentration and species diversity of airborne fungi near busy streets in Lithuanian urban areas. Ann. Agric. Environ. Med. 2003, 10, 233–239. [Google Scholar] [PubMed]
- Urzì, C.; De Leo, F.; Lo Passo, C.; Criseo, G. Intra-specific diversity of Aureobasidium pullulans strains isolated from rocks and other habitats assessed by physiological methods and by random amplified polymorphic DNA (RAPD). J. Microbiol. Methods 1999, 36, 95–105. [Google Scholar] [CrossRef]
- Simonovicová, A.; Gödyová, M.; Sevc, J. Airborne and soil microfungi as contaminants of stone in a hypogean cemetery. Int. Biodeter. Biodegr. 2004, 54, 7–11. [Google Scholar] [CrossRef]
- Mansour, M. Effects of the halophilic fungi Cladosporium sphaerospermum, Wallemia sebi, Aureobasidium pullulans and Aspergillus nidulans on halite formed on sandstone surface. Int. Biodeter. Biodegr. 2017, 117, 289–298. [Google Scholar] [CrossRef]
- Ruibal, C.; Platas, G.; Bills, G.F. High diversity and morphological convergence among melanised fungi from rock formations in the Central Mountain System of Spain. Persoonia 2008, 21, 93–110. [Google Scholar] [CrossRef]
- Moreno, P.P.; Egea, J.M. El género Lichinella Nyl. en el sureste de España y norte de Africa. Cryptogam. Bryol. Lichenol. 1992, 13, 237–259. [Google Scholar]
- Egea, J.M.; Alonso, F.L. Patrones de distribución en la flora liquénica xerófila del sureste de España. Acta Bot. Malacit. 1996, 21, 35–47. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Fu, R.; Wu, B.; Liu, X.; Xiang, M. Rock inhabiting fungi: Terminology, diversity, evolution and adaptation mechanisms. Mycology 2022, 13, 1–31. [Google Scholar] [CrossRef]
- Harutyunyan, S.; Muggia, L.; Grube, M. Black fungi in lichens from seasonally arid habitats. Stud. Mycol. 2008, 61, 83–90. [Google Scholar] [CrossRef]
- Gorbushina, A.A.; Beck, A.; Schulte, A. Microcolonial rock inhabiting fungi and lichen photobionts: Evidence for mutualistic interactions. Mycol. Res. 2005, 109, 1288–1296. [Google Scholar] [CrossRef]
- Zakharova, K.; Tesei, D.; Marzban, G.; Dijksterhuis, J.; Wyatt, T.; Sterflinger, K. Microcolonial fungi on rocks: A life in constant drought? Mycopathologia 2013, 175, 537–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sterflinger, K.; Prillinger, H. Molecular taxonomy and biodiversity of rock fungal communities in an urban environment (Vienna, Austria). Antonie van Leeuwenhoek 2001, 80, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Vondrák, J.; Říha, P.; Arup, U.; Søchting, U. The taxonomy of the Caloplaca citrina group (Teloschistaceae) in the Black Sea region; with contributions to the cryptic species concept in lichenology. Lichenologist 2009, 41, 571–604. [Google Scholar] [CrossRef] [Green Version]
- Nimis, P.L. ITALIC—The Information System on Italian Lichens, Version 5.0; Department of Biology, University of Trieste: Trieste, Italy, 2016. Available online: https://italic.units.it(accessed on 16 February 2022).
- Arup, U.; Sochting, U.; Frödén, P. A new taxonomy of the family Teloschistaceae. Nord. J. Bot. 2013, 31, 16–83. [Google Scholar] [CrossRef]
- Frolov, I.; Vondrák, J.; Kosnar, J.; Arup, U. Phylogenetic relationships within Pyrenodesmia sensu lato and the role of pigments in its taxonomic interpretation. J. Syst. Evol. 2021, 59, 454–474. [Google Scholar] [CrossRef]
- Muggia, L.; Grube, M.; Tretiach, M. A combined molecular and morphological approach to species delimitation in black-fruited, endolithic Caloplaca: High genetic and low morphological diversity. Mycol. Res. 2008, 112, 36–49. [Google Scholar] [CrossRef]
- Gaya, E.; Navarro-Rosinés, P.; Llimona, X.; Hladun, N.; Lutzoni, F. Phylogenetic reassessment of the Teloschistaceae (lichen-forming Ascomycota, Lecanoromycetes). Mycol. Res. 2008, 112, 528–546. [Google Scholar] [CrossRef]
- Barreno, E.; Merino, A. Catálogo liquénico de las calizas de Madrid (España). Lazaroa 1981, 3, 247–268. [Google Scholar]
- Ariño, X.; Ortega-Calvo, J.J.; Gomez-Bolea, A.; Saiz-Jimenez, C. Lichen colonization of the Roman pavement of Baelo Claudia (Cádiz, Spain): Biodeterioration vs. bioprotection. Sci. Total Environ. 1995, 167, 353–363. [Google Scholar] [CrossRef] [Green Version]
- Marcos Laso, B. Biodiversidad y colonización liquénica de algunos monumentos en la ciudad de Salamanca (España). Bot. Complut. 2001, 25, 93–102. [Google Scholar]
- Wilk, K. Calcicolous species of the genus Caloplaca in the Polish Western Carpathians. Pol. Bot. Stud. 2012, 29, 1–91. [Google Scholar]
- Caneva, G.; Fidanza, M.R.; Tonon, C.; Favero-Longo, S.E. Biodeterioration patterns and their interpretation for potential applications to stone conservation: A hypothesis from allelopathic inhibitory effects of lichens on the Caestia Pyramid (Rome). Sustainability 2020, 12, 1132. [Google Scholar] [CrossRef] [Green Version]
- Hawksworth, D.L. The identity of Pyrenidium actinellum Nyl. Trans. Br. Mycol. Soc. 1983, 80, 547–549. [Google Scholar] [CrossRef]
- Etayo, J.; López de Silanes, M.E. Hongos liquenícolas del norte de Portugal, especialmente del Parque Natural Montesinho. Nova Acta Cient. Compost. Biol. 2020, 27, 35–50. [Google Scholar]
- Robador, M.D.; Arroyo, F.; Perez-Rodriguez, J.L. Study and restoration of the Seville City Hall façade. Constr. Build. Mater. 2014, 53, 370–380. [Google Scholar] [CrossRef]
- Rivas, T.; Pozo-Antonio, J.S.; López de Silanes, M.E.; Ramil, A.; López, A.J. Laser versus scalpel cleaning of crustose lichens on granite. Appl. Surf. Sci. 2018, 440, 467–476. [Google Scholar] [CrossRef]
- Pozo-Antonio, J.S.; Rivas, T.; López de Silanes, M.E.; Ramil, A.; López, A.J. Dual combination of cleaning methods (scalpel, biocide, laser) to enhance lichen removal from granite. Int. Biodeterior. Biodegrad. 2022, 168, 105373. [Google Scholar] [CrossRef]
- Favero-Longo, S.E.; Viles, H.A. A review of the nature, role and control of lithobionts on stone cultural heritage: Weighing-up and managing biodeterioration and bioprotection. World J. Microbiol. Biotechnol. 2020, 36, 100. [Google Scholar] [CrossRef]
- Concha-Lozano, N.; Gaudon, P.; Pages, J.; de Billerbeck, G.; Lafon, D.; Eterradossi, O. Protective effect of endolithic fungal hyphae on oolitic limestone buildings. J. Cult. Herit. 2012, 13, 120–127. [Google Scholar] [CrossRef] [Green Version]
- Pinna, D. Biofilms and lichens on stone monuments: Do they damage or protect? Front. Microbiol. 2014, 5, 133. [Google Scholar] [CrossRef]
- Salvatori, O.; Municchia, A.C. The role of fungi and lichens in the biodeterioration of stone monuments. Open Conf. Proc. J. 2016, 7, 39–54. [Google Scholar] [CrossRef]
- Nascimbene, J.; Salvadori, O.; Nimis, P.L. Monitoring lichen recolonization on a restored calcareous statue. Sci. Total Environ. 2009, 407, 2420–2426. [Google Scholar] [CrossRef] [PubMed]
- Nascimbene, J.; Salvadori, O. Lichen recolonization on restored calcareous statues of three Venetian villas. Int. Biodeterior. Biodegrad. 2008, 62, 313–318. [Google Scholar] [CrossRef]
- Ariño, X.; Canals, A.; Gomez-Bolea, A.; Saiz-Jimenez, C. Assessment of the performance of a water-repellent/biocide treatment after 8 years. In Protection and Conservation of the Cultural Heritage of the Mediterranean Cities; Galán, E., Zezza, F., Eds.; Balkema: Lisse, The Netherlands, 2002; pp. 121–125. [Google Scholar]
- Nugari, M.P.; Salvadori, O. Biocides and treatment of stone: Limitations and future prospects. In Art, Biology and Conservation: Biodeterioration of Works of Art; Koestler, R.J., Koestler, V.H., Charola, A.E., Nieto-Fernandez, F.E., Eds.; Metropolitan Museum of Art: New York, NY, USA, 2003; pp. 519–535. [Google Scholar]
- Urzì, C.; De Leo, F. Evaluation of the efficiency of water-repellent and biocide compounds against microbial colonization of mortars. Int. Biodeterior. Biodegrad. 2007, 60, 25–34. [Google Scholar] [CrossRef]
- Pinna, D.; Salvadori, B.; Galeotti, M. Monitoring the performance of innovative and traditional biocides mixed with consolidants and water-repellents for the prevention of biological growth on stone. Sci. Total Environ. 2012, 423, 132–141. [Google Scholar] [CrossRef]
- Pinna, D.; Galeotti, M.; Perito, B.; Daly, G.; Salvadori, B. In situ long-term monitoring of recolonization by fungi and lichens after innovative and traditional conservative treatments of archaeological stones in Fiesole (Italy). Int. Biodeterior. Biodegrad. 2018, 132, 49–58. [Google Scholar] [CrossRef]


| Sample | Species | Accession Number | Group * |
|---|---|---|---|
| Brown crust (CD1) | Trebouxia aggregata | ON479828 | Chlorophyta |
| Tortula truncata | ON479827 | Bryophyta | |
| Pseudostichococcus monallantoides | ON479830 | Chlorophyta | |
| Phoma sp. | ON479831 | Fungi | |
| Naganishia albida | ON479825 | Fungi | |
| Myrmecia sp. | ON479826 | Chlorophyta | |
| Alternaria alternata | ON479829 | Fungi | |
| Black crust (CD3) | Scleroconidioma sphagnicola | ON479833 | Fungi |
| Pseudostichococcus monallantoides | ON479834 | Chlorophyta | |
| Neofusicoccum parvum | ON479835 | Fungi | |
| Lichinella cribellifera | ON479836 | Lichen | |
| Aureobasidium pullulans | ON479837 | Fungi | |
| Phoma sp. | ON479832 | Fungi | |
| Black crust (LQ1) | Trebouxia aggregata | ON479838 | Chlorophyta |
| Pseudostichococcus monallantoides | ON479839 | Chlorophyta | |
| Dothidea berberidis | ON479840 | Fungi |
| Lichen | Associated Species | Accession Number | Group * |
|---|---|---|---|
| Kuettlingeria teicholyta | Trebouxia aggregata | ON479841 | Chlorophyta |
| Pyrenodesmia chalybaea | ON479847 | Lichen | |
| Xanthoria parietina | ON479846 | Lichen | |
| Xanthoria sp. | ON479845 | Lichen | |
| Phoma sp. | ON479844 | Fungi | |
| Ceratobasidium sp. | ON479843 | Fungi | |
| Pyrenidium cf. actinellum | ON479842 | Fungi | |
| Chaetothyriales sp. | ON479848 | Fungi | |
| Rhinocladiella sp. | ON479849 | Fungi | |
| Caloplaca citrina group | Trebouxia aggregata | ON479852 | Chlorophyta |
| Xanthoria parietina | ON479850 | Lichen | |
| Xanthoria sp. | ON479851 | Lichen | |
| Pyrenodesmia variabilis | Trebouxia aggregata | ON479854 | Chlorophyta |
| Capnobotriella sp. | ON479853 | Fungi |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurado, V.; Cañaveras, J.C.; Gomez-Bolea, A.; Gonzalez-Pimentel, J.L.; Sanchez-Moral, S.; Costa, C.; Saiz-Jimenez, C. Holistic Approach to the Restoration of a Vandalized Monument: The Cross of the Inquisition, Seville City Hall, Spain. Appl. Sci. 2022, 12, 6222. https://doi.org/10.3390/app12126222
Jurado V, Cañaveras JC, Gomez-Bolea A, Gonzalez-Pimentel JL, Sanchez-Moral S, Costa C, Saiz-Jimenez C. Holistic Approach to the Restoration of a Vandalized Monument: The Cross of the Inquisition, Seville City Hall, Spain. Applied Sciences. 2022; 12(12):6222. https://doi.org/10.3390/app12126222
Chicago/Turabian StyleJurado, Valme, Juan Carlos Cañaveras, Antonio Gomez-Bolea, Jose Luis Gonzalez-Pimentel, Sergio Sanchez-Moral, Carlos Costa, and Cesareo Saiz-Jimenez. 2022. "Holistic Approach to the Restoration of a Vandalized Monument: The Cross of the Inquisition, Seville City Hall, Spain" Applied Sciences 12, no. 12: 6222. https://doi.org/10.3390/app12126222
APA StyleJurado, V., Cañaveras, J. C., Gomez-Bolea, A., Gonzalez-Pimentel, J. L., Sanchez-Moral, S., Costa, C., & Saiz-Jimenez, C. (2022). Holistic Approach to the Restoration of a Vandalized Monument: The Cross of the Inquisition, Seville City Hall, Spain. Applied Sciences, 12(12), 6222. https://doi.org/10.3390/app12126222









