Uncovering the Use of Fucoxanthin and Phycobiliproteins into Solid Matrices to Increase Their Emission Quantum Yield and Photostability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of R-PE Extracts
2.2.2. Preparation of C-PC Extracts
2.2.3. Preparation of FX Ethanolic Extracts
2.2.4. Purification of R-PE and C-PC Aqueous Extracts
2.2.5. Incorporation of R-PE, C-PC, and FX in Host Solid Matrices
2.2.6. Optical Characterization
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ferreira, R.A.S.; Correia, S.F.H.; Monguzzi, A.; Liu, X.; Meinardi, F. Spectral converters for photovoltaics—What’s ahead. Mater. Today 2020, 33, 105–121. [Google Scholar] [CrossRef] [Green Version]
- McKenna, B.; Evans, R.C. Towards efficient spectral converters through materials design for luminescent solar devices. Adv. Mater. 2017, 29, 1606491. [Google Scholar] [CrossRef]
- Jain, R.; Mathur, M.; Sikarwar, S.; Mittal, A. Removal of the hazardous dye rhodamine B through photocatalytic and adsorption treatments. J. Environ. Manag. 2007, 85, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, S.; Melikov, R.; Jalali, H.B.; Karatum, O.; Srivastava, S.B.; Conkar, D.; Firat-Karalar, E.N.; Nizamoglu, S. Ecofriendly and Efficient Luminescent Solar Concentrators Based on Fluorescent Proteins. ACS Appl. Mater. Inter. 2019, 11, 8710–8716. [Google Scholar] [CrossRef] [PubMed]
- Correia, S.F.; Bastos, A.R.; Martins, M.; Macário, I.P.; Veloso, T.; Pereira, J.L.; Coutinho, J.A.; Ventura, S.P.; André, P.S.; Ferreira, R.A. Bio-based solar energy harvesting for onsite mobile optical temperature sensing in smart cities. Adv. Sci. 2022, 2104801. [Google Scholar] [CrossRef]
- Hernández-Rodríguez, M.A.; Correia, S.F.H.; Ferreira, R.A.S.; Carlos, L.D. A perspective on sustainable luminescent solar concentrators. J. Appl. Phys. 2022, 131, 140901. [Google Scholar] [CrossRef]
- Weber, W.H.; Lambe, J. Luminescent greenhouse collector for solar radiation. Appl. Opt. 1976, 15, 2299–2300. [Google Scholar] [CrossRef]
- Meinardi, F.; Bruni, F.; Brovelli, S. Luminescent solar concentrators for building-integrated photovoltaics. Nat. Rev. Mater. 2017, 2, 17072. [Google Scholar] [CrossRef]
- Reisfeld, R.; Neuman, S. Planar solar-energy converter and concentrator based on uranyl-doped glass. Nature 1978, 274, 144–145. [Google Scholar] [CrossRef]
- Yang, C.C.; Atwater, H.A.; Baldo, M.A.; Baran, D.; Barile, C.J.; Barr, M.C.; Bates, M.; Bawendi, M.G.; Bergren, M.R.; Borhan, B.; et al. Consensus statement: Standardized reporting of power-producing luminescent solar concentrator performance. Joule 2022, 6, 8–15. [Google Scholar] [CrossRef]
- Debije, M.G.; Evans, R.C.; Griffini, G. Laboratory protocols for measuring and reporting the performance of luminescent solar concentrators. Energ. Environ. Sci. 2021, 14, 293–301. [Google Scholar] [CrossRef]
- Debije, M.G.; Rajkumar, V.A. Direct versus indirect illumination of a prototype luminescent solar concentrator. Sol. Energy 2015, 122, 334–340. [Google Scholar] [CrossRef]
- Mulder, C.L.; Theogarajan, L.; Currie, M.; Mapel, J.K.; Baldo, M.A.; Vaughn, M.; Willard, P.; Bruce, B.D.; Moss, M.W.; McLain, C.E.; et al. Luminescent Solar Concentrators Employing Phycobilisomes. Adv. Mater. 2009, 21, 3181–3185. [Google Scholar] [CrossRef]
- Bose, R.; Gonzalez, M.; Jenkins, P.; Walters, R.; Morseman, J.; Moss, M.; McLain, C.; Linsert, P.; Buchtemann, A.; Chatten, A.J.; et al. Resonance Energy Transfer in Luminescent Solar Concentrators. In Proceedings of the 35th IEEE Photovoltaic Specialists Conference, Honolulu, HI, USA, 20–25 June 2010. [Google Scholar]
- Frias, A.R.; Correia, S.F.H.; Martins, M.; Ventura, S.P.M.; Pecoraro, E.; Ribeiro, S.L.; Andre, P.S.; Ferreira, R.A.S.; Coutinho, J.A.P.; Carlos, L.D. Sustainable liquid luminescent solar concentrators. Adv. Sustain. Syst. 2019, 3, 1800134. [Google Scholar] [CrossRef]
- Frias, A.R.; Pecoraro, E.; Correia, S.F.H.; Minas, L.M.G.; Bastos, A.R.; Garcia-Revilla, S.; Balda, R.; Ribeiro, S.J.L.; Andre, P.S.; Carlos, L.D.; et al. Sustainable luminescent solar concentrators based on organic-inorganic hybrids modified with chlorophyll. J. Mater. Chem. A 2018, 6, 8712–8723. [Google Scholar] [CrossRef]
- Carlos, C.P.A.; Correia, S.F.H.; Martins, M.; Savchuk, O.A.; Coutinho, J.A.P.; André, P.S.; Nieder, J.B.; Ventura, S.P.M.; Ferreira, R.A.S. Environmentally friendly luminescent solar concentrators based on optically efficient and stable green fluorescent protein. Green. Chem. 2020, 22, 4943–4951. [Google Scholar] [CrossRef]
- Bharmoria, P.; Correia, S.F.H.; Martins, M.; Hernández-Rodríguez, M.A.; Ventura, S.P.M.; Ferreira, R.A.S.; Carlos, L.D.; Coutinho, J.A.P. Protein Co-habitation: Improving the Photo-Chemical Stability of R-Phycoerythrin in Solid State. J. Phys. Chem. Lett. 2020, 11, 6249–6255. [Google Scholar] [CrossRef]
- Keeling-Tucker, T.; Brennan, J.D. Fluorescent probes as reporters on the local structure and dynamics in sol-gel-derived nanocomposite materials. Chem. Mater. 2001, 13, 3331–3350. [Google Scholar] [CrossRef]
- Chen, Z.P.; Samuelson, L.A.; Akkara, J.; Kaplan, D.L.; Gao, H.; Kumar, J.; Marx, K.A.; Tripathy, S.K. Sol-Gel Encapsulated Light-Transducing Protein Phycoerythrin—A New Biomaterial. Chem. Mater. 1995, 7, 1779–1783. [Google Scholar] [CrossRef]
- Keeling-Tucker, T.; Rakic, M.; Spong, C.; Brennan, J.D. Controlling the material properties and biological activity of lipase within sol-gel derived bioglasses via organosilane and polymer doping. Chem. Mater. 2000, 12, 3695–3704. [Google Scholar] [CrossRef]
- Chen, Z.P.; Kaplan, D.L.; Yang, K.; Kumar, J.; Marx, K.A.; Tripathy, S.K. Phycobiliproteins encapsulated in sol-gel glass. J. Sol-Gel Sci. Technol. 1996, 7, 99–108. [Google Scholar] [CrossRef]
- Pradeep, H.N.; Nayak, C.A. Enhanced stability of C-phycocyanin colorant by extrusion encapsulation. J. Food Sci. Techol. 2019, 56, 4526–4534. [Google Scholar] [CrossRef]
- Wang, X.B.; Li, Z.Y.; Ying, W.; Chen, D.K.; Li, P.P.; Deng, Z.; Peng, X.S. Blue metal-organic framework encapsulated denatured R-phycoerythrin proteins for a white-light-emitting thin film. J. Mater. Chem. C 2020, 8, 240–246. [Google Scholar] [CrossRef]
- Sui, Y.; Gu, Y.; Lu, Y.J.; Yu, C.X.; Zheng, J.; Qi, H. Fucoxanthin@Polyvinylpyrrolidone Nanoparticles Promoted Oxidative Stress-Induced Cell Death in Caco-2 Human Colon Cancer Cells. Mar. Drugs 2021, 19, 92. [Google Scholar] [CrossRef]
- Jaswir, I.; Noviendri, D.; Taher, M.; Mohamed, F.; Octavianti, F.; Lestari, W.; Mukti, A.G.; Nirwandar, S.; Almansori, B.B.H. Optimization and Formulation of Fucoxanthin-Loaded Microsphere (F-LM) Using Response Surface Methodology (RSM) and Analysis of Its Fucoxanthin Release Profile. Molecules 2019, 24, 947. [Google Scholar] [CrossRef] [Green Version]
- Nichols, H.W. Growth media—Freshwater. In Handbook of Phycological Methods: Culture Methods and Growth Measurements; Stein, J.R., Ed.; Cambridge University Press: Cambridge, UK, 1973; pp. 7–24. [Google Scholar]
- Vaz, B.M.C.; Martins, M.; Mesquita, L.M.D.; Neves, M.C.; Fernandes, A.P.M.; Pinto, D.C.G.A.; Neves, M.G.P.M.S.; Coutinho, J.A.P.; Ventura, S.P.M. Using aqueous solutions of ionic liquids as chlorophyll eluents in solid-phase extraction processes. Chem. Eng. J. 2022, 428, 131073. [Google Scholar] [CrossRef]
- Bai, X.; Caputo, G.; Hao, Z.; Freitas, V.T.; Zhang, J.; Longo, R.L.; Malta, O.L.; Ferreira, R.A.S.; Pinna, N. Efficient and tuneable photoluminescent boehmite hybrid nanoplates lacking metal activator centres for single-phase white LEDs. Nat. Commun. 2014, 5, 5702. [Google Scholar] [CrossRef] [Green Version]
- Martins, M.; Vieira, F.A.; Correia, I.; Ferreira, R.A.S.; Abreu, H.; Coutinho, J.A.P.; Ventura, S.P.M. Recovery of phycobiliproteins from the red macroalga Gracilaria sp using ionic liquid aqueous solutions. Green. Chem. 2016, 18, 4287–4296. [Google Scholar] [CrossRef]
- Rowan, K.S. Photosynthetic Pigments of Algae, 1st ed.; Cambridge University Press Archive: Cambridge, UK, 1989. [Google Scholar]
- Egorov, V.V. Theory of the J-band: From the Frenkel exciton to charge transfer. Phys. Procedia 2009, 2, 223–326. [Google Scholar] [CrossRef] [Green Version]
- Stryjewski, W.; Barkley, M.D. Solid-State Fluorescence Lifetime Measurements. Time-Resolv. Laser Spectrosc. Biochem. II 1990, 1204, 244–246. [Google Scholar] [CrossRef]
- Glazer, A.N. Light Harvesting by Phycobilisomes. Annu. Rev. Biophys. Biol. 1985, 14, 47–77. [Google Scholar] [CrossRef] [PubMed]

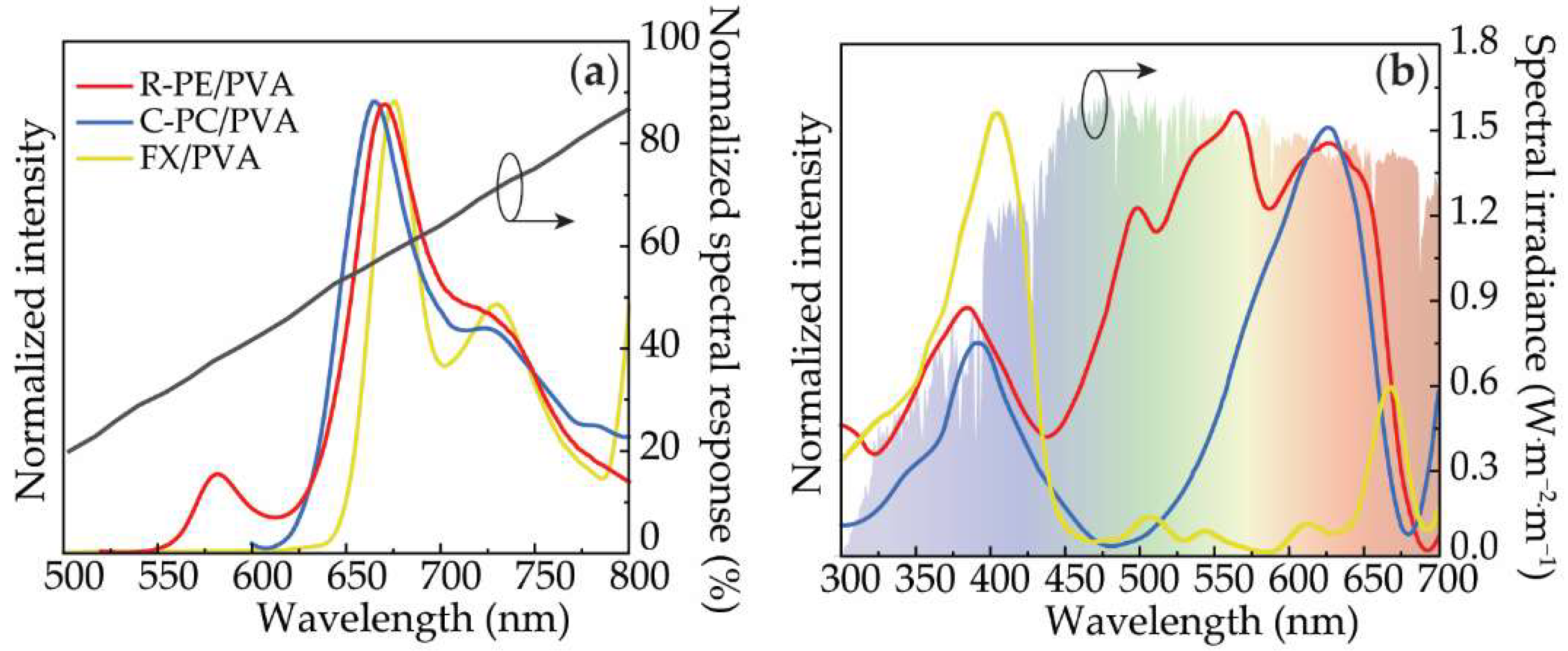
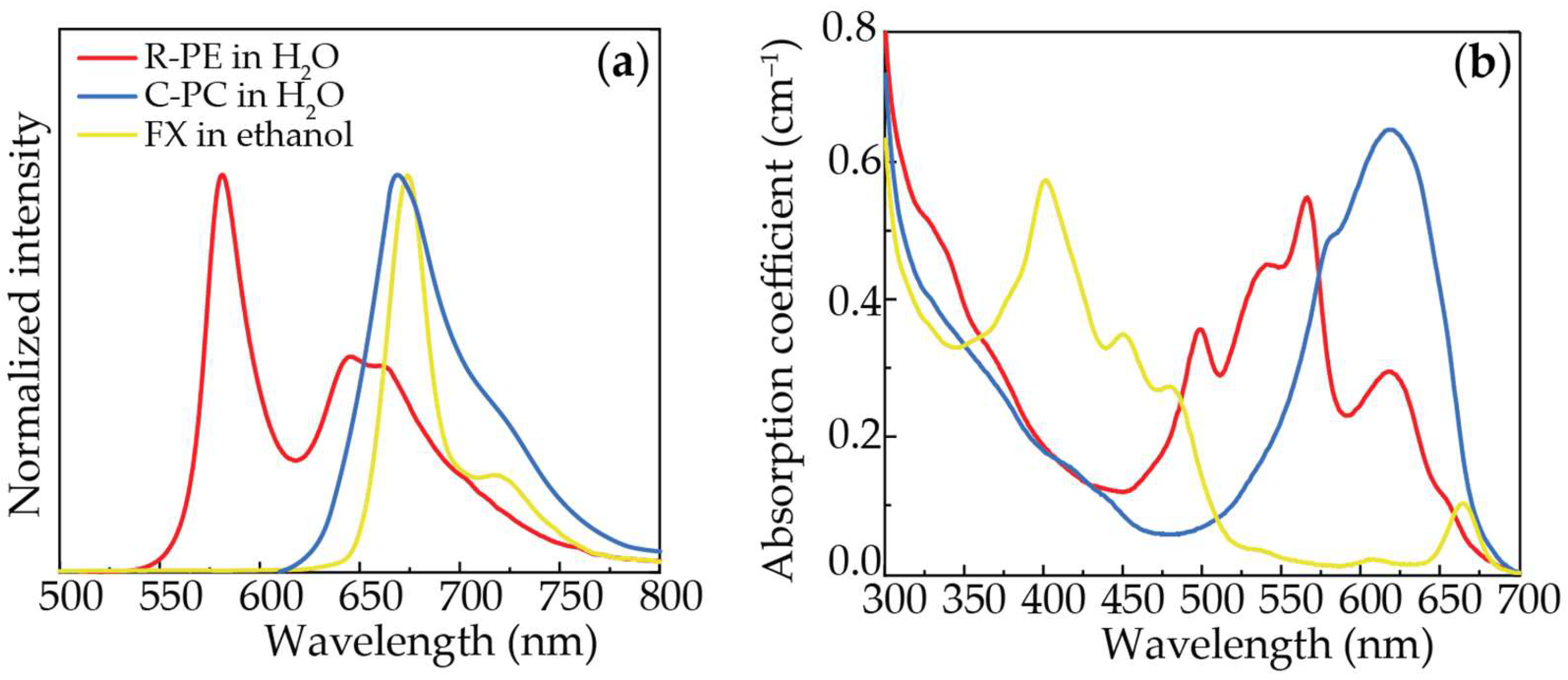
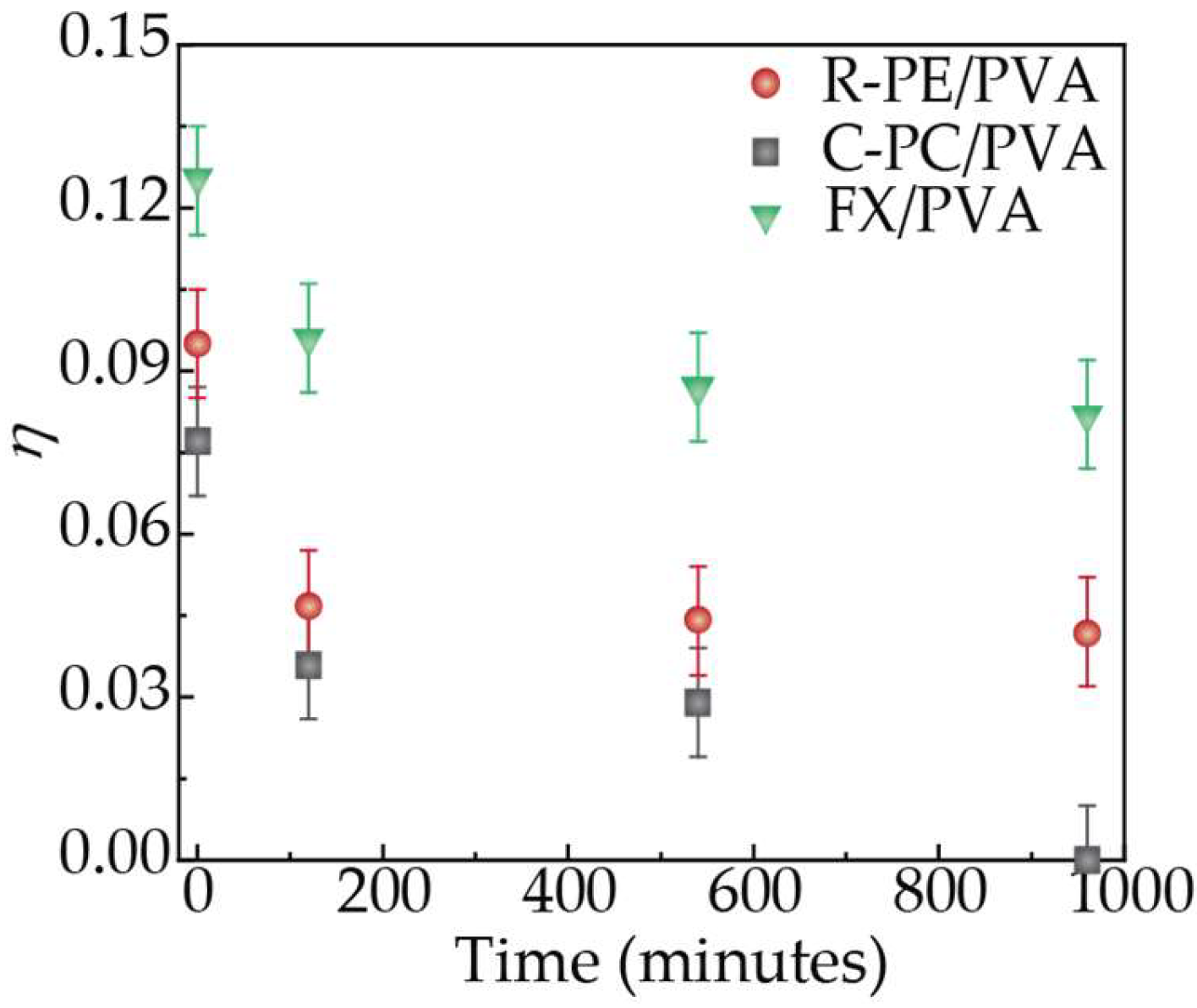
| Sample | λexc (nm) | η | τ (ns) |
|---|---|---|---|
| R-PE/PVA | 498 | 0.11 ± 0.01 | 3.74 ± 0.03 |
| C-PC/PVA | 580 | 0.09 ± 0.01 | 4.30 ± 0.04 |
| FX/PVA | 610 | 0.10 ± 0.01 | 6.68 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dias, L.M.S.; Kovaleski, G.; Fu, L.; Dias, T.R.; Macário, I.P.E.; Correia, S.F.H.; Pereira, J.L.; Coutinho, J.A.P.; Ventura, S.P.M.; Ferreira, R.A.S. Uncovering the Use of Fucoxanthin and Phycobiliproteins into Solid Matrices to Increase Their Emission Quantum Yield and Photostability. Appl. Sci. 2022, 12, 5839. https://doi.org/10.3390/app12125839
Dias LMS, Kovaleski G, Fu L, Dias TR, Macário IPE, Correia SFH, Pereira JL, Coutinho JAP, Ventura SPM, Ferreira RAS. Uncovering the Use of Fucoxanthin and Phycobiliproteins into Solid Matrices to Increase Their Emission Quantum Yield and Photostability. Applied Sciences. 2022; 12(12):5839. https://doi.org/10.3390/app12125839
Chicago/Turabian StyleDias, Lília M. S., Gabriela Kovaleski, Lianshe Fu, Tânia R. Dias, Inês P. E. Macário, Sandra F. H. Correia, Joana L. Pereira, João A. P. Coutinho, Sónia P. M. Ventura, and Rute A. S. Ferreira. 2022. "Uncovering the Use of Fucoxanthin and Phycobiliproteins into Solid Matrices to Increase Their Emission Quantum Yield and Photostability" Applied Sciences 12, no. 12: 5839. https://doi.org/10.3390/app12125839






