Performance of Apple Pomace for Gluten-Free Bread Manufacture: Effect on Physicochemical Characteristics and Nutritional Value
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Apple Pomace
2.1.2. Bread Making Ingredients (Others Than APP)
2.2. Methods
2.2.1. Apple Pomace Powder Production
2.2.2. Analytical Determinations in APP
- pH value was measured following the AOAC 981.12 method using a Crison pH meter [25].
- Fiber was determined by the Rapid Integrated Total Dietary Fiber Assay Kit (k-RINTDF) following the AOAC method 2017.06.
- Proteins were determined by Kjeldhal (AOAC 920.152) [25].
- Ash was analyzed by AOAC 940.26 [25].
- Mineral analysis: Microwave-assisted digestion of samples was carried out in a Speed wave-four equipment (Berghof), mixing 100 mg of the sample and 5 mL nitric acid (65%). The analysis was performed by ICP-MS with a helium collision cell using 24Mg, 43Ca, 55Mn, 56Fe, 63Cu, 66Zn, and 77Se isotopes and e89 as the inner standard. Calibration was performed in a concentration range from 0.05 to 500 ng/mL.
2.2.3. Bread Making Procedure
2.2.4. Analytical Determinations in Bread
- Specific volume: Breads were weighed with a precision balance (Mettler Toledo) and the volume was measured on a volumetric measuring cylinder by water displacement [32], covering each piece of bread with a plastic film, finely adapted to the loaf shape. As bread is a porous material, the plastic film prevented the samples from getting wet. Specific volume was calculated according to the formula:Specific volume (cm3/g) = Volume (cm3)/weight (g).
- Moisture: This was determined by weight difference [25]; crumb samples (cylinders 1 cm diameter) were weighed and maintained at 105 ± 2 °C (J.P. Selecta) overnight until constant weight. The water content of each sample was calculated according to the formula:where, M = Initial weight of the sample (g); m = final weight of the sample (g).Moisture (%) = (M − m) × 100/M
- Crumb texture: This was assessed instrumentally by texture profile analysis with a TA.XT2plus texture analyzer (Stable Micro System, Godalming, UK). Longitudinal cylinders (1 cm diameter) were extracted from the central part of each bread roll, with the aid of a cylindrical core sampler. The crumb sample was recovered from inside the cylinder by pushing it with a plunger. Samples’ lengths were standardized before the assay at 4 cm. Texture profile analysis (50%) was performed twice on 4 pieces of bread (n = 8), perpendicular to the axis of the cylindrical samples. Graphics were analyzed as described by other authors [33,34] and the following parameters were obtained:
- -
- Hardness: The highest force peak of the first compression cycle (N).
- -
- Cohesiveness: Ratio of the areas under the curve of the second compression cycle to the first compression cycle.
- -
- Resilience: Ratio between the areas under the curve of the withdrawal divided by the area under the curve in the downstroke, both in the first compression cycle.
- -
- Springiness: Ratio between the distances the sample is compressed in the second downstroke divided by the first downstroke.
- -
- Chewiness is calculated as cohesiveness × hardness × springiness (N).
2.2.5. Analytical Determinations in Both APP and Bread
- Water activity (aw): This was measured with a Labmaster-aw device (Novasina, Lachen, Switzerland). When measuring APP, capsules were evenly filled with pomace powder, following technical specifications. For bread samples, aw was measured similarly, by grinding samples (Imetec Dolcevita grinder) and placing the grounded bread in the measuring capsules.
- Color assessment: L*, a* and b* coordinates were measured with a spectrophotometer (CM-5, Konica Minolta, Spain), using the upper measuring site design for Petri dishes. For APP, 5 g was placed on a Petri plate covering its surface evenly; for bread samples, color measurements were taken for both crumb and crust. Crumbs were crumbled and distributed evenly on a Petri dish. When using the Petri dish to contain samples (APP or crumb), calibration was carried out with an empty Petri dish. Crust color was assessed by direct measurement on the bottom of each loaf of bread.
- Antioxidant activity: This was measured spectrophotometrically (UV/VIS mini-1240 Shimadzu Europe, Duisburg, Germany) using a modified 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) method [35]. A DPPH solution (40 ppm) was prepared mixing DPPH and methanol. Samples (APP or grinded crumb) were kept stirring in that solution for 1.5 h, covering the flask with aluminum paper to protect it from the light. The reaction mixture was a mixture of 3.9 mL DPPH in methanol (40 ppm) and 0.1 mL of the extracted solution. Absorbance was read at 515 nm after 180 min (when it was stable). Antioxidant activity was estimated with this formula:where, A0 = Absorbance of 40 ppm DPPH in methanol solution; A1 = Absorbance of the solution with the sample and 40 ppm DPPH in methanol.% antioxidant activity = 100 × (A0 − A1)/A1
2.2.6. Statistical Analyses
3. Results and Discussion
3.1. Apple Pomace Powder
3.2. Nutritional Value of GF Bread
3.3. Physicochemical Characteristics of GF Bread
3.4. Bread Color Characteristics
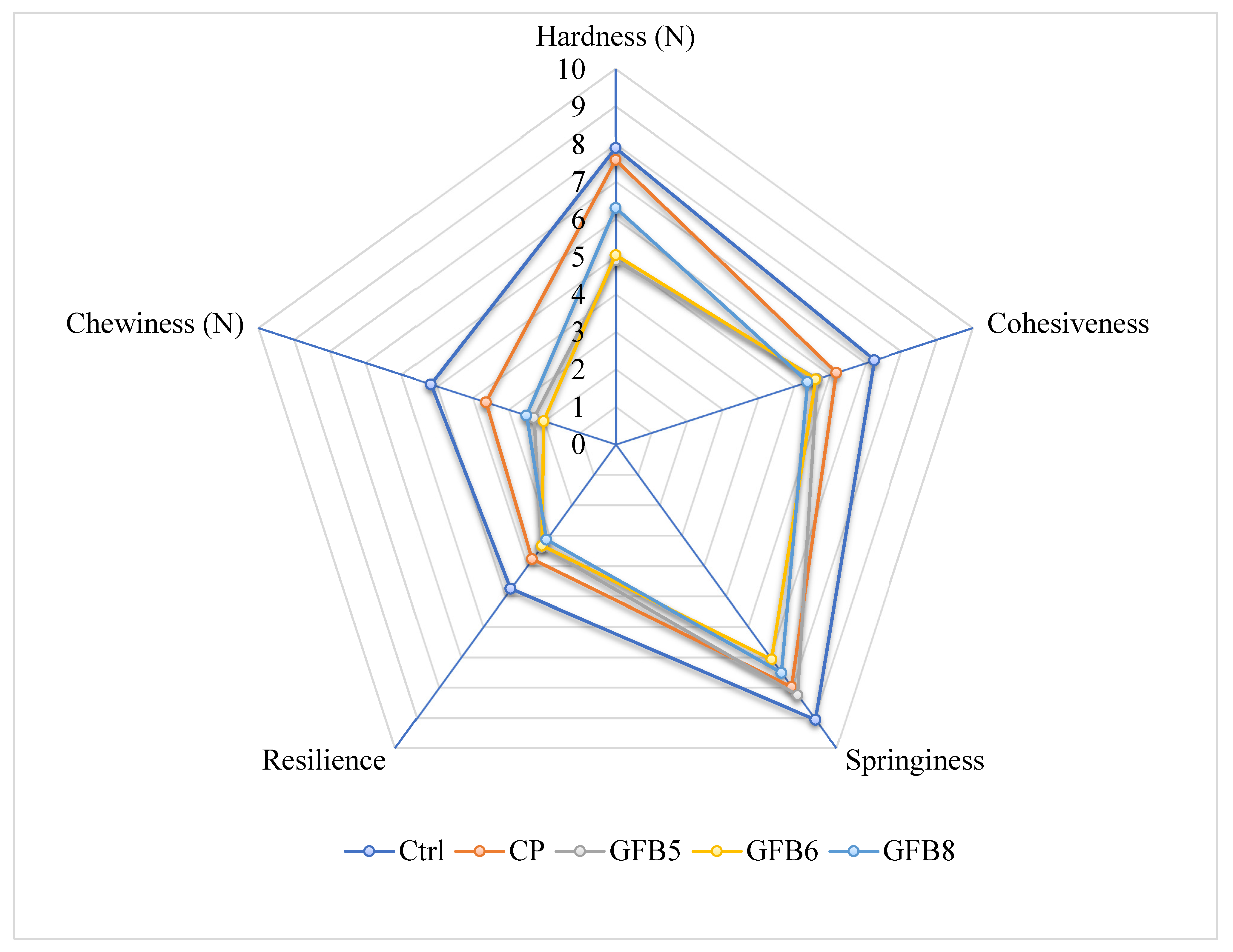
3.5. Texture Profile Analysis of Bread
3.6. Overall Remarks
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Esposito, B.; Sessa, M.R.; Sica, D.; Malandrino, O. Towards circular economy in the agri-food sector. A systematic literature review. Sustainability 2020, 12, 7401. [Google Scholar] [CrossRef]
- Schroeder, P.; Anggraeni, K.; Weber, U. The Relevance of Circular Economy Practices to the Sustainable Development Goals. J. Ind. Ecol. 2019, 23, 77–95. [Google Scholar] [CrossRef] [Green Version]
- Teigiserova, D.A.; Hamelin, L.; Thomsen, M. Towards transparent valorization of food surplus, waste and loss: Clarifying definitions, food waste hierarchy, and role in the circular economy. Sci. Total Environ. 2020, 706, 136033. [Google Scholar] [CrossRef] [PubMed]
- Nakov, G.; Brandolini, A.; Hidralgo, A. Influence of apple peel powder addition on the phsyco-chemical characteristics and nutritional quality of bread wheat cookies. Food Sci. Technol. Int. 2020, 26, 1–9. [Google Scholar] [CrossRef]
- Zlatanović, S.; Ostojić, S.; Micić, D.; Rankov, S.; Dodevska, M.; Vukosavljević, P.; Gorjanović, S. Thermal behaviour and degradation kinetics of apple pomace flours. Thermochim. Acta 2019, 673, 17–25. [Google Scholar] [CrossRef]
- Wang, X.; Kristo, E.; LaPointe, G. Adding apple pomace as a functional ingredient in stirred-type yogurt and yogurt drinks. Food Hydrocoll. 2020, 100, 105453. [Google Scholar] [CrossRef]
- Antonic, B.; Jancikova, S.; Dordevic, D.; Tremlova, B. Apple pomace as food fortification ingredient: A systematic review and meta-analysis. J. Food Sci. 2020, 85, 2977–2985. [Google Scholar] [CrossRef]
- Fernandes, P.A.R.; Ferreira, S.S.; Bastos, R.; Ferreira, I.; Cruz, M.T.; Pinto, A.; Coelho, E.; Passos, C.P.; Coimbra, M.A.; Cardoso, S.M.; et al. Apple pomace extract as a sustainable food ingredient. Antioxidants 2019, 8, 189. [Google Scholar] [CrossRef] [Green Version]
- Föste, M.; Verheyen, C.; Jekle, M.; Becker, T. Fibres of milling and fruit processing by-products in gluten-free bread making: A review of hydration properties, dough formation and quality-improving strategies. Food Chem. 2020, 306, 125451. [Google Scholar] [CrossRef]
- Makovicky, P.; Makovicky, P.; Caja, F.; Rimarova, K.; Samasca, G.; Vannucci, L. Celiac disease and gluten-free diet: Past, present, and future. Gastroenterol. Hepatol. Bed Bench 2020, 13, 1–7. [Google Scholar] [CrossRef]
- Naqash, F.; Gani, A.; Gani, A.; Masoodi, F.A. Gluten-free baking: Combating the challenges—A review. Trends Food Sci. Technol. 2017, 66, 98–107. [Google Scholar] [CrossRef]
- Cornicelli, M.; Saba, M.; Machello, N.; Silano, M. Nutritional composition of gluten-free food versus regular food sold in the Italian market. Aliment. Tract 2018, 50, 1305–1308. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, V.; González, M.P.; Martínez, M.; Samaniego-Vaesken, M.D.L.; Achón, M.; Úbeda, N.; Alonso-Aperte, E. Updated food composition database for cereal-based gluten-free products in spain: Is reformulation moving on? Nutrients 2020, 12, 2369. [Google Scholar] [CrossRef] [PubMed]
- Cardo, A.; Churruca, I.; Lasa, A.; Navarro, V.; Vázquez-Polo, M.; Perez-Junkera, G.; Larretxi, I. Nutritional imbalances in adult celiac patients following a gluten-free diet. Nutrients 2021, 13, 2877. [Google Scholar] [CrossRef]
- Krupa-Kozak, U.; Drabińska, N.; Baczek, N.; Šimková, K.; Starowicz, M.; Jeliński, T. Application of broccoli leaf powder in gluten-free bread: An innovative approach to improve its bioactive potential and technological quality. Foods 2021, 10, 819. [Google Scholar] [CrossRef]
- Miaja, M.F.; Martín, J.J.D.; Treviño, S.J.; González, M.S.; García, C.B. Study of adherence to the gluten-free diet in coeliac patients. An. Pediatr. 2020, 94, 4–11. [Google Scholar] [CrossRef]
- Miranda, J.; Lasa, A.; Bustamante, M.A.; Churruca, I.; Simon, E. Nutritional Differences Between a Gluten-free Diet and a Diet Containing Equivalent Products with Gluten. Plant Foods Hum. Nutr. 2014, 69, 182–187. [Google Scholar] [CrossRef]
- Figuerola, F.; Hurtado, M.L.; Estévez, A.M.; Chiffelle, I.; Asenjo, F. Fibre concentrates from apple pomace and citrus peel as potential fibre sources for food enrichment. Food Chem. 2005, 91, 395–401. [Google Scholar] [CrossRef]
- Skinner, R.C.; Gigliotti, J.C.; Ku, K.-M.; Tou, J.C. A comprehensive analysis of the composition, health benefits, and safety of apple pomace. Nutr. Rev. 2018, 76, 893–909. [Google Scholar] [CrossRef]
- Arslan, M.; Rakha, A.; Xiaobo, Z.; Mahmood, M.A. Complimenting gluten-free bakery products with dietary fiber: Opportunities and constraints. Trends Food Sci. Technol. 2019, 83, 194–202. [Google Scholar] [CrossRef]
- Capriles, V.D.; Santos, F.G.; Aguiar, E.V. Innovative gluten-free bread making. In Trends in Wheat and Bread Making; Galanakis, C.H., Ed.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2021; pp. 371–404. [Google Scholar] [CrossRef]
- Djeghim, F.; Bourekoua, H.; Różyło, R.; Bieńczak, A.; Tanaś, W.; Zidoune, M.N. Effect of by-products from selected fruits and vegetables on gluten-free dough rheology and bread properties. Appl. Sci. 2021, 11, 4605. [Google Scholar] [CrossRef]
- Gumul, D.; Ziobro, R.; Korus, J.; Kruczek, M. Apple pomace as a source of bioactive polyphenol compounds in gluten-free breads. Antioxidants 2021, 10, 807. [Google Scholar] [CrossRef] [PubMed]
- Lyu, F.; Luiz, S.F.; Azeredo, D.R.P.; Cruz, A.G.; Ajlouni, S.; Ranadheera, C.S. Apple pomace as a functional and healthy ingredient in food products: A review. Processes 2020, 8, 319. [Google Scholar] [CrossRef] [Green Version]
- AOAC. Official Methods of Analysis, 21st ed.; The Association of Official Analytical Chemist: Washington, DC, USA, 2019. [Google Scholar]
- Skendi, A.; Papageorgiou, M.; Varzakas, T. High Protein Substitutes for Gluten in Gluten-Free Bread. Foods 2021, 10, 1997. [Google Scholar] [CrossRef]
- Codex Alimentarius. Guidelines for the Use of Nutrition and Health Claims. 1997. Available online: https://www.fao.org/ag/humannutrition/32444-09f5545b8abe9a0c3baf01a4502ac36e4.pdf (accessed on 14 February 2022).
- Romão, B.; Botelho, R.B.A.; Alencar, E.R.; Silva, V.S.N.D.; Pacheco, M.T.B.; Zandonadi, R.P. Chemical composition and glycemic index of gluten-free bread commercialized in Brazil. Nutrients 2020, 12, 2234. [Google Scholar] [CrossRef]
- Roman, L.; Belorio, M.; Gomez, M. Gluten-Free Breads: The Gap Between Research and Commercial Reality. Compr. Rev. Food Sci. Food Saf. 2019, 18, 690–702. [Google Scholar] [CrossRef] [Green Version]
- Gómez, M.; Talegón, M.; Hera, E.D.L. Influence of mixing on quality of gluten-free bread. J. Food Qual. 2013, 36, 139–145. [Google Scholar] [CrossRef]
- Parra, A.F.R.; Ribotta, P.D.; Ferrero, C. Apple pomace in gluten-free formulations: Effect on rheology and product quality. Int. J. Food Sci. Technol. 2015, 50, 682–690. [Google Scholar] [CrossRef]
- Mandala, I.G.; Sotirakoglou, K. Effect of frozen storage and microwave reheating on some physical attributes of fresh bread containing hydrocolloids. Food Hydrocoll. 2005, 19, 709–719. [Google Scholar] [CrossRef]
- Bourne, M. Texture profile analysis. Food Technol. 1978, 32, 62–67. [Google Scholar]
- Wee, M.S.M.; Goh, A.T.; Stieger, M.; Forde, C.G. Correlation of instrumental texture properties from textural profile analysis (TPA) with eating behaviours and macronutrient composition for a wide range of solid foods. Food Funct. 2018, 9, 5301–5312. [Google Scholar] [CrossRef] [PubMed]
- Ferrentino, G.; Morozova, K.; Mosibo, O.K.; Ramezani, M.; Scampicchio, M. Biorecovery of antioxidants from apple pomace by supercritical fluid extraction. J. Clean. Prod. 2018, 186, 253–261. [Google Scholar] [CrossRef]
- Jolicoeur, C. The New Cider Maker’s Handbook: A Comprehensive Guide for Craft Producers; Chelsea Green Publishing Company: White River Junction, VT, USA, 2013. [Google Scholar]
- Zuriarrain-Ocio, A.; Zuriarrain, J.; Vidal, M.; Dueñas, M.T.; Berregi, I. Antioxidant activity and phenolic profiles of ciders from the Basque Country. Food Biosci. 2021, 41, 100887. [Google Scholar] [CrossRef]
- Li, X.; Wu, X.; Bi, J.; Liu, X.; Li, X.; Guo, C. Polyphenols accumulation effects on surface color variation in apple slices hot air drying process. LWT 2019, 108, 421–428. [Google Scholar] [CrossRef]
- Sacchetti, G.; Cocci, E.; Pinnavaia, G.; Mastrocola, D.; Rosa, M.D. Influence of processing and storage on the antioxidant activity of apple derivatives. Int. J. Food Sci. Technol. 2008, 43, 797–804. [Google Scholar] [CrossRef]
- Larretxi, I.; Churruca, I.; Navarro, V.; Miranda, J.; Lasa, A.; Bustamante, M.Á.; Simon, E. Effect of analytically measured fiber and resistant starch from gluten-free products on the diets of individuals with celiac disease. Nutrition 2020, 70, 110586. [Google Scholar] [CrossRef]
- Aguiar, E.V.; Santos, F.G.; Krupa-Kozak, U.; Capriles, V.D. Nutritional facts regarding commercially available gluten-free bread worldwide: Recent advances and future challenges. Crit. Rev. Food Sci. Nutr. 2021, 61, 1–13. [Google Scholar] [CrossRef]
- Turck, D.; Castenmiller, J.; Henauw, S.D.; Hirsch-Ernst, K.I.; Kearney, J.; Knutsen, H.K.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Pelaez, C.; et al. Dietary reference values for sodium. EFSA J. 2019, 17, e05778. [Google Scholar] [CrossRef] [Green Version]
- Regulation (EC) No. 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the Provision of Food Information to Consumers. Available online: https://eur-lex.europa.eu (accessed on 2 November 2021).
- EFSA. Dietary reference values for nutrients: Summary report. EFSA 2017, 14, e15121. [Google Scholar] [CrossRef] [Green Version]
- Regulation (EC), No. 1924/2006 of the European Parliament and of The Council on Nutrition and Health Claims Made on Foods. Available online: https://eur-lex.europa.eu (accessed on 2 November 2021).
- Al-Toma, A.; Volta, U.; Auricchio, R.; Castillejo, G.; Sanders, D.S.; Cellier, C.; Mulder, C.J.; Lundin, K.E.A. European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten-related disorders. United Eur. Gastroenterol. J. 2019, 7, 583–613. [Google Scholar] [CrossRef]
- Rondanelli, M.; Faliva, M.A.; Gasparri, C.; Peroni, G.; Naso, M.; Picciotto, G.; Riva, A.; Nichetti, M.; Infantino, V.; Alalwan, T.A.; et al. Micronutrients dietary supplementation advices for celiac patients on long-term gluten-free diet with good compliance: A review. Medicina 2019, 55, 337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parafati, L.; Restuccia, C.; Palmeri, R.; Fallico, B.; Arena, E. Characterization of prickly pear peel flour as a bioactive and functional ingredient in bread preparation. Foods 2020, 9, 1189. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, N.; Rößle, C.; Arendt, E.; Gallagher, E. Modelling the effects of orange pomace using response surface design for gluten-free bread baking. Food Chem. 2015, 166, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Curti, E.; Carini, E.; Diantom, A.; Vittadini, E. The use of potato fibre to improve bread physico-chemical properties during storage. Food Chem. 2016, 195, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Karrar, E.M.A. A review on: Antioxidant and its impact during the bread making process. Int. J. Food Sci. Nutr. 2014, 3, 592–596. [Google Scholar] [CrossRef]
- Ahmad, S.; John, S.; Bosco, D.; Ahmad, M. Effect of apple pomace on quality characteristics of brown rice based cracker. J. Saudi Soc. Agric. Sci. 2017, 16, 25–32. [Google Scholar] [CrossRef] [Green Version]
- Celik, E.E.; Gokmen, V. Formation of Maillard reaction products in bread crust like model system made of diferent whole cereal fours. Eur. Food Res. Technol. 2020, 246, 1207–1218. [Google Scholar] [CrossRef]
- Matos, M.E.; Rosell, C.M. Relationship between instrumental parameters and sensory characteristics in gluten-free breads. Eur. Food Res. Technol. 2012, 235, 107–117. [Google Scholar] [CrossRef] [Green Version]
- Jannati, N.; Hojjatoleslamy, M.; Hosseini, E.; Mozafari, H.R.; Siavoshi, M. Effect of apple pomace powder on rheological properties of dought and Sangak bread texture. Carpathian J. Food Sci. Technol. 2018, 10, 77–84. [Google Scholar]
- Masoodi, F.A.; Sharma, B.; Chauhan, G.S. Use of apple pomace as a source of dietary fiber in cakes. Plant Foods Hum. Nutr. 2002, 57, 121–128. [Google Scholar] [CrossRef]
| Ingredients | Formulations | ||||
|---|---|---|---|---|---|
| Ctrl | CP | GFB5 | GFB6 | GFB8 | |
| Water (%) | 44.0 | 44.0 | 47.8 | 47.8 | 47.8 |
| GF flour (%) | 40.8 | 37.0 | 28.3 | 27.3 | 25.3 |
| Rice Flour (%) | 4.8 | 4.8 | 4.8 | 4.8 | 4.8 |
| Corn starch (%) | 4.8 | 4.8 | 4.8 | 4.8 | 4.8 |
| Whey protein (%) | 0.0 | 3.8 | 3.8 | 3.8 | 3.8 |
| APP (%) | 0.0 | 0.0 | 5.0 | 6.0 | 8.0 |
| Oil (%) | 2.9 | 2.9 | 2.9 | 2.9 | 2.9 |
| Salt (%) | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| Yeast 1 (%) | 1.2 | 1.2 | 1.1 | 1.1 | 1.1 |
| Batches | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | ||||||
| Color | L* | 54.477 | ±0.315 a | 57.540 | ±0.705 a | 56.947 | ±0.481 a | 57.033 | ±1.097 a |
| a* | 10.897 | ±0.227 a | 10.483 | ±0.472 a | 11.497 | ±0.125 a | 11.443 | ±0.499 a | |
| b* | 25.483 | ±0.550 a | 27.057 | ±1.390 a | 29.007 | ±0.490 a | 29.207 | ±0.514 a | |
| pH | 3.967 | ±0.038 b | 4.233 | ±0.133 ab | 4.260 | ±0.021 a | 4.287 | ±0.034 a | |
| aw | 0.211 | ±0.048 a | 0.180 | ±0.012 a | 0.359 | ±0.145 a | 0.189 | ±0.021 a | |
| DPPH % | 30.171 | ±0.777 a | 27.399 | ±0.274 ab | 27.716 | ±1.189 ab | 24.232 | ±0.274 b | |
| DPPH (µMol eq./g APP) | 482.280 | ±12.424 a | 437.973 | ±4.384 ab | 443.040 | ±19.00 ab | 387.343 | ±4.384 b | |
| Batches | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | ||||||
| Minerals (µg/g) | Mg | 475.00 | (25.792) | 478.00 | (33.651) | 440.00 (30.4785) | 439.50 (10.911) | ||
| Ca | 221.00 | (9.370) | 210.00 | (16.107) | 227 (20.031) | 241.00 (16.383) | |||
| Mn | 4.030 | (0.029) | 4.01 | (0.189) | 4.055 (0.268) | 4.510 (0.166) | |||
| Fe | 18.10 | (3.058) | 11.10 | (1.276) | 22.50 (1.456) | 37.20 (1.528) | |||
| Cu | 6.79 | (0.368) | 6.69 | (0.254) | 5.540 (0.323) | 7.270 (0.265) | |||
| Zn | 3.44 | (0.330) | 3.57 | (0.375) | 6.330 (0.466) | 7.405 (0.331) | |||
| Se | <LOQ | <LOQ | <LOQ | <LOQ | |||||
| Protein (%) | 3.85 | (0.38) | 3.73 | (0.12) | 2.98 | (0.17) | 3.47 | (0.28) | |
| Ash (%) | 1.55 | (0.27) | 2.37 | (0.48) | 1.94 | (0.41) | 1.55 | (0.12) | |
| Fiber fractions | HMWDF (g/100 g) | 75.000 | (1.414) | 89.000 | (1.414) | 74 | (1.414) | 82.5 | (2.121) |
| IDF (%) | 65.31 | 73.07 | 68.93 | 64.88 | |||||
| SDFP (%) | 34.69 | 26.93 | 31.07 | 35.12 | |||||
| Energy and Nutrient Values (per 100 g of Bread) | Formulations | ||||
|---|---|---|---|---|---|
| Ctrl | CP | GFB5 | GFB6 | GFB8 | |
| Energy (kcal) | 202.15 | 203.64 | 184.30 | 183.29 | 181.49 |
| Carbohydrates (g) | 42.24 | 39.10 | 33.29 | 32.60 | 31.43 |
| Sugars (g) | 1.31 | 1.22 | 1.02 | 0.99 | 0.94 |
| Dietary fiber (g) 1 | 3.65 | 3.30 | 6.23 | 6.92 | 8.15 |
| Protein (g) | 2.71 | 5.59 | 5.15 | 5.14 | 5.12 |
| Total lipid (g) | 3.49 | 3.44 | 3.14 | 3.13 | 3.11 |
| Saturated fat (g) | 0.51 | 0.50 | 0.45 | 0.45 | 0.45 |
| Magnesium (mg) 2 | ND | ND | 2.35 | 2.86 | 3.78 |
| Calcium (mg) 2 | ND | ND | 1.06 | 1.29 | 1.71 |
| Iron (mg) 2 | ND | ND | 0.07 | 0.09 | 0.11 |
| Manganese (mg) 2 | ND | ND | 0.02 | 0.02 | 0.03 |
| Copper (mg) 2 | ND | ND | 0.03 | 0.04 | 0.05 |
| Zinc (mg) 2 | ND | ND | 0.02 | 0.02 | 0.03 |
| Salt (g) | 1.57 | 1.56 | 1.44 | 1.44 | 1.44 |
| Batch | SV (cm3/g) | WC (%) | Aw | DPPH (%) | DPPH Eq. (µM/g Bread) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ctrl | 2.517 | ±0.019 c | 48.597 | ±1.296 ab | 0.900 | ±0.019 a | 5.319 | ±0.408 b | 77.108 | ±5.902 b |
| CP | 2.383 | ±0.042 c | 47.220 | ±0.443 b | 0.835 | ±0.005 a | 6.150 | ±0.479 ab | 73.249 | ±5.707 b |
| GFB5 | 3.026 | ±0.102 a | 50.242 | ±0.309 a | 0.830 | ±0.008 a | 6.508 | ±0.139 ab | 77.513 | ±1.666 b |
| GFB6 | 2.498 | ±0.063 c | 48.145 | ±0.335 ab | 0.767 | ±0.013 a | 6.493 | ±0.103 ab | 77.359 | ±1.221 b |
| GFB8 | 2.723 | ±0.037 b | 49.454 | ±0.341 ab | 0.618 | ±0.083 b | 6.755 | ±0.333 a | 114.286 | ±5.611 a |
| Color Parameters | |||||||
|---|---|---|---|---|---|---|---|
| Batch | Zone | L* | a* | b* | |||
| Ctrl | Crust | 65.873 | ±0.540 a | 15.689 | ±0.452 b | 44.943 | ±0.231 a |
| Crumb | 78.004 | ±0.394 α | 2.278 | ±0.203 ε | 26.186 | ±0.407 α | |
| CP | Crust | 54.398 | ±0.300 b | 18.280 | ±0.061 a | 36.886 | ±0.225 b |
| Crumb | 65.996 | ±0.240 β | 4.391 | ±0.136 δ | 24.016 | ±0.255 β | |
| GFB5 | Crust | 47.209 | ±0.686 c | 14.911 | ±0.262 bc | 27.876 | ±0.239 c |
| Crumb | 47.164 | ±0.814 γ | 9.044 | ±0.120 γ | 19.722 | ±0.258 δ | |
| GFB6 | Crust | 41.549 | ±0.529 d | 14.939 | ±0.222 bc | 23.583 | ±0.658 d |
| Crumb | 47.267 | ±0.547 γ | 9.866 | ±0.112 β | 21.853 | ±0.138 γ | |
| GFB8 | Crust | 35.643 | ±1.130 e | 13.969 | ±0.355 c | 18.725 | ±0.556 e |
| Crumb | 41.776 | ±0.662 δ | 12.781 | ±0.148 α | 23.848 | ±0.586 β | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cantero, L.; Salmerón, J.; Miranda, J.; Larretxi, I.; Fernández-Gil, M.d.P.; Bustamante, M.Á.; Matias, S.; Navarro, V.; Simón, E.; Martínez, O. Performance of Apple Pomace for Gluten-Free Bread Manufacture: Effect on Physicochemical Characteristics and Nutritional Value. Appl. Sci. 2022, 12, 5934. https://doi.org/10.3390/app12125934
Cantero L, Salmerón J, Miranda J, Larretxi I, Fernández-Gil MdP, Bustamante MÁ, Matias S, Navarro V, Simón E, Martínez O. Performance of Apple Pomace for Gluten-Free Bread Manufacture: Effect on Physicochemical Characteristics and Nutritional Value. Applied Sciences. 2022; 12(12):5934. https://doi.org/10.3390/app12125934
Chicago/Turabian StyleCantero, Leire, Jesús Salmerón, Jonatan Miranda, Idoia Larretxi, María del Pilar Fernández-Gil, María Ángeles Bustamante, Silvia Matias, Virginia Navarro, Edurne Simón, and Olaia Martínez. 2022. "Performance of Apple Pomace for Gluten-Free Bread Manufacture: Effect on Physicochemical Characteristics and Nutritional Value" Applied Sciences 12, no. 12: 5934. https://doi.org/10.3390/app12125934
APA StyleCantero, L., Salmerón, J., Miranda, J., Larretxi, I., Fernández-Gil, M. d. P., Bustamante, M. Á., Matias, S., Navarro, V., Simón, E., & Martínez, O. (2022). Performance of Apple Pomace for Gluten-Free Bread Manufacture: Effect on Physicochemical Characteristics and Nutritional Value. Applied Sciences, 12(12), 5934. https://doi.org/10.3390/app12125934






