Application of the Digital Workflow in Orofacial Orthopedics and Orthodontics: Printed Appliances with Skeletal Anchorage
Abstract
:1. Introduction
2. Digital Workflow: From Intraoral Scan to the Printable Data
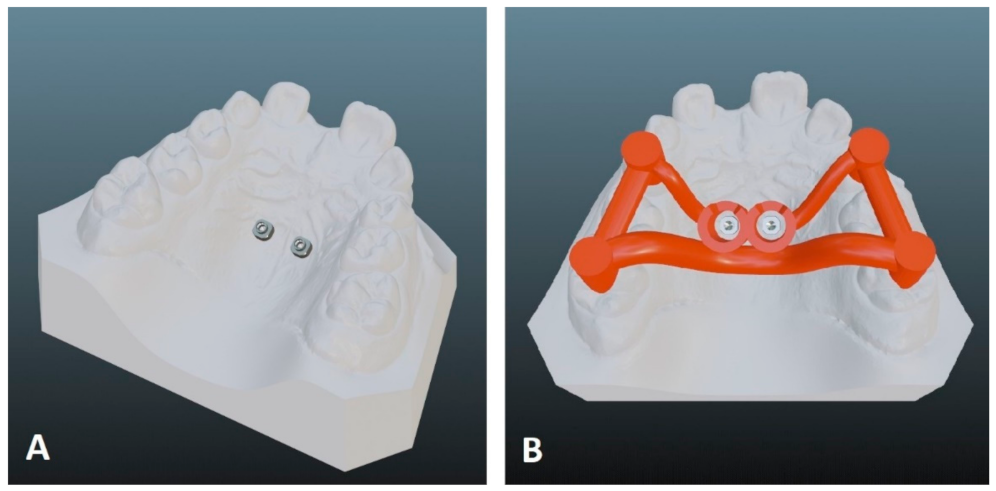
2.1. Data Acquisition
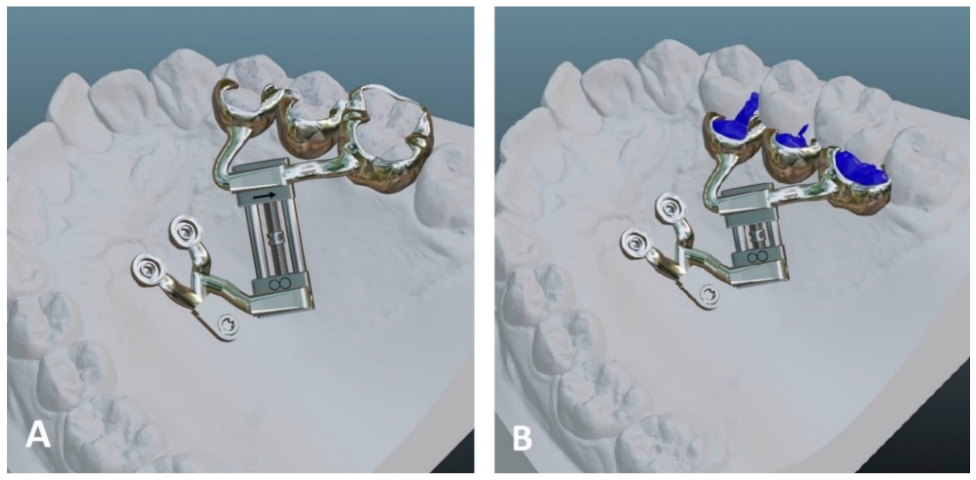
2.2. Digital Appliance Design
2.3. Data Preparation for 3D Printing
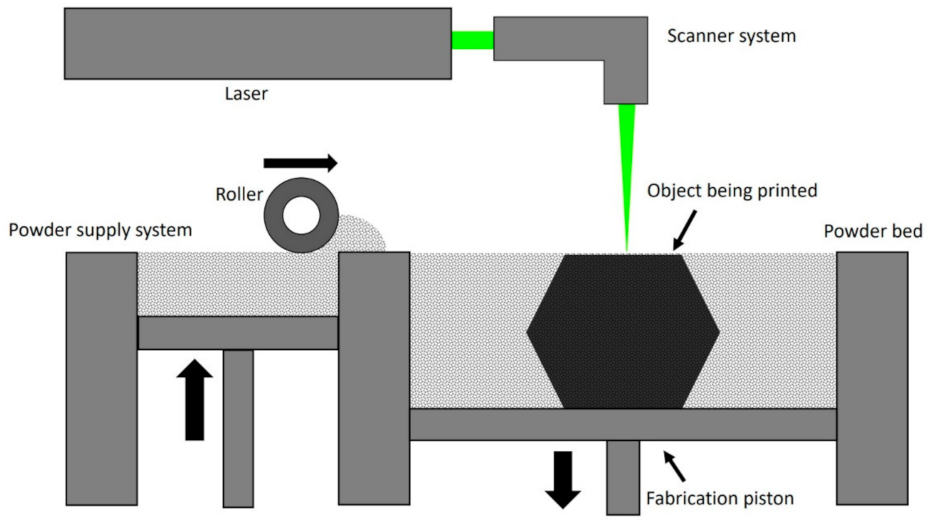
3. 3D Printing Technologies for Orthodontic Appliances
4. Sources of Error
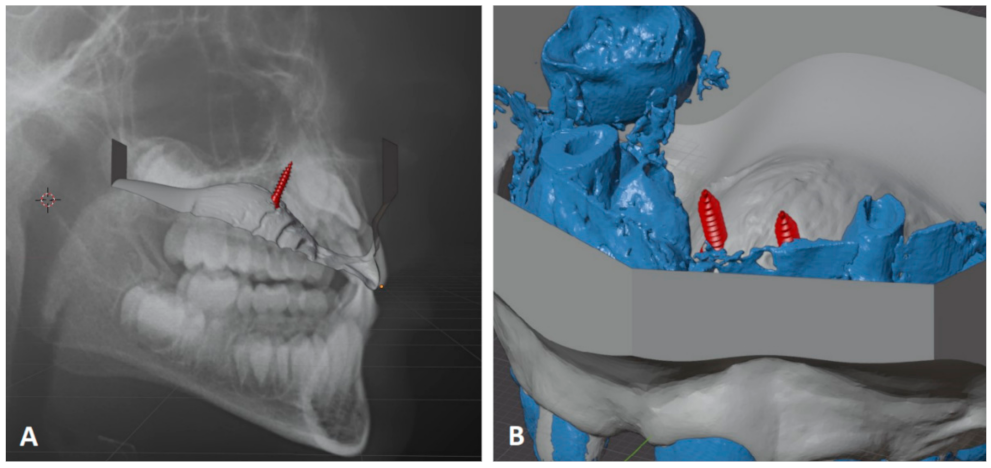
5. Clinical Application
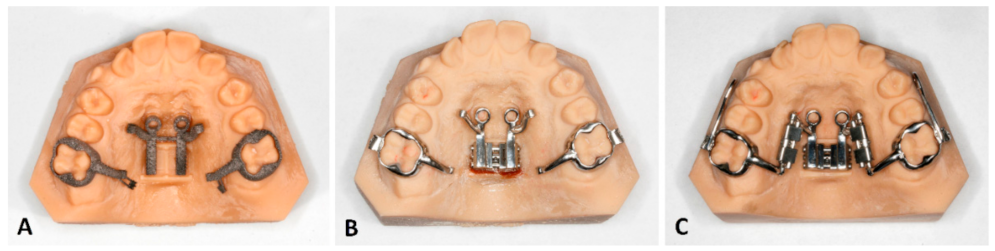
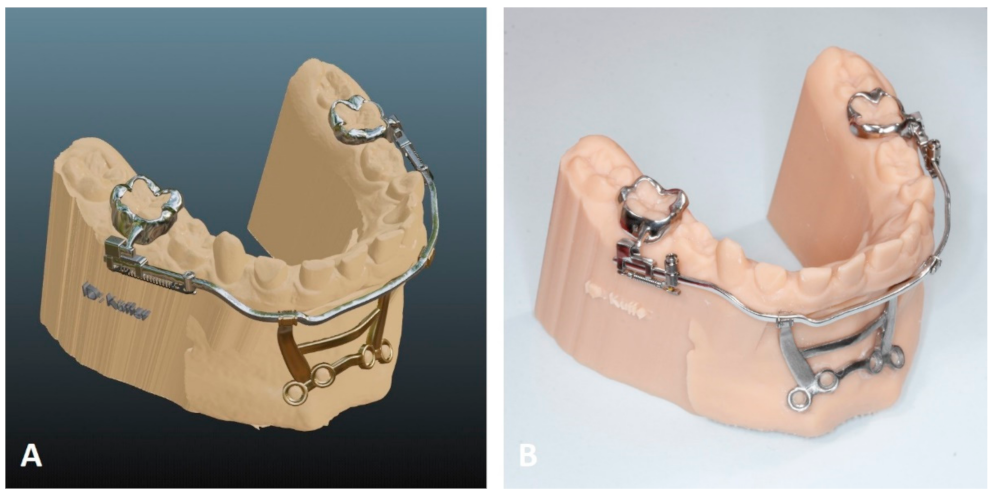
5.1. Temporary Anchorage Device-Borne Appliances
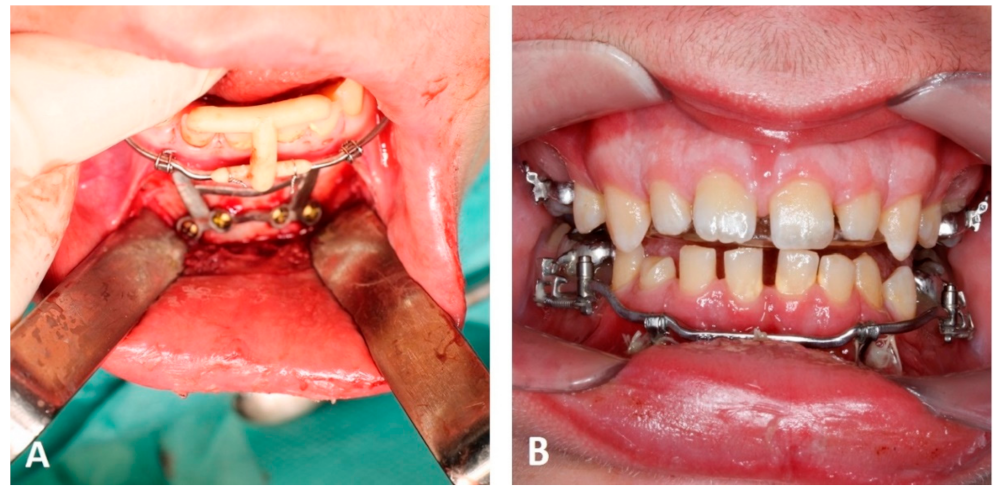
5.2. Computer-Aided Manufactured Mentoplates
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wong, K.C. 3D-printed patient-specific applications in orthopedics. Orthop. Res. Rev. 2016, 8, 57–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tack, P.; Victor, J.; Gemmel, P.; Annemans, L. 3D-printing techniques in a medical setting: A systematic literature review. Biomed. Eng. Online 2016, 15, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauermeister, A.J.; Zuriarrain, A.; Newman, M.I. Three-Dimensional Printing in Plastic and Reconstructive Surgery: A Systematic Review. Ann. Plast. Surg. 2016, 77, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Jamróz, W.; Szafraniec, J.; Kurek, M.; Jachowicz, R. 3D Printing in Pharmaceutical and Medical Applications—Recent Achievements and Challenges. Pharm. Res. 2018, 35, 176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Della Bona, A.; Cantelli, V.; Britto, V.T.; Collares, K.F.; Stansbury, J.W. 3D printing restorative materials using a stereolithographic technique: A systematic review. Dent. Mater. 2021, 37, 336–350. [Google Scholar] [CrossRef]
- Tian, Y.; Chen, C.; Xu, X.; Wang, J.; Hou, X.; Li, K.; Lu, X.; Shi, H.; Lee, E.S.; Jiang, H.B. A Review of 3D Printing in Dentistry: Technologies, Affecting Factors, and Applications. Scanning 2021, 2021, 9950131. [Google Scholar] [CrossRef]
- Graf, S.; Cornelis, M.A.; Hauber Gameiro, G.; Cattaneo, P.M. Computer-aided design and manufacture of hyrax devices: Can we really go digital? Am. J. Orthod. Dentofac. Orthop. 2017, 152, 870–874. [Google Scholar] [CrossRef] [Green Version]
- Graf, S.; Vasudavan, S.; Wilmes, B. CAD-CAM design and 3-dimensional printing of mini-implant retained orthodontic appliances. Am. J. Orthod. Dentofac. Orthop. 2018, 154, 877–882. [Google Scholar] [CrossRef] [Green Version]
- Clemente, R.; Contardo, L.; Greco, C.; Di Lenarda, R.; Perinetti, G. Class III Treatment with Skeletal and Dental Anchorage: A Review of Comparative Effects. BioMed Res. Int. 2018, 2018, 7946019. [Google Scholar] [CrossRef]
- Wilmes, B.; Olthoff, G.; Drescher, D. Comparison of skeletal and conventional anchorage methods in conjunction with pre-operative decompensation of a skeletal class III malocclusion. J. Orofac. Orthop. 2009, 70, 297–305. [Google Scholar] [CrossRef]
- Proffit, W.R.; Fields, H.W. Contemporary Orthodontics; Mosby-Year Book: St. Louis, MO, USA, 1993. [Google Scholar]
- Nienkemper, M.; Wilmes, B.; Lübberink, G.; Ludwig, B.; Drescher, D. Extrusion of impacted teeth using mini-implant mechanics. J. Clin. Orthod. 2012, 46, 150–155, quiz 183. [Google Scholar] [PubMed]
- Nienkemper, M.; Handschel, J.; Drescher, D. Systematic review of mini-implant displacement under orthodontic loading. Int. J. Oral Sci. 2014, 6, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilmes, B.; Willmann, J.; Stocker, B.; Drescher, D. Mini-Implantate zur kieferorthopädischen Verankerung im anterioren Gaumen, mediane vs. paramediane Insertion. Inf. Orthod. Kieferorthopädie 2015, 47, 243–248. [Google Scholar] [CrossRef]
- Wilmes, B.; Ludwig, B.; Vasudavan, S.; Nienkemper, M.; Drescher, D. The T-Zone: Median vs. Paramedian Insertion of Palatal Mini-Implants. J. Clin. Orthod. 2016, 50, 543–551. [Google Scholar]
- Becker, K.; Pliska, A.; Busch, C.; Wilmes, B.; Wolf, M.; Drescher, D. Efficacy of orthodontic mini implants for en masse retraction in the maxilla: A systematic review and meta-analysis. Int. J. Implant. Dent. 2018, 4, 35. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.; Wilmes, B.; Grandjean, C.; Vasudavan, S.; Drescher, D. Skeletally anchored mesialization of molars using digitized casts and two surface-matching approaches: Analysis of treatment effects. J. Orofac. Orthop. 2018, 79, 11–18. [Google Scholar] [CrossRef]
- Fritz, U.; Ehmer, A.; Diedrich, P. Clinical suitability of titanium microscrews for orthodontic anchorage-preliminary experiences. J. Orofac. Orthop. 2004, 65, 410–418. [Google Scholar] [CrossRef]
- Wilmes, B.; Drescher, D. A miniscrew system with interchangeable abutments. J. Clin. Orthod. 2008, 42, 574–580. [Google Scholar]
- Ludwig, B.; Glasl, B.; Bowman, S.J.; Wilmes, B.; Kinzinger, G.S.M.; Lisson, J.A. Anatomical guidelines for miniscrew insertion: Palatal sites. J. Clin. Orthod. 2011, 45, 433–441. [Google Scholar]
- Mohammed, H.; Wafaie, K.; Rizk, M.Z.; Almuzian, M.; Sosly, R.; Bearn, D.R. Role of anatomical sites and correlated risk factors on the survival of orthodontic miniscrew implants: A systematic review and meta-analysis. Prog. Orthod. 2018, 19, 36. [Google Scholar] [CrossRef] [Green Version]
- Nienkemper, M.; Pauls, A.; Ludwig, B.; Wilmes, B.; Drescher, D. Preprosthetic molar uprighting using skeletal anchorage. J. Clin. Orthod. 2013, 47, 433–437. [Google Scholar] [PubMed]
- Ludwig, B.; Glasl, B.; Kinzinger, G.S.M.; Lietz, T.; Lisson, J.A. Anatomical guidelines for miniscrew insertion: Vestibular interradicular sites. J. Clin. Orthod. 2011, 45, 165–173. [Google Scholar]
- Poggio, P.M.; Incorvati, C.; Velo, S.; Carano, A. “Safe zones”: A guide for miniscrew positioning in the maxillary and mandibular arch. Angle Orthod. 2006, 76, 191–197. [Google Scholar]
- Chen, Y.H.; Chang, H.H.; Chen, Y.J.; Lee, D.; Chiang, H.H.; Yao, C.C. Root contact during insertion of miniscrews for orthodontic anchorage increases the failure rate: An animal study. Clin. Oral Implant. Res. 2008, 19, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Kadioglu, O.; Büyükyilmaz, T.; Zachrisson, B.U.; Maino, B.G. Contact damage to root surfaces of premolars touching miniscrews during orthodontic treatment. Am. J. Orthod. Dentofac. Orthop. 2008, 134, 353–360. [Google Scholar] [CrossRef]
- Arqub, S.A.; Gandhi, V.; Mehta, S.; Palo, L.; Upadhyay, M.; Yadav, S. Survival estimates and risk factors for failure of palatal and buccal mini-implants. Angle Orthod. 2021, 91, 756–763. [Google Scholar] [CrossRef]
- Wilmes, B.; Nienkemper, M.; Ludwig, B.; Kau, C.H.; Drescher, D. Early Class III treatment with a hybrid hyrax-mentoplate combination. J. Clin. Orthod. 2011, 45, 15–21. [Google Scholar]
- Katyal, V.; Wilmes, B.; Nienkemper, M.; Darendeliler, M.A.; Sampson, W.; Drescher, D. The efficacy of Hybrid Hyrax-Mentoplate combination in early Class III treatment: A novel approach and pilot study. Aust. Orthod. J. 2016, 32, 88–96. [Google Scholar] [CrossRef]
- Freudenthaler, J.W.; Haas, R.; Bantleon, H.P. Bicortical titanium screws for critical orthodontic anchorage in the mandible: A preliminary report on clinical applications. Clin. Oral Implants Res. 2001, 12, 358–363. [Google Scholar] [CrossRef]
- Nienkemper, M.; Pauls, A.; Ludwig, B.; Wilmes, B.; Drescher, D. Multifunctional use of palatal mini-implants. J. Clin. Orthod. 2012, 46, 679–686. [Google Scholar]
- Becker, K.; de Gabriele, R.; Dallatana, G.; Trelenberg-Stoll, V.; Wilmes, B.; Drescher, D. 3-D-Planung für Implantate in der Kieferorthopädie; Quintessence Publishing: Berlin, Germany, 2018; pp. 147–154. [Google Scholar]
- Willmann, J.; Wilmes, B.; Becker, K.; Drescher, D. Hybrid Hyrax Direct. Kieferorthopädie 2020, 34, 249–257. [Google Scholar]
- Isidor, F. Histological evaluation of peri-implant bone at implants subjected to occlusal overload or plaque accumulation. Clin. Oral Implants Res. 1997, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Isidor, F. Loss of osseointegration caused by occlusal load of oral implants. A clinical and radiographic study in monkeys. Clin. Oral Implants Res. 1996, 7, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Jedliński, M.; Mazur, M.; Grocholewicz, K.; Janiszewska-Olszowska, J. 3D Scanners in Orthodontics-Current Knowledge and Future Perspectives—A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 1121. [Google Scholar] [CrossRef]
- Aragón, M.L.; Pontes, L.F.; Bichara, L.M.; Flores-Mir, C.; Normando, D. Validity and reliability of intraoral scanners compared to conventional gypsum models measurements: A systematic review. Eur. J. Orthod. 2016, 38, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Glisic, O.; Hoejbjerre, L.; Sonnesen, L. A comparison of patient experience, chair-side time, accuracy of dental arch measurements and costs of acquisition of dental models. Angle Orthod. 2019, 89, 868–875. [Google Scholar] [CrossRef] [Green Version]
- Groth, C.; Kravitz, N.D.; Jones, P.E.; Graham, J.W.; Redmond, W.R. Three-dimensional printing technology. J. Clin. Orthod. 2014, 48, 475–485. [Google Scholar]
- Willmann, J.H.; Wilmes, B.; Chhatwani, S.; Drescher, D. Klinische Anwendung des digitalen Workflows am Beispiel von Mini-Implantaten. Inf. Orthod. Kieferorthopädie 2020, 52, 121–127. [Google Scholar] [CrossRef]
- Möhlhenrich, S.C.; Brandt, M.; Kniha, K.; Bock, A.; Prescher, A.; Hölzle, F.; Modabber, A.; Danesh, G. Suitability of virtual plaster models superimposed with the lateral cephalogram for guided paramedian orthodontic mini-implant placement with regard to the bone support. J. Orofac. Orthop. 2020, 81, 340–349. [Google Scholar] [CrossRef]
- Kniha, K.; Brandt, M.; Bock, A.; Modabber, A.; Prescher, A.; Hölzle, F.; Danesh, G.; Möhlhenrich, S.C. Accuracy of fully guided orthodontic mini-implant placement evaluated by cone-beam computed tomography: A study involving human cadaver heads. Clin. Oral Investig. 2021, 25, 1299–1306. [Google Scholar] [CrossRef]
- Gabriele, O.; Dallatana, G.; Riva, R.; Vasudavan, S.; Wilmes, B. The easy driver for placement of palatal mini-implants and a maxillary expander in a single appointment. J. Clin. Orthod. JCO 2017, 51, 728–737. [Google Scholar] [PubMed]
- Petzold, R.; Zeilhofer, H.F.; Kalender, W.A. Rapid protyping technology in medicine-basics and applications. Comput. Med. Imaging Graph. 1999, 23, 277–284. [Google Scholar] [CrossRef]
- Möhlhenrich, S.C.; Brandt, M.; Kniha, K.; Prescher, A.; Hölzle, F.; Modabber, A.; Wolf, M.; Peters, F. Accuracy of orthodontic mini-implants placed at the anterior palate by tooth-borne or gingiva-borne guide support: A cadaveric study. Clin. Oral Investig. 2019, 23, 4425–4431. [Google Scholar] [CrossRef] [PubMed]
- Jung, B.A.; Wehrbein, H.; Heuser, L.; Kunkel, M. Vertical palatal bone dimensions on lateral cephalometry and cone-beam computed tomography: Implications for palatal implant placement. Clin. Oral Implants Res. 2011, 22, 664–668. [Google Scholar] [CrossRef] [PubMed]
- Graf, S.; Tarraf, N.E.; Kravitz, N.D. Three-dimensional metal printed orthodontic laboratory appliances. Semin. Orthod. 2021, 27, 189–193. [Google Scholar] [CrossRef]
- Mazzoli, A. Selective laser sintering in biomedical engineering. Med. Biol. Eng. Comput. 2013, 51, 245–256. [Google Scholar] [CrossRef]
- Mazzoli, A.; Germani, M.; Moriconi, G. Application of optical digitizing techniques to evaluate the shape accuracy of anatomical models derived from computed tomography data. J. Oral Maxillofac. Surg. 2007, 65, 1410–1418. [Google Scholar] [CrossRef]
- Gokuldoss, P.K.; Kolla, S.; Eckert, J. Additive Manufacturing Processes: Selective Laser Melting, Electron Beam Melting and Binder Jetting-Selection Guidelines. Materials 2017, 10, 672. [Google Scholar] [CrossRef] [Green Version]
- Al Maaz, A.; Thompson, G.A.; Drago, C.; An, H.; Berzins, D. Effect of finish line design and metal alloy on the marginal and internal gaps of selective laser melting printed copings. J. Prosthet. Dent. 2019, 122, 143–151. [Google Scholar] [CrossRef]
- Presotto, A.G.C.; Barão, V.A.R.; Bhering, C.L.B.; Mesquita, M.F. Dimensional precision of implant-supported frameworks fabricated by 3D printing. J. Prosthet. Dent. 2019, 122, 38–45. [Google Scholar] [CrossRef]
- Akçin, E.T.; Güncü, M.B.; Aktaş, G.; Aslan, Y. Effect of manufacturing techniques on the marginal and internal fit of cobalt-chromium implant-supported multiunit frameworks. J. Prosthet. Dent. 2018, 120, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Hong, M.H.; Lee, H.; Lee, C.H.; Hong, M.; Lee, J.; Lee, D.H. Reliability of Metal 3D Printing with Respect to the Marginal Fit of Fixed Dental Prostheses: A Systematic Review and Meta-Analysis. Materials 2020, 13, 4781. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Fang, Y.; Liao, Y.; Chen, G.; Gao, C.; Zhu, P. 3D printing and digital processing techniques in dentistry: A review of literature. Adv. Eng. Mater. 2019, 21, 1801013. [Google Scholar] [CrossRef]
- Tipnis, N.P.; Burgess, D.J. Sterilization of implantable polymer-based medical devices: A review. Int. J. Pharm. 2018, 544, 455–460. [Google Scholar] [CrossRef]
- Jeremy, W.; Thampi, R.; Marc, L.; Michael, K.; Quan, N. Sterilization of bedside 3D-printed devices for use in the operating room. Ann. 3D Print. Med. 2022, 5, 100045. [Google Scholar]
- Ghobeira, R.; Philips, C.; Declercq, H.; Cools, P.; De Geyter, N.; Cornelissen, R.; Morent, R. Effects of different sterilization methods on the physico-chemical and bioresponsive properties of plasma-treated polycaprolactone films. Biomed. Mater. 2017, 12, 015017. [Google Scholar] [CrossRef] [Green Version]
- Sharma, N.; Cao, S.; Msallem, B.; Kunz, C.; Brantner, P.; Honigmann, P.; Thieringer, F.M. Effects of Steam Sterilization on 3D Printed Biocompatible Resin Materials for Surgical Guides-An Accuracy Assessment Study. J. Clin. Med. 2020, 9, 1506. [Google Scholar] [CrossRef]
- Toro, M.; Cardona, A.; Restrepo, D.; Buitrago, L. Does vaporized hydrogen peroxide sterilization affect the geometrical properties of anatomic models and guides 3D printed from computed tomography images? 3D Print Med. 2021, 7, 29. [Google Scholar] [CrossRef]
- Pérez Davila, S.; González Rodríguez, L.; Chiussi, S.; Serra, J.; González, P. How to Sterilize Polylactic Acid Based Medical Devices? Polymers 2021, 13, 2115. [Google Scholar] [CrossRef]
- Melsen, B.; Costa, A. Immediate loading of implants used for orthodontic anchorage. Clin. Orthod. Res. 2000, 3, 23–28. [Google Scholar] [CrossRef]
- Rismanchian, M.; Raji, S.H.; Razavi, S.M.; Rick, D.T.; Davoudi, A. Application of Orthodontic Immediate Force on Dental Implants: Histomorphologic and Histomorphometric Assessment. Ann. Maxillofac. Surg. 2017, 7, 11–17. [Google Scholar] [PubMed]
- Rossini, G.; Parrini, S.; Castroflorio, T.; Deregibus, A.; Debernardi, C.L. Diagnostic accuracy and measurement sensitivity of digital models for orthodontic purposes: A systematic review. Am. J. Orthod. Dentofac. Orthop. 2016, 149, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Shannon, T.; Groth, C. Be your own manufacturer: 3D printing intraoral appliances. In Seminars in Orthodontics Seminars; WB Saunders: Philadelphia, PA, USA, 2021. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Küffer, M.; Drescher, D.; Becker, K. Application of the Digital Workflow in Orofacial Orthopedics and Orthodontics: Printed Appliances with Skeletal Anchorage. Appl. Sci. 2022, 12, 3820. https://doi.org/10.3390/app12083820
Küffer M, Drescher D, Becker K. Application of the Digital Workflow in Orofacial Orthopedics and Orthodontics: Printed Appliances with Skeletal Anchorage. Applied Sciences. 2022; 12(8):3820. https://doi.org/10.3390/app12083820
Chicago/Turabian StyleKüffer, Maximilian, Dieter Drescher, and Kathrin Becker. 2022. "Application of the Digital Workflow in Orofacial Orthopedics and Orthodontics: Printed Appliances with Skeletal Anchorage" Applied Sciences 12, no. 8: 3820. https://doi.org/10.3390/app12083820
APA StyleKüffer, M., Drescher, D., & Becker, K. (2022). Application of the Digital Workflow in Orofacial Orthopedics and Orthodontics: Printed Appliances with Skeletal Anchorage. Applied Sciences, 12(8), 3820. https://doi.org/10.3390/app12083820






