1. Introduction
The resorption and remodeling of the alveolar ridge in the maxilla and in the mandible are a consequence of tooth loss [
1,
2,
3,
4]. Two-thirds of this reduction occurs within the first 3 months; within 1 year, the clinical width of the alveolar ridge is reduced by approximately 50% [
1,
2,
3,
4]. The mean vertical loss of tissues at single extracted sites ranges from 1 to 4 mm, depending on the site location [
1,
2,
3,
4]. This physiologic phenomenon occurs at different rates and degrees among various individuals and, in a few cases, it can be very pronounced. This localized alveolar bone resorption may affect the possibility of placing dental implants and their aesthetic outcome, particularly in aesthetic areas and in those patients exposing visible portions of gums when speaking and smiling.
Several authors have described and developed classifications for the different types of bone defects in edentulous patients, e.g., horizontal, vertical and a mix of the two dimensions [
5,
6].
To functionally and aesthetically rehabilitate these patients with dental implants, many bone grafting techniques have been developed [
7].
Many studies had shown that bone augmentation in a horizontal direction is more predictable than procedures aimed at augmenting bone in a vertical direction [
8]. In particular, peri-operative complications for vertical augmentations range from 30 to 40% [
8,
9]. A meta-analysis reported a complication rate of 17%; however, in general, complications in the scientific literature are under-reported [
10]. There are many proposed techniques for horizontal bone augmentation. It is possible to resort to the use of a bone block graft, which can be digitally designed with modern CAD/CAM technologies as described by Dominiak et al. [
11], or a particulate bone graft.
Guided bone regeneration (GBR) is one of the most widely used procedures [
8]. In GBR, particulate autogenous bone or bone substitutes (they can also be mixed) are positioned in the recipient bed and kept in place by purposely designed membranes of other substances, e.g., zirconia or titanium meshes [
12]. Currently, bone grafts represent the gold standard in augmentation procedures, but alternative methods such as natural polymers have been proposed as materials capable of stimulating bone regeneration. According to a recent study, the combination of multiple biomaterials guarantees better physical and biomolecular characteristics than the use of bioceramics or polymers alone. Natural composites have significant potential as an effective bone scaffold to repair alveolar defects after a tooth extraction [
13].
Another natural polymer useful in oral surgery is collagen, one of the most common materials for resorbable membranes [
14].
The main purposes of the membrane is to isolate the bone graft from the soft tissues that, by being faster growing, could infiltrate the particulate bone, negatively interfering with the formation of new bone and the stabilizing of blood clots.
Initially, membranes were of a non-resorbable type and were usually made of various types of polytetrafluoroethylene known as Teflon and abbreviated as PTFE. Subsequently, various types of resorbable barriers—either collagen-based or of animal origin, or synthetic, usually made of polylactic acid (PLA) or polyglycolic acid (PLGA)—were developed. Resorbable barriers were developed for two main reasons: there was no need for a second surgery to remove a resorbable membrane and there was a clinical impression that complications from non-resorbable barriers could be reduced in both number and severity. It has been proven that when a post-operative dehiscence occurs in an area under GBR, the clinical outcome is less favorable [
15]. The use of blood derivatives such as PRP and PRF give the possibility of dealing with this complication, supporting the healing and regeneration of the site [
16,
17].
It is interesting to observe that there is a widespread opinion among clinicians that post-operative complications such as wound dehiscence and related infective consequences affecting the regeneration of new bone are more common and severe with non-resorbable membranes. At the same time, the amount and quality of newly generated bone is improved, in the absence of complications, when using non-resorbable barriers.
However, the scientific literature does not support this view [
18]. Nevertheless, attempts are ongoing to exploit the best characteristics of the combined use of both non-resorbable and resorbable barriers.
For this reason, we aimed to introduce a new technique for large-volume horizontal bone augmentation, called the “sling technique”, and to present the preliminary results obtained. With this approach, a non-resorbable membrane is placed away from the crestal incision to decrease the risk of a catastrophic infective process and to offer the best environment for bone regeneration. The occlusal part of the graft is covered with a resorbable barrier that, in the case of wound dehiscence, can locally contain and limit the infection. This is then rapidly resorbed, followed by a secondary intention healing. The rationale of the sling technique is to use a non-resorbable membrane to maintain the barrier effect and to compress the graft on the recipient bed for a longer time than a resorbable barrier, i.e., until its surgical removal.
2. Materials and Methods
This study was conducted as a case series, in compliance with the ethical standards of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Federico II University of Naples (5 March 2018; No. 347/18). Over a period ranging from April 2018 to June 2018, and in order to obtain a randomized population, we included in the study the first 10 edentulous patients affected by a maxillary or mandibular horizontal bone defect and who were unable to receive an implant-supported prosthesis due to a lack of bone. Horizontal bone defects were diagnosed through digital measurements on cone beam computed tomography (CBCT), routinely performed in order to detect the bone bio-availability. The inclusion/exclusion criteria are summarized in
Table 1 and
Table 2. Informed consent was obtained from all subjects involved in the study.
2.1. Pre-Operative Management of Patients
Cone beam computed tomography (CBCT) scans were taken to plan the regenerative procedures and to assess the number of bone defects. All patients received professional oral hygiene prior to the operation and received a prophylactic antibiotic therapy of 2 g of amoxicillin plus clavulanic acid orally 1 h prior to the intervention. Patients with chronic infections at future implant sites received 1 g of amoxicillin plus clavulanic acid 3 times a day starting 2 days before the implant surgery. Patients allergic to penicillin were given 500 mg of clarithromycin 1 h prior to the intervention or, if chronic infections were present, received 500 mg of clarithromycin twice a day starting 2 days before surgery (
Table 3).
2.2. Surgical Technique
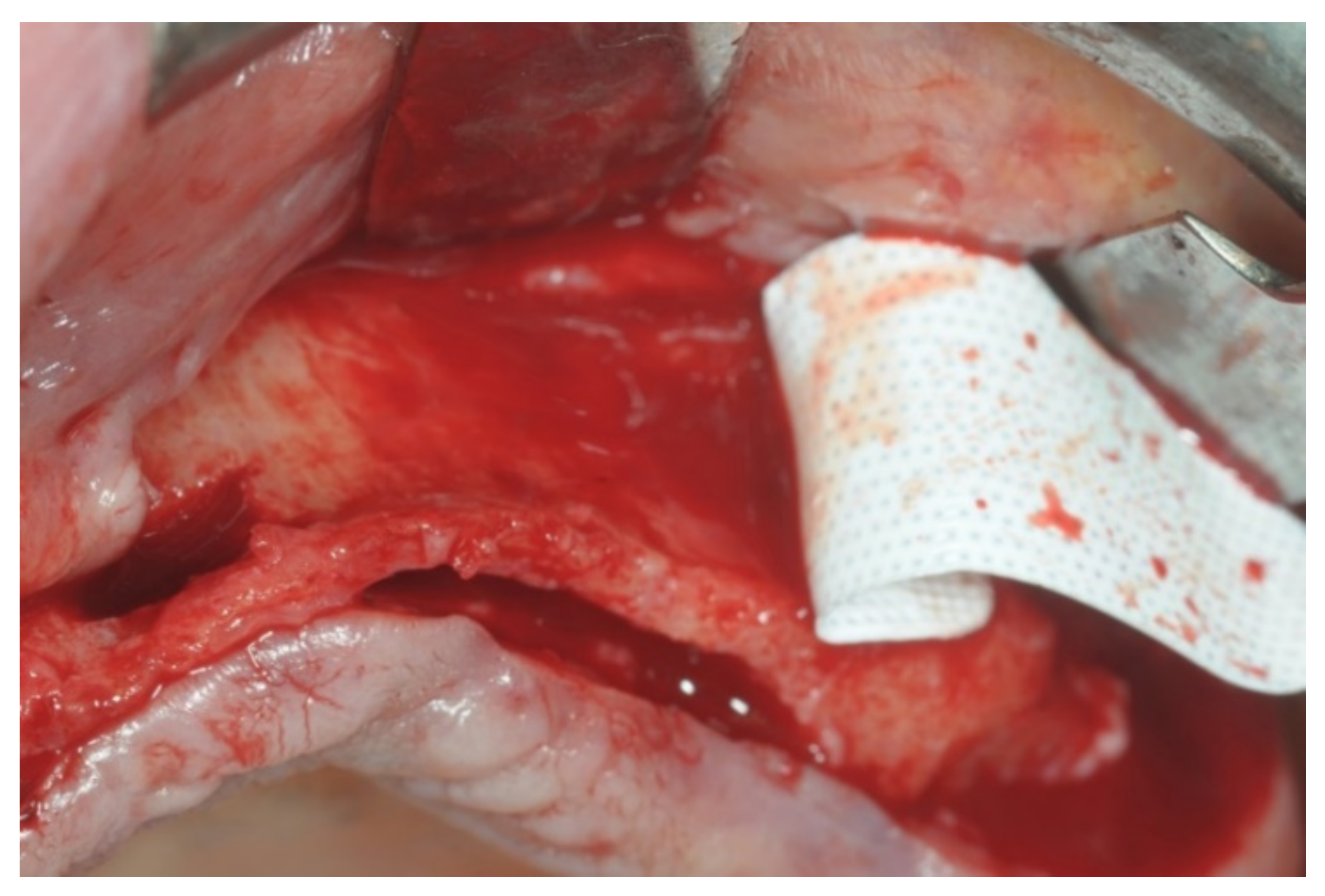
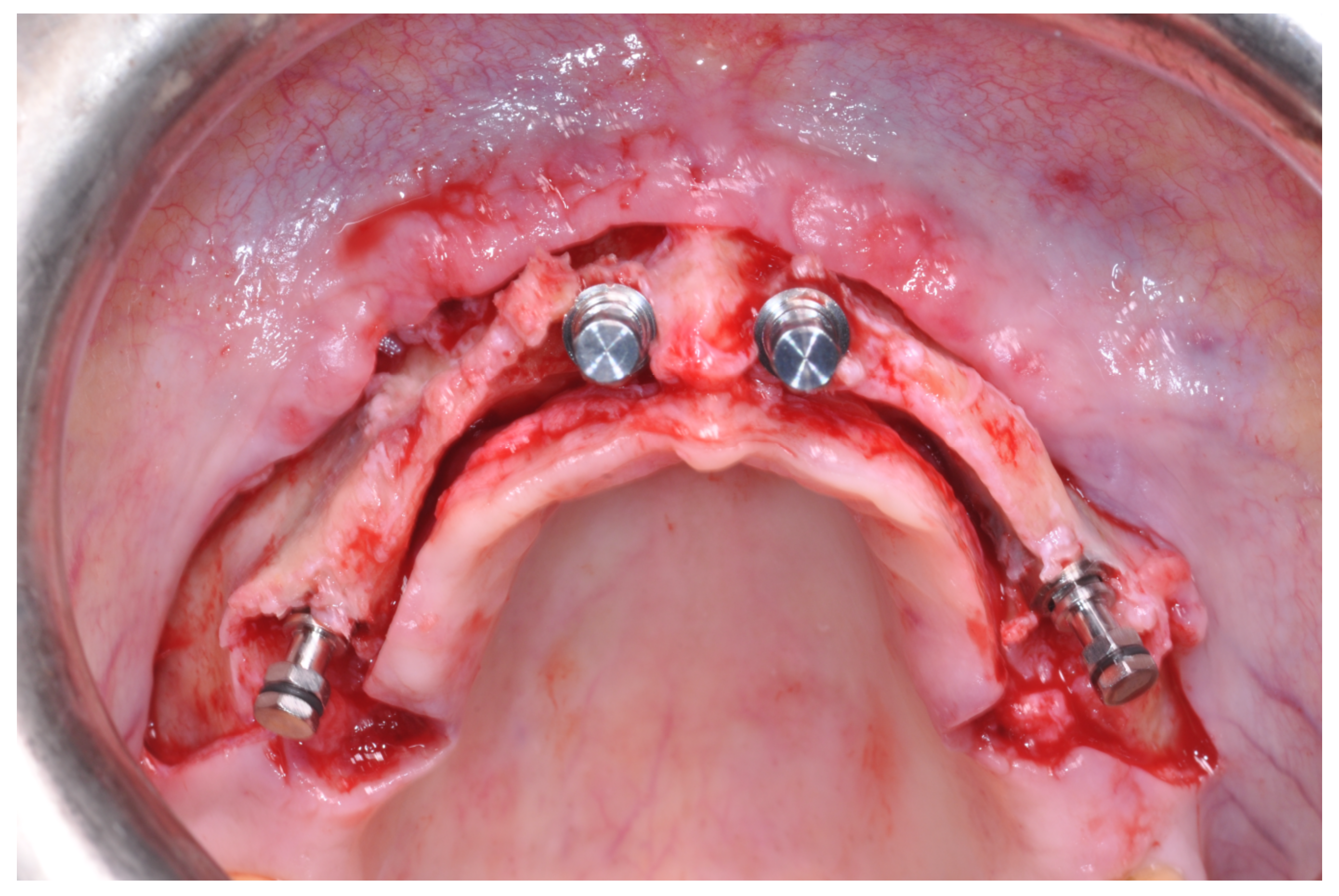
Patients rinsed with a chlorhexidine mouthwash (0.2%) for 1 min immediately prior to the intervention. Local anesthesia were administered using mepivacaine with adrenaline at ratios of 1:100,000 or 1:50,000. Crestal incisions were made with releasing incisions far away from the future membrane positioning and full thickness flaps were elevated. After the meticulous removal of all residual soft tissues in the regenerating site, copious bleeding was induced using a bone scraper (Safescraper Curve TWIST, META, Reggio Emilia, Italy) (
Figure 1). A prosthetically guided implant placement was performed following the instructions of manufacturer (Thommen Medical, Grenchen, Switzerland). Only 1 patient had implants placed 9 months after bone augmentation because the initial bone thickness did not permit the primary stabilization of the implant fixtures (
Figure 2,
Figure 3,
Figure 4,
Figure 5,
Figure 6,
Figure 7 and
Figure 8).
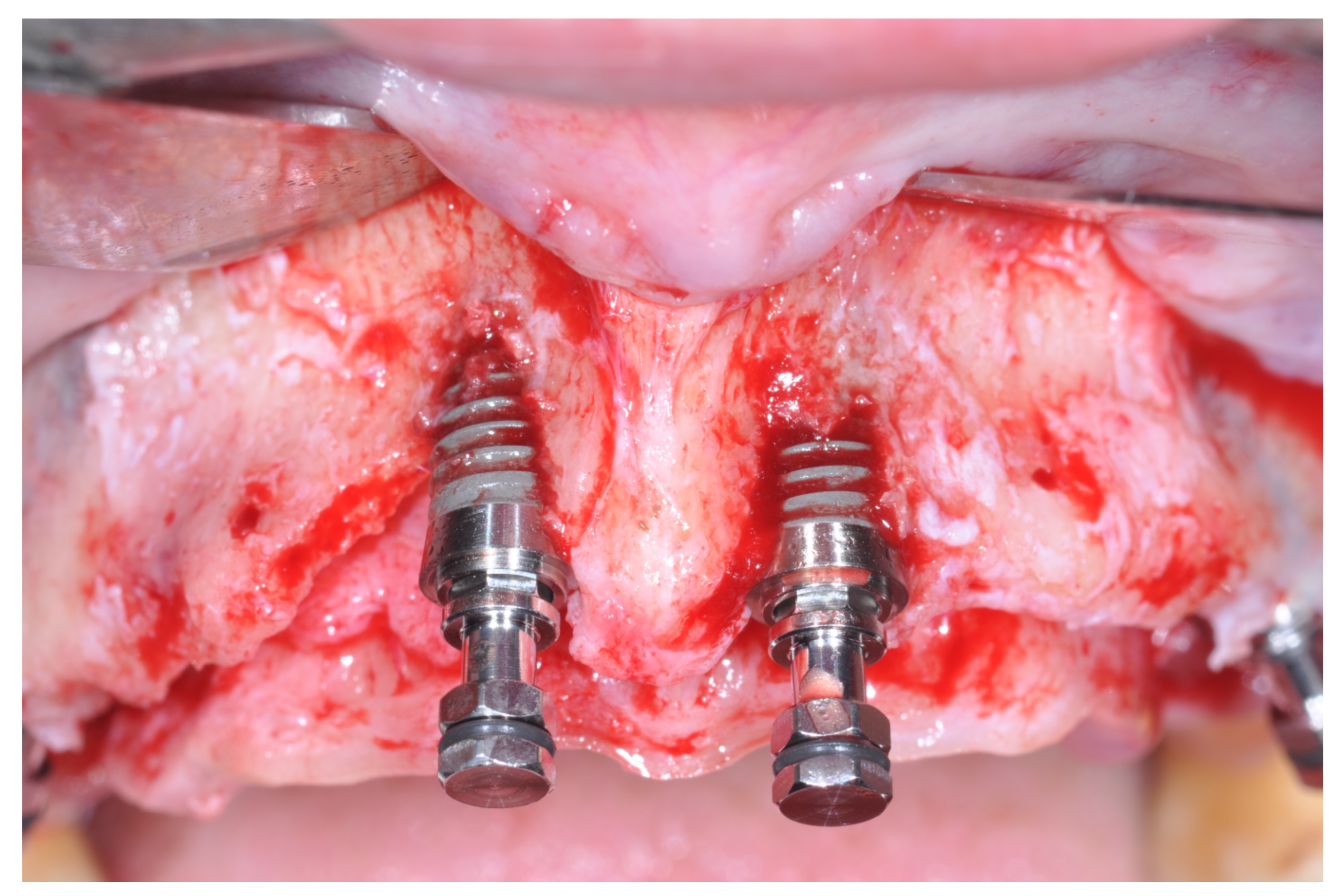
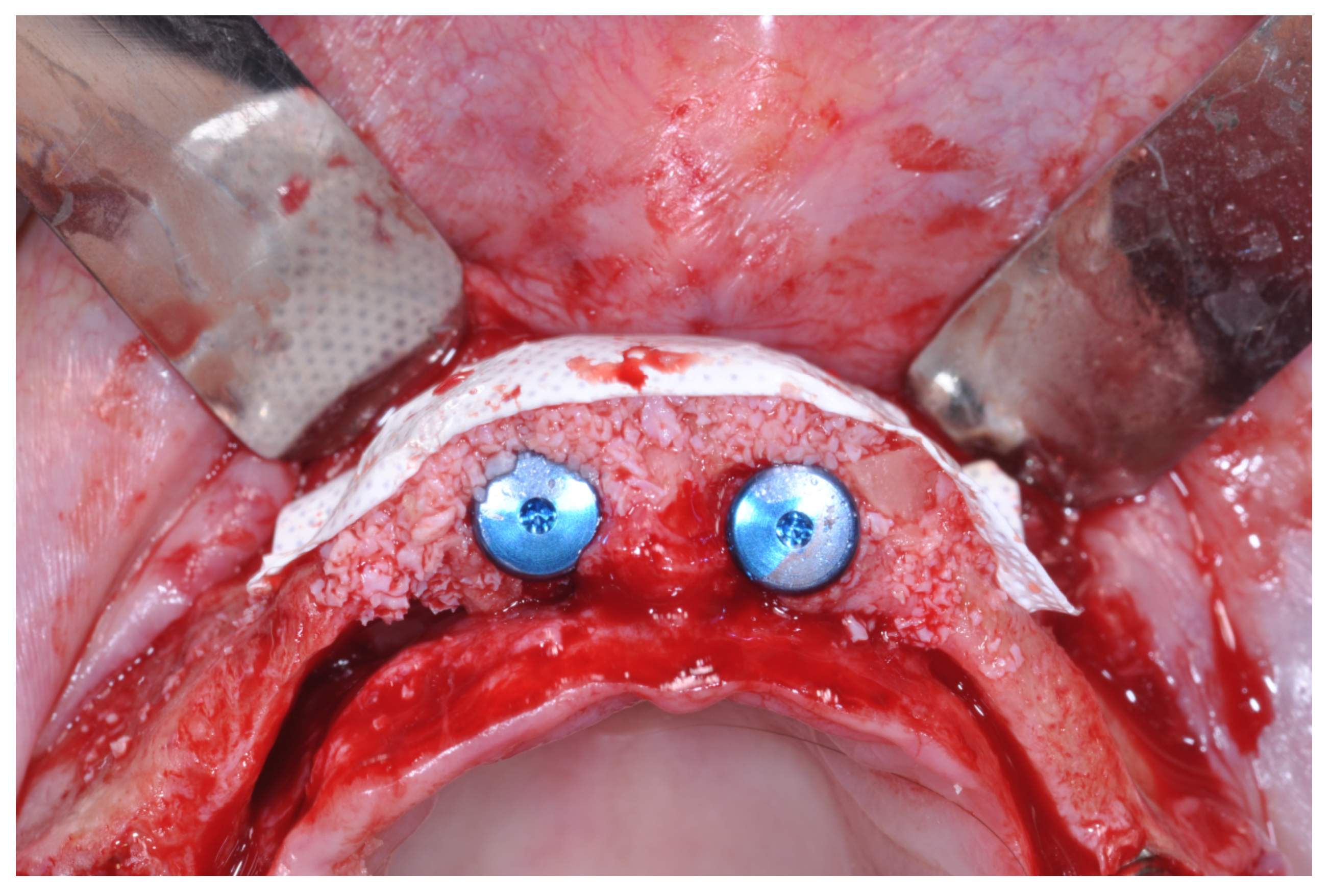
At this point, a non-resorbable high-density PTFE barrier (Cytoplast TXT-200, Osteogenics Biomedical, Lubbock, TX, USA) was designed on the basis of the bone defect shape and was distally blocked on the bone by two titanium pins (Kalos, Nike, Orbetello, Italy). A mixture of 50% autogenous bone harvested from the mandibular ramus with the use of a scraper and 50% inorganic bovine bone (Bio-Oss, Geistlich, Wolhusen, Switzerland) was placed in the recipient site and the membrane was pulled and blocked on the bone with two mesial pins. With this particular, and innovative, non-resorbable membrane management, the graft was compressed and fixed on the recipient bed (
Figure 9,
Figure 10 and
Figure 11). The occlusal portion of the graft was then covered with a layer of a collagen resorbable barrier (Bio-Gide, Geistlich) (
Figure 12) and peri-osteal incisions were performed to make the flap passive.
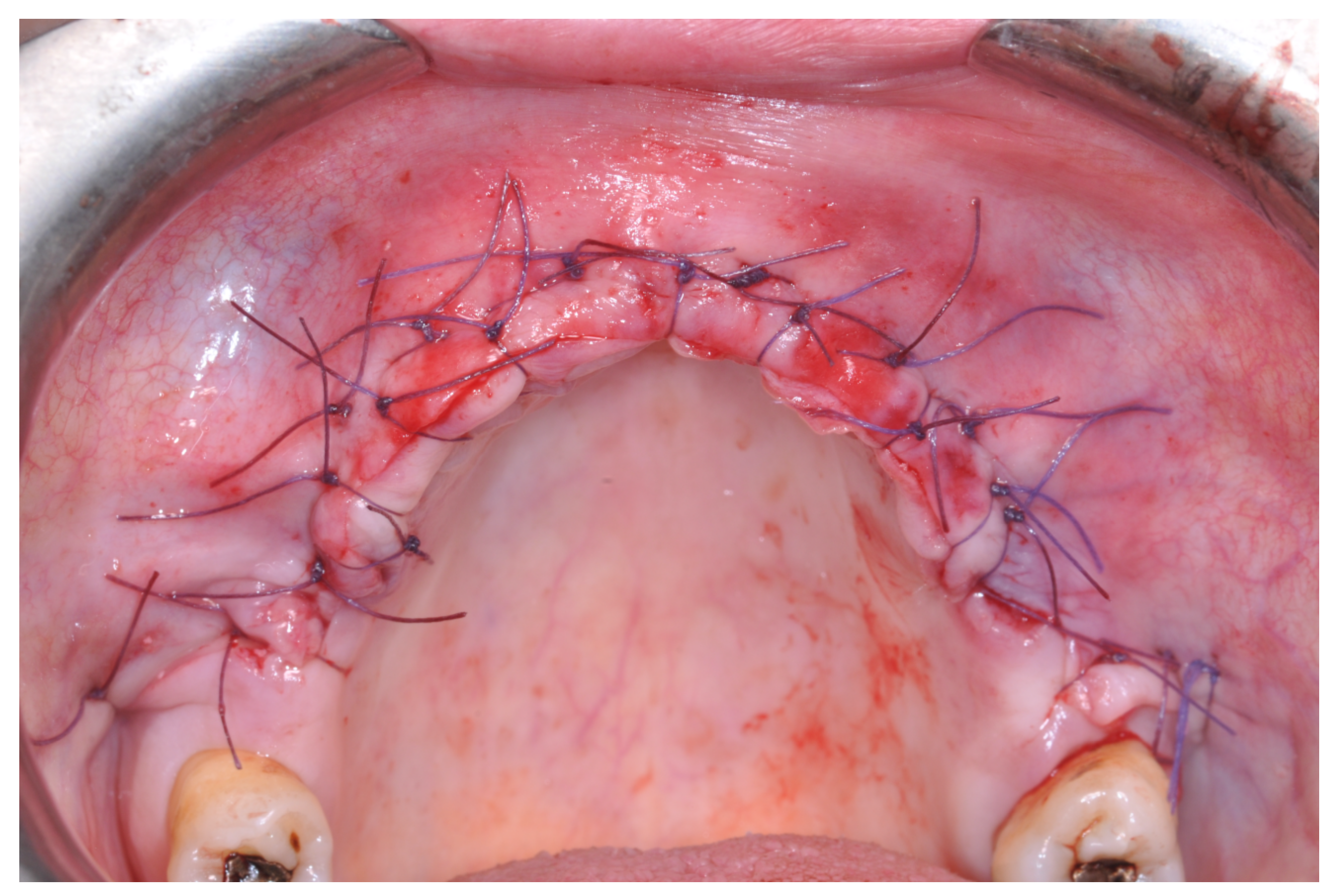
Resorbable sutures (Vicryl 6-0 SH1 needle of 17 mm 1/2c; Ethicon, New Brunswick, NJ, USA) were placed, alternating between single sutures and horizontal mattrass sutures (
Figure 13).
2.3. Post-Operative Management
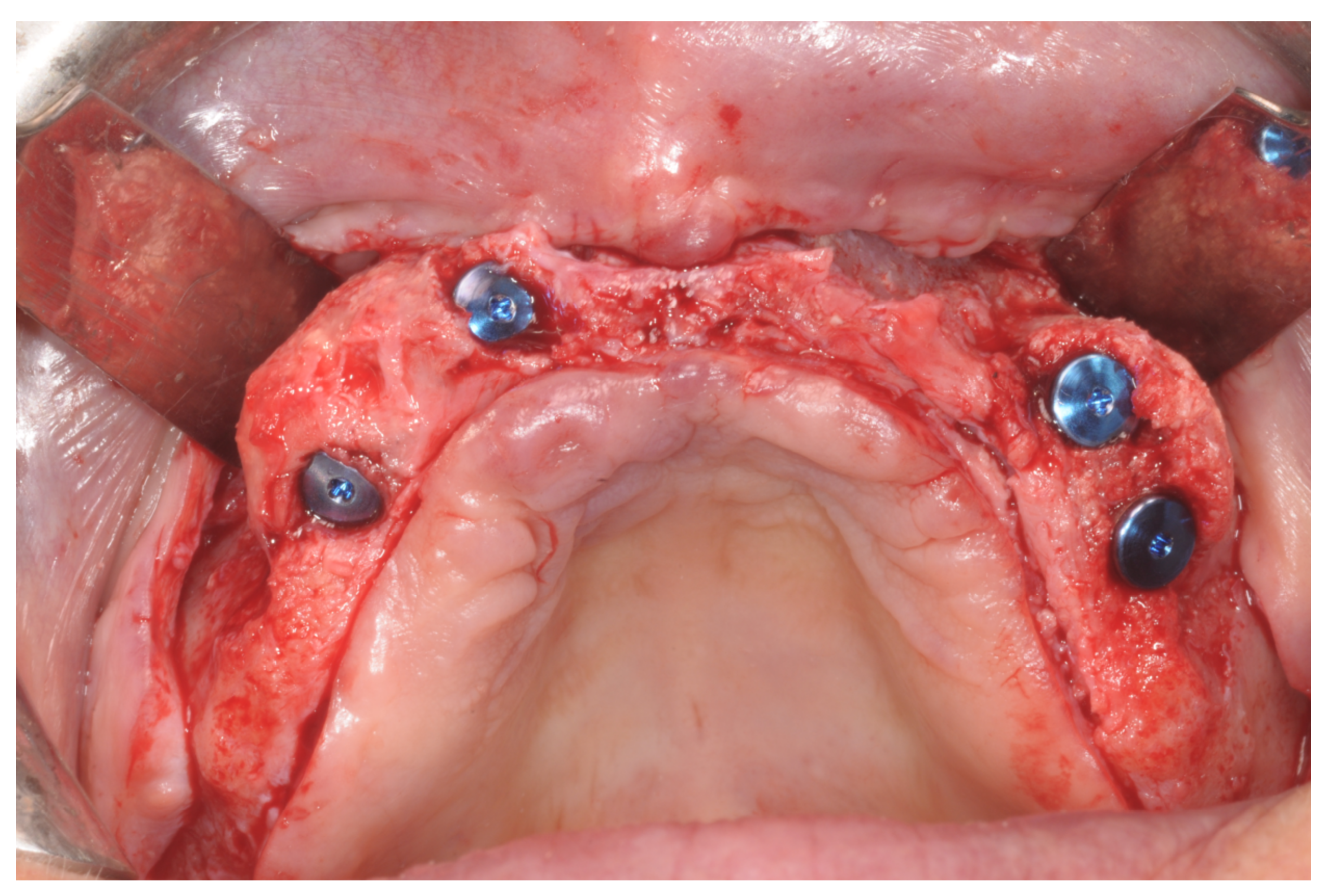
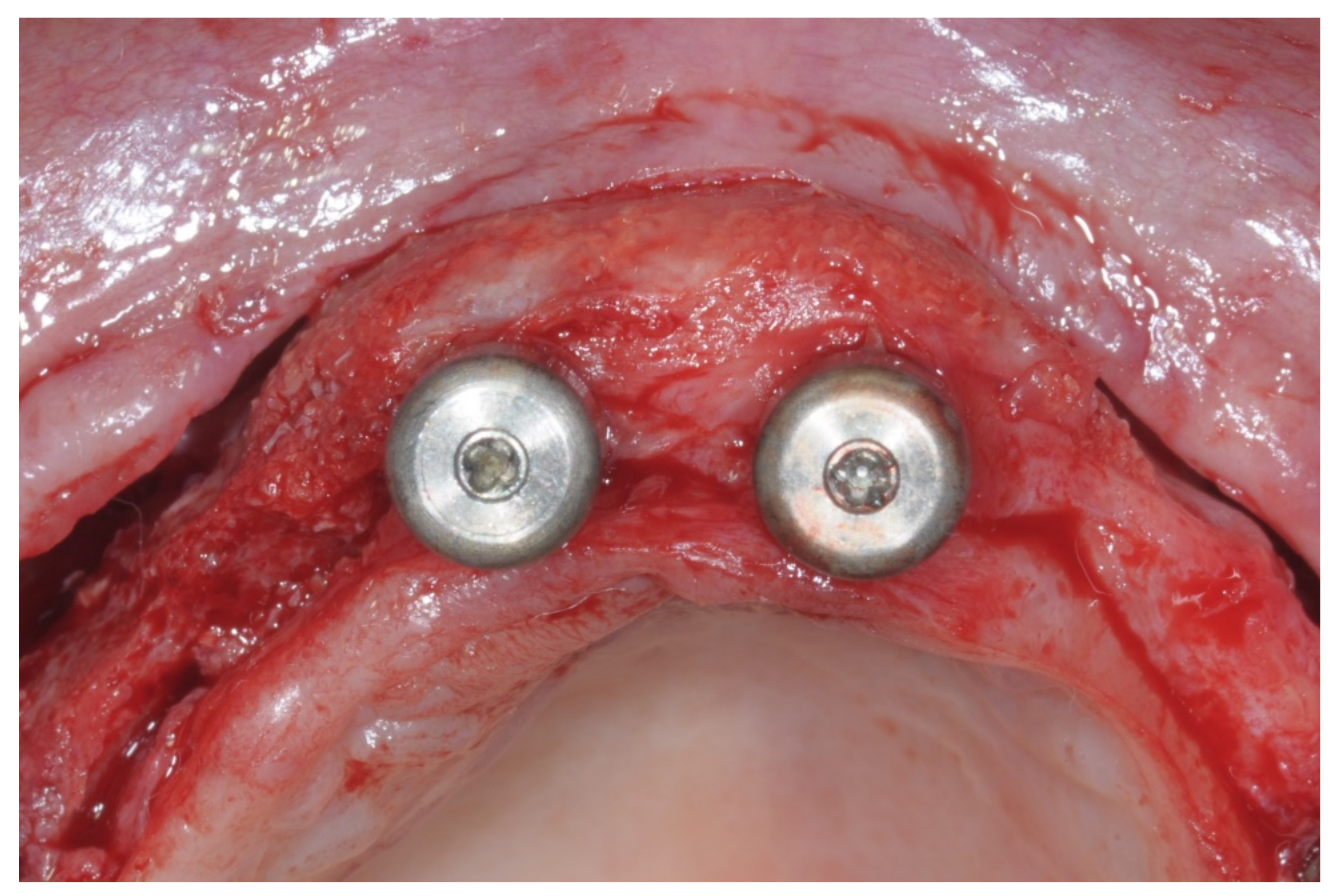
After surgery, the same antibiotic treatment was continued thrice a day for 3 days. Analgesics (ibuprofen 600 mg) were suggested to the patient to be taken twice a day during meals on demand. A soft diet was recommended until the removal of the sutures. Patients were recalled weekly for the first month and thereafter monthly to monitor the tissue healing. Patients were instructed to avoid brushing the surgical site and to rinse with 0.12% chlorhexidine mouthwash until the removal of the sutures, which was 12 days after the augmentation. After 8 to 9 months, healing screws were connected to the implants, which were surgically exposed (
Figure 14).
Impressions were taken 2 weeks after using individual trays, which were perforated to allow their seating over the transfers. Impregum F (Espe Dental, Seefeld, Germany) was the impression material [
19]. Within one week, the prosthesis framework was tried in the mouth of the patient to check the aesthetics, functions and phonetics. Metal–resin or metal–ceramic screw-retained prostheses were delivered [
13]. Intra-oral radiographs were taken to check the proper abutment seating on the implants. A group function occlusal scheme was made and the occlusion was adjusted in order to facilitate homogeneous occlusal contacts on the cantilevers when present. Oral hygiene instructions were delivered. Patients were seen again after 1 and 3 months. Patients were recalled for maintenance and occlusion checks every six months when the prostheses were removed to check implant stability and to clean them.
2.4. Outcome Measures
Any biological or prosthetic complications were grouped into three categories: (a) intra-operative and post-operative biological complications such as hemorrhages, wound dehiscence and fistulas; (b) biological complications in the maintenance such as peri-implant mucositis (heavily inflamed soft tissue without bone loss), peri-implantitis (bone loss with suppuration or heavily inflamed tissues) or fistulas; and (c) prosthetic complications such as a fracture of the implant, an abutment of the screw, the framework or the detachment of resin teeth.
Augmentation procedure failures were subjectively evaluated by the operator as an insufficient bone augmentation procedure compromising both implant placement and/or the aesthetic outcome.
Prosthesis failure was defined as a fixed prosthesis lost due to implant failure(s) or that had to be replaced for any reason.
Implant failure was defined as the presence of any mobility of the individual implant and/or any infection dictating the implant removal and/or biomechanical complications (implant fractures or deformations of the connections) rendering the implant unusable. Individual implant stability was measured 1 month and 3 months after the implant placement and thereafter every 6 months by removing the screw-retained prosthesis and rocking the implant with the handles of two instruments.
This retrospective case series aimed to include any patient treated with the “sling technique” for horizontal GBR. No sample size calculation was performed with a follow-up of at least 2 years after the augmentation. The patient was the statistical unit of the analyses. Descriptive statistics were used.
3. Results
A total of 10 edentulous patients aged from 50 to 74 years old (mean age at test, 61 years; 6 women (60%), 4 men (40%)) were enrolled on our study. Patients received 4 to 5 implants each, 11 mm long and 4 to 4.5 mm wide; 10 full-arch prostheses were delivered onto 43 implants, 33 of which were in regenerated bone.
In 9 out of 10 patients, the implants were inserted at the same time as regeneration and loaded 8–9 months later. Only in 1 patient were the implants placed 9 months after the augmentation procedure.
The follow-up focused on the time augmentation to January 2022. No patient dropped out. Three patients had various medical conditions (hypertension, controlled diabetes and a prolapse of the mitral valve) and one patient smoked more than ten cigarettes per day.
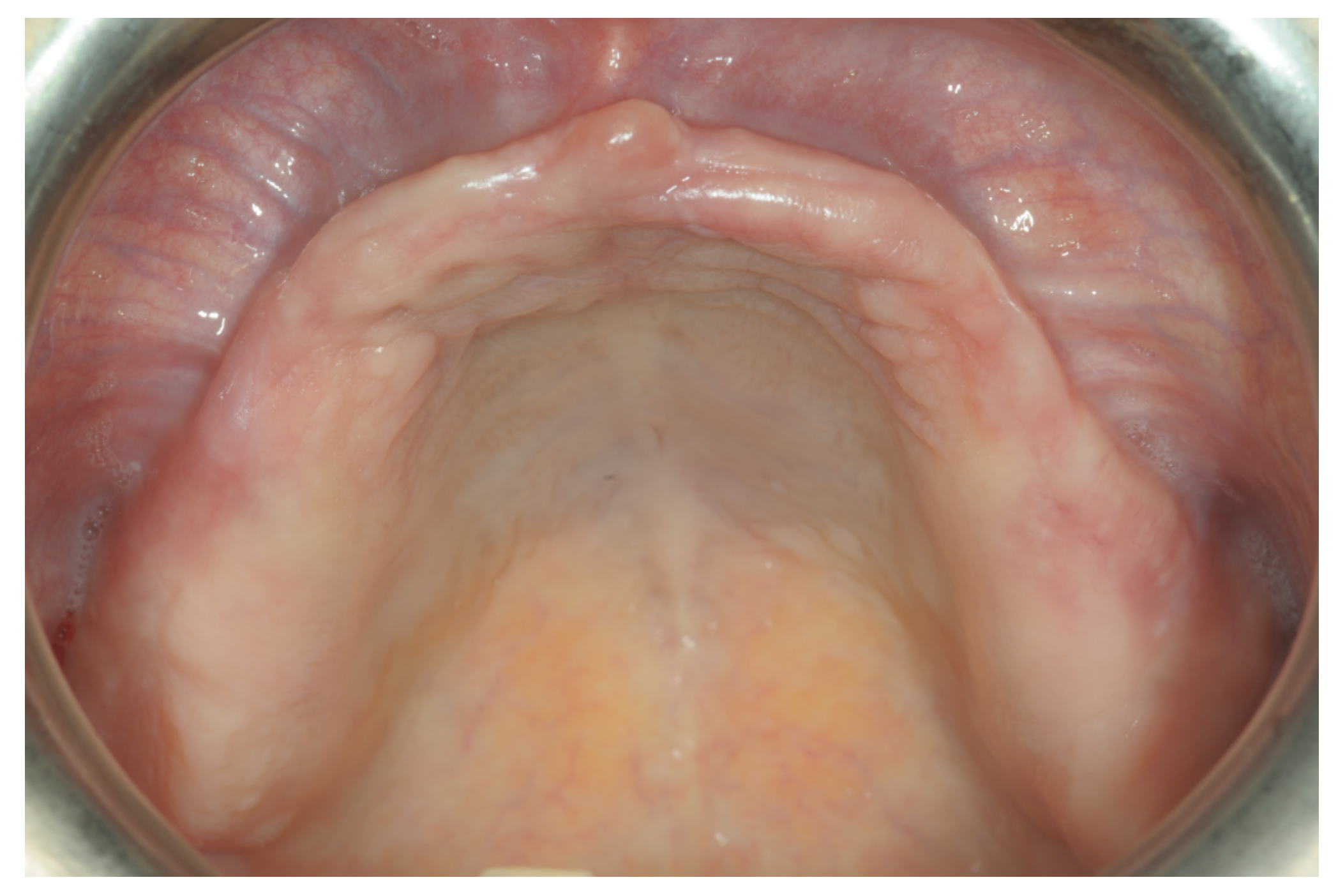
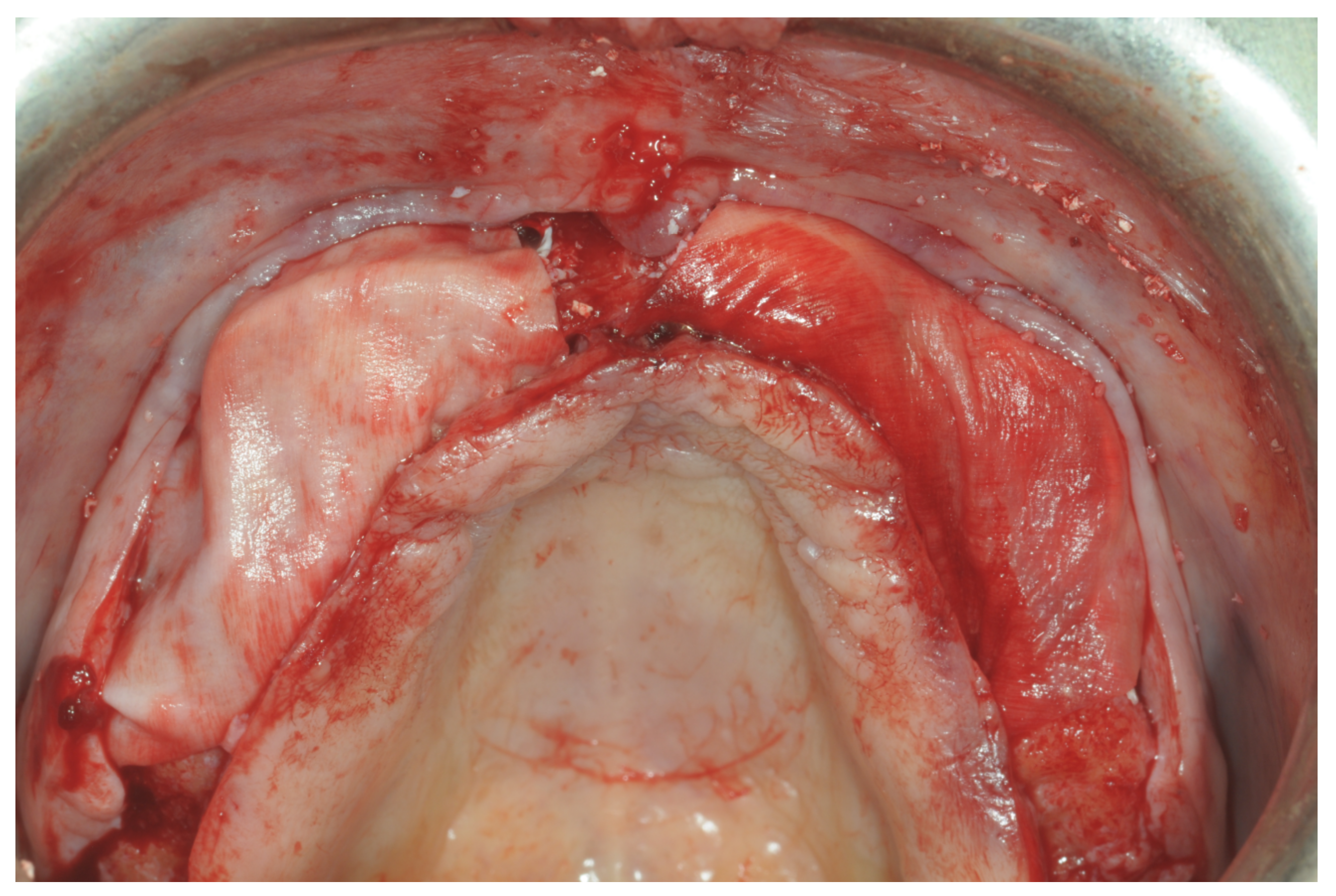
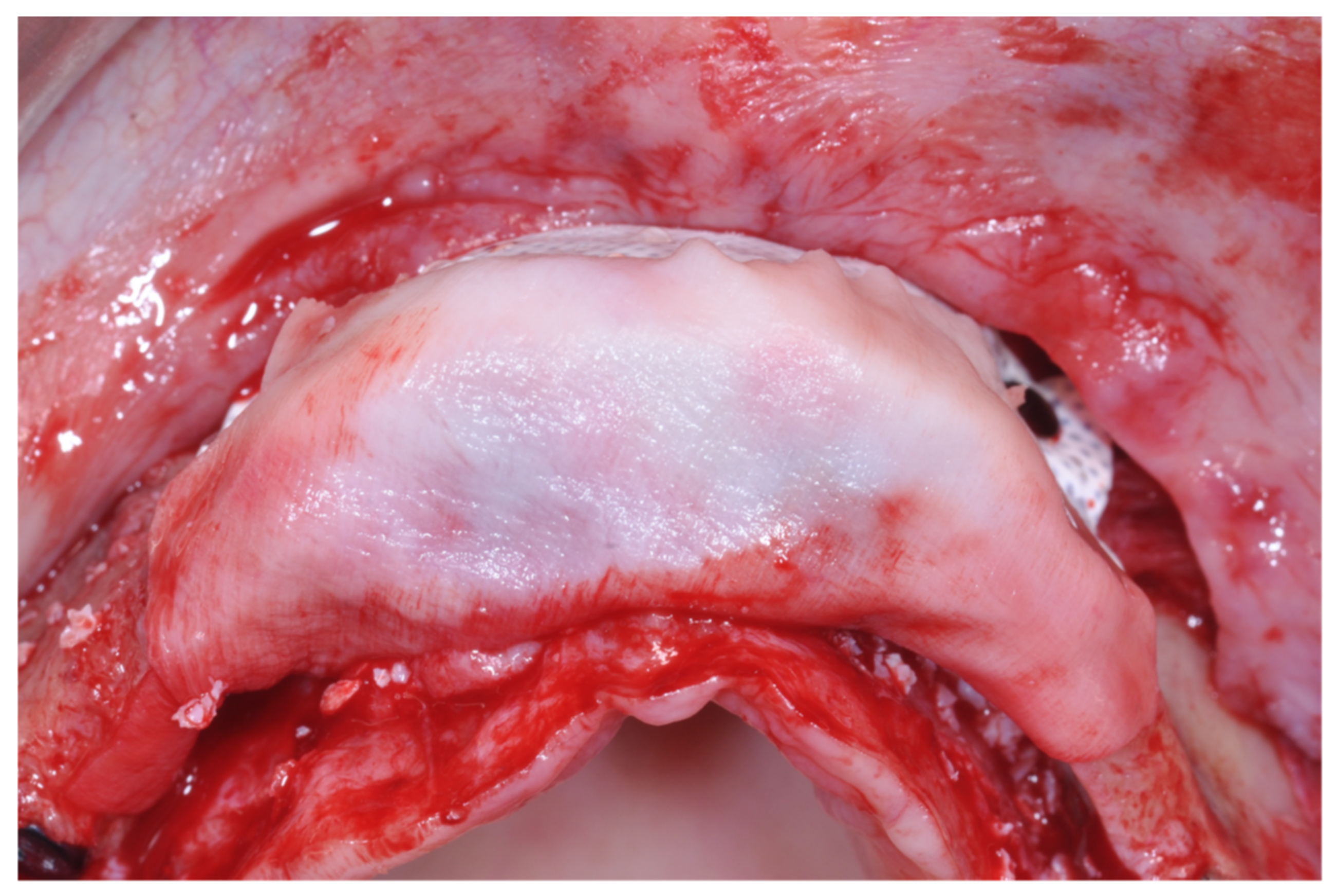
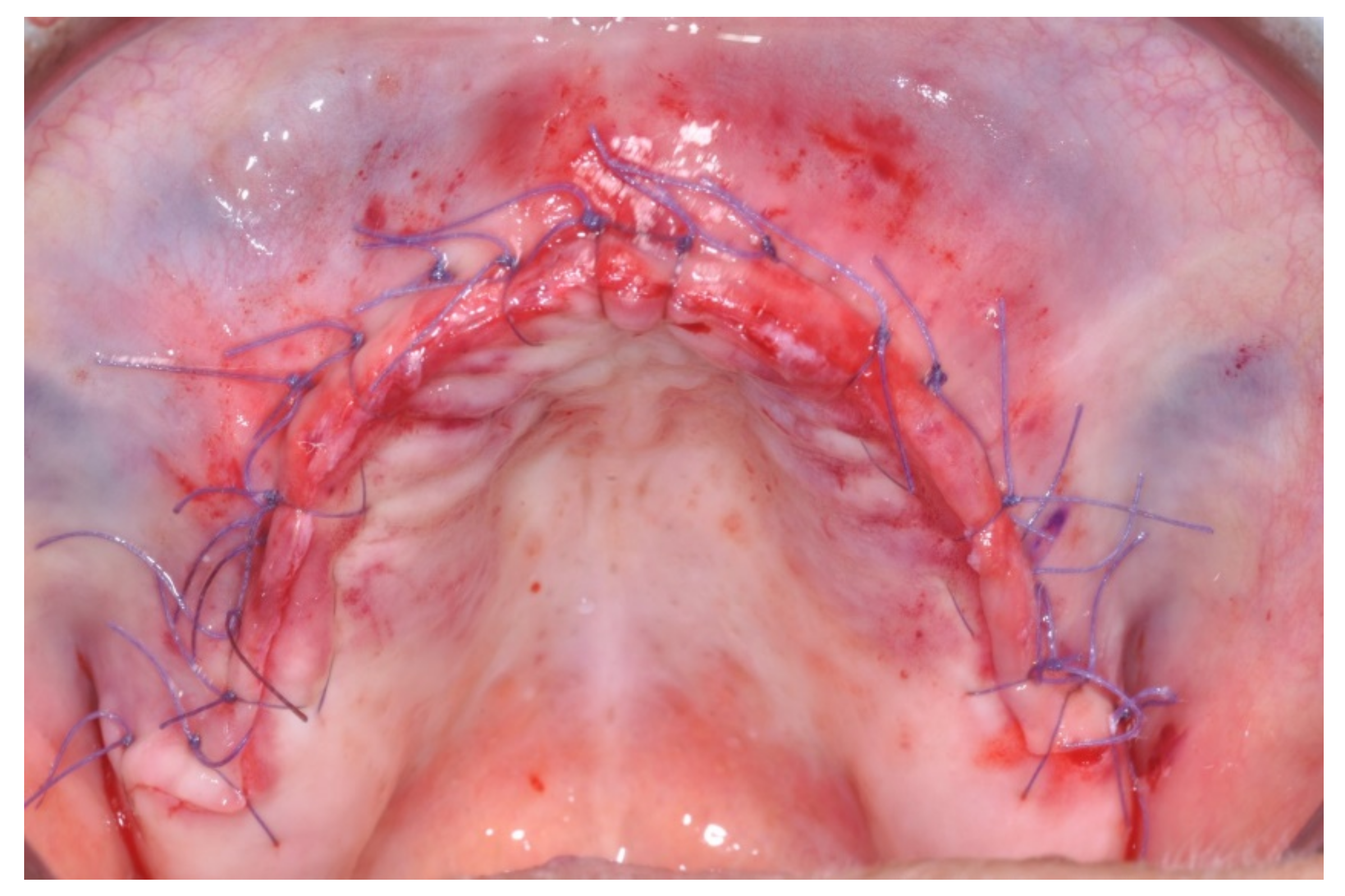
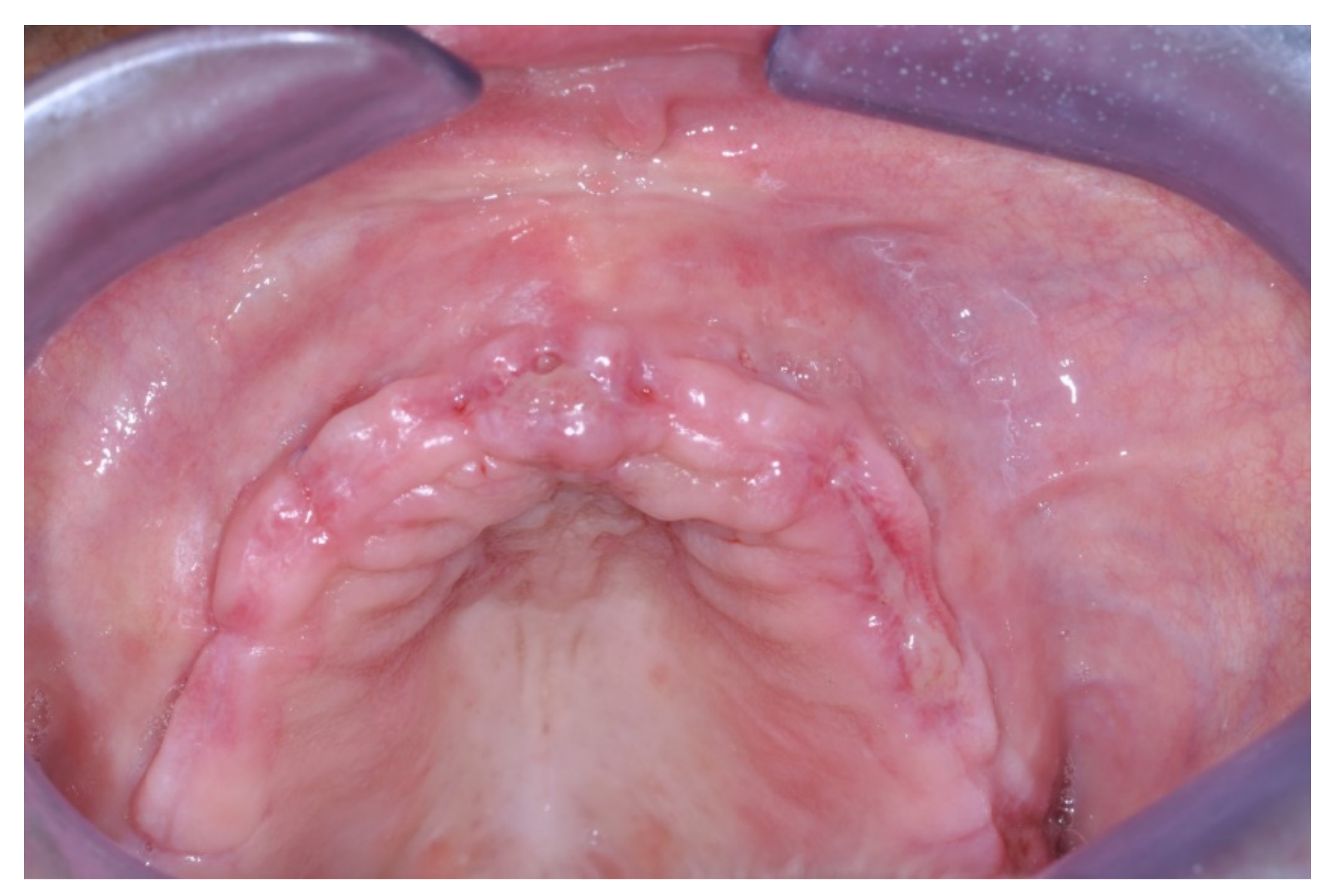
Only one minor post-operative complication occurred. It could be described as poor wound healing on the second quadrant (
Figure 15,
Figure 16 and
Figure 17), which healed without an intervention.
Healing of the sites occurred undisturbed and no biological or prosthetic complications occurred. All 33 implants placed in the regenerated sites with the “sling technique” showed 100% survival after a follow-up of more than 24 months.
After the regenerative procedure, all implants were surrounded by at least 2 mm of bone tissue.
No augmentation, implant or prosthesis failure occurred.
4. Discussion
Tooth loss and consecutive bone resorption depend on local and systemic agents and also involve psychological factors. In the elderly, depression can be more severe due to edentulous jaws and missing or decayed teeth as well as oral dryness. Therefore, people with depression or other systemic disorders may have impaired oral health [
20,
21,
22].
Guided bone regeneration (GBR) is a procedure used with the rationale to increase alveolar bone volume in areas designated for future implant placement or around previously placed implants that were deficient or inadequate. To obtain predictable results, both considerable surgical skills and the full understanding of the biological mechanism that rules this technique are mandatory [
23]. In particular, primary wound closures are required to ensure undisturbed (by external bacteria or other external injuries) wound healing as well as angiogenesis (to ensure an adequate blood supply with nutrients and bone growth factors), space maintenance/creation (to allow bone cells to grow and mature whilst excluding the faster proliferation of undesired soft tissues and to guide the bone regeneration into the detected position) and the stability of the wound (to improve the blood clot formation) [
23,
24].
Although it is a well-established technique, it is a procedure that is sensitive to the technique used [
25,
26,
27,
28]. Several bone regeneration techniques have been described such as using resorbable or non-resorbable barrier membranes [
28]. The crucial element for the success of all of them is the achievement of a good vascularization, ensuring blood supply during surgery [
26]. To reach this goal, an experienced operator is required. Several steps of these techniques, such as tension-free flap closure and the stability of the graft, need practical skills that can only be acquired after a long learning curve [
25].
In addition, numerous post-surgical complications have been described following a GBR procedure, which in most cases can compromise the outcome [
10,
29].
A recent systematic review highlighted that the most common post-surgical complication that occurs after GBR surgery is wound dehiscence, with an incidence rate of 9.9% (95% CI 6.4, 13.9,
p < 0.01). This adverse healing results in the early or late exposure of the membrane as well as contamination, infection and the partial or total loss of the graft, with a significant negative effect on bone formation gain [
30].
In a recent systematic review, Thoma et al. reported an overall membrane exposure rate of 22.7% in simultaneous GBR procedures with no significant difference between resorbable and non-resorbable membranes [
29]. These results were also confirmed by another systematic review that showed no differences in post-surgical complications based on the type of membrane used [
30].
With our case series, we aimed to report on an innovative use of the e-PTFE membrane to maximize the performance of the non-resorbable membranes and to reduce the risk of biological complications related to its exposure. Following the application of the PASS principles to perform predictable GBR procedures, one of the key factors is to stabilize the membrane and, consequently, the underlying graft materials [
23]. The primary stabilization of the graft materials is mandatory to obtain a successful regenerative procedure because micromotion of the bone particulate can lead to osteogenesis with the encapsulation of fibrous tissue [
23]. For this reason, with our “sling technique”, we maximized the compression of the graft material by stretching the non-resorbable e-PTFE membrane, pulling it from distal to mesial and fixing it with pins. In this way, the stability of blood clots improved; this is a necessary condition to recruit cytokines and growth factors as well as all the cells required to initiate the complex phenomena of bone formation and remodeling. Moreover, space maintenance is mandatory to permit the proliferation of bone-forming cells whilst excluding unwanted epithelial and connective tissue cells. The use of non-resorbable e-PTFE allows better results to be achieved when compared with resorbable membranes [
31].
For these reasons, is our opinion that a significant increase in the stabilization of blood clots and the graft material placed in the recipient site was responsible for the bone gain obtained with the “sling technique”.
No membrane exposures were detected in any of the ten patients although one minor post-operative delayed healing occurred. To minimize the risk of typical post-surgical complications linked to membrane exposure, we placed the membrane only on the vestibular side, far away from the surgical incision of the regeneration area. In this way, we avoided infectious risks linked to membrane exposure.
A review by Soldatos et al. analyzed the different types of membranes and described their limitations and methods of use. This review concluded that the most common and complex-to-manage complication of non-resorbable membranes is exposure [
31].
In the case of exposure of a non-resorbable membrane, an e-PTFE membrane has a much higher risk of complications and infections than a d-PTFE membrane [
32].
Moreover, membrane exposure during a bone augmentation procedure can compromise the amount of regeneration [
33].
After an observational period of 24 months, all 43 implants placed at the same time as the bone augmentation were completely surrounded by regenerated bone and healthy soft tissue; the survival rate was 100%.
An adequate bone thickness around the implant is mandatory to amplify the long-term success rate of an implant. Following the results of a study conducted by Spray et al., the vestibular cortical bone loss after an implant placement was greatest when the initial vestibular cortical thickness was less than 1.4 mm; it decreased to a true possibility of bone gain when the thickness increased to approximately 1.8–2 mm [
34].
Computer-guided implant surgery through 3D printed guides is a safe and feasible instrument to accurately place an implant taking into account bone thickness. Computer-guided implant placement is predictable and precise, especially when careful pre-operative digital planning is performed [
35].
Due to its nature, the “sling technique” is only applicable to horizontal GBR procedures and not to vertical GBRs, which are more risky and demanding procedures. In vertical GBRs, the membranes are required to cover a much larger bone area, which inevitably ends under the surgical incision line. Moreover, in vertical bone defects, it is necessary to increase the maintenance of the space; this can be achieved through the use of non-resorbable Ti-reinforced membranes or tenting screws [
36].
The “sling technique” does not constitute a contraindication for future orthodontic treatments. In this case series, the technique was used only on fully edentulous patients; the authors intend to evaluate the possibility of treating other clinical situations in a future study.
Limitations of the Study
One of the limitations of this new technique is the dimension of the commercialized non-resorbable barriers. We believe that the larger commercially available format (25 × 30 mm) may not be sufficient when a full hemiarch has to be regenerated (
Figure 4 and
Figure 5). The possibility of using a single larger membrane would facilitate the creation of a more homogeneous bone profile in a hemiarch, which we were able to achieve (
Figure 7). Therefore, this is a suggestion to the membrane manufacturers to also distribute membranes of larger dimensions.
The small number of treated patients and the lack of an adequate control may not allow us to draw any definitive conclusions on this procedure. Only one minor post-surgical complication occurred and the amount of bone regenerated was adequate to allow a successful implant placement as well as a related chewing function and improved aesthetics for the patient.