Impact of High-Pressure Processing (HPP) on Selected Quality and Nutritional Parameters of Cauliflower (Brassica oleracea var. Botrytis)
Abstract
1. Introduction
2. Materials and Methods
2.1. Cauliflower Raw Material
2.2. High-Pressure Processing
2.3. Microbial Analysis
2.4. Physical Parameter Measurements
2.4.1. Dry Matter
2.4.2. Drip Loss
2.4.3. Color Analysis
2.4.4. Texture Measurement
2.5. Chemical Analysis
2.5.1. Antioxidation Potential Measured with DPPH
2.5.2. Total Phenolic Content (TPC)
2.5.3. Ascorbic Acid (AA)
2.6. Statistics
3. Results
3.1. Effect of HPP on Aerobic and Anaerobic Spores
3.2. Effects of HPP on Physical Parameters of Cauliflower
3.3. Effect of HPP on the Nutrient Composition of Cauliflower
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Hodges, D.M.; Munro, K.D.; Forney, C.F.; McRae, K.B. Glucosinolate and Free Sugar Content in Cauliflower (Brassica oleracea var. botrytis cv. Freemont) during Con-trolled-Atmosphere Storage. Postharvest Biol. Technol. 2006, 40, 123–132. [Google Scholar] [CrossRef]
- Ahmed, F.A.; Ali, R.F.M. Bioactive Compounds and Antioxidant Activity of Fresh and Processed White Cauliflower. Biomed. Res. Int. 2013, 2013, 367819. [Google Scholar] [CrossRef] [PubMed]
- Collado-González, J.; Piñero, M.C.; Otálora, G.; López-Marín, J.; Del Amor, F.M. Merging Heat Stress Tolerance and Health-Promoting Properties: The Effects of Exogenous Arginine in Cauliflower (Brassica oleracea var. botrytis L.). Foods 2020, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Podsędek, A. Natural Antioxidants and Antioxidant Capacity of Brassica Vegetables: A Review. LWT-Food Sci. Technol. 2007, 40, 1–11. [Google Scholar] [CrossRef]
- Oey, I.; Lille, M.; Van Loey, A.; Hendrickx, M. Effect of High-Pressure Processing on Colour, Texture and Flavour of Fruit-and Vegetable-Based Food Products: A Review. Trends Food Sci. Technol. 2008, 19, 320–328. [Google Scholar] [CrossRef]
- Lafarga, T.; Bobo, G.; Viñas, I.; Collazo, C.; Aguiló-Aguayo, I. Effects of Thermal and Non-Thermal Processing of Cruciferous Vegetables on Glucosinolates and Its Derived Forms. J. Food Sci. Technol. 2018, 55, 1973–1981. [Google Scholar] [CrossRef]
- Roobab, U.; Aadil, R.M.; Madni, G.M.; Bekhit, A.E.-D. The Impact of Nonthermal Technologies on the Microbiological Quality of Juices: A Review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 437–457. [Google Scholar] [CrossRef]
- Farkas, D.F.; Hoover, D.G. High Pressure Processing. J. Food Sci. 2000, 65, 47–64. [Google Scholar] [CrossRef]
- Zhang, H.; Tikekar, R.V.; Ding, Q.; Gilbert, A.R.; Wimsatt, S.T. Inactivation of Foodborne Pathogens by the Synergistic Combinations of Food Processing Technologies and Food-grade Compounds. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2110–2138. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Huang, H.-W.; Hsu, C.-P.; Yang, B.B. Recent Advances in Food Processing Using High Hydrostatic Pressure Technology. Crit. Rev. Food Sci. Nutr. 2016, 56, 527–540. [Google Scholar] [CrossRef]
- Nabi, B.G.; Mukhtar, K.; Arshad, R.N.; Radicetti, E.; Tedeschi, P.; Shahbaz, M.U.; Walayat, N.; Nawaz, A.; Inam-Ur-Raheem, M.; Aadil, R.M. High-Pressure Processing for Sustainable Food Supply. Sustainability 2021, 13, 13908. [Google Scholar] [CrossRef]
- Xin, Y.; Zhang, M.; Xu, B.; Adhikari, B.; Sun, J. Research Trends in Selected Blanching Pretreatments and Quick Freezing Technologies as Applied in Fruits and Vegetables: A Review. Int. J. Refrig. 2015, 57, 11–25. [Google Scholar] [CrossRef]
- Norton, T.; Sun, D.W. Recent Advances in the Use of High Pressure as an Effective Processing Technique in the Food Industry. Food Bioprocess. Technol. 2008, 1, 2–34. [Google Scholar] [CrossRef]
- Terefe, N.S.; Buckow, R.; Versteeg, C. Quality-Related Enzymes in Fruit and Vegetable Products: Effects of Novel Food Processing Technologies, Part 1: High-Pressure Processing. Crit. Rev. Food Sci. Nutr. 2014, 54, 24–63. [Google Scholar] [CrossRef]
- Van Buggenhout, S.; Sila, D.N.; Duvetter, T.; Van Loey, A.; Hendrickx, M. Pectins in Processed Fruits and Vegetables: Part III—Texture Engineering. Compr. Rev. Food Sci. Food Saf. 2009, 8, 105–117. [Google Scholar] [CrossRef]
- Krebbers, B.; Matser, A.M.; Koets, M.; Van den Berg, R.W. Quality and Storage-Stability of High-Pressure Preserved Green Beans. J. Food Eng. 2002, 54, 27–33. [Google Scholar] [CrossRef]
- Castro, S.M.; Saraiva, J.A.; Domingues, F.M.J.; Delgadillo, I. Effect of Mild Pressure Treatments and Thermal Blanching on Yellow Bell Peppers (Capsicum annuum L.). LWT-Food Sci. Technol. 2011, 44, 363–369. [Google Scholar] [CrossRef]
- Woldemariam, H.W.; Emire, S.A. High Pressure Processing of Foods for Microbial and Mycotoxins Control: Current Trends and Future Prospects. Cogent Food Agric. 2019, 5, 1622184. [Google Scholar] [CrossRef]
- Balasubramaniam, V.M.; Martinez-Monteagudo, S.I.; Gupta, R. Principles and Application of High Pressure–Based Technologies in the Food Industry. Annu. Rev. Food Sci. Technol. 2015, 6, 435–462. [Google Scholar] [CrossRef]
- Serment-Moreno, V.; Barbosa-Cánovas, G.; Torres, J.A.; Welti-Chanes, J. High-Pressure Processing: Kinetic Models for Microbial and Enzyme Inactivation. Food Eng. Rev. 2014, 6, 56–88. [Google Scholar] [CrossRef]
- Pinton, S.C.; Bardsley, C.A.; Marik, C.M.; Boyer, R.R.; Strawn, L.K. Fate of Listeria monocytogenes on Broccoli and Cauliflower at Different Storage Temperatures. J. Food Prot. 2020, 83, 858–864. [Google Scholar] [CrossRef]
- Arroyo, G.; Sanz, P.D.; Préstamo, G. Effect of High Pressure on the Reduction of Microbial Populations in Vegetables. J. Appl. Microbiol. 1997, 82, 735–742. [Google Scholar] [CrossRef][Green Version]
- Aaby, K.; Grimsbo, I.H.; Hovda, M.B.; Rode, T.M. Effect of High Pressure and Thermal Processing on Shelf Life and Quality of Strawberry Purée and Juice. Food Chem. 2018, 260, 115–123. [Google Scholar] [CrossRef]
- Nhi, T.T.Y.; Phat, D.T.; Quyen, N.N.; Cang, M.H.; Truc, T.T.; Bach, L.G.; Muoi, N.V. Effects of Vacuum Concentration on Color, Polyphenol and Flavonoid Contents and Antioxidant Activity of Pomelo Citrus maxima (Burm. f.) Merr. Juice. IOP Conf. Ser. Mater. Sci. Eng. 2020, 991, 12060. [Google Scholar] [CrossRef]
- Koskiniemi, C.B.; Truong, V.-D.; McFeeters, R.F.; Simunovic, J. Quality Evaluation of Packaged Acidified Vegetables Subjected to Continuous Microwave Pasteurization. LWT-Food Sci. Technol. 2013, 54, 157–164. [Google Scholar] [CrossRef]
- Llorach, R.; Espín, J.C.; Tomás-Barberán, F.A.; Ferreres, F. Valorization of Cauliflower (Brassica oleracea L. var. botrytis) by-Products as a Source of Antioxidant Phenolics. J. Agric. Food Chem. 2003, 51, 2181–2187. [Google Scholar]
- Fung, D.Y.C. Food Spoilage, Preservation and Quality Control. 2009. Available online: https://www.sciencedirect.com/science/article/pii/B978012373944500122X?via%3Dihub (accessed on 23 May 2022).
- Ulmer, H.M.; Gänzle, M.G.; Vogel, R.F. Effects of High Pressure on Survival and Metabolic Activity of Lactobacillus plantarum TMW1. 460. Appl. Environ. Microbiol. 2000, 66, 3966–3973. [Google Scholar] [CrossRef]
- Dong, P.; Kong, M.; Yao, J.; Zhang, Y.; Liao, X.; Hu, X.; Zhang, Y. The Effect of High Hydrostatic Pressure on the Microbiological Quality and Physicochemical Properties of Lotus Root during Refrigerated Storage. Innov. Food Sci. Emerg. Technol. 2013, 19, 79–84. [Google Scholar] [CrossRef]
- Arroyo, G.; Sanz, P.D.; Préstamo, G. Response to High-pressure, Low-temperature Treatment in Vegetables: Determination of Survival Rates of Microbial Populations Using Flow Cytometry and Detection of Peroxidase Activity Using Confocal Microscopy. J. Appl. Microbiol. 1999, 86, 544. [Google Scholar] [CrossRef]
- Heinz, V.; Buckow, R. Food Preservation by High Pressure. J. Verbrauch. Leb. 2010, 5, 73–81. [Google Scholar] [CrossRef]
- Vervoort, L.; Van der Plancken, I.; Grauwet, T.; Verlinde, P.; Matser, A.; Hendrickx, M.; Van Loey, A. Thermal versus High Pressure Processing of Carrots: A Comparative Pilot-Scale Study on Equivalent Basis. Innov. Food Sci. Emerg. Technol. 2012, 15, 1–13. [Google Scholar] [CrossRef]
- Borch-Pedersen, K.; Mellegård, H.; Reineke, K.; Boysen, P.; Sevenich, R.; Lindbäck, T.; Aspholm, M. Effects of High Pressure on Bacillus Licheniformis Spore Germination and Inactivation. Appl. Environ. Microbiol. 2017, 83, e02108-17. [Google Scholar] [CrossRef] [PubMed]
- Ates, M.B.; Skipnes, D.; Rode, T.M.; Lekang, O.-I. Comparison of Spore Inactivation with Novel Agitating Retort, Static Retort and Combined High Pressure-Temperature Treatments. Food Control 2016, 60, 484–492. [Google Scholar] [CrossRef]
- Gao, Y.; Qiu, W.; Wu, D.; Fu, Q. Assessment of Clostridium perfringens Spore Response to High Hydrostatic Pressure and Heat with Nisin. Appl. Biochem. Biotechnol. 2011, 164, 1083–1095. [Google Scholar] [CrossRef]
- Florkiewicz, A.; Socha, R.; Filipiak-Florkiewicz, A.; Topolska, K. Sous-vide Technique as an Alternative to Traditional Cooking Methods in the Context of Antioxidant Properties of Brassica Vegetables. J. Sci. Food Agric. 2019, 99, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Cebula, S.; Kunicki, E.; Kalisz, A. Quality Changes in Curds of White, Green and Romanesco Cauliflower during Storage. Pol. J. Food Nutr. Sci. 2006, 15, 155. [Google Scholar]
- Kapusta-Duch, J.; Szeląg-Sikora, A.; Sikora, J.; Niemiec, M.; Gródek-Szostak, Z.; Kuboń, M.; Leszczyńska, T.; Borczak, B. Health-Promoting Properties of Fresh and Processed Purple Cauliflower. Sustainability 2019, 11, 4008. [Google Scholar] [CrossRef]
- Gębczyński, P.; Kmiecik, W. Effects of Traditional and Modified Technology, in the Production of Frozen Cauliflower, on the Contents of Selected Antioxidative Compounds. Food Chem. 2007, 101, 229–235. [Google Scholar] [CrossRef]
- Melse-Boonstra, A.; Verhoef, P.; Konings, E.J.M.; Van Dusseldorp, M.; Matser, A.; Hollman, P.C.H.; Meyboom, S.; Kok, F.J.; West, C.E. Influence of Processing on Total, Monoglutamate and Polyglutamate Folate Contents of Leeks, Cauliflower, and Green Beans. J. Agric. Food Chem. 2002, 50, 3473–3478. [Google Scholar] [CrossRef]
- Araya, X.I.T.; Hendrickx, M.; Verlinden, B.E.; Van Buggenhout, S.; Smale, N.J.; Stewart, C.; Mawson, A.J. Understanding Texture Changes of High Pressure Processed Fresh Carrots: A Microstructural and Biochemical Approach. J. Food Eng. 2007, 80, 873–884. [Google Scholar] [CrossRef]
- Clariana, M.; Valverde, J.; Wijngaard, H.; Mullen, A.M.; Marcos, B. High Pressure Processing of Swede (Brassica napus): Impact on Quality Properties. Innov. Food Sci. Emerg. Technol. 2011, 12, 85–92. [Google Scholar] [CrossRef]
- Matos, M.; Gutiérrez, G.; Martínez-Rey, L.; Iglesias, O.; Pazos, C. Encapsulation of Resveratrol Using Food-Grade Concentrated Double Emulsions: Emulsion Characterization and Rheological Behaviour. J. Food Eng. 2018, 226, 73–81. [Google Scholar] [CrossRef]
- Araya, X.I.T.; Smale, N.; Zabaras, D.; Winley, E.; Forde, C.; Stewart, C.M.; Mawson, A.J. Sensory Perception and Quality Attributes of High Pressure Processed Carrots in Comparison to Raw, Sous-Vide and Cooked Carrots. Innov. Food Sci. Emerg. Technol. 2009, 10, 420–433. [Google Scholar] [CrossRef]
- Alvarez-Jubete, L.; Valverde, J.; Patras, A.; Mullen, A.M.; Marcos, B. Assessing the Impact of High-Pressure Processing on Selected Physical and Biochemical Attributes of White Cabbage (Brassica oleracea L. var. capitata alba). Food Bioprocess Technol. 2014, 7, 682–692. [Google Scholar] [CrossRef]
- Hu, Q.; Li, X.; Chen, F.; Wan, R.; Yu, C.W.; Li, J.; McClements, D.J.; Deng, Z. Microencapsulation of an Essential Oil (Cinnamon Oil) by Spray Drying: Effects of Wall Materials and Storage Conditions on Microcapsule Properties. J. Food Process. Preserv. 2020, 44, e14805. [Google Scholar] [CrossRef]
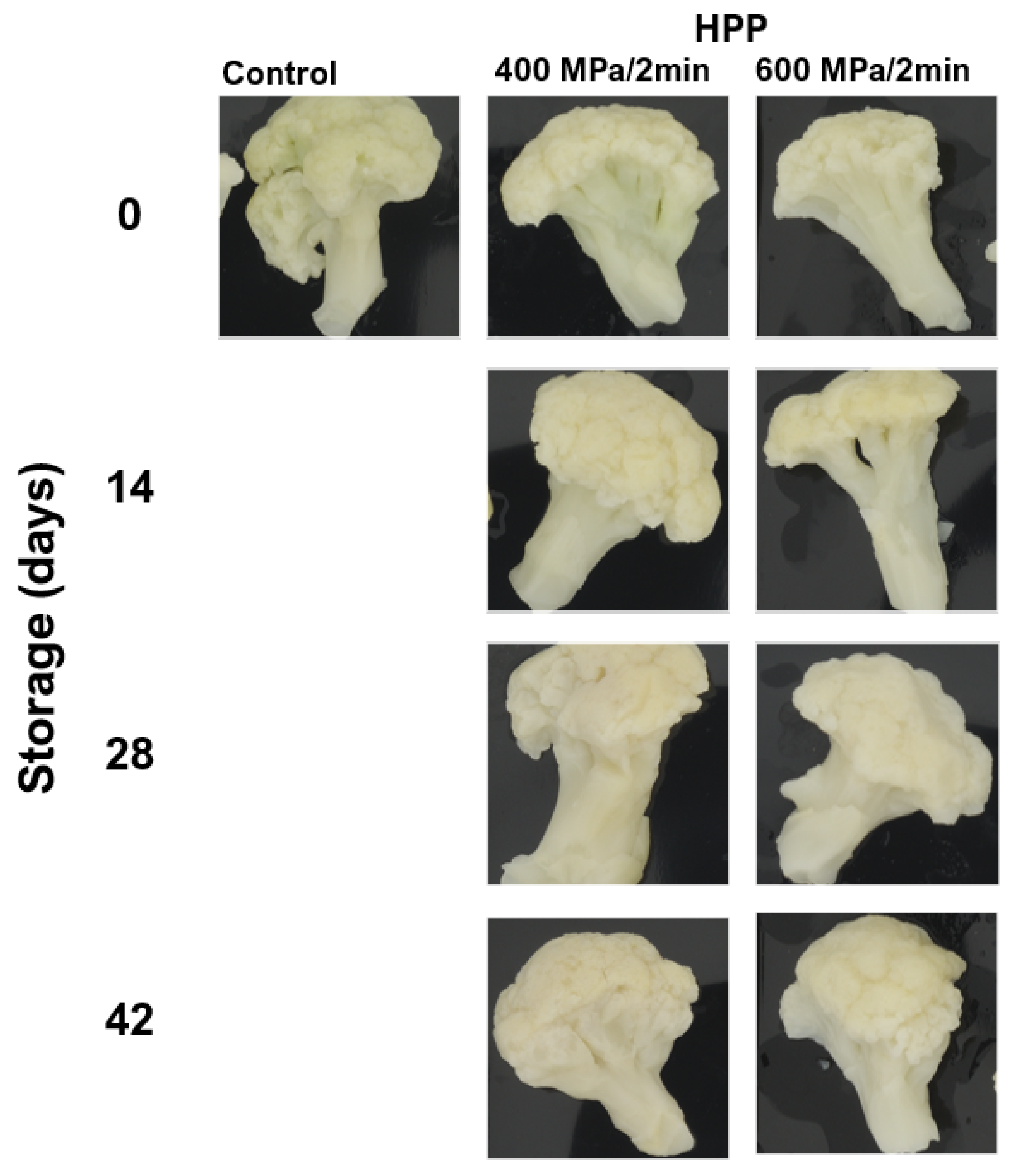
| Storage (Days) | Control | HPP | |||
|---|---|---|---|---|---|
| 0.1 MPa | 400 MPa | 600 MPa | |||
| 0 min | 2 min | 5 min | 2 min | 5 min | |
| 0 | 5.0 ± 0.2 A, a | 3.3 ± 0.2 A, b | <2.3 A, c | 2.4 ± 0.4 A, d | <2.3 A, d |
| 7 | 7.2 ± 0.9 B, a | 3.8 ± 0.3 B, c | 3.2 ± 0.3 A, c | <2.3 A, d | 2.3 ± 0.3 A, d |
| 14 | nm ** | 6.4 ± 0.5 C, b | 6.0 ± 0.7 B, b | 3.1 ± 1.0 A, c | 2.8 ± 0.4 A, B, cd |
| 28 | nm | 9.3 ± 0.5 D, a | 9.1 ± 0.5 C, a | 5.5 ± 1.7 B, b | 5.0 ± 1.9 B, C, b |
| 35 | nm | nm | nm | 6.6 ± 1.9 B, a | 6.1 ± 2.7 C, D, a |
| 42 | nm | nm | nm | 5.7 ± 2.8 B, a | 7.5 ± 2.2 D, a |
| Storage (Days) | Control 0.1 Mpa | Aerobic Spores | Anaerobic Spores | ||
|---|---|---|---|---|---|
| HPP | HPP | ||||
| 600 MPa | 600 MPa | ||||
| 0 min | 2 min | 5 min | 2 min | 5 min | |
| 0 | <2.3 | nm | nm | nm | nm |
| 35 | nm ** | <2.3 | 2.4 ± 0.9 | 2.7 ± 1.1 | 2.6 ± 0.8 |
| 42 | nm | 2.4 ± 0.8 | 2.6 ± 1.1 | 2.5 ± 0.7 | 2.6 ± 1.14 |
| Dry Matter (%) | Drip Loss (%) | Firmness (kg) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | HPP | Control | HPP | Control | HPP | ||||||||||
| Storage (Days) | 0.1 MPa | 400 MPa | 600 MPa | 0.1 MPa | 400 MPa | 600 MPa | 0.1 MPa | 400 MPa | 600 MPa | ||||||
| 0 min | 2 min | 5 min | 2 min | 5 min | 0 min | 2 min | 5 min | 2 min | 5 min | 0 min | 2 min | 5 min | 2 min | 5 min | |
| 0 | 6.9 ± 0.3 a | 6.1 ± 0.7 A, a | 6.6 ± 0.2 A, a | 6.3 ± 0.6 A, a | 5.8 ± 0.1 A, a | 18.0 ± 4.8 A, b, c | 31.7 ± 7.6 A, a | 32.3 ± 6.0 A, a | 27.2 ± 5.6 A, a, b | 33.0 ± 7.4 A, a | 15.9 ± 3.8 a | 14.4 ± 3.8 A, a | 14.5 ± 4.3 A, a | 15.6 ± 4.5 A, a | 12.5 ± 6.4 A, B, a |
| 14 | nm ** | 6.3 ± 0.4 A, b | 6.8 ± 0.4 A, ab | 6.3 ± 0.3 A, b | 6.2 ± 0.3 A, b | 17.9 ± 3.9 A, b | 32.9 ± 9.4 A, a | 28.5 ± 3.3 A, a | 30.6 ± 6.2 A, a | 29.9 ± 5.1 A, a | nm ** | 11.3 ± 5.3 A, a | 12.4 ± 4.0 A, a | 13.6 ± 3 A, a | 10.4 ± 5.8 B, a |
| 28 | nm | 6.5 ± 1.0 A, a | 6.5 ± 0.8 A, a | 6.2 ± 0.3 A, a | 6.4 ± 0.2 A, a | nm ** | 27.4 ± 6.1 A, a, b | 29.5 ± 5.8 A, a, b | 34.3 ± 6.6 A, a | 26.6 ± 4.0 A, a, b | nm | 16.6 ± 3.9 A, a | 14.3 ± 5.9 A, a | 13.1 ± 5.3 A, a | 15.7 ± 5 A, a |
| 42 | nm | 6.7 ± 0.4 A, ab | 6.6 ± 0.5 A, ab | 6.3 ± 0.1 A, ab | 6.1 ± 0.4 A, b | nm | 32.3 ± 12.7 A, a | 27.4 ± 8.2 A, a | 29.9 ± 6.0 A, a | 27.0 ± 8.3 A, a | nm | 17.1 ± 3.7 A, a | 17.3 ± 2.6 A, a | 16.0 ± 3.3 A, a | 12.6 ± 3 AB, a |
| A. Stem | |||||
|---|---|---|---|---|---|
| Storage (Days) | Control | HPP | |||
| 0.1 MPa | 400 MPa | 600 MPa | |||
| 0 min | 2 min | 5 min | 2 min | 5 min | |
| 0 | - | 2.9 ± 1.7 A, a | 3.3 ± 1.3 A, a, b | 3.2 ± 1.5 A, a, b | 3.2 ± 1.4 A, a, b |
| 14 | nm ** | 2.8 ± 1.2 A, a | 3.2 ± 1.3 A, a, b | 3.7 ± 1.4 A, a, b, c | 3.9 ± 1.9 A, c, b |
| 28 | nm | 3.5 ± 1.7 A, B, a | 3.7 ± 1.1 A, a | 3.4 ± 1.5 A, a | 3.7 ± 1.5 A, a |
| 42 | nm | 4.2 ± 2.2 B, a | 3.9 ± 1.4 A, a | 3.4 ± 1.6 A, a | 4.0 ± 1.5 A, a |
| B. Floret | |||||
| Storage (Days) | Control | HPP | |||
| 0.1 MPa | 400 MPa | 600 MPa | |||
| 0 min | 2 min | 5 min | 2 min | 5 min | |
| 0 | - | 4.0 ± 2.4 A, a | 4.7 ± 2.9 A, a, b | 4.2 ± 1.9 A, a | 4.2 ± 2.3 A, a |
| 14 | nm | 4.3 ± 1.9 A, a | 5.1 ± 2.1 A, a, b | 4.2 ± 2.0 A, a | 4.5 ± 2.1 A, B, a, b |
| 28 | nm | 4.5 ± 2.2 A, a | 4.7 ± 2.3 A, a | 4.7 ± 2.0 A, a | 5.7 ± 2.5 B, a |
| 42 | nm | 4.8 ± 2.5 A, a | 5.1 ± 2.2 A, a | 4.6 ± 2.1 A, a | 5.0 ± 2.0 A, B, a |
| DPPH Antioxidant Activity (%,) | TPC as mg Gallic Acid Equivalents (GAE)/100 g | AA (mg/100g DW) | ||||
|---|---|---|---|---|---|---|
| Control | HPP | Control | HPP | Control | HPP | |
| Storage (Days) | 0.1 MPa | 600 MPa | 0.1 MPa | 600 MPa | 0.1 MPa | 600 MPa |
| 0 min | 2 min | 0 min | 2 min | 0 min | 2 min | |
| 0 | 72.1 ± 2.5 A | 52.4 ± 6.9 C, b | 579.6 ± 19.4 A | 506.6 ± 55.6 B, a | 890.9 ± 53.6 A, B | 862.1 ± 45.6 B, a |
| 14 | nm ** | 46.5 ± 4.6 c | nm ** | 477.8 ± 47.9 a | Nm ** | 730.7 ± 22.2 b |
| 28 | nm | 68.3 ± 4.3 a | nm | 510.8 ± 24.6 a | nm | 790.4 ± 95.0 a, b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurek, M.A.; Finnseth, C.; Skipnes, D.; Rode, T.M. Impact of High-Pressure Processing (HPP) on Selected Quality and Nutritional Parameters of Cauliflower (Brassica oleracea var. Botrytis). Appl. Sci. 2022, 12, 6013. https://doi.org/10.3390/app12126013
Kurek MA, Finnseth C, Skipnes D, Rode TM. Impact of High-Pressure Processing (HPP) on Selected Quality and Nutritional Parameters of Cauliflower (Brassica oleracea var. Botrytis). Applied Sciences. 2022; 12(12):6013. https://doi.org/10.3390/app12126013
Chicago/Turabian StyleKurek, Marcin A., Christian Finnseth, Dagbjørn Skipnes, and Tone Mari Rode. 2022. "Impact of High-Pressure Processing (HPP) on Selected Quality and Nutritional Parameters of Cauliflower (Brassica oleracea var. Botrytis)" Applied Sciences 12, no. 12: 6013. https://doi.org/10.3390/app12126013
APA StyleKurek, M. A., Finnseth, C., Skipnes, D., & Rode, T. M. (2022). Impact of High-Pressure Processing (HPP) on Selected Quality and Nutritional Parameters of Cauliflower (Brassica oleracea var. Botrytis). Applied Sciences, 12(12), 6013. https://doi.org/10.3390/app12126013








