Impact of Steam Autoclaving on the Mechanical Properties of 3D-Printed Resins Used for Insertion Guides in Orthodontics and Implant Dentistry
Abstract
:1. Introduction
2. Materials and Methods
2.1. 3D-Printed Specimens Preparation
2.2. Mechanical Tests
2.3. Statistical Analysis
3. Results
3.1. Vickers Hardness
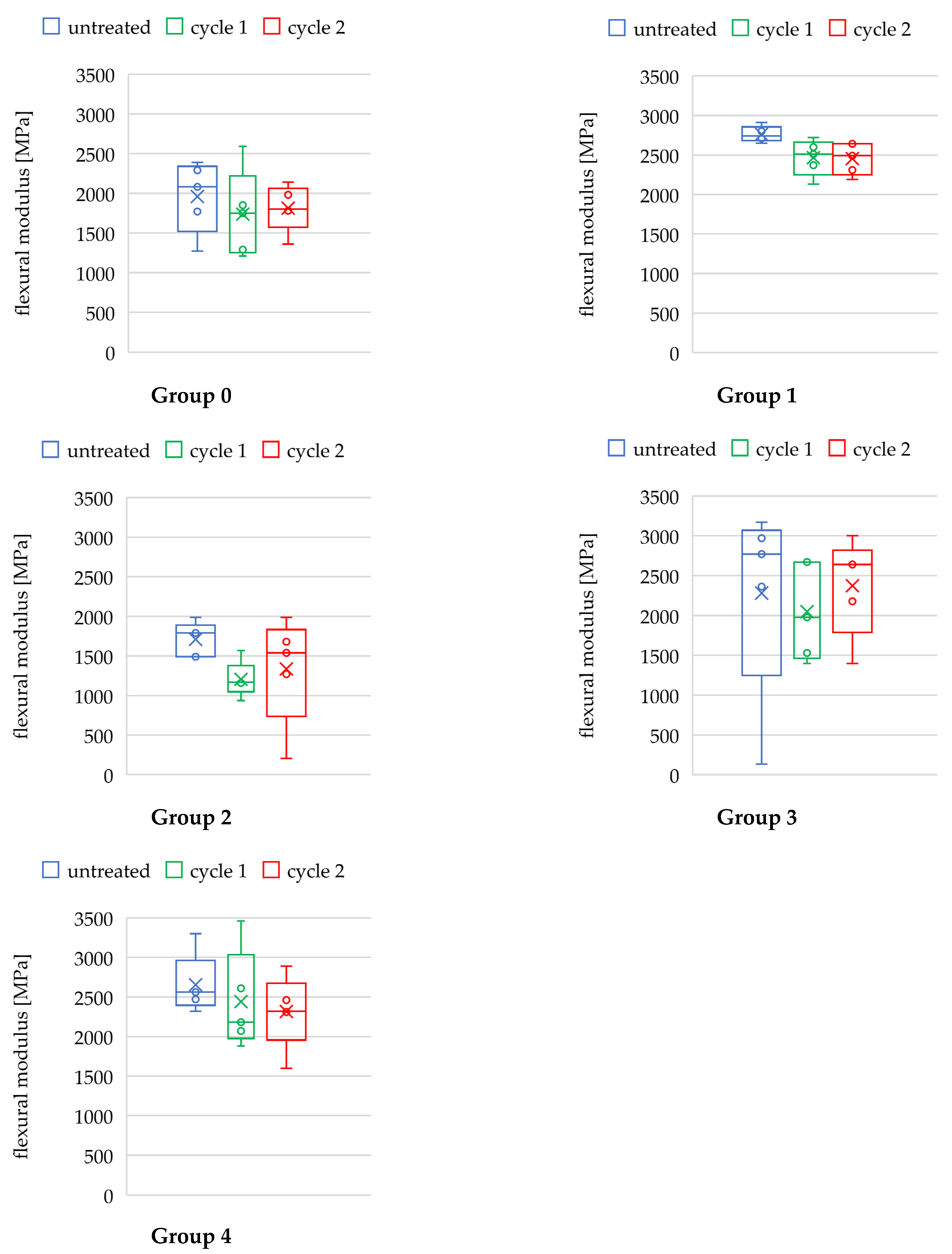
3.2. Flexural Modulus
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Louvrier, A.; Marty, P.; Barrabé, A.; Euvrard, E.; Chatelain, B.; Weber, E.; Meyer, C. How useful is 3D printing in maxillofacial surgery? J. Stomatol. Oral Maxillofac. Surg. 2017, 118, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Tattan, M.; Chambrone, L.; González-Martín, O.; Avila-Ortiz, G. Static computer-aided, partially guided, and free-handed implant placement: A systematic review and meta-analysis of randomized controlled trials. Clin. Oral Implant. Res. 2020, 31, 889–916. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Ou, Q.; Lin, X.; Wang, Y. Comparison between a Computer-Aided Surgical Template and the Free-Hand Method: A Systematic Review and Meta-Analysis. Implant Dent. 2019, 28, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Tahmaseb, A.; Wu, V.; Wismeijer, D.; Coucke, W.; Evans, C. The accuracy of static computer-aided implant surgery: A systematic review and meta-analysis. Clin. Oral Implant. Res. 2018, 29, 416–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lal, K.; White, G.S.; Morea, D.N.; Wright, R.F. Use of stereolithographic templates for surgical and prosthodontic implant planning and placement. Part I. The concept. J. Prosthodont. 2006, 15, 51–58. [Google Scholar] [CrossRef]
- Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on Medical Devices, Amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and Repealing Council Directives 90/385/EEC and 93/42/EEC. Annex 1, Chapters 1, 8. Available online: https://eur-lex.europa.eu/eli/reg/2017/745/oj (accessed on 16 February 2022).
- Raico Gallardo, Y.N.; da Silva-Olivio, I.; Mukai, E.; Morimoto, S.; Sesma, N.; Cordaro, L. Accuracy comparison of guided surgery for dental implants according to the tissue of support: A systematic review and meta-analysis. Clin. Oral Implant. Res. 2017, 28, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Cassetta, M.; Stefanelli, L.V.; Giansanti, M.; Di Mambro, A.; Calasso, S. Depth deviation and occurrence of early surgical complications or unexpected events using a single stereolithographic surgi-guide. J. Stomatol. Oral Maxillofac. Surg. 2011, 40, 1377–1387. [Google Scholar] [CrossRef]
- Schnutenhaus, S.; Edelmann, C.; Rudolph, H.; Luthardt, R.G. Retrospective study to determine the accuracy of template-guided implant placement using a novel nonradiologic evaluation method. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 121, 72–79. [Google Scholar] [CrossRef]
- Makins, S.R. Artifacts interfering with interpretation of cone beam computed tomography images. Dent. Clin. N. Am. 2014, 58, 485–495. [Google Scholar] [CrossRef]
- Tadinada, A.; Jalali, E.; Jadhav, A.; Schincaglia, G.P.; Yadav, S. Artifacts in Cone Beam Computed Tomography Image Volumes: An Illustrative Depiction. J. Mass. Dent. Soc. 2015, 64, 12–15. [Google Scholar]
- Giménez, B.; Özcan, M.; Martínez-Rus, F.; Pradíes, G. Accuracy of a digital impression system based on active wavefront sampling technology for implants considering operator experience, implant angulation, and depth. Clin. Implant Dent. Relat. Res. 2015, 17, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Joda, T.; Zarone, F.; Ferrari, M. The complete digital workflow in fixed prosthodontics: A systematic review. BMC Oral Health 2017, 17, 124. [Google Scholar] [CrossRef] [PubMed]
- Cassetta, M.; Giansanti, M.; Di Mambro, A.; Stefanelli, L.V. Accuracy of positioning of implants inserted using a mucosa-supported stereolithographic surgical guide in the edentulous maxilla and mandible. Int. J. Oral Maxillofac. Implant. 2014, 29, 1071–1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Kholy, K.; Janner, S.F.M.; Schimmel, M.; Buser, D. The influence of guided sleeve height, drilling distance, and drilling key length on the accuracy of static computer-assisted implant surgery. Clin. Implant Dent. Relat. Res. 2019, 21, 101–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassetta, M.; Altieri, F.; Di Giorgio, R.; Barbato, E. Palatal orthodontic miniscrew insertion using a CAD-CAM surgical guide: Description of a technique. Int. J. Clin. Oral Maxillofac. Surg. 2018, 47, 1195–1198. [Google Scholar] [CrossRef] [PubMed]
- Jedliński, M.; Janiszewska-Olszowska, J.; Mazur, M.; Ottolenghi, L.; Grocholewicz, K.; Galluccio, G. Guided Insertion of Temporary Anchorage Device in Form of Orthodontic Titanium Miniscrews with Customized 3D Templates—A Systematic Review with Meta-Analysis of Clinical Studies. Coatings 2021, 11, 1488. [Google Scholar] [CrossRef]
- Becker, K.; Unland, J.; Wilmes, B.; Tarraf, N.E.; Drescher, D. Is there an ideal insertion angle and position for orthodontic mini-implants in the anterior palate? A CBCT study in humans. Am. J. Orthod. Dentofac. Orthop. 2019, 156, 345–354. [Google Scholar] [CrossRef]
- Küffer, M.; Drescher, D.; Becker, K. Application of the Digital Workflow in Orofacial Orthopedics and Orthodontics: Printed Appliances with Skeletal Anchorage. J. Appl. Sci. 2022, 12, 3820. [Google Scholar] [CrossRef]
- D’haese, J.; Ackhurst, J.; Wismeijer, D.; De Bruyn, H.; Tahmaseb, A. Current state of the art of computer-guided implant surgery. Periodontology 2000 2017, 73, 121–133. [Google Scholar] [CrossRef]
- Wegmüller, L.; Halbeisen, F.; Sharma, N.; Kühl, S.; Thieringer, F.M. Consumer vs. High-End 3D Printers for Guided Implant Surgery-An In Vitro Accuracy Assessment Study of Different 3D Printing Technologies. J. Clin. Med. 2021, 10, 4894. [Google Scholar] [CrossRef]
- Herschdorfer, L.; Negreiros, W.M.; Gallucci, G.O.; Hamilton, A. Comparison of the accuracy of implants placed with CAD-CAM surgical templates manufactured with various 3D printers: An in vitro study. J. Prosthet. Dent. 2021, 125, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Chen LLin, W.S.; Polido, W.D.; Eckert, G.J.; Morton, D. Accuracy, reproducibility, and dimensional stability of additively manufactured surgical templates. J. Prosthet. Dent. 2019, 122, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Kohn, W.G.; Collins, A.S.; Cleveland, J.L.; Harte, J.A.; Eklund, K.J.; Malvitz, D.M.; Centers for Disease Control and Prevention (CDC). Guidelines for infection control in dental health-care settings—2003. MMWR Recomm. Rep. 2003, 52, 1–61. [Google Scholar] [PubMed]
- Tallarico, M.; Lumbau, A.I.; Park, C.J.; Puddu, A.; Sanseverino, F.; Amarena, R.; Meloni, S.M. In vitro evaluation of bioburden, three-dimensional stability, and accuracy of surgical templates without metallic sleeves after routinely infection control activities. Clin. Implant Dent. Relat. Res. 2021, 23, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Marei, H.F.; Alshaia, A.; Alarifi, S.; Almasoud, N.; Abdelhady, A. Effect of Steam Heat Sterilization on the Accuracy of 3D Printed Surgical Guides. Implant Dent. 2019, 28, 372–377. [Google Scholar] [CrossRef]
- Török, G.; Gombocz, P.; Bognár, E.; Nagy, P.; Dinya, E.; Kispélyi, B.; Hermann, P. Effects of disinfection and sterilization on the dimensional changes and mechanical properties of 3D printed surgical guides for implant therapy—Pilot study. BMC Oral Health 2020, 20, 19. [Google Scholar] [CrossRef]
- Quintana, R.; Choi, J.W.; Puebla, K.; Wicker, R. Effects of build orientation on tensile strength for stereolithography-manufactured ASTM D-638 type I specimens. Int. J. Adv. Manuf. Technol. 2010, 46, 201–215. [Google Scholar] [CrossRef]
- Pop, S.I.; Dudescu, M.; Mihali, S.G.; Păcurar, M.; Bratu, D.C. Effects of Disinfection and Steam Sterilization on the Mechanical Properties of 3D SLA- and DLP-Printed Surgical Guides for Orthodontic Implant Placement. Polymers 2022, 14, 2107. [Google Scholar] [CrossRef]
- Bayarsaikhan, E.; Lim, J.H.; Shin, S.H.; Park, K.H.; Park, Y.B.; Lee, J.H.; Kim, J.E. Effects of Postcuring Temperature on the Mechanical Properties and Biocompatibility of Three-Dimensional Printed Dental Resin Material. Polymers 2021, 13, 1180. [Google Scholar] [CrossRef]
- Da Silva, F.C.; Kimpara, E.T.; Mancini, M.N.; Balducci, I.; Jorge, A.O.; Koga-Ito, C.Y. Effectiveness of six different disinfectants on removing five microbial species and effects on the topographic characteristics of acrylic resin. J. Prosthodont. 2008, 17, 627–633. [Google Scholar] [CrossRef]
- Sennhenn-Kirchner, S.; Weustermann, S.; Mergeryan, H.; Jacobs, H.G.; Borg-von Zepelin, M.; Kirchner, B. Preoperative sterilization and disinfection of drill guide templates. Clin. Oral Investig. 2008, 12, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.N.; Palenik, C.J.; Blanchard, S.B. Microbial contamination and the sterilization/disinfection of surgical guides used in the placement of endosteal implants. Int. J. Oral Maxillofac. Implant. 2011, 26, 274–281. [Google Scholar]
- Smith, A.J.; Bagg, J.; Hurrell, D.; McHugh, S. Sterilization of re-usable instruments in general dental practice. Br. Dent. J. 2007, 203, E16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahasneh, A.M.; Alakhras, M.; Khabour, O.F.; Al-Sa’di, A.G.; Al-Mousa, D.S. Practices of Infection Control among Dental Care Providers: A Cross Sectional Study. Clin. Cosmet. Investig. Dent. 2020, 12, 281–289. [Google Scholar] [CrossRef]
- Dagher, J.; Sfeir, C.; Abdallah, A.; Majzoub, Z. Sterilization and Biologic Monitoring in Private Dental Clinics in Lebanon. J. Contemp. Dent. Pract. 2018, 19, 853–861. [Google Scholar]
- Röhm-Rodowald, E.; Jakimiak, B.; Chojecka, A.; Zmuda-Baranowska, M.; Kanclerski, K. Ocena procesów dekontaminacji: Mycia, dezynfekcji i sterylizacji w praktyce stomatologicznej w Polsce w latach 2011–2012 [Assessment of decontamination processes: Cleaning, disinfection and sterilization in dental practice in Poland in the years 2011–2012]. Prz. Epidemiol. 2012, 66, 635–641. [Google Scholar]
- Jindal, P.; Juneja, M.; Bajaj, D.; Siena, F.L.; Breedon, P. Effects of post-curing conditions on mechanical properties of 3D printed clear dental aligners. Rapid Prototyp. J. 2020, 26, 1337–1344. [Google Scholar] [CrossRef]
- Jakubovics, N.; Greenwood, M.; Meechan, J.G. General medicine and surgery for dental practitioners: Part 4. Infections and infection control. Br. Dent. J. 2014, 217, 73–77. [Google Scholar] [CrossRef]

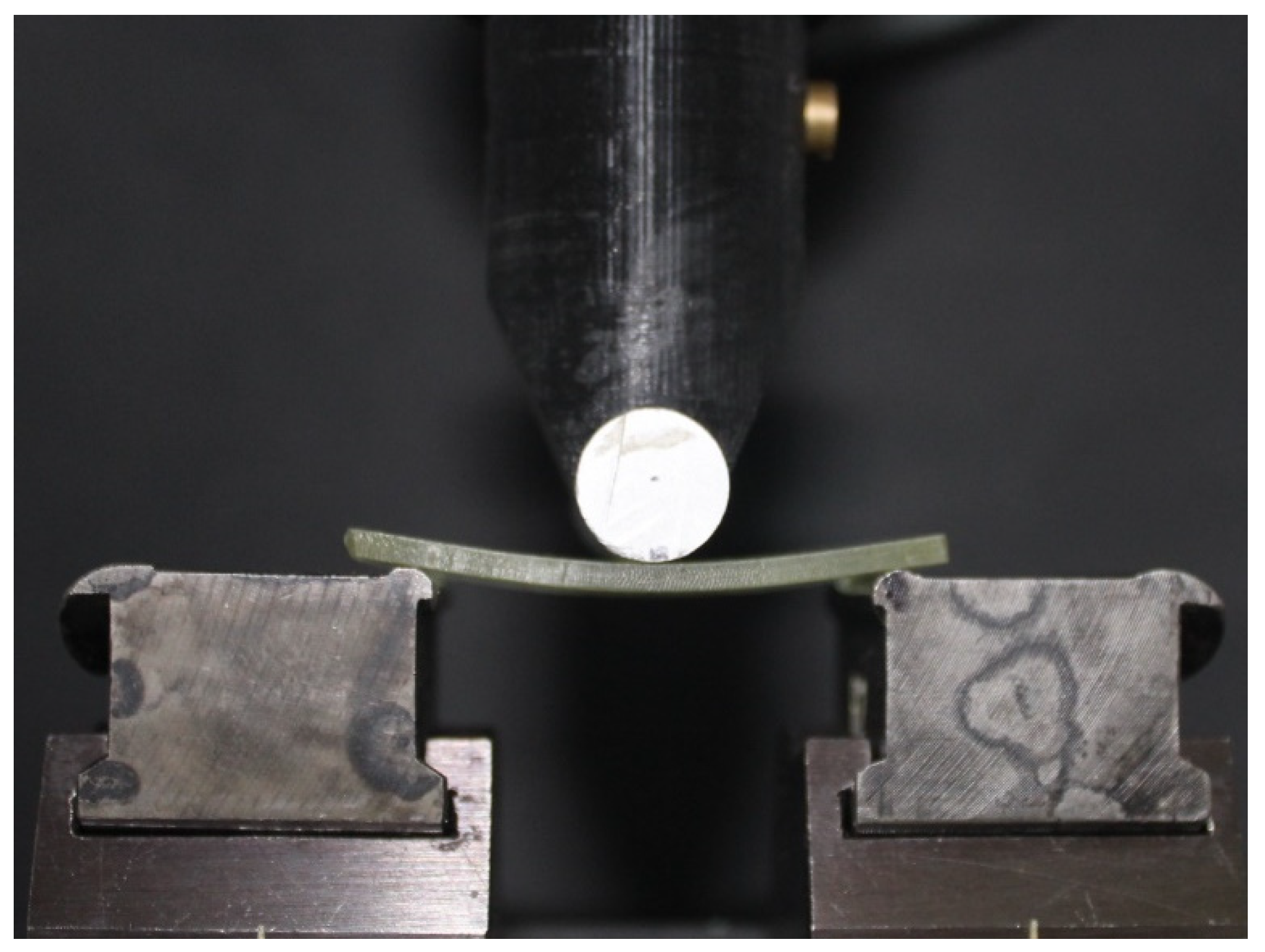

| Group § | Printing Method | Printer, Manufacturer | Resin, Manufacturer |
|---|---|---|---|
| 0 | DLP | Micro Plus XL, Envisiontec Inc. | E-Guide, Envisiontec Inc. |
| 1 | DLP | NextDent 5100, Vertex-Dental B.V. | NextDent SG, Vertex-Dental B.V. |
| 2 | DLP | ASIGA MAX, Pluradent GmbH & Co. KG | Optiprint Guide, dentona AG |
| 3 | SLA | Form 3, Formlabs Inc. | Dental SG, Formlabs Inc. |
| 4 | LCD-SLA | Slash Plus, UniZ Technology LLC. | zSG Amber, UniZ Technology LLC. |
| Vickers Hardness [HV0.5] | Kruskal–Wallis | Bonferroni-Corrected Post Hoc Test (Mann–Whitney-U, If pH < 0.05) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group § | ICC | Subgroup §§ | M ± SD | H (2) | pH | Comparison | U | z | pU | r |
| 0 | 0.993 | untreated | 13.50 ± 2.62 | 35.896 | <0.001 *** | untreated vs. cycle 1 | 8 | −4.334 | <0.001 *** | −0.791 |
| cycle 1 | 6.94 ± 2.21 | untreated vs. cycle 2 | 9 | −4.293 | <0.001 *** | −0.784 | ||||
| cycle 2 | 19.57 ± 2.04 | cycle 1 vs. cycle 2 | 1 | −4.625 | <0.001 *** | −0.844 | ||||
| 1 | 0.582 | untreated | 29.16 ± 6.83 | 5.847 | 0.054 | - | - | - | - | - |
| cycle 1 | 27.67 ± 5.42 | - | - | - | - | - | ||||
| cycle 2 | 25.30 ± 1.65 | - | - | - | - | - | ||||
| 2 | 0.945 | untreated | 14.14 ± 2.33 | 26.303 | <0.001 *** | untreated vs. cycle 1 | 75 | −1.555 | 0.359 | −0.284 |
| cycle 1 | 16.00 ± 3.06 | untreated vs. cycle 2 | 26 | −3.588 | 0.001 ** | −0.655 | ||||
| cycle 2 | 10.52 ± 1.05 | cycle 1 vs. cycle 2 | 3 | −4.542 | <0.001 *** | −0.829 | ||||
| 3 | 0.925 | untreated | 17.12 ± 1.96 | 22.659 | <0.001 *** | untreated vs. cycle 1 | 42 | −2.924 | 0.010 * | −0.534 |
| cycle 1 | 20.80 ± 2.95 | untreated vs. cycle 2 | 1 | −4.625 | <0.001 *** | −0.844 | ||||
| cycle 2 | 23.84 ± 2.24 | cycle 1 vs. cycle 2 | 60 | −2.178 | 0.088 | −0.398 | ||||
| 4 | 0.670 | untreated | 15.67 ± 2.41 | 1.844 | 0.398 | - | - | - | - | - |
| cycle 1 | 16.96 ± 2.92 | - | - | - | - | - | ||||
| cycle 2 | 14.74 ± 1.25 | - | - | - | - | - | ||||
| Flexural Modulus Ef [MPa] | ANOVA | Bonferroni-Corrected Post Hoc Test (Students-t, If pA < 0.05) | |||||
|---|---|---|---|---|---|---|---|
| Group § | Subgroup §§ | M ± SD | F (2,12) | pF | Comparison | t (8) | pt |
| 0 | untreated | 1960 ± 405 | 0.322 | 0.731 | - | - | - |
| cycle 1 | 1738 ± 494 | - | - | - | |||
| cycle 2 | 1812 ± 261 | - | - | - | |||
| 1 | untreated | 2762 ± 88 | 4.492 | 0.035 * | untreated vs. cycle 1 | 2.671 | 0.085 |
| cycle 1 | 2466 ± 203 | untreated vs. cycle 2 | 3.080 | 0.045 * | |||
| cycle 2 | 2454 ± 179 | cycle 1 vs. cycle 2 | 0.089 | >0.999 | |||
| 2 | untreated | 1710 ± 194 | 1.810 | 0.206 | - | - | - |
| cycle 1 | 1205 ± 204 | - | - | - | |||
| cycle 2 | 1337 ± 613 | - | - | - | |||
| 3 | untreated | 2280 ± 1108 | 0.181 | 0.837 | - | - | - |
| cycle 1 | 2050 ± 542 | - | - | - | |||
| cycle 2 | 2372 ± 551 | - | - | - | |||
| 4 | untreated | 2654 ± 338 | 0.580 | 0.575 | - | - | - |
| cycle 1 | 2440 ± 564 | - | - | - | |||
| cycle 2 | 2367 ± 536 | - | - | - | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirschner, A.; David, S.; Brunello, G.; Keilig, L.; Drescher, D.; Bourauel, C.; Becker, K. Impact of Steam Autoclaving on the Mechanical Properties of 3D-Printed Resins Used for Insertion Guides in Orthodontics and Implant Dentistry. Appl. Sci. 2022, 12, 6195. https://doi.org/10.3390/app12126195
Kirschner A, David S, Brunello G, Keilig L, Drescher D, Bourauel C, Becker K. Impact of Steam Autoclaving on the Mechanical Properties of 3D-Printed Resins Used for Insertion Guides in Orthodontics and Implant Dentistry. Applied Sciences. 2022; 12(12):6195. https://doi.org/10.3390/app12126195
Chicago/Turabian StyleKirschner, Anna, Samuel David, Giulia Brunello, Ludger Keilig, Dieter Drescher, Christoph Bourauel, and Kathrin Becker. 2022. "Impact of Steam Autoclaving on the Mechanical Properties of 3D-Printed Resins Used for Insertion Guides in Orthodontics and Implant Dentistry" Applied Sciences 12, no. 12: 6195. https://doi.org/10.3390/app12126195
APA StyleKirschner, A., David, S., Brunello, G., Keilig, L., Drescher, D., Bourauel, C., & Becker, K. (2022). Impact of Steam Autoclaving on the Mechanical Properties of 3D-Printed Resins Used for Insertion Guides in Orthodontics and Implant Dentistry. Applied Sciences, 12(12), 6195. https://doi.org/10.3390/app12126195








