Maintaining, Managing, and Tele-Monitoring a Nutritionally Adequate Mediterranean Gluten-Free Diet and Proper Lifestyle in Adult Patients
Abstract
1. Introduction
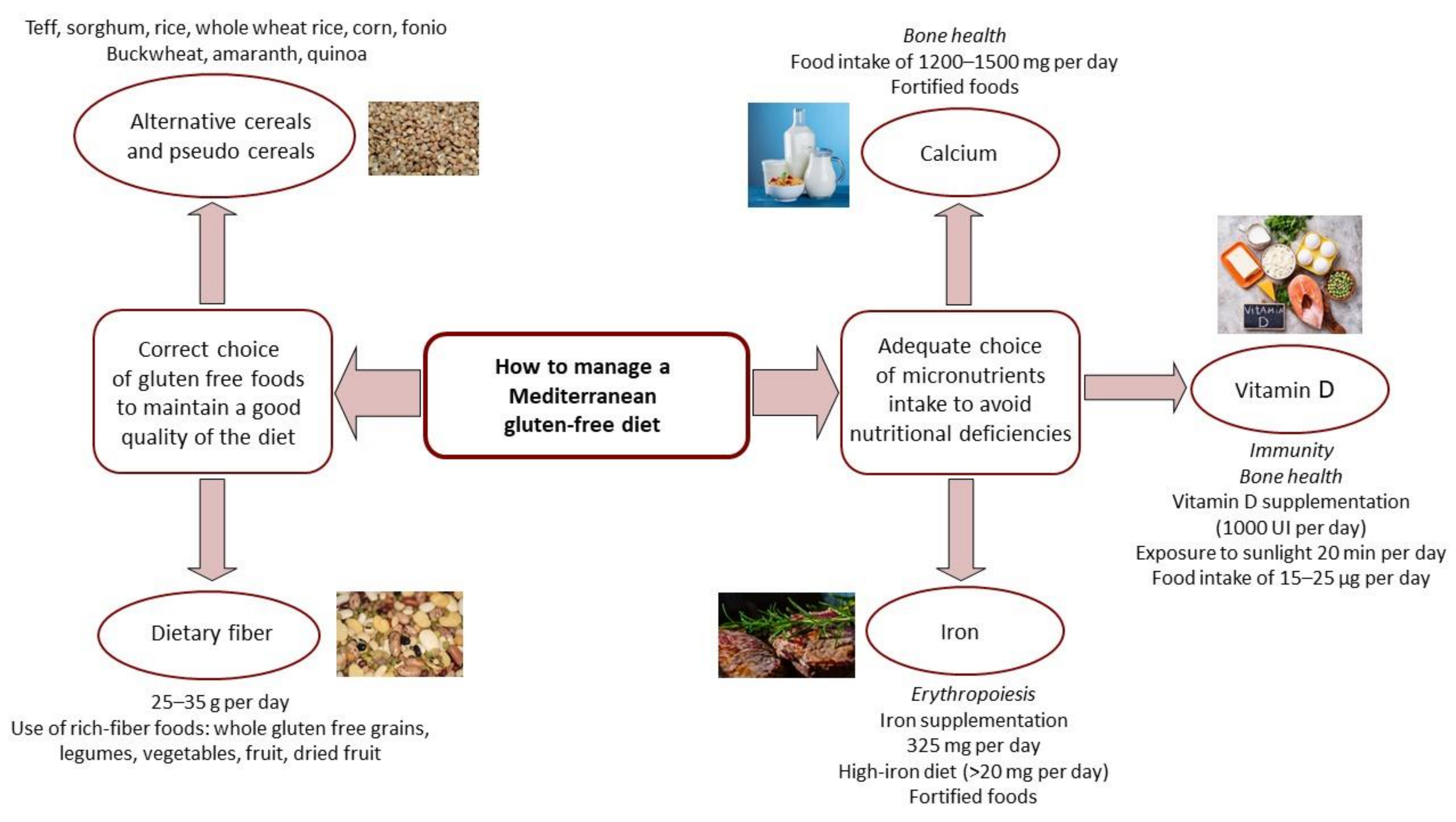
2. Mediterranean Gluten-Free Diet and Nutritional Support
3. Importance of a Healthy Lifestyle and a Natural Med-GFD
4. Self-Evaluation of GFD Correctness: Gluten Detection Tests
5. Telemedicine to Monitor the GFD
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elli, L.; Ferretti, F.; Orlando, S.; Vecchi, M.; Monguzzi, E.; Roncoroni, L.; Schuppan, D. Management of celiac disease in daily clinical practice. Eur. J. Intern. Med. 2018, 61, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Elli, L.; Villalta, D.; Roncoroni, L.; Barisani, D.; Ferrero, S.; Pellegrini, N.; Bardella, M.T.; Valiante, F.; Tomba, C.; Carroccio, A.; et al. Nomenclature and diagnosis of gluten-related disorders: A position statement by the Italian Association of Hospital Gastroenterologists and Endoscopists (AIGO). Dig. Liver Dis. 2016, 49, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Martínez-González, M.A.; Tong, T.Y.; Forouhi, N.G.; Khandelwal, S.; Prabhakaran, D.; Mozaffarian, D.; de Lorgeril, M. Definitions and potential health benefits of the Mediterranean diet: Views from experts around the world. BMC Med. 2014, 12, 112. [Google Scholar] [CrossRef] [PubMed]
- Elli, L.; Barisani, D.; Vaira, V.; Bardella, M.T.; Topa, M.; Vecchi, M.; Doneda, L.; Scricciolo, A.; Lombardo, V.; Roncoroni, L. How to manage celiac disease and gluten-free diet during the COVID-19 era: Proposals from a tertiary referral center in a high-incidence scenario. BMC Gastroenterol. 2020, 20, 387. [Google Scholar] [CrossRef]
- Costantino, A.; Roncoroni, L.; Noviello, D.; Nandi, N.; Lombardo, V.; Scricciolo, A.; Scaramella, L.; Vecchi, M.; Elli, L. Nutritional and Gastroenterological Monitoring of Patients with Celiac Disease During COVID-19 Pandemic: The Emerging Role of Telemedicine and Point-of-Care Gluten Detection Tests. Front. Nutr. 2021, 8, 622514. [Google Scholar] [CrossRef]
- Costantino, A.; Bortoluzzi, F.; Giuffrè, M.; Vassallo, R.; Montalbano, L.M.; Monica, F.; Canova, D.; Checchin, D.; Fedeli, P.; Marmo, R.; et al. Correct use of telemedicine in gastroenterology, hepatology, and endoscopy during and after the COVID-19 pandemic: Recommendations from the Italian association of hospital gastroenterologists and endoscopists (AIGO). Dig. Liver Dis. 2021, 53, 1221–1227. [Google Scholar] [CrossRef]
- Olivares, M.; Neef, A.; Castillejo, G.; De Palma, G.; Varea, V.; Capilla, A.; Palau, F.; Nova, E.; Marcos, A.; Polanco, I.; et al. The HLA-DQ2 genotype selects for early intestinal microbiota composition in infants at high risk of developing coeliac disease. Gut 2014, 64, 406–417. [Google Scholar] [CrossRef]
- Bodkhe, R.; Shetty, S.; Dhotre, D.; Verma, A.K.; Bhatia, K.; Mishra, A.; Kaur, G.; Pande, P.; Bangarusamy, D.K.; Santosh, B.P.; et al. Comparison of Small Gut and Whole Gut Microbiota of First-Degree Relatives with Adult Celiac Disease Patients and Controls. Front. Microbiol. 2019, 10, 164. [Google Scholar] [CrossRef]
- Mohan, M.; Chow, C.-E.T.; Ryan, C.N.; Chan, L.S.; Dufour, J.; Aye, P.P.; Blanchard, J.; Moehs, C.P.; Sestak, K. Dietary Gluten-Induced Gut Dysbiosis Is Accompanied by Selective Upregulation of microRNAs with Intestinal Tight Junction and Bacteria-Binding Motifs in Rhesus Macaque Model of Celiac Disease. Nutrients 2016, 8, 684. [Google Scholar] [CrossRef]
- Barone, M.; Della Valle, N.; Rosania, R.; Facciorusso, A.; Trotta, A.; Cantatore, F.P.; Falco, S.; Pignatiello, S.; Viggiani, M.T.; Amoruso, A.; et al. A comparison of the nutritional status between adult celiac patients on a long-term, strictly gluten-free diet and healthy subjects. Eur. J. Clin. Nutr. 2015, 70, 23–27. [Google Scholar] [CrossRef]
- Cena, H.; Calder, P.C. Defining a Healthy Diet: Evidence for the Role of Contemporary Dietary Patterns in Health and Disease. Nutrients 2020, 12, 334. [Google Scholar] [CrossRef] [PubMed]
- Salas-Salvadó, J.; Díaz-López, A.; Ruiz-Canela, M.; Basora, J.; Fitó, M.; Corella, D. Effect of a Lifestyle Intervention Program With Energy-Restricted Mediterranean Diet and Exercise on Weight Loss and Cardiovascular Risk Factors: One-Year Results of the PREDIMED-Plus Trial. Diabetes Care 2019, 42, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Menotti, A.; Puddu, P. How the Seven Countries Study contributed to the definition and development of the Mediterranean diet concept: A 50-year journey. Nutr. Metab. Cardiovasc. Dis. 2014, 25, 245–252. [Google Scholar] [CrossRef]
- Rijkers, G.T. Nutrition, immunity and human health. Br. J. Nutr. 2013, 114, 1329–1330. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vici, G.; Belli, L.; Biondi, M.; Polzonetti, V. Gluten free diet and nutrient deficiencies: A review. Clin. Nutr. 2016, 35, 1236–1241. [Google Scholar] [CrossRef]
- Theethira, T.G.; Dennis, M. Celiac Disease and the Gluten-Free Diet: Consequences and Recommendations for Improvement. Dig. Dis. 2015, 33, 175–182. [Google Scholar] [CrossRef]
- Melini, V.; Melini, F. Gluten-Free Diet: Gaps and Needs for a Healthier Diet. Nutrients 2019, 11, 170. [Google Scholar] [CrossRef] [PubMed]
- Anania, C.; Pacifico, L.; Olivero, F.; Perla, F.M.; Chiesa, C. Cardiometabolic risk factors in children with celiac disease on a gluten-free diet. World J. Clin. Pediatr. 2017, 6, 143–148. [Google Scholar] [CrossRef]
- Bascuñán, K.A.; Elli, L.; Vecchi, M.; Scricciolo, A.; Mascaretti, F.; Parisi, M.; Doneda, L.; Lombardo, V.; Araya, M.; Roncoroni, L. Mediterranean Gluten-Free Diet: Is It a Fair Bet for the Treatment of Gluten-Related Disorders? Front. Nutr. 2020, 7, 81. [Google Scholar] [CrossRef]
- Bascuñán, K.A.; Araya, M.; Roncoroni, L.; Doneda, L.; Elli, L. Dietary Gluten as a Conditioning Factor of the Gut Microbiota in Celiac Disease. Adv. Nutr. 2019, 11, 160–174. [Google Scholar] [CrossRef]
- Norsa, L.; Shamir, R.; Zevit, N.; Verduci, E.; Hartman, C.; Ghisleni, D.; Riva, E.; Giovannini, M. Cardiovascular disease risk factor profiles in children with celiac disease on gluten-free diets. World J. Gastroenterol. 2013, 19, 5658–5664. [Google Scholar] [CrossRef] [PubMed]
- Emilsson, L.; Lebwohl, B.; Sundström, J.; Ludvigsson, J.F. Cardiovascular disease in patients with coeliac disease: A systematic review and meta-analysis. Dig. Liver Dis. 2015, 47, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Scricciolo, A.; Elli, L.; Doneda, L.; Bascuñán, K.A.; Branchi, F.; Ferretti, F.; Vecchi, M.; Roncoroni, L. Efficacy of a High-Iron Dietary Intervention in Women with Celiac Disease and Iron Deficiency without Anemia: A Clinical Trial. Nutrients 2020, 12, 2122. [Google Scholar] [CrossRef]
- Holden, J.M.; Lemar, L.E. Assessing vitamin D contents in foods and supplements: Challenges and needs. Am. J. Clin. Nutr. 2008, 88, 551S–553S. [Google Scholar] [CrossRef] [PubMed]
- Hernigou, P.; Sitbon, J.; Dubory, A.; Auregan, J.C. Vitamin D history part III: The “modern times”—New questions for orthopaedic practice: Deficiency, cell therapy, osteomalacia, fractures, supplementation, infections. Int. Orthop. 2019, 43, 1755–1771. [Google Scholar] [CrossRef] [PubMed]
- Cardwell, G.; Bornman, J.F.; James, A.P.; Black, L.J. A Review of Mushrooms as a Potential Source of Dietary Vitamin D. Nutrients 2018, 10, 1498. [Google Scholar] [CrossRef] [PubMed]
- Food and Nutrition Center (CREA). Food Composition Tables. 2019. Available online: https://www.alimentinutrizione.it/sezioni/tabelle-nutrizionali (accessed on 12 December 2020).
- Italian Society of Human Nutrition (SINU). Reference Intake Levels of Nutrients and Energy (LARN IV); SINU: Milan, Italy, 2014. [Google Scholar]
- National Institute for Health and Care Excellence (NICE). COVID-19 Rapid Guideline: Vitamin D. Available online: https://www.nice.org.uk/guidance/ng187 (accessed on 15 October 2021).
- Matsui, M.S. Vitamin D Update. Curr. Dermatol. Rep. 2020, 9, 323–330. [Google Scholar] [CrossRef]
- Berry, E.M. Sustainable Food Systems and the Mediterranean Diet. Nutrients 2019, 11, 2229. [Google Scholar] [CrossRef]
- Abdullah, M.M.; Jones, J.; Jones, P.J. Economic benefits of the Mediterranean-style diet consumption in Canada and the United States. Food Nutr. Res. 2015, 59, 27541. [Google Scholar] [CrossRef]
- Saulle, R.; Semyonov, L.; La Torre, G. Cost and Cost-Effectiveness of the Mediterranean Diet: Results of a Systematic Review. Nutrients 2013, 5, 4566–4586. [Google Scholar] [CrossRef]
- Bonaccio, M.; Costanzo, S.; Di Castelnuovo, A.; Persichillo, M.; De Curtis, A.; Olivieri, M.; Cerletti, C.; Donati, M.B.; de Gaetano, G.; Iacoviello, L. The CASSIOPEA Study (Economic Crisis and Adherence to the Mediterranean diet: Possible impact on biOmarkers of inflammation and metabolic PhEnotypes in the cohort of the Moli-sAni Study): Rationale, design and characteristics of participants. Nutr. Metab. Cardiovasc. Dis. 2020, 31, 1053–1062. [Google Scholar] [CrossRef]
- Polak, R.; Phillips, E.M.; Campbell, A. Legumes: Health Benefits and Culinary Approaches to Increase Intake. Clin. Diabetes 2015, 33, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Maphosa, Y.; Jideani, V. The Role of Legumes in Human Nutrition. In Functional Food—Improve Health through Adequate Food Maria Chavarri Hueda; Department of Food Science and Technology, Cape Peninsula University of Technology: Bellville, South Africa, 2017; pp. 103–122. ISBN 978-953-51-3440-4. [Google Scholar]
- Han, F.; Moughan, P.J.; Li, J.; Stroebinger, N.; Pang, S. The Complementarity of Amino Acids in Cooked Pulse/Cereal Blends and Effects on DIAAS. Plants 2021, 10, 1999. [Google Scholar] [CrossRef] [PubMed]
- Bennett, G.; Young, E.; Butler, I.; Coe, S. The Impact of Lockdown During the COVID-19 Outbreak on Dietary Habits in Various Population Groups: A Scoping Review. Front. Nutr. 2021, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, L.; Gualtieri, P.; Pivari, F.; Soldati, L.; Attinà, A.; Cinelli, G.; Leggeri, C.; Caparello, G.; Barrea, L.; Scerbo, F.; et al. Eating habits and lifestyle changes during COVID-19 lockdown: An Italian survey. J. Transl. Med. 2020, 18, 229. [Google Scholar] [CrossRef] [PubMed]
- Chtourou, H.; Trabelsi, K.; H’Mida, C.; Boukhris, O.; Glenn, J.M.; Brach, M.; Bentlage, E.; Bott, N.; Shephard, R.J.; Ammar, A.; et al. Staying Physically Active During the Quarantine and Self-Isolation Period for Controlling and Mitigating the COVID-19 Pandemic: A Systematic Overview of the Literature. Front. Psychol. 2020, 11, 1708. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Suárez, V.J.; Beltrán-Velasco, A.I.; Ramos-Campo, D.J.; Mielgo-Ayuso, J.; Nikolaidis, P.A.; Belando, N.; Tornero-Aguilera, J.F. Physical activity and COVID-19. The basis for an efficient intervention in times of COVID-19 pandemic. Physiol. Behav. 2021, 244, 113667. [Google Scholar] [CrossRef]
- Khoramipour, K.; Basereh, A.; Hekmatikar, A.A.; Castell, L.; Ruhee, R.T.; Suzuki, K. Physical activity and nutrition guidelines to help with the fight against COVID-19. J. Sports Sci. 2020, 39, 101–107. [Google Scholar] [CrossRef]
- de Moreno, M.L.; Cebolla, Á.; Muñoz-Suano, A.; Carrillo-Carrion, C.; Comino, I.; Pizarro, Á. Detection of gluten immunogenic peptides in the urine of patients with coeliac disease reveals transgressions in the gluten-free diet and incomplete mucosal healing. Gut 2017, 66, 250–257. [Google Scholar] [CrossRef]
- Costa, A.F.; Sugai, E.; de la Paz Temprano, M.; Niveloni, S.I.; Vázquez, H.; Moreno, M.L.; Domínguez-Flores, M.R.; Muñoz-Suano, A.; Smecuol, E.; Stefanolo, J.P.; et al. Gluten immunogenic peptide excretion detects dietary transgressions in treated celiac disease patients. World J. Gastroenterol. 2019, 25, 1409–1420. [Google Scholar] [CrossRef]
- Wieser, H.; Segura, V.; Ruiz-Carnicer, Á.; Sousa, C.; Comino, I. Food Safety and Cross-Contamination of Gluten-Free Products: A Narrative Review. Nutrients 2021, 13, 2244. [Google Scholar] [CrossRef] [PubMed]
- Rej, A.; Elli, L.; Sanders, D.S. Persisting Villous Atrophy and Adherence in Celiac Disease: What Does the Patient Want? What Should a Clinician Advise? Am. J. Gastroenterol. 2021, 116, 946–948. [Google Scholar] [CrossRef] [PubMed]
- Soler, M.; Estevez, M.-C.; Moreno, M.D.L.; Cebolla, A.; Lechuga, L. Label-free SPR detection of gluten peptides in urine for non-invasive celiac disease follow-up. Biosens. Bioelectron. 2016, 79, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Kanters, T.A.; Bouwmans-Frijters, C.; Van Der Linden, N.; Tan, S.S.; Roijen, L.H.-V. Update of the Dutch manual for costing studies in health care. PLoS ONE 2017, 12, e0187477. [Google Scholar] [CrossRef]
- Siniscalchi, M.; Zingone, F.; Savarino, E.V.; D’Odorico, A.; Ciacci, C. COVID-19 pandemic perception in adults with celiac disease: An impulse to implement the use of telemedicine: COVID-19 and CeD. Dig. Liver Dis. 2020, 52, 1071–1075. [Google Scholar] [CrossRef]
- Wyatt, J.C.; Sullivan, F. eHealth and the future: Promise or peril? BMJ 2005, 331, 1391–1393. [Google Scholar] [CrossRef]
- Vriezinga, S.; Borghorst, A.; Marle, E.V.D.A.-V.; Benninga, M.; George, E.; Hendriks, D.; Hopman, E.; de Meij, T.; Jong, A.V.D.M.-D.; Putter, H.; et al. E-Healthcare for Celiac Disease—A Multicenter Randomized Controlled Trial. J. Pediatr. 2017, 195, 154–160.e7. [Google Scholar] [CrossRef]
- Mehta, P.; Stahl, M.G.; Germone, M.M.; Nagle, S.; Guigli, R.; Thomas, J.; Shull, M.; Liu, E. Telehealth and Nutrition Support During the COVID-19 Pandemic. J. Acad. Nutr. Diet. 2020, 120, 1953–1957. [Google Scholar] [CrossRef]
- Kelly, J.T.; Reidlinger, D.P.; Hoffmann, T.C.; Campbell, K.L. Telehealth methods to deliver dietary interventions in adults with chronic disease: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2016, 104, 1693–1702. [Google Scholar] [CrossRef]
- Knotowicz, H.; Haas, A.; Coe, S.; Furuta, G.T.; Mehta, P. Opportunities for Innovation and Improved Care Using Telehealth for Nutritional Interventions. Gastroenterology 2019, 157, 594–597. [Google Scholar] [CrossRef]
- Kelly, J.T.; Conley, M.; Hoffmann, T.; Craig, J.; Tong, A.; Reidlinger, D.P.; Reeves, M.M.; Howard, K.; Krishnasamy, R.; Kurtkoti, J.; et al. A Coaching Program to Improve Dietary Intake of Patients with CKD. Clin. J. Am. Soc. Nephrol. 2020, 15, 330–340. [Google Scholar] [CrossRef] [PubMed]
- American Telemedicine Association (ATA). Telehealth Basic. Available online: https://www.americantelemed.org/resource/why-telemedicine/ (accessed on 23 January 2021).
- Rubio-Tapia, A.; Hill, I.D.; Kelly, C.P.; Calderwood, A.H.; Murray, J.A. ACG Clinical Guidelines: Diagnosis and Management of Celiac Disease. Am. J. Gastroenterol. 2013, 108, 656–676. [Google Scholar] [CrossRef] [PubMed]
- Coenen, S.; Nijns, E.; Weyts, E.; Geens, P.; Bosch, B.V.D.; Vermeire, S.; Ferrante, M.; Vanhaecht, K.; Van Assche, G. Development and feasibility of a telemonitoring tool with full integration in the electronic medical record: A proof of concept study for patients with inflammatory bowel disease in remission on biological therapy. Scand. J. Gastroenterol. 2020, 55, 287–293. [Google Scholar] [CrossRef] [PubMed]
| Proposed Steps | Healthcare Professionals | Assessments |
|---|---|---|
| Suspected gluten-related disorder | Physician | Clinical history Family history Assessment of symptoms and general health conditions Indication for serum antibody analysis |
| Gluten-related disorder diagnosis | Physician | Education on gluten-related disorder Supplementation to treat/prevent nutritional deficiencies Referral to nutritionist Search for deficiencies, complications, and other diagnoses |
| Gluten-related disorder follow-up | Physician | Assessment of general health status Evaluation of persistent symptoms and signs Evaluation and management of clinical findings Request for serum antibodies within 6 months |
| Gluten-related disorder diagnosis | Nutritionist | Nutritional evaluation with self-reported body measures Food education on gluten-free diet Assessment and treatment of nutritional deficiencies Activities, customs, and daily habits that influence diet Food preferences and rejections GFD eating plan design |
| Gluten-related disorder follow-up | Nutritionist | Nutritional evaluation with self-reported body measures Adherence to the GFD (by questionnaire) To assess specific health-related quality of life (by questionnaire) Viability and monitoring of the gluten-free eating plan Evaluation of urinary gluten peptides |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scricciolo, A.; Bascuñán, K.A.; Araya, M.; Sanders, D.S.; Trott, N.; Elli, L.; Bardella, M.T.; Doneda, L.; Lombardo, V.; Nandi, N.; et al. Maintaining, Managing, and Tele-Monitoring a Nutritionally Adequate Mediterranean Gluten-Free Diet and Proper Lifestyle in Adult Patients. Appl. Sci. 2022, 12, 1578. https://doi.org/10.3390/app12031578
Scricciolo A, Bascuñán KA, Araya M, Sanders DS, Trott N, Elli L, Bardella MT, Doneda L, Lombardo V, Nandi N, et al. Maintaining, Managing, and Tele-Monitoring a Nutritionally Adequate Mediterranean Gluten-Free Diet and Proper Lifestyle in Adult Patients. Applied Sciences. 2022; 12(3):1578. https://doi.org/10.3390/app12031578
Chicago/Turabian StyleScricciolo, Alice, Karla A. Bascuñán, Magdalena Araya, David S. Sanders, Nick Trott, Luca Elli, Maria Teresa Bardella, Luisa Doneda, Vincenza Lombardo, Nicoletta Nandi, and et al. 2022. "Maintaining, Managing, and Tele-Monitoring a Nutritionally Adequate Mediterranean Gluten-Free Diet and Proper Lifestyle in Adult Patients" Applied Sciences 12, no. 3: 1578. https://doi.org/10.3390/app12031578
APA StyleScricciolo, A., Bascuñán, K. A., Araya, M., Sanders, D. S., Trott, N., Elli, L., Bardella, M. T., Doneda, L., Lombardo, V., Nandi, N., Vecchi, M., & Roncoroni, L. (2022). Maintaining, Managing, and Tele-Monitoring a Nutritionally Adequate Mediterranean Gluten-Free Diet and Proper Lifestyle in Adult Patients. Applied Sciences, 12(3), 1578. https://doi.org/10.3390/app12031578








